Abstract
Objectives
Physical exercise has beneficial effects on cardiovascular risk factors. Knowledge about the effect of exercise intensity, specifically walking speed, on cardiovascular risk factors is limited. We report the relationship between walking speed and changes in cardiovascular risk factors in participants of a 12-day walking tour to Santiago de Compostela.
Design
Prospective cohort study.
Setting
Single-centre study with healthy middle-aged volunteers.
Participants
Healthy middle-aged men (n=15) and women (n=14). Subjects using lipid-lowering medication were excluded.
Intervention
Participants walked 281±10 km of the classical route to Santiago de Compostela in 12 days in 2009.
Primary and secondary outcome measures
Walking speed was recorded and blood pressure, weight, waist circumference, lipids and glucose were measured every other day. Changes in risk factors were compared between gender-pooled groups with faster and slower walking speed. Second, the relationship between walking speed and changes in risk factors was quantified using a linear mixed effects model.
Results
In the faster walking speed (4.6±0.2 km/h) group, high-density lipoprotein cholesterol (HDL-c) increased more than in the slower walking speed (4.1±0.2 km/h) group (difference in change between groups: 0.20; 95% CI −0.02 to 0.42 mmol/l), while low-density lipoprotein cholesterol (LDL-c) and total cholesterol decreased more in the slower walking speed group (differences in changes between groups: LDL-c: −0.50; 95% CI −0.88 to −0.12 mmol/l and total cholesterol: −0.75; 95% CI −1.19 to −0.31 mmol/l). A 1 km/h higher walking speed was related to an increase in HDL-c (0.24; 95% CI 0.12 to 0.30 mmol/l), LDL-c (0.18; 95% CI −0.16 to 0.42 mmol/l) and total cholesterol (0.36; 95% CI 0.12 to 0.60 mmol/l), adjusted for age, gender, smoking, body mass index and heart rate, during the whole walking tour.
Conclusions
Walking the same distance faster improves HDL-c more, while LDL-c and total cholesterol decrease more with lower walking speed independent of changes in body weight in healthy middle-aged subjects.
Article summary
Article focus
Physical exercise has beneficial effects on cardiovascular risk factors; however, the knowledge about the effect of exercise intensity, specifically walking speed, on cardiovascular risk factors is limited.
We report the relationship between walking speed and changes in cardiovascular risk factors in participants of a 12-day walking tour to Santiago de Compostela.
Key messages
- In subjects walking a 12-day walking tour to Santiago de Compostela, with long daily stages:
- walking the same distance with higher walking speed was related to a higher increase in HDL-c, while walking with lower walking speed was related to larger decreases in LDL-c and total cholesterol, adjusted for age, gender, smoking, body mass index and heart rate.
- there was no relationship between walking speed and changes in weight, waist circumference, blood pressure, triglycerides or glucose.
Strengths and limitations of this study
All subjects walked the same overall distance, walking speed was measured and measurements of cardiovascular risk factors were conducted every other day.
This is a small study with 29 participants walking 281 km in 12 days. Whether the results of this study can be extrapolated to less exercise, and other types of exercise is not known.
Introduction
Exercise has an inverse dose–response relationship with all-cause mortality and is related to a lower risk of cardiovascular disease and type 2 diabetes.1 2 An important part of these long-term beneficial effects of exercise is caused by improvement of classical cardiovascular risk factors as physical activity lowers body weight, lowers blood pressure, decreases insulin resistance and glucose intolerance, lowers plasma triglycerides and increases high-density lipoprotein cholesterol (HDL-c).3 For these reasons, physical exercise is widely recommended in guidelines for treatment and prevention of cardiovascular diseases.4–6 Guidelines recommend a minimum weekly physical activity equal to 150 min of brisk walking; however, it is not specified at what intensity this exercise should be preferably conducted.4–6 Brisk walking or shorter periods of exercise at a higher intensity (eg, running) are considered equally effective.4–6 However, the results from studies evaluating the effects of exercise intensity on cardiovascular risk factors are conflicting. Several randomised clinical trials report no differences between various intensities of exercise and conclude that the total amount of exercise is more important than exercise intensity.7–10 Other studies conclude that exercise at a higher intensity results in more beneficial changes in cardiovascular risk factors compared with exercise at a lower intensity,11–15 although not all studies adequately control for differences in the total amount of exercise.12 13
Walking is one of the most accessible forms of physical exercise and is, together with gardening, the major component of leisure time physical activity.16 Walking speed is an easy parameter to express exercise intensity and can be measured outside a laboratory with limited resources. Results from large epidemiologic studies show a relationship between increased walking speed and a decreased risk for cardiovascular disease and diabetes.17–20 However, in these studies, the walking speed was not measured but assessed using questionnaires where study participants estimated their usual walking speed in broad categories such as ‘easy’, ‘average’ or ‘brisk’. Furthermore, these studies did not evaluate the effects of walking speed on cardiovascular risk factors.
In the Santiago study, 29 healthy middle-aged men and women walked an equal distance consisting of 281 km at their own individual preferred speed during 12 days in Spain.21 Marked inter-individual differences in changes in cardiovascular risk factors were observed, predominantly in plasma lipids.21 In the present study, we evaluated the influence of the measured walking speed on changes in plasma lipids, blood pressure, weight, waist circumference and glucose.
Subjects and methods
Subjects and exercise
Healthy male and female participants between 40 and 70 years of age were recruited by an announcement in the magazine of the Dutch Saint James Fellowship. The cohort size of 30 participants was based on a sample size calculation to detect a difference in endothelial function in the original Santiago study.21 Subjects diagnosed as having diabetes mellitus, uncontrolled hypertension or a history of cardiovascular disease were excluded, as well as subjects using lipid-lowering medication. There were 49 subjects responding to the advertisement and applied for participation in the intervention group of the Santiago study. One subject was not eligible because of a history of diabetes mellitus, and one subject was not eligible because of uncontrolled hypertension (systolic blood pressure >170 mm Hg). From the remaining 47 eligible subjects, the first 15 men and 15 women were recruited for participation. After signing informed consent form, but before start of the intervention period, one female subject ended participation for personal reasons. The design of the SANTIAGO study is described in more detail elsewhere.21 Briefly, the SANTIAGO study is a non-randomised intervention study on the immediate and longer term effects of long daily periods of walking on vascular function and cardiovascular risk factors. Participants already intended to walk part of the Santiago de Compostela pilgrimage. The intervention consisted of walking part of the Camino Francés, the classical pilgrimage route to Santiago de Compostela,22 from 28th June to 10th July 2009, and covering 281 km between Hospital de Órbigo and Santiago de Compostela in Spain. Mean daily walked distance was 23±1 km, mean daily walked time 5.39±0.36 h and mean steps per day 31 058±2154. All participants completed the 12-day walking tour. For the present study, the data of the 29 persons (15 men, 14 women) in the intervention group were used. The SANTIAGO study was approved by the Medical Ethics Committee of the UMC Utrecht. All participants gave written informed consent before inclusion.
Measurement of walking speed
All participants used a diary to record their exact time of departure, time of arrival and resting time, and the daily walking time was calculated. Participants walked at their individually preferred speed and were unaware that the effects of their walking speed would become subject of evaluation. All participants carried a pedometer (Digiwalker SW-200; Yamax USA Inc., San Antonio, TX, USA), measuring the number of steps daily. The participants were instructed to wear the pedometer at their belt or waistband at the left or right side of the body. From these data, the walking speed was calculated in kilometres per hour by dividing the total distance covered during the study by the total walking time without including the resting time. Walking speed was also expressed in steps per hour by dividing the total number of steps by the total walking time.
Measurement of cardiovascular risk factors
Measurements were conducted in Spain, at the start, after arrival and at every other day in between during the walking tour. All measurements were conducted in the fasted state, before the start of the walking distance that day. Measurements included weight, waist circumference and blood pressure. Weight was measured without shoes on the same balance during the whole study. Waist circumference was measured in standing position with a tape measure just above the iliac spine. Blood pressure was calculated as the mean of three recordings in seated position at the arm with the highest value at the baseline visit, using an automated blood pressure device (Omron 705 IT; Hoofddorp, The Netherlands). Furthermore, blood was obtained with a finger prick, for immediate analysis of total cholesterol, HDL-c, triglycerides and glucose with a portable LDX analyser (Cholestech Corporation, Hayward, CA, USA). Low-density lipoprotein cholesterol (LDL-c) was calculated. No information about dietary intake at baseline or during the study was obtained. Participants were not instructed on their diet.
Data analyses
Continuous variables are expressed as mean±SD when normal distributed and as median (IQR) in case of skewed distribution. Categorical variables are expressed as percentage (%). To analyse the role of walking speed on the change in cardiovascular risk factors, we first compared the changes in cardiovascular risk factors between participants walking with faster speed and participants walking with slower speed. As there is no generally accepted cut-off point for faster or slower walking speed, the study population was divided based on median walking speed, which also has the advantage of creating groups of equal size. To prevent over-representation of male participants in the high-speed group, initially men and women were classified separately as walking with faster or slower speed according to the median speed of their sex. Thereafter, men and women classified as faster walking speed were pooled in the faster walking speed group, and men and women classified as slower walking speed were pooled in the slower walking speed group.
Second, a linear mixed effects model was used. In this model, the relationship between walking speed and changes in cardiovascular risk factors was adjusted for differences in baseline values of cardiovascular risk factors (using a random intercept) and for changes in cardiovascular risk factors due to the progression of the walking tour (using a fixed time-dependent variable). To investigate the effect of walking speed, an interaction variable of walking speed and progression of the walking tour (represented by the fixed time-dependent variable) was added to the model. The β coefficients with 95% CIs of these interaction terms are reported, denoting the change in the specific risk factor per 2 days which is related to an increase in walking speed of 1 km/h or 1000 steps/h. In model I, the unadjusted relationship between walking speed and changes in cardiovascular risk factors during the walking tour is presented. In model II, adjustments were made for the potential confounding variables age and gender. In model III, additional adjustments were made for current smoking, heart rate at baseline as the best available measure for physical fitness and baseline body mass index (BMI). The main results are based upon this model. We conducted an exploratory analysis with additional adjustment for changes in body weight, to see if changes in body weight during the walking tour were in the causal pathway of the relationship between walking speed and changes in blood lipids. In a sensitivity analysis, we additionally adjusted model III for baseline characteristics with large differences between the low-speed group and the high-speed group: systolic and diastolic blood pressure, HDL-c, LDL-c and triglycerides.
For all analyses, SPSS V.15.0.1 was used.
Results
Baseline characteristics
The faster walking speed group consisted of eight men and seven women, 60.9±3.5 years old, who walked with an average speed of 4.6±0.2 km/h, while the slower walking speed group comprised seven men and seven women, 58.1±6.6 years old (p value for age between groups =0.17), with a mean walking speed of 4.1±0.2 km/h (p value for walking speed between groups <0.01) (table 1). The median speed of the men (n=8) in the faster walking speed group was 4.62 (IQR 4.57–4.92), of the women in the faster walking speed group (n=7) 4.52 (IQR 4.24–4.62), of the men in the slower walking speed group (n=7) 4.23 (IQR 4.01–4.33) and of the women in the slower walking speed group (n=7) 4.08 (IQR 3.94–4.10) km/h. Walking speed varied during the 12-day pilgrimage from 4.37 (IQR 4.21–4.80) to 5.01 (IQR 4.78–5.16) in the faster walking speed group and from 3.77 (IQR 3.50–4.07) to 4.30 (IQR 4.29–4.51) in the slower walking speed group. Both groups walked a similar overall distance (284±7 and 278±11 km, respectively, p=0.13). At baseline, the systolic and diastolic blood pressure (148±18/87±10 vs 138±8/81±9 mm Hg, p values, respectively, 0.16 and 0.11) and heart rate (69±10 vs 63±10 beats/min, p=0.14) were higher in the faster walking speed group compared with the slower walking speed group, and BMI was lower (24.2±2.2 vs 27.0±2.7 kg/m2, p<0.01). The baseline lipid profile was more favourable in the faster walking speed group than in the slower walking speed group (HDL-c 1.45±0.39 vs 1.24±0.36 mmol/l, p=0.14; LDL-c 3.4±0.5 vs 3.7±0.8 mmol/l, p=0.22 and triglycerides 1.1±0.5 vs 1.5±0.9 mmol/l, p=0.12, respectively).
Table 1.
Baseline characteristics for all participants and according to walking speed
| Faster walking speed group (n=15) | Slower walking speed group (n=14) | All participants (n=29) | |
| Mean walking speed (km/h) | 4.6±0.2 | 4.1±0.2 | 4.4±0.3 |
| Walking speed range (km/h) | 4.2–5.0 | 3.8–4.5 | 3.8–5.0 |
| Number of steps/h | 6309±582 | 5547±437 | 5941±639 |
| Total walking time (hours) | 62±3 | 68±3 | 65±4 |
| Total walking distance (km) | 284±7 | 278±11 | 281±10 |
| Male subjects | 8 (53%) | 7 (50%) | 15 (52%) |
| Age (years) | 60.9±3.5 | 58.1±6.6 | 59.5±5.3 |
| Current smoking | 3 (20%) | 2 (14%) | 5 (17%) |
| Systolic blood pressure (mm Hg) | 148±18 | 138±18 | 143±19 |
| Diastolic blood pressure (mm Hg) | 87±10 | 81±9 | 84±10 |
| Heart rate (beats/minute) | 69±10 | 63±10 | 66±11 |
| BMI (kg/m2) | 24.2±2.2 | 27.0±2.7 | 25.5±2.8 |
| Waist circumference (cm) | 88±10 | 92±11 | 90±10 |
| Glucose (mmol/l) | 5.2±0.6 | 5.2±0.4 | 5.2±0.5 |
| Total cholesterol (mmol/l) | 5.3±0.7 | 5.6±0.8 | 5.5±0.8 |
| LDL cholesterol (mmol/l) | 3.4±0.5 | 3.7±0.8 | 3.5±0.7 |
| HDL cholesterol (mmol/l) | 1.45±0.39 | 1.24±0.36 | 1.35±0.38 |
| Triglycerides (mmol/l) | 1.1±0.5 | 1.5±0.9 | 1.3±0.8 |
| Total cholesterol:HDL-c ratio | 3.8±1.0 | 5.0±2.1 | 4.4±1.7 |
| LDL-c:HDL-c ratio | 2.5±0.7 | 3.3±1.5 | 2.9±1.2 |
Baseline characteristics are shown according to walking speed and for all participants together. In order to avoid predominantly male subjects in the faster walking speed group, the faster walking speed group is gender pooled and consists of the eight men and seven women with a walking speed higher than the median speed for their gender.
BMI, body mass index; LDL, low-density lipoprotein; LDL-c, low-density lipoprotein cholesterol; HDL, high-density lipoprotein.
Changes in cardiovascular risk factors according to high or low walking speed
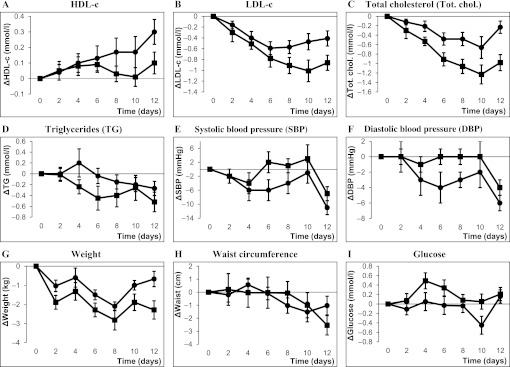
The whole study population together showed decreases in weight (−1.4±1.8 kg), waist circumference (−1.8±2.9 cm), LDL-c (−0.60±0.60 mmol/l), total cholesterol (−0.60±0.70 mmol/l), triglycerides (−0.39±0.58 mmol/l) and systolic (−9±9 mm Hg) and diastolic (−5±4 mm Hg) blood pressure during the walking tour, while HDL-c increased (0.20±0.30 mmol/l).21 Most of these changes were short lived; after 2 months, there was only a significant difference in change of weight (−2.0 kg; 95% CI −3.2 to −0.8) in the participants walking the pilgrimage compared with controls who did not walk the pilgrimage, while there were no differences in changes in the other cardiovascular risk factors between the groups.21 In figure 1A–I, the changes in cardiovascular risk factors for the faster and slower walking speed groups during the walking period are shown. The HDL-c in the faster walking speed group increased more than in the slower walking speed group (difference in change between the groups 0.20; 95% CI −0.02 to 0.42 mmol/l) (figure 1A). In the slower walking speed group, the decreases in LDL-c and total cholesterol were larger than in the faster walking speed group (differences in changes in LDL-c between the groups −0.50, 95% CI −0.88 to −0.12 and for total cholesterol −0.75, 95% CI −1.19 to −0.31) (figure 1B,C). Furthermore, weight decreased more in the slower walking speed group (difference in change between the groups −1.6, 95% CI −2.9 to −0.3 kg) (figure 1G). The decreases in blood pressure were larger in the faster walking speed group compared with the slower walking speed group, although this difference was not statistically significant (difference in change between the groups −4, 95% CI −11 to 3 mm Hg for systolic and −2, 95% CI −5 to 1 mm Hg for diastolic blood pressure).
Figure 1.
(A–I) Changes in cardiovascular risk factors during the walking tour according to walking speed. Changes in cardiovascular risk factors from baseline values during the walking tour for the faster walking speed group (–●–) and the slower walking speed group (–■–). Measurements were conducted at day 0 and every other day. Data are presented as mean with SEM.
The quantitative influence of walking speed on the change in cardiovascular risk factors
A 1 km/h higher walking speed is related to an increase in HDL-c of 0.04 mmol/l (95% CI 0.02 to 0.05) per 2 days walking (table 2). For the whole 12-day walking tour, the increase in HDL-c related to a 1 km/h higher walking speed is then six times 0.04 mmol/l (0.24 mmol/l, 95% CI 0.12 to 0.30). Furthermore, a 1 km/h higher walking speed is related to an increase in LDL-c of 0.03 (95% CI −0.01 to 0.07) mmol/l per 2 days walking and for total cholesterol, this is 0.06 (95% CI 0.02 to 0.10) mmol/l per 2 days walking. For the whole walking tour, a 1 km/h higher walking speed is related to a LDL-c increase of 0.18 mmol/l (95% CI −0.16 to 0.42) and an increase in total cholesterol of 0.36 mmol/l (95% CI 0.12 to 0.60) mmol/l. Lower or higher walking speed was not related to differences in blood pressure, weight, waist circumference, triglycerides or glucose (table 2).
Table 2.
The effect of walking speed in km/h on the changes per 2 days in cardiovascular risk factors
| HDL cholesterol | LDL cholesterol | Total cholesterol | Triglycerides | Systolic BP | |
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| Model I | 0.03 (0.02 to 0.05)* | 0.02 (−0.02 to 0.06) | 0.05 (0.01 to 0.09)* | −0.02 (−0.06 to 0.03) | 0.03 (−0.74 to 0.80) |
| Model II | 0.04 (0.02 to 0.05)* | 0.02 (−0.02 to 0.06) | 0.05 (0.01 to 0.10)* | −0.01 (−0.06 to 0.03) | −0.07 (−0.84 to 0.70) |
| Model III | 0.04 (0.02 to 0.05)* | 0.03 (−0.01 to 0.07) | 0.06 (0.02 to 0.10)* | 0.00 (−0.05 to 0.04) | −0.07 (−0.85 to 0.70) |
| Diastolic BP | Weight | Waist circ. | Glucose | |
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| Model I | 0.01 (−0.43 to 0.45) | 0.06 (−0.06 to 0.18) | 0.15 (−0.25 to 0.56) | −0.01 (−0.05 to 0.03) |
| Model II | −0.01 (−0.45 to 0.42) | 0.05 (−0.07 to 0.18) | 0.07 (−0.33 to 0.47) | −0.02 (−0.06 to 0.02) |
| Model III | −0.03 (−0.47 to 0.41) | 0.06 (−0.06 to 0.19) | 0.18 (−0.21 to 0.57) | 0.00 (−0.04 to 0.04) |
The regression coefficient β (with 95% CI) denotes the mean change in the risk factor per 2 days which is associated with a 1 km/h higher walking speed. For example, a 1 km/h higher walking speed is associated with an increase in HDL cholesterol of 0.04 (95% CI 0.02 to 0.05) mmol/l (model III) per 2 days, translating to 0.24 (95% CI 0.12 to 0.30) mmol/l during the whole 12-day walking tour. Model I = crude, model II = age and gender and model III = age, gender, current smoking, BMI and heart rate at baseline.
p<0.05.
BMI, body mass index; BP, blood pressure; waist circ., waist circumference; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Similar analyses were performed with walking speed expressed in steps per hour instead of kilometres per hour, with similar results. A 1000 steps/h faster walking speed was associated with increases in HDL-c of 0.01 mmol/l (95% CI 0.00 to 0.02), LDL-c of 0.02 mmol/l (95% CI 0.00 to 0.04) and total cholesterol of 0.03 mmol/l (95% CI 0.00 to 0.05) per 2 days of walking (table 3). Exploratory adjustment of the relationship between walking speed and changes in total cholesterol, LDL-c, HDL-c and triglycerides for changes in body weight did not change the results. Adjusting all analyses for the total walked distance did not change the results, as expected, as differences in total walking distance between subjects were very small. In a sensitivity analysis, we additionally adjusted for baseline values of LDL-c, HDL-c, triglycerides and systolic and diastolic blood pressure, which did not change the results markedly.
Table 3.
The effect of walking speed in 1000 steps/h on the changes per 2 days in cardiovascular risk factors
| HDL cholesterol | LDL cholesterol | Total cholesterol | Triglycerides | Systolic BP | |
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| Model I | 0.01 (0.00 to 0.02)* | 0.02 (0.00 to 0.04)* | 0.02 (0.00 to 0.05)* | −0.01 (−0.03 to 0.01) | −0.41 (−0.81 to −0.01)* |
| Model II | 0.01 (0.00 to 0.02)* | 0.02 (0.00 to 0.04)* | 0.02 (0.00 to 0.05)* | −0.01 (−0.03 to 0.01) | −0.40 (−0.79 to −0.00)* |
| Model III | 0.01 (0.00 to 0.02)* | 0.02 (0.00 to 0.04)* | 0.03 (0.00 to 0.05)* | 0.00 (−0.03 to 0.02) | −0.36 (−0.76 to 0.04) |
| Diastolic BP | Weight | Waist circ. | Glucose | |
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| Model I | −0.10 (−0.33 to 0.13) | 0.01 (−0.05 to 0.08) | 0.09 (−0.12 to 0.30) | 0.00 (−0.03 to 0.02) |
| Model II | −0.09 (−0.32 to 0.14) | 0.01 (−0.05 to 0.08) | 0.09 (−0.12 to 0.30) | −0.01 (−0.03 to 0.02) |
| Model III | −0.06 (−0.29 to 0.17) | 0.01 (−0.05 to 0.08) | 0.12 (−0.08 to 0.32) | 0.00 (−0.02 to 0.02) |
The regression coefficient β (with 95% CI) denotes the mean change in the risk factor per 2 days which is associated with a 1000 steps/h higher walking speed. For example, a 1000 steps/h higher walking speed is associated with an increase in HDL cholesterol of 0.01 (95% CI 0.00 to 0.02) mmol/l (model III) per 2 days, translating to 0.06 (95% CI 0.00 to 0.12) mmol/l during the whole 12-day walking tour. Model I = crude, model II = age and gender and model III = age, gender, current smoking, BMI and heart rate at baseline.
p<0.05.
BMI, body mass index; BP, blood pressure; waist circ., waist circumference; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Discussion
In the present study, it is shown that walking speed significantly relates to changes in the lipid profile in healthy middle-aged men and women walking 12 days to Santiago de Compostela. A higher walking speed was related to a higher increase in HDL-c and attenuated decrease in LDL-c and total cholesterol, a relationship that was not explained by changes in body weight. Differences in walking speed were not related to changes in blood pressure, weight, waist circumference, triglycerides or glucose.
Several well-designed randomised controlled trials, controlling for exercise volume, report no effects of exercise intensity on plasma lipoproteins or on other cardiovascular risk factors.7–10 These trials describe long-term changes (after 3–8 months) in cardiovascular risk factors and the total weekly amount of exercise is limited (not more than 3 h or 1000–1200 calories/week).7–10 The present study describes changes in cardiovascular risk factors during exercise, and the daily amount of exercise in the current study was almost twice the amount of weekly exercise in the trials described above (5.39±0.36 h daily in the present study).
Possibly, the changes in lipoproteins related to the walking speed described in the current study are present for a limited time span shortly after very large bouts of exercise and are therefore not seen in the studies described above. Other randomised trials report larger decreases in weight, waist circumference and diastolic blood pressure,13 or larger increases in HDL-c,12 for higher compared with lower intensity exercise, but these studies did not control for differences in the total amount of exercise, so the reported effects could be due to the higher exercise volume instead of the higher intensity. In the present study, all participants walked almost the same distance and in addition, we adjusted the analyses for the small differences in total walking distance, which did not change the results.
There is no doubt that physical exercise should be advised to everyone who is capable to exercise, as physical exercise has multiple beneficial health effects.1–3 Furthermore, more exercise is better, as there is a clear inverse dose–response relationship between exercise and all-cause mortality.2 However, what walking speed is optimal for improving the lipid profile is not sure. Should we advise people to walk with high speed or with low speed when the goal is improvement of the lipid profile? In the present study, walking with higher speed increases HDL-c more, but at the expense of less LDL-c decrease, and walking with lower speed leads to less HDL-c increase but a more profound LDL-c decrease. Does the extra increase in HDL-c related to a higher walking speed outweighs the less decrease in LDL-c? This question cannot be answered with the results of the current study. In general, the primary lipid target in the prevention and treatment of cardiovascular disease is LDL-c, which is best reached with lower walking speed, according to the results of the present study. However, in large prospective cohort studies in the healthy population, an increased walking speed assessed by a questionnaire has been related to a lower risk for coronary heart disease and diabetes, independent of walking volume.17–20 This finding can lead to the speculation that the extra increase in HDL-c related to a higher walking speed could be more important than the less decrease in LDL-c. However, drawing conclusions from the combined findings of these two completely different types of studies is a step to far.
Several physiological mechanisms can be considered to explain the exercise-induced and intensity-independent changes in LDL-c and HDL-c. Exercise-induced changes in LDL-c may be due to dilution as a result of an increase in plasma volume,23 a decrease in body weight or a change in body fat distribution,24 an upregulated expression of hepatic LDL receptors,25 an increased cholesterol transfer from apoA-containing particles (LDL-c, very-low-density lipoprotein) to HDL particles26 and the use of cholesterol for cellular metabolism and repair due to muscle damage immediately after intense exercise.23 Exercise-induced HDL-c changes may be explained by the increased acceptance of free cholesterol from peripheral tissues by nascent HDL particles,27 increased HDL particle maturation by cholesterol esterification due to increased lecithin:cholesterol acyltransferase,28 increased breakdown of triglyceride-rich particles resulting from an increased lipoprotein lipase activity, leading to uptake of the cholesterol content by HDL-c particles,29 which could lead to prolonged HDL particle survival,30 and finally a decrease in cholesteryl ester transfer protein leading to a reduced shift of cholesterol esters from HDL to non-HDL lipoproteins.31 Which of these mechanisms is responsible for the observed increases in HDL-c and LDL-c related to higher walking speed in the present study is unknown. We did not measure (markers of) plasma volume changes, which could possibly be of influence on the results. However, as the reported results are linear during 12 days, and the measurements were conducted early in the morning, more than 12 h after the ending of the previous walking stage, we believe the influence of changes in plasma volume on the results to be small. Furthermore, we showed in an exploratory analysis that the relationship between walking speed and changes in blood lipids were not explained by changes in body weight. As the differences between the slower and faster walking speed groups occurred rapidly, within several days, and the amount of daily exercise was large, it is conceivable that consumption of cholesterol, from both HDL and LDL particles, for cellular metabolism and cellular repair due to muscle damage contributes to the observed changes. This explanation is more likely than other more long-term metabolic adaptations. The overall duration of exercise could have a higher impact than the small differences in intensity of this exercise on the amount of cholesterol needed for cellular metabolism and repair of muscle damage, leading to less increase in HDL-c and more decrease in LDL-c with longer exercise at a lower walking speed.
Walking a pilgrimage requires a considerable amount of time, a thorough preparation and a good physical and mental health. Our findings can be generalised to healthy middle-aged men and women who satisfy these conditions, and possibly to other types of exercise, consisting of prolonged daily periods of moderate intensity. However, the results of the present study are based on a relatively small group of subjects walking 281 km in 12 days. Therefore, no statistical interaction tests and no subgroup analyses could be performed. Whether the relationship between walking speed and the change in lipoproteins can be extrapolated to smaller amounts or other types of exercise is not known. The current study reports pragmatic research about exercise in real-life; however, more research needs to be done in a controlled lab-based setting in order to fully explore and understand the results of this study. A strength of this study is the equal amount of exercise, in this case the total walking distance, for all participants, eliminating this factor as a possible confounder in the relationship between walking speed and changes in cardiovascular risk factors. Furthermore, walking speed was measured and not assessed with a questionnaire like in many cohort studies, and the consistent results for walking speed expressed in kilometres per hour and steps per hour strengthen our findings.
We also acknowledge study limitations. Participants walking with slower speed were metabolically unhealthier as baseline than subjects walking with faster speed. Whether the worse baseline metabolic profile (such as higher BMI) is the cause of the slower walking speed achieved, or the consequence of, for example, a lower physical fitness which also results in a slower walking speed, is unclear and cannot be determined from the present study. Therefore, we adjusted the mixed linear effect models for baseline differences between the faster and slower walking speed groups, which did not change the results. Furthermore, we were not able to adjust for differences in the dietary pattern or cardiorespiratory fitness level of the participants, as these variables were not measured. However, by adjusting for the heart rate at baseline as a proxy for cardiorespiratory fitness and for other variables related to cardiorespiratory fitness or unhealthy dietary intake such as age, gender, BMI and smoking, residual confounding of cardiorespiratory fitness or dietary intake is unlikely.
In conclusion, during a 12-day walking tour to Santiago de Compostela with long daily walking stages, walking the same distance with a higher walking speed was related to a more pronounced increase in HDL-c, but to less decrease in LDL-c and total cholesterol, independent of changes in body weight, in healthy middle-aged men and women.
Supplementary Material
Acknowledgments
The authors thank Mr W Wesseldijk for his extensive contribution in organising the Santiago study.
Footnotes
To cite: Bemelmans RHH, Blommaert PP, Wassink AMJ, et al. The relationship between walking speed and changes in cardiovascular risk factors during a 12-day walking tour to Santiago de Compostela: a cohort study. BMJ Open 2012;2:e000875. doi:10.1136/bmjopen-2012-000875
Contributors: RHHB, PPB and FLJV were involved in the design and conduction of the study, analysis and interpretation of the data and writing of the manuscript. AMJW, BC, WS and YvdG were involved in the interpretation of the data and contributed important intellectual content to the first draft of the manuscript.
Funding: The SANTIAGO study is an investigator-driven study, funded by the University Medical Center Utrecht. No external sponsor was involved. The funding body had no role in the study design; in the collection, analysis and interpretation of the data; in the writing of the report and in the decision to submit the paper for publication.
Competing interests: None.
Ethics approval: Ethics approval was provided by Medical Ethical Committee of University Center Utrecht, Utrecht, The Netherlands.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Not applicable, no additional data present.
References
- 1.Wannamethee SG, Shaper AG, Alberti KG. Physical activity, metabolic factors, and the incidence of coronary heart disease and type 2 diabetes. Arch Intern Med 2000;160:2108–16 [DOI] [PubMed] [Google Scholar]
- 2.Lee IM, Skerrett PJ. Physical activity and all-cause mortality: what is the dose-response relation? Med Sci Sports Exerc 2001;33:S459–71 [DOI] [PubMed] [Google Scholar]
- 3.Thompson PD, Buchner D, Pina IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the council on clinical cardiology (subcommittee on exercise, rehabilitation, and prevention) and the council on nutrition, physical activity, and metabolism (subcommittee on physical activity). Circulation 2003;107:3109–16 [DOI] [PubMed] [Google Scholar]
- 4.Graham I, Atar D, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Eur Heart J 2007;28:2375–414 [DOI] [PubMed] [Google Scholar]
- 5.Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women–2011 update: a guideline from the American Heart Association. J Am Coll Cardiol 2011;57:1404–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American heart Association. Circulation 2007;116:1081–93 [DOI] [PubMed] [Google Scholar]
- 7.Kraus WE, Houmard JA, Duscha BD, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med 2002;347:1483–92 [DOI] [PubMed] [Google Scholar]
- 8.Cho JK, Lee SH, Lee JY, et al. Randomized controlled trial of training intensity in adiposity. Int J Sports Med 2011;32:468–75 [DOI] [PubMed] [Google Scholar]
- 9.Crouse SF, O'Brien BC, Grandjean PW, et al. Training intensity, blood lipids, and apolipoproteins in men with high cholesterol. J Appl Physiol 1997;82:270–7 [DOI] [PubMed] [Google Scholar]
- 10.Slentz CA, Duscha BD, Johnson JL, et al. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE—a randomized controlled study. Arch Intern Med 2004;164:31–9 [DOI] [PubMed] [Google Scholar]
- 11.Swain DP, Franklin BA. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol 2006;97:141–7 [DOI] [PubMed] [Google Scholar]
- 12.Duncan GE, Anton SD, Sydeman SJ, et al. Prescribing exercise at varied levels of intensity and frequency: a randomized trial. Arch Intern Med 2005;165:2362–9 [DOI] [PubMed] [Google Scholar]
- 13.Cornelissen VA, Arnout J, Holvoet P, et al. Influence of exercise at lower and higher intensity on blood pressure and cardiovascular risk factors at older age. J Hypertens 2009;27:753–62 [DOI] [PubMed] [Google Scholar]
- 14.Leon AS, Sanchez OA. Response of blood lipids to exercise training alone or combined with dietary intervention. Med Sci Sports Exerc 2001;33:S502–15 [DOI] [PubMed] [Google Scholar]
- 15.Tambalis K, Panagiotakos DB, Kavouras SA, et al. Responses of blood lipids to aerobic, resistance, and combined aerobic with resistance exercise training: a systematic review of current evidence. Angiology 2009;60:614–32 [DOI] [PubMed] [Google Scholar]
- 16.Crespo CJ, Keteyian SJ, Heath GW, et al. Leisure-time physical activity among US adults. Results from the third national health and nutrition examination survey. Arch Intern Med 1996;156:93–8 [PubMed] [Google Scholar]
- 17.Manson JE, Greenland P, LaCroix AZ, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med 2002;347:716–25 [DOI] [PubMed] [Google Scholar]
- 18.Hu FB, Sigal RJ, Rich-Edwards JW, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA 1999;282:1433–9 [DOI] [PubMed] [Google Scholar]
- 19.Tanasescu M, Leitzmann MF, Rimm EB, et al. Exercise type and intensity in relation to coronary heart disease in men. JAMA 2002;288:1994–2000 [DOI] [PubMed] [Google Scholar]
- 20.Manson JE, Hu FB, Rich-Edwards JW, et al. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med 1999;341:650–8 [DOI] [PubMed] [Google Scholar]
- 21.Bemelmans RH, Coll B, Faber DR, et al. Vascular and metabolic effects of 12 days intensive walking to Santiago de Compostela. Atherosclerosis 2010;212:621–7 [DOI] [PubMed] [Google Scholar]
- 22.Bisset W. The Camino Francés. London, United Kingdom: CSJ, 2009 [Google Scholar]
- 23.Ferguson MA, Alderson NL, Trost SG, et al. Effects of four different single exercise sessions on lipids, lipoproteins, and lipoprotein lipase. J Appl Physiol 1998;85:1169–74 [DOI] [PubMed] [Google Scholar]
- 24.Andersen RE, Wadden TA, Bartlett SJ, et al. Effects of lifestyle activity vs structured aerobic exercise in obese women: a randomized trial. JAMA 1999;281:335–40 [DOI] [PubMed] [Google Scholar]
- 25.Vinagre CG, Ficker ES, Finazzo C, et al. Enhanced removal from the plasma of LDL-like nanoemulsion cholesteryl ester in trained men compared with sedentary healthy men. J Appl Physiol 2007;103:1166–71 [DOI] [PubMed] [Google Scholar]
- 26.Butcher LR, Thomas A, Backx K, et al. Low-intensity exercise exerts beneficial effects on plasma lipids via PPARgamma. Med Sci Sports Exerc 2008;40:1263–70 [DOI] [PubMed] [Google Scholar]
- 27.Leaf DA. The effect of physical exercise on reverse cholesterol transport. Metabolism 2003;52:950–7 [DOI] [PubMed] [Google Scholar]
- 28.Durstine JL, Grandjean PW, Cox CA, et al. Lipids, lipoproteins, and exercise. J Cardiopulm Rehabil 2002;22:385–98 [DOI] [PubMed] [Google Scholar]
- 29.Grandjean PW, Crouse SF, O'Brien BC, et al. The effects of menopausal status and exercise training on serum lipids and the activities of intravascular enzymes related to lipid transport. Metabolism 1998;47:377–83 [DOI] [PubMed] [Google Scholar]
- 30.Thompson PD, Cullinane EM, Sady SP, et al. High density lipoprotein metabolism in endurance athletes and sedentary men. Circulation 1991;84:140–52 [DOI] [PubMed] [Google Scholar]
- 31.Seip RL, Moulin P, Cocke T, et al. Exercise training decreases plasma cholesteryl ester transfer protein. Arterioscler Thromb 1993;13:1359–67 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.