Abstract
Wnt signaling is essential for the regulation of cell polarity and cell fate in the early embryogenesis of many animal species. Multiple Wnt genes and its pathway members are expressed in the mouse early embryo, raising the question whether they play any roles in preimplantation development. Dishevelled is an important transducer of divergent Wnt pathways. Here we show that three of the mouse Dishevelled proteins are not only expressed in oocytes and during preimplantation development, but also display distinct spatio-temporal localization. Interestingly, as embryos reach blastocyst stage, Dishevelled 2 becomes increasingly associated with cell membrane in trophectoderm cells, while at E4.5, Dishevelled 3 is highly enriched in the cytoplasm of ICM cells. These changes are coincident with an increase in the active form of β-catenin, p120catenin transcription and decrease of Kaiso expression, indicating an upregulation of Wnt signaling activity before implantation. When Dishevelled-GFP fusion proteins are overexpressed in single blastomeres of the 4-cell stage embryo, the progeny of this cell show reduction in cell adhesiveness and a rounded shape at the blastocyst stage. This suggests that perturbing Dvl function interferes with cell-cell adhesion through the non-canonical Wnt pathway in blastocysts.
Keywords: Dishevelled, mouse, blastocyst, preimplantation, development
Introduction
Wnt signaling is one of the earliest signaling pathways activated to regulate the establishment of the basic embryo axis and cell fate decisions in many organisms (Brannon et al., 1997; Hobmayer et al., 2000; Liu et al., 1999). In the canonical Wnt pathway, the phosphorylation and degradation of cytoplasmic β-catenin is prevented in response to Wnt signals (Kishida et al., 1999). Increased levels of cytoplasmic β-catenin can then translocate into the nucleus and activate the transcription of Wnt responsive genes (Kishida et al., 1999). The non-canonical Wnt pathways have been shown to control cell polarity through regulating cell adhesion and oriented cell divisions (Gong et al., 2004; Moon et al., 1993). Dishevelled is an evolutionarily conserved multi-domain molecule responsible for routing the Wnt signal to the correct downstream cellular machineries (Boutros and Mlodzik, 1999; Wharton, 2003). It has been demonstrated that in early Xenopus and sea urchin embryos, the distribution of Dishevelled protein is important for the correct regional activation of Wnt signaling (Miller et al., 1999; Weitzel et al., 2004). In mammals, three Dishevelled genes have been identified. To distinguish them from the Dishevelled (Dsh) in non-mammals, they are abbreviated as Dvl (Sussman et al., 1994). The mRNA transcripts of all three Dvl genes are widely expressed during post-gastrulation mouse development (Klingensmith et al., 1996; Sussman et al., 1994; Tsang et al., 1996), however their expression pattern before gastrulation has not been characterized. Recently, several studies have reported that many Wnt ligands are expressed in mouse preimplantation embryos (Kemp et al., 2005; Lloyd et al., 2003; Mohamed et al., 2004b; Wang et al., 2004). To examine the activity of this divergent signaling pathway, we followed the expression and spatio-temporal localization of the three Dvl homologues throughout preimplantation development. We found Dvl proteins showed an interesting and distinct pattern of relocation from early to late blastocyst stage (embryonic day 3 to day 4 respectively). These changes were coincident with other signs of elevated Wnt signaling activity, such as an increase of dephosphorylated (active) β-catenin. Using real-time imaging, we found that overexpression of Dvl proteins perturbed cell adhesion properties at blastocyst stage. These results suggest that the Wnt pathways start to play a role as the blastocyst develops.
Experimental Procedures
Mouse embryo collection and culture
Oocytes and embryos for western blot and immunostaining were collected from F1 females mated with F1 males (both C57BL/6xCBA). GV stage oocytes were collected by puncturing the follicles of ovaries from 6-8 weeks old females. Metaphase II-arrested oocytes were released from ampullae from mice superovulated with intraperitoneal injection of 7.5 IU of pregnant mares serum gondotrophin (PMSG) followed 48 hours later by 7.5 IU of human chorionic gonadotrophin (hCG). Embryos were also obtained from superovulated females. The timing and method for collection were as follows: 4-cell and 8-cell embryos, by dissecting the oval duct at 56 hours and 64 hours post-hCG. E3.5, E4.25 and E4.5 blastocysts are flushed out from uterus using a 1 ml syringe and a G23 needle at 96, 114 and 120 hours post-hCG. All the collections are done in M2 medium. 4-cell embryos for microinjection and imaging experiments were cultured in KSOM.
RT-PCR
Reverse transcription reaction was carried out as described in (Brady and Iscove, 1993) using AMV-and MMLV-reverse transcriptase (Roche). The primers of mouse Dvl1-3, β-actin, Axin2 and the size of the respective PCR products are listed in table 1. PCR reactions were performed with Taq DNA polymerase (Roche) using the following parameters: first 10 cycles of 94°C denature (15 sec), 65-55°C touch-down annealing (30 sec) and 72°C extension (30 sec) followed by 25 cycles of 94°C denature (15 sec), 55°C annealing and 72°C extension (30 sec). The identities of the PCR products were confirmed by DNA sequencing (Biochemistry DNA seq. Facility, Cambridge,UK).
Table 1.
RT-PCR primers.
| Accession No. | Primers | Size | |
|---|---|---|---|
|
| |||
| mDvl1 | MMU10115 | Forward 5′- GCTACTATGTCTTTGGCGACCTGTG-3′ Reverse 5′-TGCTCTTGCTCCCTTCACTCTG-3′ |
249 |
| mDvl2 | MMU24160 | Forward 5′-GGCAGTGGCAGTGAGTCAGAAC-3′ Reverse 5′-GGGGTGGAGGCATCATAACTACC-3′ |
217 |
| mDvl3 | NM_007889 | Forward 5′-AGTCAGCACAGTGAAGGCAGTCG-3′ Reverse 5′-ATCAGCATCGGGGGACCATAGAGAG-3′ |
305 |
|
| |||
| Actin | NM_007393 | Forward 5′-CATCCGTAAAGACCTCTATGCCAAC-3′ Reverse 5′ CAAAGAAAGGGTGTAAAACGCAGC-3′ |
300 |
|
| |||
| Kaiso | AF097416 | Forward 5′- CAAGGTTGATGCTGGAAAAGAGC -3′ Reverse 5′- AAGTCTACTCAGAAAACTATGTCCCTGC - 3′ |
439 |
|
| |||
| p120 catenin |
NM_007615 | Forward 5′- TTCTTACCCACTGTTGACCCCC -3′ Reverse 5′- TTCCCTGTAGCCTTTCAGAGGTG -3′ |
251 |
Antibodies, Western Blot and Immunofluorescence
Antibodies used in this study are as follows. Dvl1 antibody (Rabbit polyclonal) was a gift from Dr. Mei (Luo et al., 2002). Dvl2 and 3 antibodies (rabbit polyclonal), Affiniti Research Products, Exeter, UK. The rabbit anti β-catenin polyclonal antibody and the monoclonal mouse anti active β-catenin antibody 8E7 were from Sigma and Upstate respectively. Clathrin antibody is from Abcam.
For western blots, oocytes and embryos were washed with phosphate-buffered saline (PBS) and lysed in the sample buffer (BioRad). Proteins were separated on 10 % polyacrylamide gels, electrotransfered onto nitrocellulose membranes, blotted with Dvl antibodies and subjected to ECL detection (Amersham Pharmacia Biotech).
For immunofluorescence, embryos were briefly exposed to acidic Tyrode’s solution to remove the zona pellucida. They were fixed in fixation buffer (130 mM KCl, 25 mM HEPES pH=6.9, 3 mM MgCl2, 4% paraformaldhyde, 0.15 % glutaraldehyde, 0.06 % Triton X-100) followed by permeabilization in PBS containing 0.2 % Triton X-100. They were then blocked with 3 % bovine serum albumin (BSA) in PBS 0.1% Tween-20 (PBST) at 4 °C overnight and incubated with an appropriate primary antibodies. DNA was stained with TOTO3 (Molecular Probes). Fluorescence was detected on a Bio-Rad MRC-1024 laser scanning confocal microscope with a 60x oil objective. Images were analyzed with IMARIS software. For every developmental stage, 3 independent experiments with 8 to 10 embryos in each experiment were examined.
For specificity control of Dvl2 and 3 antibodies, they were first incubated with their epitope peptides (50-fold excess) for 1 hour at 37 °C respectively, then used for western blot and immunofluorescence. The epitope peptide of Dvl2, CGAGRTGRPEERAPES corresponds to the amino acid 600-614 of mouse Dvl2 protein. The epitope peptide of Dvl3, CGSDRRKEKDPKAGDS corresponding to the amino acid 582-596 of mouse Dvl3 protein (Affiniti Research Products, Exeter, UK).
cDNA constructs, mRNA synthesis and microinjection
The Dvl1, 2 and 3-GFP fusion proteins were engineered by PCR. The DNA fragments of the coding region of Dvls were amplified from the cDNA in pBluescriptSK with T7 primer as the forward primer and reverse primer for Dvl1, 5′-CGGGTTAACCATGATGTCCACAAAGAACTCAC-3′; Dvl2, 5′-GGGGATCCCATAACATCCACAAAAAACTCAC-3′ and Dvl3, 5′-CGGGTTAACCATCACATCCACAAAGAACTCAC-3′. The stop codon of Dvl1 and 3 was substituted with an Hpa I restriction enzyme site, while for Dvl2, a Bam HI site was created to replace the stop codon. The DNA fragments were then digested with EcoR I and Hpa I/Bam HI restriction enzymes, and ligated into the EcoR I and Sma I/Bam HI site of the pRN3P MmGFP vector (Zernicka-Goetz et al., 1997). The cDNAs of the fusion proteins were sequenced to ensure the fidelity of the PCR amplification.
Capped RNA was transcribed in vitro as described in Na et al. (Na et al., 2003). Micro-injection embryos was performed on a Leica DM IRB inverted microscope equipped with Eppendorf micromanipulator and transinjector as described in Zernicka-Goetz et al (Zernicka-Goetz et al., 1997). After injection, embryos were cultured in KSOM medium at 37°C with 5% CO2 in air.
Live imaging experiments
For imaging the course of blastocyst formation, embryos were placed in a drop of KSOM (without phenol red) medium in a glass-bottomed dish (MatTek) covered with mineral oil. The dish was placed on the stage of a Zeiss Axiovert 200M microscope equipped with a 37°C incubator and supplied with 5% CO2 in air. Digital time-lapse images were acquired with a 20x Plan Apofluar objective (N/A 0.75) in both EGFP and transmitted light channels using a Hammamasu Orca ER CCD camera controlled by the AQM Advance 6 imaging software package (Kinetic Imaging).
For Concanavalin A staining, compacted 8-cell stage embryos were incubated in 20 μg/ml tetramethylrhodamine conjugated Concanavalin A (Molecular Probes) in M2 for 2 minutes, followed by washing in M2 several times. Live embryos were placed in M2 (without Phenol Red) in a glass-bottomed dish (MatTek) covered with mineral oil. Multi-channel (red/green/transmitted light), multi-section images were acquired on a Bio-Rad MRC-1024 laser scanning inverted confocal microscope with a 60x oil objective.
BAT-gal Wnt reporter transgenic mice and lacZ staining
BAT-gal Wnt reporter transgenic mice were obtained from Dr. Maretto (Maretto et al., 2003) and maintained as homozygous line on F1 background. For lacZ detection, blastocysts or dissected E5.5 and E6.25 embryos were fixed in 4% paraformaldehyde for 10 minutes at room temperature. Then they were incubated with X-gal solution. For E5.5 and E6.25 embryos, the reaction was stopped after 16 and 2 hours respectively after positive staining was visible. For embryos at the blastocyst stage, they were incubated for up to one week with changes of fresh substrate every 2 days.
Results
The Expression and Localization of Dvl proteins preimplantation mouse embryos
Three Dvl homologues (Dvl1-3) have been identified in the mouse (Klingensmith et al., 1996; Sussman et al., 1994; Tsang et al., 1996), and their transcripts have been detected by microarray studies in mouse oocytes and preimplantation embryos (Knowles et al., 2003; Wang et al., 2004). Using RT-PCR, we also detected the mRNA of all three Dvl homologues at these stages, confirming the microarray results (Fig. 1A). Our western blot analysis demonstrated that Dvl1-3 proteins are present in germinal vesicle (GV) and metaphase II (MII) oocytes. From the 8-cell to blastocyst stage, the amount of Dvl1 protein (85 kDa) showed a marked increase, while Dvl2 (95 kDa) remained constant. In contrast, we found a decline in Dvl3 (93kDa) protein between these two stages (Fig. 1B). To confirm the specificity of the antibodies used, we probed the blot with pre-immune rabbit serum, Dvl2 and 3 antibodies after absorption with their epitope peptides respectively. Specific bands of the three Dvl proteins were no longer present (Fig. 1B). Further verification of the specificity of the antibodies used in this study is shown in supplementary figure 1.
Figure 1.
Dvl expression in oocyte and preimplantation embryos. (A) RT-PCR of Dvl 1-3 in MII oocyte, zygote, 4-cell embryo and E3.5 blastocyst. The identities of the PCR products were confirmed by DNA sequencing. (B) Western blot of Dvl1-3 in GV, MII oocytes, 2-cell, 8-cell embryo and E3.5 blastocysts. Lysates from 200 embryos at indicated stages were loaded per lane. β-catenin is shown as a loading control. When Dvl1 pre-immune serum, Dvl2 and 3 Abs, after incubation with their epitope peptides, were used to probe the same blot, the specific bands of Dvl1, 2 and 3 were not present. (C) 8-cell and blastocyst stage embryos were immunostained for Dvl1 (a, d), Dvl2 (b, e) and Dvl3 (c, f) (all in red). DNA, blue. Note that Dvl1 and 2 localized to vesicle like structure (arrow heads in a and b). While in E3.25 blastocyst, Dvl1 shows more punctate staining (arrow head in d). In blastocysts, Dvl2 also distributed to the cell membrane (arrow head in e). (D) In 8-cell embryos, Dvl1 (similarly, Dvl2, data not shown) co-localizes with clathrin-coated vesicles (arrow head).
We next used confocal microscopy to examine the subcellular localization of Dvl proteins in preimplantation embryos. At the 8-cell stage, Dvl1 and 2 were both found associated with larger vesicle-like structures, mostly at the cell cortex (Fig. 1C a and b). Double immunostaining revealed that these vesicles also contained clathrin (Fig. 1D) When embryos reached the blastocyst stage, Dvl1 showed enhanced punctate staining in the trophectoderm and inner cell mass (ICM) cells (Fig. 1C d), while Dvl2 increasingly associated with plasma membranes (Fig. 2C e). In contrast to Dvl1 and 2, Dvl3 was diffusely distributed in the cytoplasm in both 8-cell and early blastocyst embryos (Fig. 1C c and f). Together, these results demonstrated that all three Dvl proteins are present throughout preimplantation development.
Figure 2.
Dvl protein distribution in blastocyst on embryonic day 4. A. Morphology of freshly flushed E4.0 expanded blastocyst (a) and E4.5 implanting blastocysts (b and c) B. a-c, E4.25 blastocysts, confocal middle section. d-f, E4.5 blastocysts, confocal middle section. g-i, E4.5 blastocysts, 3-D projection. Dvls, red. DNA, blue. MTE, mural trophectoderm. ICM, inner cell mass. Arrow heads indicate the specific accumulation of Dvl2 on the cell membrane and Dvl3 in the cytoplasm of the ICM cells. C. Enlargement of Dvl1, 2 and 3 subcellular localization in one blastomere. Arrow heads hightlight the cyplamic puncta and membrane concentration of Dvl proteins.
Changes in Dvl protein localization in the late blastocyst stage coincide with an increase of active β-catenin and alterations of the expression of Kaiso and p120catenin transcripts
At embryonic day four, mouse blastocysts become expanded and “hatch” from the zona pellucida (Fig. 2A a). Approximately half a day later, they will initiate implantation into the uterus (Fig. 2A b and c). We, and other groups, have observed that embryos often attach initially to uterus wall through association of the mural trophectoderm (MTE), or sometimes, one side of the embryo (Carson et al., 2000; Paria et al., 2002; Tan et al., 2005). This is reflected by the MTE cells becoming round and “bubbly”, (a sign of attachment and initiation of invasion into the uterus wall), earlier than the polar trophectoderm (PTE) cells (Fig. 2A b and c). We examined the distribution of Dvl proteins at these stages. Interestingly, Dvl2 showed an even stronger membrane localization, particularly in the invading MTE cells (Fig. 2B b, e and h), while Dvl3, on the other hand, displayed a markedly enhanced punctate localization in the cytoplasm of the ICM cells (Fig2. B c, f and i). Dvl proteins are known to translocate to the cell membrane and form cytoplasmic punctae in response to the stimuli of Wnt signals. Subsequently, they can inhibit the activity of the Axin/GSK3β/APC complex and prevent the phosphorylation and degradation of cytoplasmic β-catenin (Boutros and Mlodzik, 1999; Kishida et al., 1999; Wharton, 2003). We therefore examined the levels of β-catenin phosphorylation by western bloting. Indeed, a mouse monoclonal antibody that specifically recognizes the active form (dephosphorylated) of β-catenin detected a much stronger band in E4.5 than in E3.5 blastocysts, while the level of total β-catenin remained similar at these stages (Fig. 3A). At E4.5, the level of Dvl3 protein also showed a similar increase (Fig. 3A).
Figure 3.
(A) Western blot analysis showed increased levels of dephosphorylated β-catenin and Dvl3 proteins in E4.5 blastocysts. 120 embryos per lane. The stage is as listed. Arrows indicate bands for β-catenin (100 kDa) and Dvl3 (93 kDa). (B) lacZ staining of F1 E4.0 (a), BAT-gal E4.0 (b), E5.5 (c) and E6.25 (d) embryos, positive signal was only detected in the extraembryonic visceral endoderm at E5.5 and the site where future primitive streak is going to form at E6.25. (C) RT-PCR analysis of Kaiso and p120catenin expression. The identities of all the PCR products were confirmed by DNA sequencing.
To examine whether canonical Wnt pathway becomes active at this time point, we performed lacZ staining on E4.0 embryos from Wnt reporter transgenic mice (BAT-gal) (Maretto et al., 2003). However, we did not detect any lacZ signal even after prolonged incubation with the substrate (Fig. 3B a and b). The earliest lacZ staining appeared at the extraembryonic visceral endoderm at E5.5 (Fig. 3B c) and, at E6.25, a strong lacZ signal was detected at the site where the future primitive streak will form (Fig. 3B d). These findings are in agreement with the observations that the canonical Wnt pathway becomes active only shortly after implantation (Mohamed et al., 2004a). Recently, it has been reported that Kaiso, a methylation-dependent transcription repressor prevents the premature activation of Wnt target genes in Xenopus embryos (Park et al., 2005). This repression can be relieved by its binding to p120catenin (Park et al., 2005). To investigate the possibility that Kaiso may be involved in the repression of canonical Wnt signaling in preimplantation mouse embryos, we examined its expression by RT-PCR. This revealed that the transcript of Kaiso is highly expressed from the 4-cell to early blastocyst (E3.5) stage, but hardly detectable in late blastocysts (E4.5) (Fig. 3C), while the transcript of p120catenin showed an increase at E4.5 (Fig. 3C). These results suggest that the canonical Wnt pathway may be kept quiescent by Kaiso mediated transcription repression during preimplantation development. When embryos reach the late blastocyst stage, Wnt signaling activity begins to rise, resulting in the changes of Dvl protein localization and the increase of dephosphylated β-catenin.
Overexpression of Dvls perturbs cell adhesion and embryo polarity at blastocyst stage
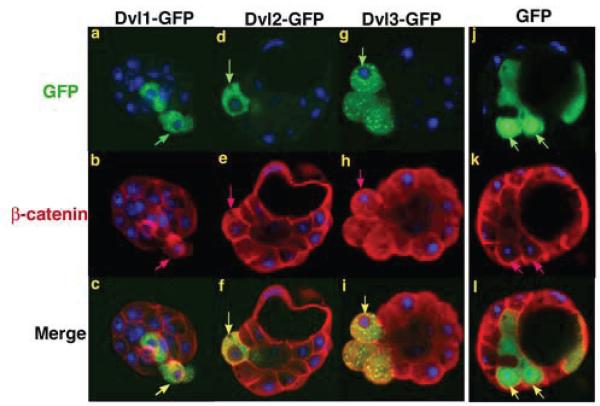
In the non-canonical Wnt pathway, Dvl proteins are involved in the regulation of cell polarity and cell adhesion functions (Fanto and McNeill, 2004). Overexpression of Dishevelled or its mutant form in Xenopus and Zebrafish gastrula embryos lead to defects in convergence extension movements (Gong et al., 2004; Wallingford et al., 2000). To examine whether perturbing Dvl function may affect cell adhesion during mouse preimplantation development, we ectopically overexpressed Dvls by injecting one random blastomere of the late 4-cell or early 8-cell stage embryos with Dvl1, Dvl2 or Dvl3-GFP mRNA. The injected embryos were then cultured to the blastocyst stage followed by fixation and examination by confocal microscopy. Dvl1, Dvl2 and Dvl3-GFP fusion proteins were found to distribute diffusely in the cytoplasm and accumulate in small vesicles in a manner similar to the localization of the endogenous proteins. Strikingly, blastomeres with high levels of either Dvl-GFP showed a more rounded morphology and tended to protrude from the embryo (Fig. 4 a, d and g)., in some cases, even became excluded from the embryo (Fig. 4 a). Moreover, β-catenin was found to be enriched in the cytoplasm and translocated into the nuclei in extreme cases (12/25 embryos showed increased cytoplasmic β-catenin, while 3/25 had nuclear accumulation of β-catenin) (Fig. 4 b, c, e, f, h and i). This indicates a marked change in cell adhesion properties. In contrast, in control embryos injected only with GFP mRNA, none of the green blastomeres showed abnormal β-catenin localization compared to neighbouring cells (n=20, from 3 independent experiments) (Fig. 4 j-l). To examine whether the overexpression of Dvl-GFPs can activate canonical Wnt pathway in the pre-implantation mouse embryos, we carried out similar micro-injection experiment on individual 4-cell blastomeres of BAT-gal Wnt reporter embryos. Upon microinjection, the embryos were cultured to the blastocyst stage, fixed and subjected to lacZ staining. We found that even after prolonged incubation with the substrate, no lacZ signal was detected in any embryo (10 –15 embryos in each group from two independent experiments) (Fig. 5). Thus, we conclude that the rounding of the cell expressing high level of Dvl-GFP is not due to the activation of canonical pathway.
Figure 4.
Overexpression of Dvl proteins in preimplantation embryos led to blastomere extrusion and the accumulation of β-catenin in the cytoplasm. Dvl1, 2 or 3-GFP were microinjected into one blastomere of late 4-cell or early 8-cell embryos. These embryos were cultured to blastocyst stage and stained for β-catenin. Dvl-GFPs, green; β-catenin, red; DNA, blue. Blastomeres with high level of Dvl-GFPs also showed increased β-catenin in the cytoplasm and sometimes in the nucleus (c, arrows). Note that the blastomeres with high levels of Dvl-GFP are more rounded (a-i, arrows). They also tend to bulge out from the rest of the embryo.
Figure 5.
Overexpression of Dvl1-3-GFP did not activate canonical Wnt pathway in blastocysts. A. mRNAs encoding GFP (a) or Dvl1-3-GFP (b, c and d respectively) were injected into one blastomere of 4-cell embryos from BAT-gal Wnt reporter mice. B. The injected embryos were cultured to blastocyst stage and staining for lacZ. No signal was detected in all cases even after one-week incubation with the substrate.
We then attempted to ascertain when in development high levels of Dvl experession began to affect the adhesive properties of the cell. One possibility is that overexpression of Dvls could affect embryo compaction at the 8-cell stage. In this event the injected blastomere and its progeny should remain with a round shape until the blastocyst stage. Alternatively, Dvl could interfere with cell adhesion at later stages to cause such a phenotype. We therefore first examined the compaction process in embryos injected with Dvl-GFPs during the 8-cell stage. We found that non-injected control blastomeres and blastomeres showing high levels of Dvl-GFP proteins lost their spherical shape equally and compacted (30/30 embryos from 3 independent experiments). To verify whether the polarization of these blastomeres was normal, we stained embryos with Rhodamine conjugated concanavaline A, which specifically binds to the apical microvilli (Johnson and Ziomek, 1981). Concanavaline A displayed similar apical concentration in blastomeres with or without high levels of Dvl-GFPs (Fig. 6) (20/20 embryos from 3 independent experiments). This suggests that the round appearance of the blastomeres resulting from overexpression of Dvl is not due to compaction failure or defects in the generation of cell polarity, but must develop at a later developmental stage. To determine when this might be, we used time-lapse video recording to monitor the course of blastocyst formation in embryos with clones of cells expressing high levels of Dvl-GFP. In embryos injected with GFP mRNA alone, the outer layer of cells showing strong GFP expression became flattened upon the expansion of the blastoceol. Moreover, the GFP expressing clones were coherent throughout the process (20/20 embryos from 2 independent experiments) (Fig. 7A). In contrast, cells expressing high levels of Dvl-GFP proteins were more rounded. Some cells comprising the blastocoel wall failed to flatten or were excluded into the blastocoel after division (Fig. 7B). In another example, cells of the Dvl-GFP clone became scattered upon the formation and the expansion of the blastocyst (Fig. 7C).
Figure 6.
Overexpression of Dvl-GFPs does not affect compaction. GFP (A) or Dvl1, 2 ,3-GFP (B) mRNA were microinjected into one blastomere of late 4-cell or early 8-cell embryos. They were cultured for further 6 hours when non-injected sibling embryos were compacted. Embryos were then briefly exposed to Rhodamine conjugated Concanavalin A and subjected to confocal live imaging. Concanavalin A staining is shown in red, Dvl-GFPs is in green and transmitted light channel is in blue. Arrow head (A) and arrows (B) indicate the apical staining of microvilli.
Figure 7.
Overexpression of Dvl-GFPs perturbs cell flattening and adhesion in blastocyst. Late 4-cell or early 8-cell embryos were injected in one blastomere with GFP or Dvl-GFPs and cultured for 24 hours (by which time the uninjected sibling embryos started to form blastocyst). Embryos were then subject to multi-channel, multi-section and time-lapse filming for 8 hours to record the process of blastocyst formation. Both GFP channel and brightfield channel images were shown. Time is as indicated. (A) Embryos injected with GFP formed normal blastocyst. (B and C) Blastomeres expressing Dvl-GFPs are more rounded. One blastomere failed to flatten (B, arrow head), another was excluded into the blastocoel (B, arrow). In C, the progeny of Dvl-GFP expressing blastomeres became scattered.
Thus, the above data suggests that high concentration of Dvl proteins leads to a reduction in cell adhesiveness and subsequently cell rounding.
Discussion
Dishevelled proteins indicate a change in Wnt signalling activity in preimplantation mouse embryos
In this study, we found that three mouse Dvl proteins are present in oocytes and preimplantation embryos and show a dynamic pattern of localization. The levels of both Dvl1 and 2 proteins increase from the 8-cell to blastocyst stage. Although the level of Dvl3 protein decline initially, it increases in E4.5 blastocysts. At the 8-cell stage Dvl 1 and 2 localize to larger cytoplasmic vesicles containing clathrin. This is in agreement with Wnts binding to the cell surface 7 transmembrane spanning receptor, Frizzled and undergoing endocytosis by clathrin coated vesicles which also contains Dvls (Chen et al., 2003). When embryos reach the blastocyst stage, Dvls display significantly enhanced localization in cytoplasmic punctae and show association with cell membranes. Taken together these findings suggest the presence of Wnt ligands in early, preimplantation mouse embryos, although Wnts may not be signaling actively at this stage since there is very little dephosphorylated β-catenin. However, the significant change of distribution of Dvls in blastocysts suggests that there is an upregulation of Wnt activity from this time point onwards, as increased cytoplasmic punctae and membrane localization associate with the activation of Wnt signaling (Capelluto et al., 2002; Chen et al., 2003; Ciani et al., 2004; Rothbacher et al., 2000; Torres and Nelson, 2000). In accordance with the behaviour of Dvls, we find that the amount of dephosphorylated β-catenin increases, while the expression of Kaiso, a transcriptional suppressor of Wnt responsive genes (Park et al., 2005), decreases significantly at the blastocyst stage. On the other hand, the canonical Wnt pathway is not active by the blastocyst stage, but only after implantation as revealed by lacZ staining of a BAT-gal Wnt reporter line. Our findings suggest that during early preimplantation development, Kaiso and other transcription repressors such as polycomb group of proteins may have a role in the repression of Wnt responsive genes (Lee et al., 2006), preventing them from being activated prematurely. Such inhibition may become attenuated at the late blastocyst stage to permit rapid growth and differentiation following implantation, while the magnitude of activation is not yet sufficient to lead to the expression of the Wnt reporter. This hypothesis is consistent with findings of an increasing number of Wnt ligands expressed in blastocysts; particularly, Wnt5a and Wnt11, which are up-regulated only in the late blastocyst in response to uterine factors (Lloyd et al., 2003; Mohamed et al., 2004b; Wang et al., 2004).
The specific enrichment of Dvl2 on trophectoderm cell membrane and Dvl3 in the ICM cells are particularly interesting. Dvl2 has been shown to be responsible for Wnt induced cell motility (Endo et al., 2005). During early pregnancy, Wnt4 is highly expressed in the uterine stroma (Paria et al., 2001). Canonical Wnt/β-catenin signaling becomes activated in the uterus from E4.0 (Mohamed et al., 2005). Thus, the strong membrane localization of Dvl2 in the outgrowing trophectoderm cells could be due to both the uterine and embryonic Wnt activity and may facilitate the invasion of trophectoderm cells into the uterus. During early mouse development, β-catenin function is required in the epiblast for the establishment of anterior-posterior axis and the induction of the mesoderm (Huelsken et al., 2000). The high level of Dvl3 protein in the ICM could stabilize cytoplasmic β-catenin and facilitate its signal tranduction function. Thus, it is possible that Dvl3 plays a role in the modification of cytoplasmic level of β-catenin in the ICM cells of peri-implantation embryos. This may be crucial for the subsequent development and differentiation of the ICM cell following implantation.
In early Xenopus and sea urchin embryos, the polarized distribution of Dishevelled protein predicts the future body axis (Miller et al., 1999; Weitzel et al., 2004). However, in mouse pre-implantation embryos, we did not observe asymmetrical distribution of Dvls that can be related to this. Thus, the early mouse embryo may use a different mechanism to set up its axial asymmetry that does not involve Dvls.
We have used RNAi to knockdown Dvl1-3, however, this did not cause any obvious abnormalities in the preimplantation development, although we observed earlier defects than Dvl1 and 2 double knockout mutants in postimplantation embryos (Soares et al., 2005). This is possibly due to the presence of the maternal proteins and other compensatory mechanisms at this period of development. Thus, whether or not Dvl plays a critical role at these early stages cannot be fully assessed without eliminating maternal provision of Dvl proteins.
Dvl protein may participate in the regulation of cell adhesion in early mouse embryos
In addition to their roles in the canonical Wnt signaling pathway, Dvls are also important regulators of cell polarity and cell adhesion through non-canonical Wnt pathways (Fanto and McNeill, 2004; Moon et al., 1993). In our experiments, overexpression Dvl-GFP fusion proteins in one blastomere of 4-cell stage embryos could lead to a dramatic change in the morphology and adhesion properties of its progeny: they show a more rounded shape and form less coherent clones in blastocysts. Although this is accompanied by marked increase of cytoplasmic β-catenin in the progeny of the injected blastomere, high levels of cytoplasmic Dvl-GFP do not activate the canonical pathway, judging by absence of any lacZ signal in BAT-gal Wnt reporter embryos. At least two explanations can be considered to account for this phenotype. First, a high concentration of Dvl may activate RhoA (Habas et al., 2001) and change the organization of cytoskeleton, thus affect the cell shape. Second, β-catenin has been proposed to exist in different subcellular pools (bound to membrane E-cadherin and in the cytoplasm and nucleus), the proportions being regulated by competitive interactions (Gottardi and Gumbiner, 2001). As an increased level of Dvl leads to an accumulation of cytoplasmic β-catenin, this suggests a change in the balance between the cytoplasmic pool and membrane bound pool of β-catenin that in turn would attenuate cell-cell adhesion at the blastocyst stage. Thus, it is possible to imagine how perturbation of both of the above pathways could contribute to the cell adhesion defect phenotype we observed in blastocysts.
Our study opens up several new questions. For example, does the spatio-temporal change of localization of the different Dvl proteins we report here correlate with the activities of different Wnts and Wnt antagonists recently shown to display distinct localizations in mouse blastocysts (Kemp et al., 2005)? What causes the upregulation of Wnt activity at late blastocyst stage; what is the molecular machinery involved; and what is its influence on postimplantion development? Finding answers to these questions will help to unravel the complex signalling networks that pattern the early mouse embryo and specify cell fate decisions.
Supplementary Material
Supplementary Figure 1. (A) Dvl homologue-specific antibodies do not cross-react. Xenopus embryos injected with Dvl1-GFP, Dvl2-GFP and Dvl3 separately were used for western blot by Dvl antibodies. Protein equivalent to 1/4 of Xenopus embryo was loaded per lane. Dvl1 antibody only recognized bands corresponding to the size of Dvl1-GFP fusion protein (125 kDa) and possibly partially degraded fusion protein but not other Dvl proteins. Similar results were obtained with Dvl2 antibody, which only revealed Dvl2-GFP (135 kDa) and some partially degraded Dvl2-GFP fusion protein. Dvl3 antibody specifically revealed a band of 93 kDa corresponding to Dvl3 protein.
(B) Negative controls for Dvl immunofluorescence. Pre-immune serum of Dvl1, Dvl2 and 3 antibodies after absorption with their epitope peptides did not show any staining in E4.25 blastocysts (a-c).
(C) Positive control for Dvl1-3 antibodies. mRNA encoding Dvl1-3 GFP were injected into one blastomere of 4-cell embryo, they were cultured for further 24 hours, fixed and stained using Dvl1-3 antibodies. The GFP fluorescence co-localizes with enhanced antibody staining in all Dvl-GFP positive embryos (5 embryos examined in each group). Green, GFP; red, Dvl staining; blue, DNA. Arrows highlight co-localizations.
Acknowledgements
We are grateful to the Wellcome Trust for Senior Research Fellowship and BBSRC grant to MZG which both supported this work. Jie Na is supported by the Royal Society Relocation Fellowship in 2006. We thank Daniel Sussman for mouse Dishevelled 1, 2 and 3 cDNA, Lin Mei for Dvl1 antibody, Randall Moon for initial discussion of this project, Jerome Jullien and Ilenia Simeoni in John Gurdon’s lab for assistance in injection of Xenopus embryos.
References
- Boutros M, Mlodzik M. Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech Dev. 1999;83:27–37. doi: 10.1016/s0925-4773(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Brady G, Iscove NN. Construction of cDNA libraries from single cells. Methods Enzymol. 1993;225:611–23. doi: 10.1016/0076-6879(93)25039-5. [DOI] [PubMed] [Google Scholar]
- Brannon M, Gomperts M, Sumoy L, Moon RT, Kimelman D. A beta-catenin/XTcf-3 complex binds to the siamois promoter to regulate dorsal axis specification in Xenopus. Genes Dev. 1997;11:2359–70. doi: 10.1101/gad.11.18.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelluto DG, Kutateladze TG, Habas R, Finkielstein CV, He X, Overduin M. The DIX domain targets dishevelled to actin stress fibres and vesicular membranes. Nature. 2002;419:726–9. doi: 10.1038/nature01056. [DOI] [PubMed] [Google Scholar]
- Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K. Embryo implantation. Dev Biol. 2000;223:217–37. doi: 10.1006/dbio.2000.9767. [DOI] [PubMed] [Google Scholar]
- Chen W, ten Berge D, Brown J, Ahn S, Hu LA, Miller WE, Caron MG, Barak LS, Nusse R, Lefkowitz RJ. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science. 2003;301:1391–4. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- Ciani L, Krylova O, Smalley MJ, Dale TC, Salinas PC. A divergent canonical WNT-signaling pathway regulates microtubule dynamics: Dishevelled signals locally to stabilize microtubules. J Cell Biol. 2004;164:243–53. doi: 10.1083/jcb.200309096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y, Wolf V, Muraiso K, Kamijo K, Soon L, Uren A, Barshishat-Kupper M, Rubin JS. Wnt-3a-dependent cell motility involves RhoA activation and is specifically regulated by dishevelled-2. J Biol Chem. 2005;280:777–86. doi: 10.1074/jbc.M406391200. [DOI] [PubMed] [Google Scholar]
- Fanto M, McNeill H. Planar polarity from flies to vertebrates. J Cell Sci. 2004;117:527–33. doi: 10.1242/jcs.00973. [DOI] [PubMed] [Google Scholar]
- Gong Y, Mo C, Fraser SE. Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature. 2004;430:689–93. doi: 10.1038/nature02796. [DOI] [PubMed] [Google Scholar]
- Gottardi CJ, Gumbiner BM. Adhesion signaling: how beta-catenin interacts with its partners. Curr Biol. 2001;11:R792–4. doi: 10.1016/s0960-9822(01)00473-0. [DOI] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–54. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- Hobmayer B, Rentzsch F, Kuhn K, Happel CM, von Laue CC, Snyder P, Rothbacher U, Holstein TW. WNT signalling molecules act in axis formation in the diploblastic metazoan Hydra. Nature. 2000;407:186–9. doi: 10.1038/35025063. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W. Requirement for beta-catenin in anterior-posterior axis formation in mice. J Cell Biol. 2000;148:567–78. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Ziomek CA. The foundation of two distinct cell lineages within the mouse morula. Cell. 1981;24:71–80. doi: 10.1016/0092-8674(81)90502-x. [DOI] [PubMed] [Google Scholar]
- Kemp C, Willems E, Abdo S, Lambiv L, Leyns L. Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Developmental Dynamics. 2005 doi: 10.1002/dvdy.20408. Article online in advance of print. [DOI] [PubMed] [Google Scholar]
- Kishida S, Yamamoto H, Hino S, Ikeda S, Kishida M, Kikuchi A. DIX domains of Dvl and axin are necessary for protein interactions and their ability to regulate beta-catenin stability. Mol Cell Biol. 1999;19:4414–22. doi: 10.1128/mcb.19.6.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingensmith J, Yang Y, Axelrod JD, Beier DR, Perrimon N, Sussman DJ. Conservation of dishevelled structure and function between flies and mice: isolation and characterization of Dvl2. Mech Dev. 1996;58:15–26. doi: 10.1016/s0925-4773(96)00549-7. [DOI] [PubMed] [Google Scholar]
- Knowles BB, Evsikov AV, de Vries WN, Peaston AE, Solter D. Molecular control of the oocyte to embryo transition. Philos Trans R Soc Lond B Biol Sci. 2003;358:1381–7. doi: 10.1098/rstb.2003.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, Chevalier B, Johnstone SE, Cole MF, Isono K, Koseki H, Fuchikami T, Abe K, Murray HL, Zucker JP, Yuan B, Bell GW, Herbolsheimer E, Hannett NM, Sun K, Odom DT, Otte AP, Volkert TL, Bartel DP, Melton DA, Gifford DK, Jaenisch R, Young RA. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell. 2006;125:301–13. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–5. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Lloyd S, Fleming TP, Collins JE. Expression of Wnt genes during mouse preimplantation development. Gene Expr Patterns. 2003;3:309–12. doi: 10.1016/s1567-133x(03)00046-2. [DOI] [PubMed] [Google Scholar]
- Luo ZG, Wang Q, Zhou JZ, Wang J, Luo Z, Liu M, He X, Wynshaw-Boris A, Xiong WC, Lu B, Mei L. Regulation of AChR clustering by Dishevelled interacting with MuSK and PAK1. Neuron. 2002;35:489–505. doi: 10.1016/s0896-6273(02)00783-3. [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JR, Rowning BA, Larabell CA, Yang-Snyder JA, Bates RL, Moon RT. Establishment of the dorsal-ventral axis in Xenopus embryos coincides with the dorsal enrichment of dishevelled that is dependent on cortical rotation. J Cell Biol. 1999;146:427–37. doi: 10.1083/jcb.146.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed OA, Clarke HJ, Dufort D. beta-catenin signaling marks the prospective site of primitive streak formation in the mouse embryo. Dev Dyn. 2004a;231:416–24. doi: 10.1002/dvdy.20135. [DOI] [PubMed] [Google Scholar]
- Mohamed OA, Dufort D, Clarke HJ. Expression and estradiol regulation of Wnt genes in the mouse blastocyst identify a candidate pathway for embryo-maternal signaling at implantation. Biol Reprod. 2004b;71:417–24. doi: 10.1095/biolreprod.103.025692. [DOI] [PubMed] [Google Scholar]
- Mohamed OA, Jonnaert M, Labelle-Dumais C, Kuroda K, Clarke HJ, Dufort D. Uterine Wnt/beta-catenin signaling is required for implantation. Proc Natl Acad Sci U S A. 2005;102:8579–84. doi: 10.1073/pnas.0500612102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RT, DeMarais A, Olson DJ. Responses to Wnt signals in vertebrate embryos may involve changes in cell adhesion and cell movement. J Cell Sci Suppl. 1993;17:183–8. doi: 10.1242/jcs.1993.supplement_17.26. [DOI] [PubMed] [Google Scholar]
- Na J, Marsden M, DeSimone DW. Differential regulation of cell adhesive functions by integrin alpha subunit cytoplasmic tails in vivo. J Cell Sci. 2003;116:2333–43. doi: 10.1242/jcs.00445. [DOI] [PubMed] [Google Scholar]
- Paria BC, Ma W, Tan J, Raja S, Das SK, Dey SK, Hogan BL. Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc Natl Acad Sci U S A. 2001;98:1047–52. doi: 10.1073/pnas.98.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paria BC, Reese J, Das SK, Dey SK. Deciphering the cross-talk of implantation: advances and challenges. Science. 2002;296:2185–8. doi: 10.1126/science.1071601. [DOI] [PubMed] [Google Scholar]
- Park JI, Kim SW, Lyons JP, Ji H, Nguyen TT, Cho K, Barton MC, Deroo T, Vleminckx K, McCrea PD. Kaiso/p120-catenin and TCF/beta-catenin complexes coordinately regulate canonical Wnt gene targets. Dev Cell. 2005;8:843–54. doi: 10.1016/j.devcel.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Rothbacher U, Laurent MN, Deardorff MA, Klein PS, Cho KW, Fraser SE. Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. Embo J. 2000;19:1010–22. doi: 10.1093/emboj/19.5.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares ML, Haraguchi S, Torres-Padilla ME, Kalmar T, Carpenter L, Bell G, Morrison A, Ring CJ, Clarke NJ, Glover DM, Zernicka-Goetz M. Functional studies of signaling pathways in peri-implantation development of the mouse embryo by RNAi. BMC Dev Biol. 2005;5:28. doi: 10.1186/1471-213X-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. (A) Dvl homologue-specific antibodies do not cross-react. Xenopus embryos injected with Dvl1-GFP, Dvl2-GFP and Dvl3 separately were used for western blot by Dvl antibodies. Protein equivalent to 1/4 of Xenopus embryo was loaded per lane. Dvl1 antibody only recognized bands corresponding to the size of Dvl1-GFP fusion protein (125 kDa) and possibly partially degraded fusion protein but not other Dvl proteins. Similar results were obtained with Dvl2 antibody, which only revealed Dvl2-GFP (135 kDa) and some partially degraded Dvl2-GFP fusion protein. Dvl3 antibody specifically revealed a band of 93 kDa corresponding to Dvl3 protein.
(B) Negative controls for Dvl immunofluorescence. Pre-immune serum of Dvl1, Dvl2 and 3 antibodies after absorption with their epitope peptides did not show any staining in E4.25 blastocysts (a-c).
(C) Positive control for Dvl1-3 antibodies. mRNA encoding Dvl1-3 GFP were injected into one blastomere of 4-cell embryo, they were cultured for further 24 hours, fixed and stained using Dvl1-3 antibodies. The GFP fluorescence co-localizes with enhanced antibody staining in all Dvl-GFP positive embryos (5 embryos examined in each group). Green, GFP; red, Dvl staining; blue, DNA. Arrows highlight co-localizations.