Abstract
Prospective community-based studies have provided fundamental insights into the epidemiology of influenza in temperate regions, but few comparable studies have been undertaken in the tropics. The authors conducted prospective influenza surveillance and intermittent seroprevalence surveys in a household-based cohort in Vietnam between December 2007 and April 2010, resulting in 1,793 person-seasons of influenza surveillance. Age- and sex-standardized estimates of the risk of acquiring any influenza infection per season in persons 5 years of age or older were 21.1% (95% confidence interval: 17.4, 24.7) in season 1, 26.4% (95% confidence interval: 22.6, 30.2) in season 2, and 17.0% (95% confidence interval: 13.6, 20.4) in season 3. Some individuals experienced multiple episodes of infection with different influenza types/subtypes in the same season (n = 27) or reinfection with the same subtype in different seasons (n = 22). The highest risk of influenza infection was in persons 5–9 years old, in whom the risk of influenza infection per season was 41.8%. Although the highest infection risk was in school-aged children, there were important heterogeneities in the age of infection by subtype and season. These heterogeneities could influence the impact of school closure and childhood vaccination on influenza transmission in tropical areas, such as Vietnam.
Keywords: communicable disease control; disease transmission, infectious; incidence; influenza, human; tropical climate
Until relatively recently, influenza was conceptualized as a problem of developed countries, with little consideration given to the frequency and burden of influenza in low-income and tropical countries. This all changed with the widespread reemergence of highly pathogenic avian influenza A/H5N1 virus in 2004 and further intensified with analyses suggesting that Southeast Asia may be the region where influenza A/H3N2 (hereafter referred to as H3N2) viruses undergo evolution before subsequent spread to higher latitudes of the northern and southern hemispheres (1, 2). As a result, influenza surveillance and control in Southeast Asia have come to be perceived as important to global public health, with substantial investment in influenza surveillance, antiviral stockpiling, vaccine development, and epidemic preparedness taking place in that region (3, 4).
Despite this interest in influenza in Southeast Asia, very few data are available about influenza transmission at the community level. To plan responses to both seasonal and pandemic influenza outbreaks and the optimal application of interventions, such as vaccination, antiviral prophylaxis, or school closure, it is first necessary to have a detailed understanding of how influenza is transmitted within the community (5). Community-based studies with follow-up over several years have been a key source of information on the transmission behavior of influenza. These studies have provided important insights into the epidemiology of respiratory infections and crucial information for the design of public-health interventions. They have demonstrated that preschool and school-aged children have the highest rate of respiratory illnesses (6–9), that mothers have higher attack rates than do fathers (6, 10), that children play an important role in the introduction of infection into families (9, 11–13), and that there are high rates of serologic evidence of infection without corresponding disease (5, 14, 15). However, those studies largely took place between the 1940s and early 1980s in the United States, and very few comparable community-based or household-based studies have been undertaken in the tropics. Population densities, family structures, behaviors, mobility, material conditions, health, and climate are different in Southeast Asia compared with the United States, so the epidemiology of influenza might also differ in important ways. In studies of influenza in Southeast Asia, investigators have largely assessed the incidence of influenza-associated clinical illness at health-care facilities or analyzed surveillance and health-care utilization data (16–20). Such studies are important in quantifying the clinical burden of influenza but cannot provide a full understanding of the epidemiology and transmission of influenza. Recent studies of the pandemic influenza A/H1N1/2009 (hereafter referred to as H1N1) virus have mostly relied on single cross-sectional serology to infer infection rates, but this is a less robust method of identifying recent infection than is the detection of increases in antibody titers in paired sera (21). We therefore established a household-based cohort to quantify the burden of influenza infection in a semirural community of northern Vietnam and to gain insights into the epidemiology of influenza in the tropics.
MATERIALS AND METHODS
Study design and setting
A full description of the materials and methods is provided in the Web Appendix (available at http://aje.oxfordjournals.org/) and only a brief description is provided here. In 2007, a prospective, household-based community cohort was established in Thanh Ha Commune, Thanh Liem District, Ha Nam Province, Vietnam. The primary sampling unit of study was the household, and all households in the Commune were eligible for inclusion in the study. Households were randomly selected from a list of all households using a random number table. If a randomly selected household declined to participate, the next nearest household was approached until a household was successfully recruited. All permanent residents of the household were eligible for inclusion and were asked to participate.
Blood sampling
Participants who were 5 years of age or older at the time of sampling were asked to provide blood at recruitment and at 3 additional time points. Recruitment blood samples were drawn between December 1 and 7, 2007 (bleed 1). Subsequent draws took place between December 9 and 15, 2008 (bleed 2), June 2 and 4, 2009 (bleed 3), and on the April 3, 2010 (bleed 4). The bleeding time points were not decided a priori but were chosen when national influenza surveillance data indicated that influenza circulation was minimal. The 4 sets of samples provided 3 sets of paired sera.
Surveillance of influenza-like illness
Trained hamlet health workers undertook weekly active surveillance of each participating household for episodes of influenza-like illness (ILI) and for changes in household composition. ILI was defined as an illness that included an orally measured body temperature of 38°C or higher and either a cough or a sore throat. Any participant who reported an ILI was asked to provide a nose swab and a throat swab and to complete a 10-day symptom diary.
Definition of exposure and outcome variables
For the purpose of analysis, an influenza season was defined as the period between consecutive bleeds, and an influenza transmission period was defined as the period when influenza was known to be circulating based on clinical cases confirmed using reverse transcription polymerase chain reaction (RT-PCR). Influenza infection was defined as either the detection of influenza RNA in a swab sample using RT-PCR or a 4-fold or greater rise in hemagglutination inhibition antibody titer in paired sera, with a second titer of at least 1:40. If paired sera were not available, a single high titer of at least 1:160 for seasonal influenza or of 1:80 or higher in someone who was less than 40 years of age for pandemic influenza H1N1 was also considered to indicate recent influenza infection. Influenza illness was defined as either the detection of influenza-specific RNA in a swab using RT-PCR and a report of concurrent ILI or serologic evidence of recent influenza infection (see above) plus an ILI episode that occurred during a known period of transmission of the relevant influenza subtype.
Laboratory methods
Tests for influenza viruses were performed on all nasal and throat swab specimens by using RT-PCR. Influenza hemagglutination inhibition assays were performed using standard methods. Samples that were negative on hemagglutination inhibition assay in the lowest dilution (1:10) were assigned a titer of 1:5 for the purposes of computing seroconversion.
Statistical methods
Absolute observed risks of ILI (for subjects under ILI surveillance) and of influenza infection (for subjects under influenza infection surveillance) were calculated per season. Participants were considered to be under ILI surveillance for a particular season if they were under weekly ILI surveillance throughout the influenza transmission period and were considered to be under influenza infection surveillance if they additionally contributed a postseason blood sample.
We used survey analysis methodology to derive risk estimates and associated 95% confidence intervals standardized to the age and sex structure of the Vietnamese rural population based on the 2009 Population and Housing Census. This provides valid inference accounting for effects of the survey design, which was based on cluster sampling by household, and biases in the provision of blood samples. As children under 5 years of age were not asked to give blood samples, standardization for influenza risks was to the portion of the census population who were 5 years of age or older. Standardization was implemented by raking, that is, by repeatedly reweighting the data to match the population age or sex distribution until convergence (22).
Seven potential risk factors for influenza infection were predefined. To assess these factors, we pooled data from all 3 seasons and modeled the overall risk of an influenza infection using a logistic mixed-effects model that included the season, a random household effect (to account for potential clustering within households), a random subject effect (to account for potential within-subject correlation between seasons), and the respective risk factor as explanatory variables. All analyses were performed using R, version 2.10.1 (R Foundation for Statistical Computing, Vienna, Austria) and the companion R packages survey 3.22-3 (for survey sampling) and lme4 0.999375-35 (for mixed models) (23).
RESULTS
A total of 940 individuals from 270 households were recruited from a study base of 2,127 enumerated households. The household refusal rate was approximately 10%, but we did not keep a record of the number of refusals or the reasons for refusal. The baseline characteristics of the 940 individuals and 270 households are shown in Table 1. None of the participants had ever received an influenza vaccination. The age distribution of the cohort was significantly different from that of both the Ha Nam province and the national rural population (chi-squared tests; both P < 0.001). This was largely due to an over-representation of persons who were 10–19 years old and an under-representation of persons who were 20–34 years old in the cohort (Web Figure 1). The household size distribution of the cohort matched well with that of the Red River Delta rural population (chi-squared goodness-of-fit test, P = 0.86).
Table 1.
Characteristics of Participants and Households at Recruitment, Ha Nam, Vietnam, 2007–2010
| Characteristic | No. of Participants | Total No. Assessed | % |
| Entire study population | |||
| Age, years | |||
| 0–4 | 83 | 929 | 8.9 |
| 5–9 | 70 | 929 | 7.5 |
| 10–19 | 209 | 929 | 22.5 |
| 20–39 | 246 | 929 | 26.5 |
| 40–59 | 241 | 929 | 25.9 |
| ≥60 | 80 | 929 | 8.6 |
| Female sex | 508 | 932 | 54.5 |
| Chronic diseasea | 5 | 869 | 0.6 |
| Adults (age ≥18 years) | 592 | ||
| Caring for children at home or at work | |||
| Never | 284 | 569 | 49.9 |
| Sometimes | 100 | 569 | 17.6 |
| Most days | 185 | 569 | 32.5 |
| Current smoker | 107 | 560 | 19.1 |
| Cigarettes smoked per day | |||
| ≤5 | 49 | 103 | 47.6 |
| 6–10 | 45 | 103 | 43.7 |
| 11–20 | 9 | 103 | 8.7 |
| Households | 270 | ||
| No. of people in the household | |||
| 1 | 28 | 270 | 10.4 |
| 2 | 41 | 270 | 15.2 |
| 3 | 65 | 270 | 24.1 |
| 4 | 74 | 270 | 27.4 |
| 5 | 42 | 270 | 15.6 |
| ≥6 | 20 | 270 | 7.4 |
| Home crowding (>2 people per room) | 46 | 237 | 19.4 |
| School-aged children in household (5–17 years of age) | 156 | 264 | 59.1 |
There were 2 participants with chronic lung disease, 2 with chronic heart disease, and 1 with chronic liver disease.
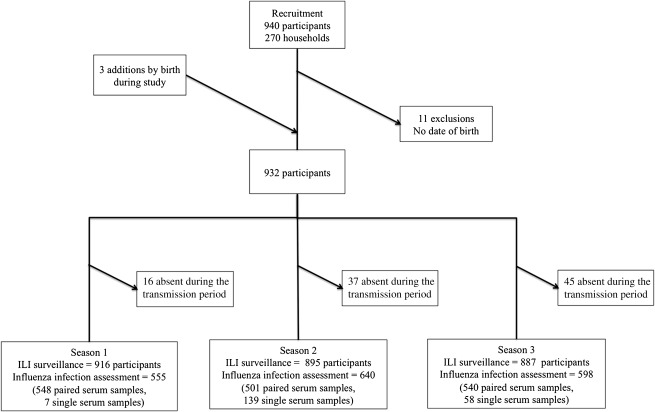
The cohort was studied for 3 consecutive influenza seasons, from December 2007 through April 2010. Data on age were missing for 11 of the original cohort members, so they were excluded from all further analysis. Three children were born into participating households during the study. The final total cohort size was 932 people (Figure 1). Some participants were absent from the study site during periods of influenza transmission and were therefore excluded from analysis of the relevant season. Figure 1 shows the number of participants included in each season’s analysis; a total of 1,793 person-seasons of influenza infection surveillance were available. The completeness of bleeds varied by age and sex, with the most complete blood sampling in females in bleed 1 (85%) and the least complete in males in bleed 2 (55%) (Web Figure 2).
Figure 1.
Criteria for inclusion of study participants in an assessment of influenza-like illness (ILI) and infection status by influenza season, Ha Nam, Vietnam, 2007–2010.
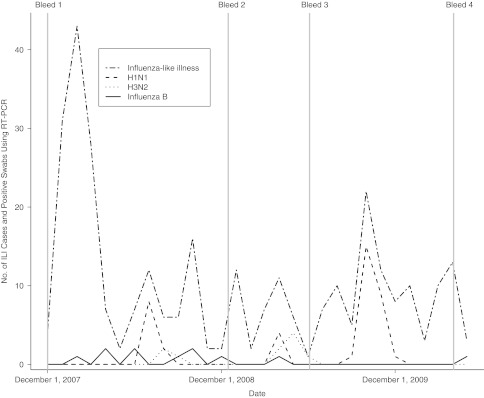
The temporal relation among periods of ILI and influenza activity confirmed using RT-PCR and the bleeding time points is shown in Figure 2. Three clear peaks of influenza A activity were detected: in summer 2008 (influenza transmission period 1: July 1, 2008–September 30, 2008), spring 2009 (influenza transmission period 2: April 1, 2009–June 05, 2009), and autumn 2009 (influenza transmission period 3: September 1, 2009–December 31, 2009). Clear peaks in influenza B activity were not seen. Cocirculation of influenza B, H1N1, and H3N2 viruses was detected in summer 2008 and spring 2009.
Figure 2.
Timeline of influenza-like illness (ILI) cases, influenza illnesses confirmed using reverse transcription polymerase chain reaction (RT-PCR), and cross-sectional bleeds, Ha Nam, Vietnam, 2007–2010.
Table 2 shows the number of reported ILI episodes in participants under ILI surveillance, the age- and sex-standardized ILI risk per season, and the number of swabs determined to be positive for influenza using RT-PCR in participants who reported an ILI. The standardized risk of ILI per season ranged from 14.1% in season 1 to 4.9% in season 3. The maximum risk of RT-PCR-confirmed influenza illness occurred among participants who were 5–19 years old in season 3; 5% of this age group was affected. In season 1, the influenza A virus strains detected in the cohort were A/H1N1/Brisbane/59/2007-like and A/H3N2/Brisbane/10/2007-like; in season 2, they were A/H1N1/Brisbane/59/2007-like and A/H3N2/Perth/16/2009-like; and in season 3, it was A/H1N1/California/7/2009-like. There was cocirculation of influenza B Yamagata lineage and Victoria lineage in both season 1 and season 2, with a predominance of Yamagata lineage in season 1 and Victoria lineage in season 2. In seasons 1 and 2, the overall rate of successful detection of influenza viruses from respiratory swabs was 18.4%, with the detection rate being highest in children who were 4 years of age or younger (50%) and declining with increasing age to 8.9% in persons 40 years of age or older.
Table 2.
Episodes of Influenza-Like Illness and Influenza Virus Detections by Season and Age Group, Ha Nam, Vietnam, 2007–2010
| Age at the Beginning of the Season, years |
|||||||||||||||
| Season and Variable | 0–4 |
5–19 |
20–39 |
≥40 |
All |
||||||||||
| No. of Participants | % | 95% CI | No. of Participants | % | 95% CI | No. of Participants | % | 95% CI | No. of Participants | % | 95% CI | No. | % | 95% CI | |
| Season 1 | 84 | 273 | 240 | 319 | 916 | ||||||||||
| No. reporting an ILI episode | 4 | 4.8 | 42 | 15.4 | 31 | 12.9 | 57 | 17.9 | 134 | 14.6 | |||||
| Standardized ILI risk per seasona | 5.3 | 0.3, 10.2 | 15.5 | 10.5, 20.4 | 11.7 | 7.9, 15.4 | 17.8 | 13.5, 22.1 | 14.1 | 11.3, 16.8 | |||||
| Influenza A/H1N1 virus detected using RT- PCR | 1 | 1.2 | 6 | 2.2 | 2 | 0.8 | 1 | 0.3 | 10 | 1.1 | |||||
| Influenza A/H3N2 virus detected using RT- PCR | 0 | 0.0 | 1 | 0.4 | 1 | 0.4 | 1 | 0.3 | 3 | 0.3 | |||||
| Influenza B virus detected using RT- PCR | 1 | 1.2 | 4 | 1.5 | 1 | 0.4 | 3 | 0.9 | 9 | 1.0 | |||||
| Season 2 | 59 | 284 | 226 | 326 | 895 | ||||||||||
| No. reporting an ILI episode | 0 | 0.0 | 16 | 5.6 | 13 | 5.8 | 22 | 6.7 | 51 | 5.7 | |||||
| Standardized ILI risk per seasona | 0.0 | 5.7 | 2.9, 8.5 | 4.6 | 2.1, 7.1 | 6.9 | 3.8, 9.9 | 5.2 | 3.5, 6.9 | ||||||
| Influenza A/H1N1 virus detected using RT- PCR | 0 | 0.0 | 4 | 1.4 | 0 | 0.0 | 0 | 0.0 | 4 | 0.4 | |||||
| Influenza A/H3N2 virus detected using RT- PCR | 0 | 0.0 | 3 | 1.1 | 2 | 0.9 | 2 | 0.6 | 7 | 0.8 | |||||
| Influenza B virus detected using RT- PCR | 0 | 0.0 | 1 | 0.4 | 0 | 0.0 | 0 | 0.0 | 1 | 0.1 | |||||
| Season 3 | 54 | 279 | 225 | 329 | 887 | ||||||||||
| No. reporting an ILI episodeb | 3 | 5.6 | 21 | 7.5 | 11 | 4.9 | 10 | 3.0 | 45 | 5.1 | |||||
| Standardized ILI risk per seasona | 5.8 | 0.0, 12.2 | 7.5 | 4.3, 10.6 | 4.4 | 1.4, 7.4 | 2.7 | 1.0, 4.3 | 4.9 | 3.1, 6.6 | |||||
| Pandemic influenza A virus detected | 0 | 0.0 | 14 | 5.0 | 7 | 3.1 | 3 | 0.9 | 24 | 2.7 | |||||
Abbreviations: CI, confidence interval; ILI, influenza-like illness; RT-PCR, reverse transcription polymerase chain reaction.
Standardized to age and sex distribution of the Vietnamese national rural population according to the 2009 Population and Housing Census.
Five subjects reported 2 ILI episodes during the season.
Unadjusted and standardized estimates of influenza infection and influenza illness rates per season are shown in Table 3. This analysis is restricted to those participants who were under ILI surveillance and who also provided at least 1 end-of-season blood sample (Figure 1). Standardized estimates of the risk of acquiring any influenza infection per season in persons 5 years of age or older were 21.1% in season 1, 26.4% in season 2, and 17% in season 3. H3N2 infection was more common in season 2 than in season 1 after a change in the circulating virus strain from A/H3N2/Brisbane/10/2007-like to A/H3N2/Perth/16/2009-like.
Table 3.
Unadjusted and Standardized Risks of Influenza Infection and Influenza Illness by Season in Persons 5 Years of Age or Older, Ha Nam, Vietnam, 2007–2010
| Season and Influenza Type/Subtype | No. of Seroconversions | No. of Single High Titers | Positive RT-PCRa | Observed Influenza Infections |
Standardizedb Influenza Infection Risk |
Observed Influenza Illnesses |
Standardizedb Influenza Illness Risk |
||||||
| No. of Participants | Total No. Assessed | % | % | 95% CI | No. of Participants | Total No. Assessed | % | % | 95% CI | ||||
| Season 1 (n = 555) | |||||||||||||
| Anyc | 116 | 0 | 17 (4) | 120 | 555 | 21.6 | 21.1 | 17.4, 24.7 | 28 | 555 | 5.0 | 4.5 | 2.8, 6.3 |
| Influenza A/H1N1 | 36 | 0 | 8 (4) | 40 | 555 | 7.2 | 7.4 | 5.0, 9.8 | 8 | 555 | 1.4 | 1.3 | 0.3, 2.3 |
| Influenza A/H3N2 | 13 | 0 | 3 (0) | 13 | 555 | 2.3 | 2.3 | 0.8, 3.8 | 3 | 555 | 0.5 | 0.6 | 0.0, 1.3 |
| Influenza B | 69 | 0 | 6 (1) | 70 | 555 | 12.6 | 12.0 | 8.8, 15.2 | 17 | 555 | 3.1 | 2.6 | 1.3, 4.0 |
| Season 2 (n = 640) | |||||||||||||
| Anyd | 152 | 23 | 12 (3) | 178 | 640 | 27.8 | 26.4 | 22.6, 30.2 | 17 | 640 | 2.7 | 2.4 | 1.2, 3.7 |
| Influenza A/H1N1 | 46 | 7 | 4 (2) | 55 | 640 | 8.6 | 8.3 | 6.1, 10.5 | 5 | 640 | 0.8 | 0.8 | 0.1, 1.6 |
| Influenza A/H3N2 | 71 | 12 | 7 (1) | 84 | 640 | 13.1 | 11.8 | 9.0, 14.6 | 8 | 640 | 1.2 | 1.0 | 0.2, 1.9 |
| Influenza B | 59 | 7 | 1 (1) | 67 | 640 | 10.5 | 10.2 | 7.7, 12.7 | 4 | 640 | 0.6 | 0.6 | 0.0, 1.1 |
| Season 3 (n = 598) | |||||||||||||
| Influenza A/H1N1 | 98 | 6 | 18 (5) | 109 | 598 | 18.2 | 17.0 | 13.6, 20.4 | 17 | 598 | 2.8 | 2.6 | 1.3, 3.9 |
Abbreviations: CI, confidence interval; RT-PCR, reverse transcription polymerase chain reaction.
Numbers in parentheses refer to samples determined to be positive using RT-PCR without documented seroconversion or single high titer.
Standardized to age and sex distribution of the Vietnamese national rural population according to the 2009 Population and Housing Census.
One subject had both influenza A/H1N1 and influenza A/H3N2 seroconversion, 1 subject had both influenza A/H1N1 and influenza B seroconversion, and 1 subject was H1N1-postive according to RT-PCR (but no seroconversion) and had influenza B seroconversion.
Four subjects were infected with all 3 influenza subtypes, 5 with H1N1 and H3N2, 7 with H1N1 and influenza B, and 8 with H3N2 and influenza B.
In all, 427 participants could be assessed for influenza infection over all 3 seasons, 242 (56.7%) of whom showed evidence of at least 1 acute influenza infection over the whole study period. After adjustment for the household-based sampling design and standardization to the age and sex structure of the Vietnam rural population, the estimated risk of any influenza infection in people 5 years of age or older over the entire 3-season period was 55.4% (95% confidence interval (CI): 49.6, 61.2). In all seasons, the estimated influenza illness risks were substantially lower than the infection risks. The percentages of identified influenza infections in which an influenza illness was also detected were 14% (13 of 95) for H1N1, 11% (11 of 97) for H3N2, 15% (21 of 137) for influenza B, and 16% (17 of 109) for H1N1/2009.
Multiple episodes of influenza infection by different influenza types/subtypes in the same season (multiple infections) were identified in 27 individuals; 23 had evidence of infection by 2 types/subtypes and 4 had evidence of infection by all 3 types/subtypes (Table 3). Reinfection with the same influenza type/subtype in season 2 as in season 1 was detected in 8 participants (H3N2 = 1 older adult (≥40 years of age); seasonal H1N1 = 2 children between 5 and 14 years of age; and influenza B = 4 children between 10 and 14 years of age and 1 older adult). In addition, 14 participants who had been infected with seasonal H1N1 in season 1 (1 child 5–9 years of age and 3 adults) or season 2 (6 children 5–14 years of age and 4 adults) were also infected with pandemic H1N1 in season 3.
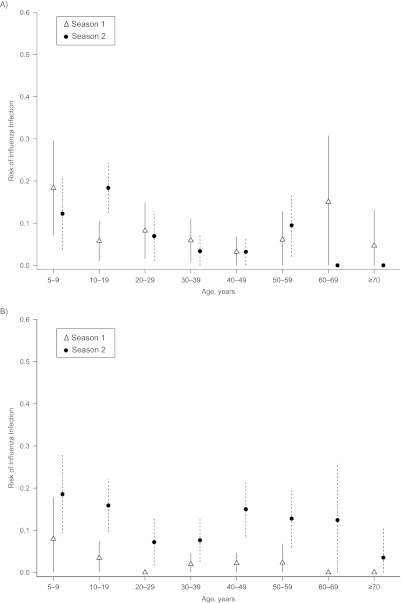
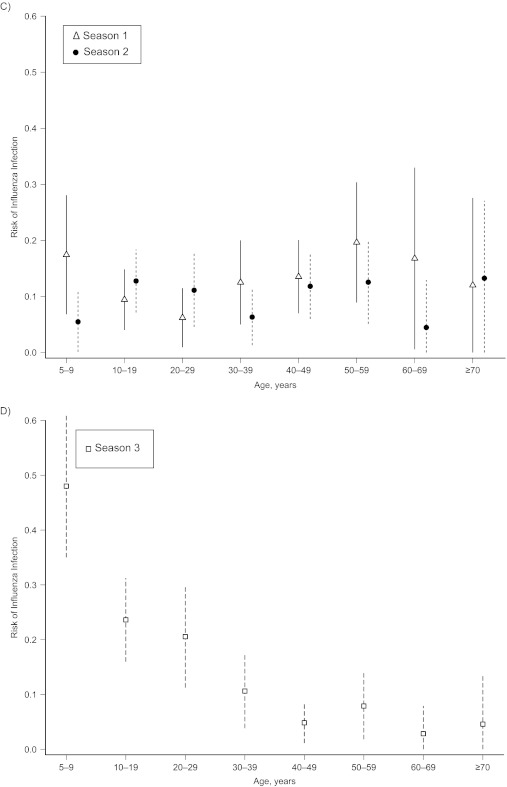
Influenza infection risk varied by age most clearly in season 3, when pandemic influenza H1N1 first circulated in the cohort and infected a large proportion of children and young adults (Figure 3). Age patterns in infection risk were less marked for interpandemic strains. The highest risk of infection with H3N2 (seasons 1 and 2) and H1N1 (season 1 only) was in children 5–9 years of age. In season 2, the highest risk of H1N1 infection was in people 10–19 years of age. To assess the significance of the apparent age-dependent peaks of H3N2 infections in season 2, we applied the same methodology as we did for Table 4 (without a random effect for patient because there is only 1 patient record per season). In season 2, we observed significantly higher H3N2 risks in children less than 10 years of age (odds ratio = 3.47, 95% CI: 1.37, 8.79; P = 0.009) and persons 10–20 years of age (odds ratio = 2.3, 95% CI: 1.02, 5.17; P = 0.043) than in individuals who were 20–39 years old. The second peak for persons 40 years of age or older was borderline significant, with an odds ratio of 2.12 (95% CI: 0.99, 4.54; P = 0.052). Web Figure 3 and the Web Table show the proportions of participants with influenza infection per season by age group and type/subtype compared with those from previously published household-based cohort studies.
Figure 3.
Risk of influenza infection by season, influenza subtype, and age group, Ha Nam, Vietnam, 2007–2010. Models were adjusted for household clustered design and standardized to the age and sex distribution of the Vietnam national rural population 5 years of age or older. A) Seasonal influenza A/H1N1; B) influenza A/H3N2; C) influenza B; and D) pandemic influenza A/H1N1/2009. Bars, 95% confidence interval.
Table 4.
Risk Factors for Influenza Infection Aggregated Over Influenza Subtypes and Seasons, Ha Nam, Vietnam, 2007–2010
| Aggregateda Observed Absolute Influenza Infection Risk per Season |
Univariate Association |
P Valueb | Multivariate Associationc |
P Valueb | |||||
| Covariate and Category | No. of Influenza Infections | Person-Seasons | % | OR | 95% CI | OR | 95% CI | ||
| Age, years | |||||||||
| 5–9 | 79 | 189 | 41.8 | 3.65 | 2.50, 5.34 | <0.001d | 4.15 | 2.41, 7.13 | <0.001 |
| 10–19 | 120 | 408 | 29.4 | 2.11 | 1.52, 2.91 | 2.34 | 1.44, 3.79 | ||
| 20–39 | 88 | 513 | 17.2 | Baseline | |||||
| ≥40 | 120 | 683 | 17.6 | 1.06 | 0.77, 1.45 | 1.10 | 0.77, 1.58 | ||
| Sexe | |||||||||
| Male | 164 | 745 | 22.0 | ||||||
| Female | 243 | 1,048 | 23.2 | 1.07 | 0.85, 1.35 | 0.55 | 1.06 | 0.81, 1.38 | 0.66 |
| No. of persons in the household | |||||||||
| 1–2 | 31 | 185 | 16.8 | 0.64 | 0.39, 1.04 | 0.25 | 0.76 | 0.43, 1.33 | 0.81 |
| 3 | 83 | 350 | 23.7 | ||||||
| 4 | 138 | 590 | 23.7 | 1.00 | 0.71, 1.42 | 0.98 | 0.69, 1.40 | ||
| ≥5 | 155 | 668 | 23.2 | 0.98 | 0.70, 1.39 | 0.98 | 0.61, 1.56 | ||
| Home crowding (>2 people per room) | |||||||||
| No | 250 | 1,132 | 22.1 | ||||||
| Yes | 119 | 507 | 23.5 | 1.10 | 0.84, 1.44 | 0.50 | 0.90 | 0.60, 1.35 | 0.62 |
| Caring for children at work or homef | |||||||||
| No | 84 | 549 | 15.3 | 0.18 | |||||
| Sometimes | 36 | 201 | 17.9 | 1.23 | 0.77, 1.95 | 1.40 | 0.89, 2.21 | ||
| Most days | 88 | 435 | 20.2 | 1.40 | 0.98, 2.01 | 0.18 | 1.37 | 0.93, 2.00 | |
| School-aged children (5–17 years of age) in householdf | |||||||||
| No | 83 | 462 | 18.0 | ||||||
| Yes | 131 | 767 | 17.1 | 0.94 | 0.67, 1.32 | 0.72 | 0.87 | 0.61, 1.26 | 0.47 |
| Smokingf | |||||||||
| No | 172 | 970 | 17.7 | ||||||
| Yes | 32 | 200 | 16.0 | 0.87 | 0.56, 1.34 | 0.53 | 0.89 | 0.54, 1.45 | 0.63 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Wald-type test for the significance of the whole factor.
Aggregated over all seasons and influenza subtypes (but only pandemic influenza was assessed in season 3).
Adjusted for all other covariates in the model. Covariates that are reported in adults only for the univariate associations were included as indicator variables with value 0 for children.
Also significant for H1N1 alone (P < 0.001), H3N2 alone (P = 0.02), and pandemic H1N1 alone (P < 0.001) but not for influenza B alone (P = 0.33).
Univariate analysis of sex for adults only was also not significant.
Variables studied only in adults 18 years of age or older.
Risk factors for influenza infection were explored in univariate and multivariate analysis (Table 4). Age was significantly associated with the risk of influenza infection in both univariate and multivariate analysis. This association was also observed for interpandemic H1N1 and H3N2 and pandemic H1N1 when they were analyzed separately but not for influenza B (Table 4). The highest risk of influenza infection was in children 5–9 years of age, in whom the observed absolute risk of influenza infection per season was 41.8%. The lowest infection risk was in adults who were 20–39 years old. There was no observed sex effect (Table 4, Web Figure 4), and no other covariates were significantly associated with influenza infection risk in either univariate or multivariate analyses.
DISCUSSION
The present study is one of the first to prospectively quantify the incidence of influenza infection in the same individuals over multiple seasons in a tropical setting. It demonstrates that influenza infection is common, with an average estimated risk of influenza infection in a single season of between 17% and 26%, with approximately 57% of people experiencing at least 1 acute influenza infection in a 3-year period. These estimates are minimum estimates because we used the hemagglutination inhibition assay, which is less sensitive than the microneutralization assay, and conservative definitions of laboratory evidence of influenza infection (24). Although varying study designs, laboratory methods, data availability, and periods of influenza emergence and reemergence confounded direct comparison with earlier family studies in temperate settings, the levels of infection we identified were similar, as shown in Web Figure 3 (9, 10, 14, 15, 25–29). Although the rates we observed were generally in the lower range of those reported in other household studies, most of these previous studies recruited only households with infants or young children and did not standardize the results to the general population structure. For example, children 5–14 years old constituted 47% of the Cleveland Family Study during the H2N2 pandemic (14). Also, we were not able to obtain blood samples from children who were less than 5 years of age, a subgroup expected to have high rates of infection. The 17% infection rate for pandemic influenza H1N1 in our study was similar to rates in contemporary seroepidemiology reports from other areas (30–34).
As found in other longitudinal studies, multiple infections in the same season with different influenza types/subtypes and reinfection with the same subtype in consecutive seasons do occur, and although more common in children, they can occur in persons of any age (8, 15, 35–38).
Between 11% and 16% of influenza infections resulted in an illness that was detected by weekly active ILI surveillance. Our figures of the proportion of infections that cause clinically detected illness were lower than estimates obtained by Monto (39), who found that at least 15%–25% of H3N2 infections and 19%–34% of influenza B infections resulted in clinical disease. Although this might have been a real effect, perhaps influenced by the slightly greater proportion of participants 40 years of age or older in our study compared with the Tecumseh study, it may have also represented a reporting bias, with a greater propensity for participants to report illnesses in Tecumseh in the 1970s than in our study site.
The data reveal clear variations in the risk of influenza by age and influenza subtype. As observed elsewhere, the 2009 H1N1 pandemic resulted in very high infection rates in young children, and those rates dropped sharply with age. The high rates of H1N1/2009 in persons 5–29 years of age, which exceed those seen for any other subtype and season, may be explained by the immunologic naivety of this age group to this antigenic hemagglutinin variant. The low rates of infection with pandemic H1N1 in older adults indicate that long-lived and cross-protective immunity against H1N1 might be induced either by repeated infection or by infection with an antigenically related virus (40, 41). Similar long-lived protection was observed when H1N1 reemerged in 1977 after an absence of 20 years (26, 39).
H3N2 infections in season 2 (when a drifted variant circulated) were highest in school-aged children, with a second peak in older adults. In a recent cross-sectional seroprevalence study from Canada, Skowronski et al. (42) also found a second peak in H3N2 titers in people who were 60 years of age or older, and there is ample evidence that adults experience higher rates of infection and reinfection with H3N2 that with other influenza types/subtypes (28, 43–47). These serologic measures of risk also translate into clinical illness, with H3N2 more commonly causing clinical illness in adults in the community and in institutional care compared with other influenza viruses (48–52). We observed a fairly constant risk of influenza B infection across the whole age range. This contrasts with some earlier studies in temperate countries in which influenza B risk peaked in preschool or school-aged children (15, 26, 27, 29). Although this pattern may be due to the absence of an influenza B epidemic during the study period, the influenza B infection rates were moderately high and the age distribution could therefore be the consequence of prolonged circulation of influenza B viruses without the opportunity for a build-up of a large cohort of susceptible children.
One possible explanation for the more even age distribution of the risk of interpandemic influenza infection in our study compared with some historic studies in temperate climates is that temporal changes in climate suitability and school-related contact behaviors have a lesser effect on transmission probabilities in the tropics, resulting in less intense school-based transmission and proportionately greater community-based transmission. Multiple epidemics per year and more prolonged virus circulation might also limit the pool of susceptible children. In this respect, it is relevant that, in contrast to community studies conducted in temperate settings, we identified neither an increased risk of influenza infection in women compared with men nor an association between caring for children or the presence of a school-aged child in the house and the risk of influenza in adults. This may have important implications for the impact of school closure and childhood vaccination on the transmission of interpandemic influenza in tropical areas such as Vietnam. Although school closure was reported to be effective in reducing transmission of H1N1/2009 in Hong Kong, our study shows that the age distribution of H1N1/2009 infection was not characteristic of interpandemic influenza in the tropics (53). Longitudinal studies like the present study that follow individuals of all ages over multiple seasons with serial serology provide not only the most robust estimates of true influenza infection incidence but also information that is critical for understanding influenza epidemiology in the tropics and for planning effective influenza control strategies.
Supplementary Material
Acknowledgments
Author affiliations: Oxford University Clinical Research Unit and Wellcome Trust Major Overseas Programme, Hanoi, Vietnam (Peter Horby, Annette Fox); Oxford University Clinical Research Unit and Wellcome Trust Major Overseas Programme, Ho Chi Minh City, Vietnam (Jeremy Farrar, Marcel Wolbers); National Institute of Hygiene and Epidemiology, Hanoi, Vietnam (Le Quynh Mai, Pham Quang Thai, Nguyen Thi Thu Yen, Le Thi Thanh, Nguyen Le Khanh Hang, Tran Nhu Duong, Nguyen Tran Hien); and Ha Nam Centre for Preventive Medicine, Ha Nam, Vietnam (Dang Dinh Thoang).
This work was supported by the United Kingdom Wellcome Trust (grants 081613/Z/06/Z and 077078/Z/05/Z).
The authors thank the hamlet health workers who conducted the interviews and surveillance and the Ministry of Health of Vietnam for their continuing support of the research collaboration between the Oxford University Clinical Research Unit and the National Institute for Hygiene and Epidemiology. The authors also thank Dr. Neal Alexander for providing advice on study design and data analysis.
The United Kingdom Wellcome Trust had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- ILI
influenza-like infection
- RT-PCR
reverse transcription polymerase chain reaction
References
- 1.Rambaut A, Pybus OG, Nelson MI, et al. The genomic and epidemiological dynamics of human influenza A virus. Nature. 2008;453(7195):615–619. doi: 10.1038/nature06945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell CA, Jones TC, Barr IG, et al. The global circulation of seasonal influenza A (H3N2) viruses. Science. 2008;320(5874):340–346. doi: 10.1126/science.1154137. [DOI] [PubMed] [Google Scholar]
- 3.Hoa LK, Hiep LV, Be LV. Development of pandemic influenza vaccine production capacity in Viet Nam. Vaccine. 2011;29(suppl 1):A34–A36. doi: 10.1016/j.vaccine.2011.04.118. [DOI] [PubMed] [Google Scholar]
- 4.Hanvoravongchai P, Adisasmito W, Chau PN, et al. Pandemic influenza preparedness and health systems challenges in Asia: results from rapid analyses in 6 Asian countries. BMC Public Health. 2010;10(1):322. doi: 10.1186/1471-2458-10-322. (doi:10.1186/1471-2458-10-322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16(2):351–373. doi: 10.1093/oxfordjournals.epirev.a036158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badger GF, Dingle JH, Feller AE, et al. A study of illness in a group of Cleveland families: II: incidence of the common respiratory diseases. Am J Hyg. 1953;58(1):31–40. doi: 10.1093/oxfordjournals.aje.a119588. [DOI] [PubMed] [Google Scholar]
- 7.Hope-Simpson RE, Higgins PG. A respiratory virus study in Great Britain: review and evaluation. Prog Med Virol. 1969;11:354–407. [PubMed] [Google Scholar]
- 8.Fox JP, Hall CE, Cooney MK, et al. The Seattle virus watch: II: objectives, study population and its observation, data processing and summary of illnesses. Am J Epidemiol. 1972;96(4):270–285. doi: 10.1093/oxfordjournals.aje.a121458. [DOI] [PubMed] [Google Scholar]
- 9.Monto AS, Kioumehr F. The Tecumseh Study of Respiratory Illness: IX: occurrence of influenza in the community, 1966–1971. Am J Epidemiol. 1975;102(6):553–563. doi: 10.1093/oxfordjournals.aje.a112193. [DOI] [PubMed] [Google Scholar]
- 10.Hall CE, Brandt CD, Frothingham TE, et al. The virus watch program: a continuing surveillance of viral infections in metropolitan New York families. IX: a comparison of infections with several respiratory pathogens in New York and New Orleans families. Am J Epidemiol. 1971;94(4):367–385. doi: 10.1093/oxfordjournals.aje.a121332. [DOI] [PubMed] [Google Scholar]
- 11.Badger GF, Dingle JH, Feller AE, et al. A study of illness in a group of Cleveland families: IV: the spread of respiratory infections within the home. Am J Hyg. 1953;58(2):174–178. doi: 10.1093/oxfordjournals.aje.a119598. [DOI] [PubMed] [Google Scholar]
- 12.Cauchemez S, Carrat F, Viboud C, et al. A Bayesian MCMC approach to study transmission of influenza: application to household longitudinal data. Stat Med. 2004;23(22):3469–3487. doi: 10.1002/sim.1912. [DOI] [PubMed] [Google Scholar]
- 13.Viboud C, Boëlle PY, Cauchemez S, et al. Risk factors of influenza transmission in households. Br J Gen Pract. 2004;54(506):684–689. [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan WS, Jr, Denny FW, Jr, Badger GF, et al. A study of illness in a group of Cleveland families: XVII: the occurrence of Asian influenza. Am J Hyg. 1958;68(2):190–212. doi: 10.1093/oxfordjournals.aje.a119962. [DOI] [PubMed] [Google Scholar]
- 15.Hall CE, Cooney MK, Fox JP. The Seattle virus watch: IV: comparative epidemiologic observations of infections with influenza A and B viruses, 1965–1969, in families with young children. Am J Epidemiol. 1973;98(5):365–380. doi: 10.1093/oxfordjournals.aje.a121566. [DOI] [PubMed] [Google Scholar]
- 16.Wong CM, Yang L, Chan KP, et al. Influenza-associated hospitalization in a subtropical city. PLoS Med. 2006;3(4):e121. doi: 10.1371/journal.pmed.0030121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simmerman JM, Uyeki TM. The burden of influenza in East and South-East Asia: a review of the English language literature. Influenza Other Respi Viruses. 2008;2(3):81–92. doi: 10.1111/j.1750-2659.2008.00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasegawa G, Kyaw Y, Danjuan L, et al. Influenza virus infections in Yangon, Myanmar. J Clin Virol. 2006;37(3):233–234. doi: 10.1016/j.jcv.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Beckett CG, Kosasih H, Ma’roef C, et al. Influenza surveillance in Indonesia: 1999–2003. Clin Infect Dis. 2004;39(4):443–449. doi: 10.1086/422314. [DOI] [PubMed] [Google Scholar]
- 20.Clague B, Chamany S, Burapat C, et al. A household survey to assess the burden of influenza in rural Thailand. Southeast Asian J Trop Med Public Health. 2006;37(3):488–493. [PubMed] [Google Scholar]
- 21.Lee VJ, Chen MI, Yap J, et al. Comparability of different methods for estimating influenza infection rates over a single epidemic wave. Am J Epidemiol. 2011;174(4):468–478. doi: 10.1093/aje/kwr113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lumley T. Complex Surveys: A Guide to Analysis Using R. Philadelphia, PA: John Wiley & Sons, Inc; 2010. [Google Scholar]
- 23.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. ( http://www.r-project.org/). (Accessed January 26, 2012) [Google Scholar]
- 24.Miller E, Hoschler K, Hardelid P, et al. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet. 2010;375(9720):1100–1108. doi: 10.1016/S0140-6736(09)62126-7. [DOI] [PubMed] [Google Scholar]
- 25.Jordan WS, Jr, Badger GF, Dingle JH. A study of illness in a group of Cleveland families: XVI: the epidemiology of influenza, 1948–1953. Am J Hyg. 1958;68(2):169–189. doi: 10.1093/oxfordjournals.aje.a119961. [DOI] [PubMed] [Google Scholar]
- 26.Fox JP, Hall CE, Cooney MK, et al. Influenza virus infections in Seattle families, 1975–1979. I. Study design, methods and the occurrence of infections by time and age. Am J Epidemiol. 1982;116(2):212–227. doi: 10.1093/oxfordjournals.aje.a113407. [DOI] [PubMed] [Google Scholar]
- 27.Monto AS, Sullivan KM. Acute respiratory illness in the community: frequency of illness and the agents involved. Epidemiol Infect. 1993;110(1):145–160. doi: 10.1017/s0950268800050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frank AL, Taber LH, Wells JM. Comparison of infection rates and severity of illness for influenza A subtypes H1N1 and H3N2. J Infect Dis. 1985;151(1):73–80. doi: 10.1093/infdis/151.1.73. [DOI] [PubMed] [Google Scholar]
- 29.Frank AL, Taber LH, Glezen WP, et al. Influenza B virus infections in the community and the family: the epidemics of 1976–1977 and 1979–1980 in Houston, Texas. Am J Epidemiol. 1983;118(3):313–325. doi: 10.1093/oxfordjournals.aje.a113638. [DOI] [PubMed] [Google Scholar]
- 30.Miller E, Hoschler K, Hardelid P, et al. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Health Technol Assess. 2010;6736(9):1–9. doi: 10.1016/S0140-6736(09)62126-7. [DOI] [PubMed] [Google Scholar]
- 31.Wu JT, Ma ES, Lee CK, et al. The infection attack rate and severity of 2009 pandemic H1N1 influenza in Hong Kong. Clin Infect Dis. 2010;51(10):1184–1191. doi: 10.1086/656740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen MI, Lee VJ, Lim WY, et al. 2009 influenza A(H1N1) seroconversion rates and risk factors among distinct adult cohorts in Singapore. JAMA. 2010;303(14):1383–1391. doi: 10.1001/jama.2010.404. [DOI] [PubMed] [Google Scholar]
- 33.Deng Y, Pang XH, Yang P, et al. Serological survey of 2009 H1N1 influenza in residents of Beijing, China. Epidemiol Infect. 2011;139(1):52–58. doi: 10.1017/S0950268810002189. [DOI] [PubMed] [Google Scholar]
- 34.Riley S, Kwok KO, Wu KM, et al. Epidemiological characteristics of 2009 (H1N1) pandemic influenza based on paired sera from a longitudinal community cohort study. PLoS Med. 2011;8(6):e1000442. doi: 10.1371/journal.pmed.1000442. (doi:10.1371/journal.pmed.1000442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frank AL, Taber LH. Variation in frequency of natural reinfection with influenza A viruses. J Med Virol. 1983;12(1):17–23. doi: 10.1002/jmv.1890120103. [DOI] [PubMed] [Google Scholar]
- 36.Frank AL, Taber LH, Wells JM. Individuals infected with two subtypes of influenza A virus in the same season. J Infect Dis. 1983;147(1):120–124. doi: 10.1093/infdis/147.1.120. [DOI] [PubMed] [Google Scholar]
- 37.Frank AL, Taber LH, Porter CM. Influenza B virus reinfection. Am J Epidemiol. 1987;125(4):576–586. doi: 10.1093/oxfordjournals.aje.a114571. [DOI] [PubMed] [Google Scholar]
- 38.Sonoguchi T, Sakoh M, Kunita N, et al. Reinfection with influenza A (H2N2, H3N2, and H1N1) viruses in soldiers and students in Japan. J Infect Dis. 1986;153(1):33–40. doi: 10.1093/infdis/153.1.33. [DOI] [PubMed] [Google Scholar]
- 39.Monto AS, Koopman JS, Longini IM., Jr Tecumseh study of illness: XIII: influenza infection and disease, 1976–1981. Am J Epidemiol. 1985;121(6):811–822. doi: 10.1093/oxfordjournals.aje.a114052. [DOI] [PubMed] [Google Scholar]
- 40.Duvvuri VR, Moghadas SM, Guo H, et al. Highly conserved cross-reactive CD4+ T-cell HA-epitopes of seasonal and the 2009 pandemic influenza viruses. Influenza Other Respi Viruses. 2010;4(5):249–258. doi: 10.1111/j.1750-2659.2010.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greenbaum JA, Kotturi MF, Kim Y, et al. Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population. Proc Natl Acad Sci U S A. 2009;106(48):20365–20370. doi: 10.1073/pnas.0911580106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skowronski DM, Hottes TS, McElhaney JE, et al. Immuno-epidemiologic correlates of pandemic H1N1 surveillance observations: higher antibody and lower cell-mediated immune responses with advanced age. J Infect Dis. 2011;203(2):158–167. doi: 10.1093/infdis/jiq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sohier R, Henry M. Epidemiological data on Hong Kong influenza in France. Bull World Health Organ. 1969;41(3):402–404. [PMC free article] [PubMed] [Google Scholar]
- 44.Hope-Simpson RE. First outbreak of Hong Kong influenza in a general practice population in Great Britain: a field and laboratory study. Br Med J. 1970;3(5714):74–77. doi: 10.1136/bmj.3.5714.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zdanov VM, Antonova IV. The Hong Kong influenza virus epidemic in the USSR. Bull World Health Organ. 1969;41(3):381–386. [PMC free article] [PubMed] [Google Scholar]
- 46.Willers H, Höpken W. Epidemiology of influenza in Lower Saxony during the period 1968–1978 with particular emphasis on subtypes A(H3N2) and A(H1N1) in winter 1977–78. Med Microbiol Immunol. 1979;167(1):21–27. doi: 10.1007/BF02123292. [DOI] [PubMed] [Google Scholar]
- 47.Davis LE, Caldwell GG, Lynch RE, et al. Hong Kong influenza: the epidemiologic features of a high school family study analyzed and compared with a similar study during the 1957 Asian influenza epidemic. Am J Epidemiol. 1970;92(4):240–247. doi: 10.1093/oxfordjournals.aje.a121203. [DOI] [PubMed] [Google Scholar]
- 48.Hope-Simpson RE. Age and secular distributions of virus-proven influenza patients in successive epidemics 1961–1976 in Cirencester: epidemiological significance discussed. J Hyg (Lond) 1984;92(3):303–336. doi: 10.1017/s0022172400064548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Camacho A, Ballesteros S, Graham AL, et al. Explaining rapid reinfections in multiple-wave influenza outbreaks: Tristan da Cunha 1971 epidemic as a case study. Proc Biol Sci. 2011;278(1725):3635–3643. doi: 10.1098/rspb.2011.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glezen WP, Keitel WA, Taber LH, et al. Age distribution of patients with medically-attended illnesses caused by sequential variants of influenza A/H1N1: comparison to age-specific infection rates, 1978–1989. Am J Epidemiol. 1991;133(3):296–304. doi: 10.1093/oxfordjournals.aje.a115874. [DOI] [PubMed] [Google Scholar]
- 51.Khiabanian H, Farrell GM, St George K, et al. Differences in patient age distribution between influenza A subtypes. PLoS One. 2009;4(8):e6832. doi: 10.1371/journal.pone.0006832. (doi:10.1371/journal.pone.0006832) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee BE, Mukhi SN, Drews SJ. Association between patient age and influenza A subtype during influenza outbreaks. Infect Control Hosp Epidemiol. 2010;31(5):535–537. doi: 10.1086/652159. [DOI] [PubMed] [Google Scholar]
- 53.Wu JT, Cowling BJ, Lau EH, et al. School closure and mitigation of pandemic (H1N1) 2009, Hong Kong. Emerg Infect Dis. 2010;16(3):538–541. doi: 10.3201/eid1603.091216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.