Abstract
Antihormonal and chemotherapy are standard treatments for nonorgan-confined prostate cancer. The effectivity of these therapies is limited and the development of alternative approaches is necessary. In the present study, we report on the use of the multikinase inhibitor sorafenib in a panel of prostate cancer cell lines and their derivatives which mimic endocrine and chemotherapy resistance. 3H-thymidine incorporation assays revealed that sorafenib causes a dose-dependent inhibition of proliferation of all cell lines associated with downregulation of cyclin-dependent kinase 2 and cyclin D1 expression. Apoptosis was induced at 2 μM of sorafenib in androgen-sensitive cells, whereas a higher dose of the drug was needed in castration-resistant cell lines. Sorafenib stimulated apoptosis in prostate cancer cell lines through downregulation of myeloid cell leukemia-1 (MCL-1) expression and Akt phosphorylation. Although concentrations of sorafenib required for the antitumor effect in therapy-resistant sublines were higher than those needed in parental cells, the drug showed efficacy in cells which became resistant to bicalutamide and docetaxel respectively. Most interestingly, we show that sorafenib has an inhibitory effect on androgen receptor (AR) and prostate-specific antigen expression. In cells in which AR expression was downregulated by short interfering RNA, the treatment with sorafenib increased apoptosis in an additive manner. In summary, the results of the present study indicate that there is a potential to use sorafenib in prostate cancers as an adjuvant therapy option to current androgen ablation treatments, but also in progressed prostate cancers that become unresponsive to standard therapies.
Introduction
Prostate cancer is the most common malignancy in Western countries and the second leading cause of cancer-related deaths in males (Jemal et al. 2010). Patients diagnosed with localized disease can be cured by either surgery or radiation therapy. In contrast, advanced stages of the tumor are subjected to androgen ablation treatment in order to reduce the tumor-promoting effect of androgens. Standard therapy approaches include administration of LH releasing hormone analogs, nonsteroidal antiandrogens (e.g. bicalutamide), or surgical castration. However, androgen-ablated tumors eventually develop resistance to this therapy and progress toward castration-resistant prostate cancer (CRPC), for which only palliative treatment is available. Androgen receptor (AR) was shown to play a critical role in progression of prostate cancer (Grossmann et al. 2001). Activated AR interacts with androgen response elements in the promoters of target genes including prostate-specific antigen (PSA), thereby regulating their transcription. PSA is the most frequently used marker for monitoring response to prostate cancer treatment. Chemotherapy for prostate cancer has been used for a number of years, however only limited improvement in survival was observed in CRPC with docetaxel-based therapies (Tannock et al. 2004). Nevertheless, apart from a relatively short extension of survival, ∼50% of patients initially do not respond to docetaxel treatment and are exposed to significant toxicity. Therefore, novel targeted approaches are in need to optimize the currently available therapies for patients with androgen-sensitive and CRPC.
One aim of therapies for various cancers including that of the prostate is to increase the percentage of tumor cells undergoing apoptosis. Increased expression of endogenous inhibitors of programmed cell death is one of the reasons for the development of therapy resistance. One of these inhibitors is myeloid cell leukemia-1 (MCL-1), an antiapoptotic member of the Bcl-2 family, which was originally identified as an early gene induced during differentiation of ML-1 myeloid leukemia cells (Kozopas et al. 1993). MCL-1 is overexpressed in various human malignancies and has been implicated in resistance to anticancer drugs (Craig 2002). Elevated expression of MCL-1 in prostate cancer tissue compared to normal or hyperplastic tissue or prostate intraepithelial neoplasia (Krajewska et al. 1996) suggests an involvement of this protein in tumor initiation and progression. Previously, we demonstrated the importance of MCL-1 in mediating the prosurvival activity of interleukin 6 (IL6) in prostate cancer (Cavarretta et al. 2007). In view of its active role in protecting prostate cancer cells from induction of apoptosis (Cavarretta et al. 2007), targeting MCL-1 could be considered a valid therapeutic approach.
Another potential therapy target is Akt (protein kinase B), a serine–threonine protein kinase, which plays a central role in phosphoinositide-3-kinase-mediated signaling. Its activation has been implicated in prostate cancer cell survival as well as in progression to castration resistance and refractoriness to chemotherapy (Nesterov et al. 2001). Akt is frequently activated in advanced prostate cancer due to deletion or mutation of the PTEN tumor suppressor gene (Sircar et al. 2009). In clinical and preclinical studies, overexpression and activation of Akt have been associated with high preoperative levels of PSA, higher Gleason grades, shorter relapses, and resistance to treatment (Sircar et al. 2009). Activated Akt phosphorylates and thereby inactivates its downstream target glycogen synthase kinase-3β (GSK-3β). Consequently, GSK-3β-mediated phosphorylation of MCL-1 promotes its binding to the E3 ligase β-TrCP and degradation of MCL-1 by the proteasome (Ding et al. 2007). Furthermore, it has recently been reported that Akt activity can positively regulate AR protein levels (Ha et al. 2011).
Sorafenib (Nexavar, BAY 43-9006) is an oral multikinase inhibitor that was initially developed in an attempt to block Raf kinase, a well-studied serine–threonine kinase regulating cell survival (Wilhelm et al. 2004). It was revealed that sorafenib also targets a number of receptor tyrosine kinases involved in neoangiogenesis including vascular endothelial growth factor receptor, platelet-derived growth factor receptor, FLT3, Ret, and c-Kit (Wilhelm et al. 2004). Moreover, sorafenib was found to induce apoptosis in several human cancer cell lines by downregulating the expression levels of MCL-1 (Rahmani et al. 2005). Sorafenib has shown promising preclinical activity against a variety of tumor types and is approved for the treatment of hepatocellular and renal cell carcinoma (Kane et al. 2006, Lang 2008). In prostate cancer, it was shown that sorafenib treatment has a positive outcome in clinical studies in combination with antiangiogenic agents in CRPC (Steinbild et al. 2007, Chi et al. 2008, Dahut et al. 2008). Although sorafenib is undergoing phase II clinical evaluation for treatment of prostate cancer, molecular events following inhibition of its targets and regulation of the apoptotic pathways have not been studied systematically. We also hypothesized that sorafenib has a potential in the treatment of endocrine- and chemotherapy-resistant prostate cancer.
In this study, we demonstrate that sorafenib exerts antiproliferative and proapoptotic activities in human prostate cancer cells by targeting several regulators of cell cycle progression and survival. We also evaluated the antitumor efficacy of sorafenib in bicalutamide- and docetaxel-resistant cell lines in order to test the anticancer potential of sorafenib in therapy-resistant prostate cancer.
Materials and methods
Cell lines
Prostate cancer cells PC3, LNCaP, and 22Rv1 were obtained from ATCC (Rockville, MD, USA). Cell line authenticity was confirmed by short tandem repeat analysis. The LNCaP subline LNCaP-IL6+ was derived in the presence of IL6, as described elsewhere (Hobisch et al. 2001). The therapy-resistant model LNCaP-Bic was obtained by long-term treatment of LNCaP cells with 10 pM R1881 and 1 μM bicalutamide (Hobisch et al. 2006). The LNCaP-abl subline was described previously (Culig et al. 1999). PC3-DR cells were established by continuously treating PC3 cells in a dose escalation manner with docetaxel until reaching a concentration of 12.5 nM in analogy to Patterson et al. (2006). PC3 cells were cultured in RPMI 1640 containing 10% FCS, 1% antibiotics, and glutaMax. For LNCaP and 22Rv1 cell lines, media were additionally supplemented with 1 mM sodium pyruvate, 4.5 g/l glucose, and 10 mM HEPES buffer (pH 7.2). LNCaP-IL6+ cells were maintained in the presence of 5 ng/ml of IL6. PC3-DR cells were cultured in RPMI 1640 containing 10% FCS, 1% antibiotics, and glutaMax supplemented with 12.5 nM docetaxel. All treatments with sorafenib were performed for 48 h in modified HITES medium (RPMI medium supplemented with 10 nM hydrocortisone, 10 nM estradiol, and 1× insulin–transferrin–selenium (Life Technologies, Vienna, Austria)).
Chemicals and plasmids
Sorafenib tosylate (BAY 43-9006) was provided by Bayer and dissolved in dimethylsulfoxide (DMSO) to a stock concentration of 10 mM. Bicalutamide (Casodex was kindly provided by Astrazeneca (Macclesfield, UK) and dissolved in DMSO to a stock concentration of 10 mM. Controls were treated with the corresponding volume of the vehicle. The MCL-1 expression vector was purchased from OriGene (Rockville, MD, USA).
Proliferation assays
LNCaP-IL6+, LNCaP-Bic, PC3, and PC3-DR cells were seeded at a density of 6×103 per well, and LNCaP and 22Rv1 cells were seeded at a density of 1×104 per well in triplicates onto 96 well plates. Plates for LNCaP cells were previously coated with poly-d-lysine hydrobromide (30 μg/ml; Sigma–Aldrich). On the next day, the cells were treated with increasing concentrations of sorafenib (0–2 μM) alone or in combination with docetaxel or bicalutamide for 48 h in modified HITES medium. The cells were incubated for the last 16 h of treatment with 37 kBq/well 3H-thymidine and DNA was measured as described before (Puhr et al. 2010).
Western blotting
Western blot analysis was performed as described previously (Cavarretta et al. 2007). The following antibodies were used for western blots: anti-MCL-1 (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:100 000; Chemicon InternationalInc., Billerica, MA, USA), anti-phospho Akt (S473; 1:1000; Cell Signaling Technology, Danvers, MA, USA), anti-Akt (1:1000; Cell Signaling Technology), anti-phospho GSK-3β (S9; 1:500; Cell Signaling Technology), anti-GSK-3β (1:1000; Cell Signaling Technology), anti-AR (1:500; Santa Cruz Biotechnology), anti-cyclin-dependent kinase 2 (CDK2; 1:1000; Santa Cruz Biotechnology), and anti-cyclin D1 (1:1000; Neomarkers Inc., Fremont, CA, USA).
Short interfering RNA transfection
LNCaP and 22Rv1 cells were plated at low density in the presence of 10% FCS onto six well tissue culture plates previously coated with poly-d-lysine hydrobromide (30 μg/ml, for experiments with LNCaP cells; Sigma–Aldrich). One day later, the cells were transfected using Lipofectamine 2000 in serum- and antibiotics-free medium with 10 nM ligand-binding domain (LBD) short interfering RNA (siRNA) according to the manufacturer's protocol (Invitrogen). The target sequence for AR LBD was published previously (Desiniotis et al. 2010). A nontargeting siRNA pool was used as a negative control and purchased from Dharmacon (Lafayette, CO, USA). Six hours after transfection, medium was changed to full growth conditions for overnight. On the next day, treatment with sorafenib (2 μM) was performed for 48 h in serum-free HITES medium. Cells were harvested for western blot analysis and caspase 3/7 activity assay.
Apoptosis assay
Cells were seeded onto six wells and treated with sorafenib (0–4 μM) alone or in combination as described above. After 48 h, the cells were harvested and centrifuged. Apoptosis was measured by using the PE Annexin V Apoptosis Detection Kit I in combination with flow cytometry (Becton Dickinson, Schwechat, Austria) according to the manufacturer's protocols. Assays for caspase 3/7 activity were performed with the Caspase-Glo 3/7 assay kit (Promega) according to the manufacturer's protocols (Santer et al. 2011).
PSA measurements
Supernatants of LNCaP and LNCaP-Bic cells after the treatment with sorafenib or bicalutamide for 48 h were collected and PSA concentration was determined on an Advia Centaur XP Immunoassay System (Siemens, Vienna, Austria). The cells were trypsinized and counted with a Casy Counter (Schärfe System GmbH, Reutlingen, Germany). Secreted PSA concentrations were normalized to cell number.
Statistical analysis
Student's t-test was used to assess significant differences between the control and the indicated treated group and was encoded as follows: *P<0.05; **P<0.01; ***P<0.001.
Results
Sorafenib inhibits proliferation of prostate cancer cells in a dose-dependent manner and targets cell cycle control proteins
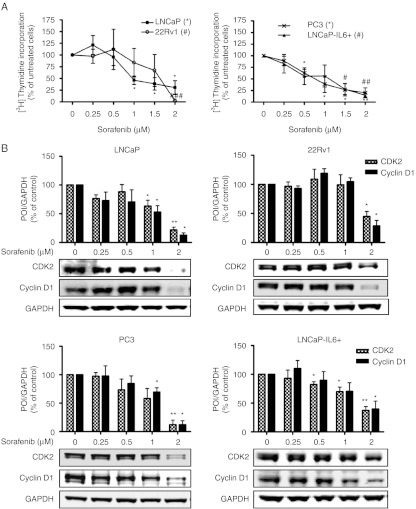
In the first attempt we analyzed the consequences of sorafenib treatment on prostate cancer cell proliferation and expression of cell cycle regulatory proteins. AR-positive (LNCaP and 22Rv1) and -negative (PC3 and LNCaP-IL6+) cell lines were cultured in the presence of increasing doses of sorafenib for 48 h. Proliferation was analyzed using 3H-thymidine incorporation assay and protein expression was determined by western blotting. An inhibitory effect of sorafenib on proliferation of androgen-sensitive as well as castration-resistant cell lines in a dose-dependent manner was observable (Fig. 1A). Interestingly, subphysiological concentrations of sorafenib (0.5–2 μM; Wilhelm et al. 2004) were sufficient to reduce proliferation of LNCaP, PC3, and LNCaP-IL6+ cells significantly. Sensitivity of 22Rv1 to sorafenib was slightly decreased compared to other cell lines analyzed. Moreover, we observed a dose-dependent downregulation of cell cycle regulators CDK2 and cyclin D1 in all cell lines after 48 h of treatment (Fig. 1B), thus supporting the antiproliferative role of sorafenib.
Figure 1.
Dose-dependent inhibition of proliferation in prostate cancer cell lines by sorafenib. LNCaP, 22Rv1, PC3, and LNCaP-IL6+ cells were exposed to increasing concentrations of sorafenib in HITES medium for 48 h. (A) Proliferation was assessed by 3H-thymidine incorporation. (B) Protein expression of CDK2 and cyclin D1 was detected by western blotting. Bands were scanned densitometrically and normalized to expression levels of GAPDH. Representative western blots from at least three independent experiments are shown. (A and B) Statistical significances are calculated against the DMSO-treated cells and values indicated are mean±s.e.m., n≥3. */# P<0.05; **/## P<0.01; ***/### P<0.001. POI, protein of interest.
Sorafenib induces apoptosis in prostate cancer cells and downregulates MCL-1 and the Akt pathway
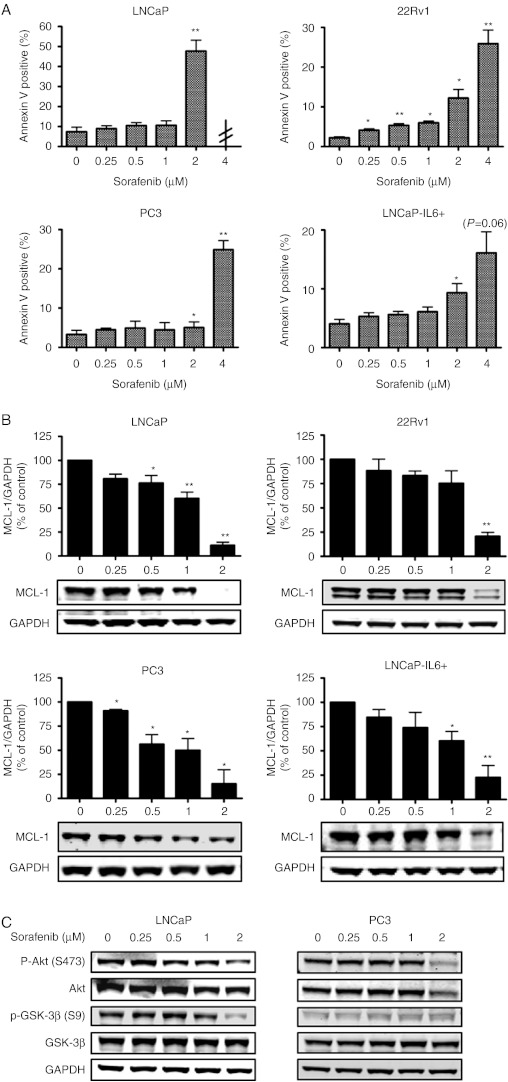
To corroborate a possible apoptosis-inducing effect of sorafenib on prostate cancer cells, we performed flow cytometry using annexin V staining and caspase 3/7 activity assays (Fig. 2A and Supplementary Figure 1, see section on supplementary data given at the end of this article). Cells were exposed to increasing concentrations (0–4 μM) of sorafenib for 48 h. A physiological concentration of sorafenib (2 μM) was sufficient to induce apoptosis in LNCaP and 22Rv1 cells significantly, while 4 μM of sorafenib were required in PC3 and LNCaP-IL6+ cells. LNCaP cells treated with 4 μM sorafenib underwent massive apoptosis resulting in an insufficient number of cells to perform assays. Taken together, these results demonstrate that AR-positive cell lines are more responsive to sorafenib-induced apoptosis than their counterparts which do not express the AR.
Figure 2.
Differential sensitivities of prostate cancer cell lines to sorafenib-mediated apoptosis through downregulation of MCL-1 and Akt pathway. LNCaP, 22Rv1, PC3, and LNCaP-IL6+ cells were exposed to increasing concentrations of sorafenib in HITES medium for 48 h. (A) Apoptosis was determined by PE/Annexin V staining and flow cytometry. LNCaP cells treated with 4 μM sorafenib underwent massive apoptosis resulting in an insufficient number of cells to perform assays. (B) Expression of MCL-1 was determined by western blotting. Bands were scanned densitometrically and normalized to expression levels of GAPDH. (C) Expression levels and phosphorylation status of Akt and GSK-3β in LNCaP and PC3 cells were determined by western blotting. GAPDH served as loading control. (A and B) Statistical significances are calculated against the DMSO-treated cells and values indicated are mean±s.e.m., n≥3. * P<0.05; ** P<0.01; *** P<0.001. Representative western blots from at least three independent experiments are shown.
The antiapoptotic protein MCL-1 has been identified as one of the main targets of sorafenib in several cancers (Rahmani et al. 2005). Western blotting was performed to investigate whether MCL-1 is implicated in sorafenib-mediated apoptosis in prostate cancer cell lines. As shown in Fig. 2B, all cell lines expressed MCL-1 protein and sorafenib reduced its expression in a dose-dependent manner. In order to further study the role of MCL-1 in the induction of cell death by sorafenib, we have transfected PC3 cells with the MCL-1 expression vector and determined caspase 3/7 activity after treatment with sorafenib (Supplementary Figure 2, see section on supplementary data given at the end of this article). We confirmed overexpression of MCL-1, however the definitive answer to this question could not be given since 4 μM of sorafenib treatment were sufficient to decrease MCL-1 expression.
We examined whether sorafenib can regulate phosphorylation of Akt and its direct downstream target GSK-3β in LNCaP and PC3 cells. Indeed, Akt phosphorylation at S473 was decreased by sorafenib in both cell lines as shown by western blot (Fig. 2C). Additionally, PC3 cells showed a decreased expression of nonphosphorylated Akt. Consequently, a reduced phosphorylation of GSK-3β was observable in LNCaP, while total GSK-3β expression was unaffected. In PC3 cells, GSK-3β phosphorylation at S9 was less prominent and nonphosphorylated GSK-3β was not influenced by sorafenib. Together, our data suggest that sorafenib is able to inactivate signaling through the Akt pathway.
Inhibitory effects of sorafenib in therapy-resistant models of human prostate cancer
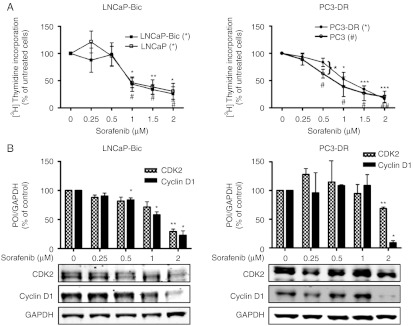
Next, we evaluated the effects of sorafenib in the therapy-resistant cell models LNCaP-Bic and PC3-DR. Both cell lines that represent bicalutamide- or docetaxel-resistant prostate cancer were treated with increasing concentrations of sorafenib (0–2 μM; Fig. 3). LNCaP-Bic cells showed the same sensitivity with regard to growth inhibition as measured by 3H-thymidine incorporation and downregulation of CDK2 and cyclin D1 by sorafenib as parental LNCaP cells. Compared to PC3 cells, a decreased sensitivity of the PC3-DR derivative to low concentrations of sorafenib (0.5–1 μM) was observed, whereas doses higher than 1 μM resulted in a similar inhibition of proliferation and decrease of CDK2 and cyclin D1. We hypothesized that docetaxel potentiates the effect of sorafenib in parental PC3 cells. Interestingly, there was no concentration-dependent effect of addition of docetaxel after sorafenib on proliferation and apoptosis of PC3 cells (Supplementary Figure 3, see section on supplementary data given at the end of this article).
Figure 3.
Antiproliferative effects of sorafenib in therapy-resistant models of human prostate cancer. LNCaP-Bic and PC3-DR were exposed to increasing concentrations of sorafenib in HITES medium for 48 h. (A) Proliferation was assessed by 3H-thymidine incorporation. For comparison purposes results from Fig. 1A (LNCaP and PC3) are shown again. (B) Protein expression of CDK2 and cyclin D1 was detected by western blotting. Bands were scanned densitometrically and normalized to expression levels of GAPDH. Representative western blots from at least three independent experiments are shown. Statistical significances are calculated against the DMSO-treated cells and values indicated are mean±s.e.m., n≥3. */# P<0.05; **/## P<0.01; ***/### P<0.001. POI, protein of interest.
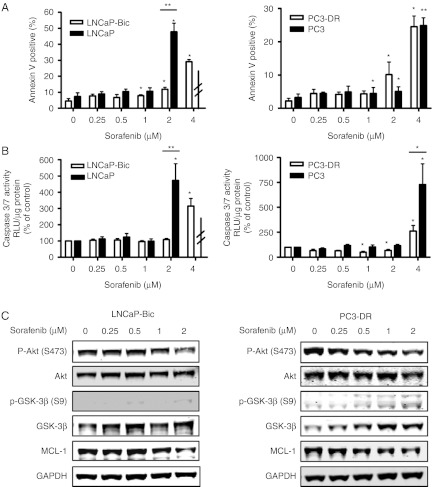
On the other hand, in apoptosis assays both models showed different responses to sorafenib compared to parental cells (Fig. 4A and B). The concentration of 4 μM sorafenib was in need to induce apoptosis in LNCaP-Bic cells, while 2 μM was sufficient for parental LNCaP cells. Similarly, the PC3-DR subline showed a decreased sensitivity to sorafenib compared to parental PC3 cells. Again, expression levels of MCL-1 and phosphorylated and total Akt and GSK-3β were analyzed (Fig. 4C and Supplementary Figure 4, see section on supplementary data given at the end of this article). In both cell lines, phosphorylation of Akt was reduced by higher sorafenib concentrations. Interestingly, phosphorylation of GSK-3β was completely lost in LNCaP-Bic leading to the hypothesis for a role for GSK-3β in therapy resistance development. Altogether, these results show a decreased sensitivity of the therapy-resistant cell models to sorafenib compared to parental cell lines.
Figure 4.
Therapy-resistant cells have decreased apoptotic sensitivity to sorafenib. LNCaP-Bic and PC3-DR were exposed to increasing concentrations of sorafenib in HITES medium for 48 h. (A) Apoptosis was determined by PE/Annexin V staining and flow cytometry. For comparison purposes results from Fig. 2A (LNCaP and PC3) are shown again. (B) Activity of the executioner caspases 3 and 7 after addition of the specific substrate. (C) Expression levels and phosphorylation status of MCL-1, Akt, and GSK-3β were determined by western blotting. Representative western blots from at least three independent experiments are shown. (A and B) Statistical significances are calculated against the DMSO-treated cells or parental cells and values indicated are mean±s.e.m., n≥3. * P<0.05; ** P<0.01; *** P<0.001. RLU, relative light units.
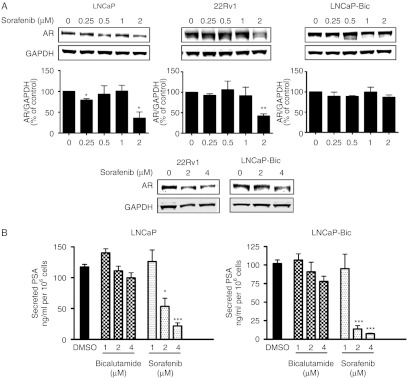
Sorafenib inhibits expression of AR and reduces PSA levels in androgen-sensitive cell lines
Modulation of AR signaling by the Her-2 tyrosine kinase has been reported (Craft et al. 1999). However, little is known about the regulation of AR signaling by tyrosine kinase inhibitors. LNCaP cells were more sensitive to sorafenib than LNCaP-Bic or LNCaP-abl cells (Fig. 4 and Supplementary Figure 5, see section on supplementary data given at the end of this article). Increased AR expression in LNCaP-abl cells was demonstrated in a previous publication of our laboratory (Culig et al. 1999). Thus, we hypothesized that AR is a target of sorafenib in prostate cancer cells. To clarify possible effects of sorafenib on AR, receptor expression levels were measured in LNCaP, 22Rv1, and LNCaP-Bic cells (Fig. 5A). In LNCaP and 22Rv1 cells, AR levels were decreased in the presence of 2 μM sorafenib (Fig. 5A). In LNCaP-Bic cells which express higher levels of AR, AR protein level was downregulated only by 4 μM of sorafenib. Moreover, concentration of secreted PSA was measured in all but the CRPC cell line 22Rv1 that lack detectable levels of secreted PSA under basal culture conditions (Tepper et al. 2002; Fig. 5B). In both LNCaP and LNCaP-Bic cells, secreted PSA levels were dramatically reduced in the presence of sorafenib. Intriguingly, sorafenib showed a higher ability to decrease PSA than bicalutamide at the same concentrations (1–4 μM).
Figure 5.
Sorafenib suppresses AR expression and decreases PSA secretion. (A) LNCaP, 22Rv1, and LNCaP-Bic were exposed to increasing concentration of sorafenib in HITES medium for 48 h. (A) Protein expression of AR was detected by western blotting. Bands were scanned densitometrically and normalized to expression levels of GAPDH. Representative western blots from at least three independent experiments are shown. (B) LNCaP and LNCaP-Bic cells were treated with sorafenib or bicalutamide in HITES medium for 48 h. Secreted PSA in the supernatants was measured and normalized to the respective cell number. (A and B) Statistical significances are calculated against the DMSO-treated cells and values indicated are mean±s.e.m., n≥3. * P<0.05; ** P<0.01; *** P<0.001.
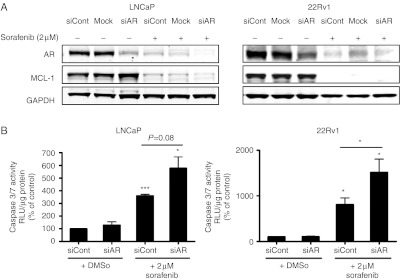
Downregulation of AR by siRNA enhances sorafenib-induced increase of caspase 3/7 activity
In regard to a possible clinical application of sorafenib for prostate cancer in combination with existing androgen-ablation therapies, we analyzed whether AR inhibition and sorafenib treatment have an additive effect. LNCaP and 22Rv1 cells were transfected with 10 nM AR–LBD siRNA or control siRNA and treated with 2 μM of sorafenib or vehicle (Fig. 6A). AR–LBD siRNA efficiently downregulated AR expression levels by 70–90% but did not affect expression levels of MCL-1. In the presence of 2 μM of sorafenib, both AR and MCL-1 were downregulated as expected. AR expression was almost absent in the specific siRNA- and sorafenib-treated samples. Apoptosis was induced in both cell lines after 48 h of sorafenib treatment as measured by caspase 3/7 assays (Fig. 6B). Moreover, a significant increase of apoptosis could be observed in 22Rv1 cells with decreased AR expression levels and treated with sorafenib compared to cells with reduced AR expression only. In contrast to the experiments in which AR was downregulated by siRNA, cotreatment of LNCaP cells with sorafenib and bicalutamide did not cause additional inhibition of proliferation or stimulation of apoptosis (Supplementary Figure 3, see section on supplementary data given at the end of this article).
Figure 6.
Downregulation of AR by siRNA enhances apoptotic sensitivity of androgen-sensitive cells to sorafenib. LNCaP and 22Rv1 cells were transfected with 10 nM AR LBD siRNA and exposed on the next day to 2 μM sorafenib or DMSO in HITES medium for 48 h. (A) Protein expression of AR was detected by western blotting. GAPDH served as a loading control. Representative western blots from at least three independent experiments are shown. (B) Activity of the executioner caspases 3 and 7 after addition of the specific substrate. Values indicated are mean±s.e.m., n≥3. * P<0.05; ** P<0.01; *** P<0.001. RLU, relative light units.
Altogether, these data demonstrate that inhibition of AR expression and sorafenib treatment have additive effects in apoptosis induction.
Discussion
In this study, we evaluated the therapeutic potential of sorafenib on several preclinical models of advanced prostate cancer including antiandrogen- and chemotherapy-resistant sublines. Our results demonstrated that physiological concentrations of sorafenib induce a dose-dependent inhibition of proliferation by downregulating key G1/S transition proteins CDK2 and cyclin D1 in all cell lines. Furthermore, sorafenib treatment enhanced apoptosis by targeting the Akt/GSK-3β prosurvival pathway and the antiapoptotic MCL-1. The antitumor activity of sorafenib by similar underlying molecular mechanisms in parental as well as in therapy-resistant cell lines indicates that sorafenib could be considered as an adjuvant treatment option in combination with current androgen ablation therapies, but could also have beneficial effects in the progressed stages of therapy-resistant prostate cancer.
In vitro potential of sorafenib in therapy-resistant prostate cancer is determined by inhibition of AR expression
Interestingly, AR-positive cell lines LNCaP and 22Rv1 were more responsive to sorafenib-induced apoptosis than LNCaP-IL6+ or PC3 cells. Furthermore, 22Rv1 cells showed a diminished increase of apoptotic cells in comparison to LNCaP after treatment with 2 μM of sorafenib. An explanation for this could be the fact that CRPC 22Rv1 cells display a decreased sensitivity to androgen in comparison to LNCaP due to an insertional mutation in the AR locus (Tepper et al. 2002). 22Rv1 cells express low levels of PSA mRNA and do not express detectable levels of PSA protein in androgen-depleted medium or after androgenic stimulation (Tepper et al. 2002). The AR pathway may be less important for the survival of 22Rv1 cells compared to LNCaP cells, thus explaining the difference in sensitivity to sorafenib with regard to apoptosis. The different responsiveness of androgen-sensitive and -insensitive cells could be explained by our findings obtained in experiments in which we investigated regulation of the AR signaling pathway by sorafenib. In this study, we report for the first time that sorafenib suppressed AR protein expression and decreased PSA levels. It is interesting to note that the dual epidermal growth factor receptor/Her-2 inhibitor PKI-166 reduced AR expression and transcriptional activity (Mellinghoff et al. 2004). It is established that cancer progression toward castration resistance occurs in the presence of a functional androgen signaling pathway (Feldman & Feldman 2001). AR overexpression may occur due to AR gene amplification or increased stabilization of its mRNA or protein (Visakorpi et al. 1995). The state-of-the-art antiandrogen therapy is based on administration of AR antagonists such as hydroxyflutamide or bicalutamide. The use of these agents may be compromised because of emergence of receptor mutations during therapy or increased expression of cofactors which potentiate agonistic effects of hydroxyflutamide, such as CREB-binding protein (CBP) or gelsolin (Culig et al. 2005). For this reason, a novel AR antagonist, such as MDV3100, which acts by a different mechanism in comparison to bicalutamide by blocking AR nuclear translocation, impairing DNA binding to androgen response elements and recruitment of coactivators, is currently being tested in clinical trials (Tran et al. 2009). In contrast to MDV3100, sorafenib diminishes AR expression. Inhibitory effects of sorafenib on expression of other steroid receptors have not been reported so far. Our data may initiate studies in other endocrine-related cancers in which possible effects of sorafenib on steroid receptors could be investigated. Although the possibility that the observed effect of sorafenib is a consequence of cell death that cannot be completely ruled out, it has to be mentioned that higher concentrations of sorafenib are required for induction of apoptosis in two LNCaP sublines which express increased AR levels (Culig et al. 1999), thus supporting the conception that AR inhibition by sorafenib precedes cell death.
Our results also justify considerations about the development of a more efficient combination therapy in prostate cancer with sorafenib as one of the compounds used. Additive effects of AR siRNA and sorafenib support the combination therapy approach and may lead to a reduction of doses of sorafenib which cause a therapeutical benefit. Interestingly, in contrast to the experiments performed with AR siRNA there was no additional effect of cotreatment of LNCaP cells with sorafenib and bicalutamide which interferes with AR function. Sorafenib has already shown enhanced antitumor activity combined with other agents such as docetaxel, vitamin K, TRAIL, or radiation treatment in multiple cancers (Huang & Sinicrope 2010, Ulivi et al. 2010, Wei et al. 2010, Yadav et al. 2011). Importantly, the combinatorial effects of sorafenib and other drugs may strongly depend on the drug sequence employed (Ulivi et al. 2010). For instance, drug metabolism may be regulated in a different manner after various drug administration sequences.
Efficiency of sorafenib in endocrine- and chemotherapy-resistant models
In order to test the hypothesis that there is a rationale for administration of sorafenib in prostate cancer that is resistant to endocrine or chemotherapy, we treated the sublines LNCaP-Bic and PC3-DR, resistant to bicalutamide and docetaxel respectively. Importantly, there was no major difference in proliferative responsiveness to sorafenib between parental and antiandrogen-resistant cells. This was not surprising since cell cycle regulatory proteins were similarly inhibited in both parental and therapy-resistant sublines. AR expression was also reduced by sorafenib in LNCaP-Bic, however higher concentrations of sorafenib were required to achieve this effect. Likewise, induction of apoptosis in the androgen-independent LNCaP-Bic subline was only observed after treatment with higher drug doses. AR expression increased in LNCaP-Bic cells in comparison to those reported in a previous study (Hobisch et al. 2006); however, higher passages of the resistant subline were used in the present work.
According to the data available in the literature, the development of docetaxel resistance in prostate cancer is a complex cell line-specific process (Madan et al. 2011). Examples of the upregulated proteins in docetaxel resistance include but are not limited to Pim-1 kinase, chemokine CCL2, and class III β tubulin (Zemskova et al. 2008, Ploussard et al. 2010, Qian et al. 2010). Identification of additional mechanisms being responsible for resistance of the sublines derived in our laboratory is at present under investigation. However, although efficacy of growth inhibition and apoptosis induction of PC3-DR is somewhat reduced compared to parental cells, it is important to note that PC3-DR could still be inhibited by sorafenib but no longer by docetaxel. This finding may have clinical implications especially when keeping in mind that the duration of docetaxel response in prostate cancer patients is limited to several months.
Antiapoptotic pathways in prostate cancer cells are inhibited by sorafenib
In concordance to findings observed in other tumors, inhibition of Akt phosphorylation by sorafenib was also seen in our experiments in LNCaP and PC3 cells (Chapuy et al. 2011). The Akt signaling pathway is frequently activated in advanced prostate cancer due to deletion or mutation of the PTEN tumor suppressor gene (Sircar et al. 2009). In cell culture models, Akt is constitutively active in LNCaP and PC3 cells due to PTEN mutation (LNCaP) or deletion (PC3; Vlietstra et al. 1998). In line with those data, Kreisberg et al. (2004) showed that phosphorylation of Akt S473 is a predictor of poor clinical outcome in prostate cancer. Moreover, it is known that the Akt downstream target GSK-3β mediates degradation of MCL-1 by the proteasome. Interestingly, differences in phosphorylation of GSK-3β in prostate cancer after sorafenib treatment were observed in a cell type-dependent manner. GSK-3β is phosphorylated and inactivated by phosphorylated Akt. Consequently, phosphorylation of GSK-3β may lead to upregulation of MCL-1 in multiple tumor cell lines and primary cancer samples (Maurer et al. 2006). As an implication of sorafenib treatment, downregulation of MCL-1 could be achieved by a decrease of total or inactivated, i.e. phosphorylated GSK-3β. It is known that MCL-1 is expressed at high levels in prostate cancer and is important for mediating a survival function of the proinflammatory cytokine IL6 (Krajewska et al. 1996, Cavarretta et al. 2007). Taken together, our results suggest the sorafenib-mediated modulation of the Akt/GSK-3β/MCL-1 pathway in prostate cancer is clinically relevant. Although the results of our overexpression experiments cannot definitively answer the question whether the presence of MCL-1 is required for the antiapoptotic effect of sorafenib in prostate cancer cells, there is an evidence in the scientific literature supporting this view. First, in K562 chronic myelogenous leukemia cells overexpression of MCL-1 inhibited sorafenib-induced apoptosis (Yu et al. 2005). In addition, in a recent study performed in androgen-insensitive prostate cancer cell lines sorafenib sensitized tumor cells to (−)-gossypol through MCL-1 inhibition (Lian et al. 2012).
The perspective for further development of sorafenib-based prostate cancer treatments
Three preclinical studies have addressed the drug response of sorafenib on prostate cancer cells in vitro (Dahut et al. 2008, Huang et al. 2010, Ullen et al. 2010). In contrast to our work, those reports were focused on antiangiogenic and cytotoxic effects of sorafenib. Moreover, they were performed in a single prostate cancer cell line using concentrations of the drug which were higher than the physiological concentrations of 2–5 μM measured in sera of patients after administration of 400 mg twice daily (Dahut et al. 2008). In one of those previous studies, decreased phosphorylation of MAP kinases by sorafenib in PC3 and DU145 cells was observed (Ullen et al. 2010) confirming the results in colon, pancreas, and breast cancer cell lines (Wilhelm et al. 2004). However, other signaling pathways were not investigated after sorafenib treatment in prostate cancer in previous reports.
Our results may have implications for development of clinical prostate cancer therapies. Tannock et al. (2004) documented that docetaxel-based chemotherapy in combination with prednisone improved median overall survival of patients with CRPC by 2.4 months. However, because of limited benefits and significant toxicity of docetaxel therapy, the search for a more efficient treatment for CRPC is continued. On the basis of a recent publication by de Bono et al. (2011) that administration of the inhibitor of androgen synthesis abiraterone in combination with prednisone in patients pretreated with docetaxel prolonged survival to 450 vs 332 days, it could be concluded that targeting the androgen signaling pathway in docetaxel-resistant prostate cancer in vivo is nevertheless a worthy therapeutic goal. The question whether a combinatorial treatment on the basis of androgenic and multiple kinase inhibition by sorafenib has a benefit in patients with therapy-resistant prostate cancer needs to be addressed in the future.
Clinical studies have reported benefits following treatment with tyrosine kinase inhibitors erlotinib and sunitinib in prostate cancer patients (Gravis et al. 2008, Sonpavde et al. 2008). In other clinical trials, the investigators reported on a small number of patients in which stabilization of the disease by sorafenib was achieved (Chi et al. 2008, Dahut et al. 2008, Steinbild et al. 2007, Aragon-Ching et al. 2009). On the other hand, difficulties in correlating clinical response and PSA measurements were observed. In the context of the final analysis of a phase II trial, Aragon-Ching et al. (2009) suggested that a selected population of patients may benefit from sorafenib treatment. The absence of adequate biomarkers for monitoring the therapeutic success may be the reason why it is difficult to match preclinical findings with clinical effects. It should be mentioned that PSA measurements in vitro could not be simply extrapolated in vivo since the patients' data also reflect the disruption of the basement membrane. In a recently reported phase II clinical trial with sorafenib and bicalutamide in patients with CRPC 47% of patients presented with either PSA decrease or stable disease (Beardsley et al. 2012). Those clinical findings could be partly explained by our results showing differences in responsiveness of prostate cancer parental cells and sublines representing advanced disease stages to sorafenib.
In summary, we demonstrate that the multitargeting effects of sorafenib induce growth inhibition and apoptosis in a variety of prostate cancer cell lines. Most importantly, we found that sorafenib affects AR expression and signaling, which is a previously unknown mechanism of sorafenib. Our data also suggest that maximal effect of sorafenib may be expected in androgen-sensitive prostate cancer prior to the development of resistance to castration and chemotherapy. However, there may be also a rationale for the use of sorafenib in docetaxel-resistant carcinoma of the prostate. The evidence for differential response of prostate cancer cell lines may explain why sorafenib is beneficial in a selected population of patients in clinical trials.
Supplementary data
This is linked to the online version of the paper at http://dx.doi.org/10.1530/ERC-11-0298.
Author contribution statement
S J Oh performed research, analyzed data, wrote the first version of the paper; H H H Erb performed research, analyzed data; A Hobisch designed research; F R Santer performed and supervised research, analyzed data, prepared the final version of the paper; Z Culig designed and supervised research, and prepared the final version of the paper. All authors have participated in writing and approved the final version of the paper.
Acknowledgements
We thank Ms Tanja Fuchs and Birgit Stenzel for PSA measurements. We are grateful to all members of the Culig laboratory for their discussions during preparation of the manuscript, Dr Walther Parson for cell authentication, Dr Dennis Healy and Mr Gerhard Briesch for providing sorafenib.
Footnotes
(F R Santer and Z Culig joint senior authors)
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by the Austrian Science Fund (FWF, grant number L544 to Z Culig), Austrian National Bank (OENB, grant number 13952 to Z Culig), and Bayer Austria. Research support by Bayer Austria (to Z Culig) was received.
References
- Aragon-Ching JB, Jain L, Gulley JL, Arlen PM, Wright JJ, Steinberg SM, Draper D, Venitz J, Jones E, Chen CC, et al. Final analysis of a phase II trial using sorafenib for metastatic castration-resistant prostate cancer. British Journal of Urology International. 2009;103:1636–1640. doi: 10.1111/j.1464-410X.2008.08327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley EK, Hotte SJ, North S, Ellard SL, Winquist E, Kollmannsberger C, Mukherjee SD, Chi KN. A phase II study of sorafenib in combination with bicalutamide in patients with chemotherapy-naive castration resistant prostate cancer. Investigational New Drugs. 2012:[in press]. doi: 10.1007/s10637-011-9722-5. [DOI] [PubMed] [Google Scholar]
- de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB, Jr, Saad F, et al. Abiraterone and increased survival in metastatic prostate cancer. New England Journal of Medicine. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavarretta IT, Neuwirt H, Untergasser G, Moser PL, Zaki MH, Steiner H, Rumpold H, Fuchs D, Hobisch A, Nemeth JA, et al. The antiapoptotic effect of IL-6 autocrine loop in a cellular model of advanced prostate cancer is mediated by Mcl-1. Oncogene. 2007;26:2822–2832. doi: 10.1038/sj.onc.1210097. [DOI] [PubMed] [Google Scholar]
- Chapuy B, Schuelper N, Panse M, Dohm A, Hand E, Schroers R, Truemper L, Wulff GG. Multikinase inhibitor sorafenib exerts cytocidal efficacy against non-Hodgkin lymphomas associated with inhibition of MAPK14 and AKT phosphorylation. British Journal of Haematology. 2011;152:401–412. doi: 10.1111/j.1365-2141.2010.08526.x. [DOI] [PubMed] [Google Scholar]
- Chi KN, Ellard SL, Hotte SJ, Czaykowski P, Moore M, Ruether JD, Schell AJ, Taylor S, Hansen C, Gauthier I, et al. A phase II study of sorafenib in patients with chemo-naive castration-resistant prostate cancer. Annals of Oncology. 2008;19:746–751. doi: 10.1093/annonc/mdm554. [DOI] [PubMed] [Google Scholar]
- Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nature Medicine. 1999;5:280–285. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- Craig RW. MCL1 provides a window on the role of the BCL2 family in cell proliferation, differentiation and tumorigenesis. Leukemia. 2002;16:444–454. doi: 10.1038/sj.leu.2402416. [DOI] [PubMed] [Google Scholar]
- Culig Z, Hoffmann J, Erdel M, Eder IE, Hobisch A, Hittmair A, Bartsch G, Utermann G, Schneider MR, Parczyk K, et al. Switch from antagonist to agonist of the androgen receptor blocker bicalutamide is associated with prostate tumour progression in a new model system. British Journal of Cancer. 1999;81:242–251. doi: 10.1038/sj.bjc.6690684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culig Z, Steiner H, Bartsch G, Hobisch A. Mechanisms of endocrine therapy-responsive and -unresponsive prostate tumours. Endocrine-Related Cancer. 2005;12:229–244. doi: 10.1677/erc.1.00775a. [DOI] [PubMed] [Google Scholar]
- Dahut WL, Scripture C, Posadas E, Jain L, Gulley JL, Arlen PM, Wright JJ, Yu Y, Cao L, Steinberg SM, et al. A phase II clinical trial of sorafenib in androgen-independent prostate cancer. Clinical Cancer Research. 2008;14:209–214. doi: 10.1158/1078-0432.CCR-07-1355. [DOI] [PubMed] [Google Scholar]
- Desiniotis A, Schafer G, Klocker H, Eder IE. Enhanced antiproliferative and proapoptotic effects on prostate cancer cells by simultaneously inhibiting androgen receptor and cAMP-dependent protein kinase A. International Journal of Cancer. 2010;126:775–789. doi: 10.1002/ijc.24806. [DOI] [PubMed] [Google Scholar]
- Ding Q, He X, Hsu JM, Xia W, Chen CT, Li LY, Lee DF, Liu JC, Zhong Q, Wang X, et al. Degradation of Mcl-1 by beta-TrCP mediates glycogen synthase kinase 3-induced tumor suppression and chemosensitization. Molecular and Cellular Biology. 2007;27:4006–4017. doi: 10.1128/MCB.00620-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nature Reviews. Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- Gravis G, Bladou F, Salem N, Goncalves A, Esterni B, Walz J, Bagattini S, Marcy M, Brunelle S, Viens P. Results from a monocentric phase II trial of erlotinib in patients with metastatic prostate cancer. Annals of Oncology. 2008;19:1624–1628. doi: 10.1093/annonc/mdn174. [DOI] [PubMed] [Google Scholar]
- Grossmann ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. Journal of the National Cancer Institute. 2001;93:1687–1697. doi: 10.1093/jnci/93.22.1687. [DOI] [PubMed] [Google Scholar]
- Ha S, Ruoff R, Kahoud N, Franke TF, Logan SK. Androgen receptor levels are upregulated by Akt in prostate cancer. Endocrine-Related Cancer. 2011;18:245–255. doi: 10.1530/ERC-10-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobisch A, Ramoner R, Fuchs D, Godoy-Tundidor S, Bartsch G, Klocker H, Culig Z. Prostate cancer cells (LNCaP) generated after long-term interleukin 6 (IL-6) treatment express IL-6 and acquire an IL-6 partially resistant phenotype. Clinical Cancer Research. 2001;7:2941–2948. [PubMed] [Google Scholar]
- Hobisch A, Fritzer A, Comuzzi B, Fiechtl M, Malinowska K, Steiner H, Bartsch G, Culig Z. The androgen receptor pathway is by-passed in prostate cancer cells generated after prolonged treatment with bicalutamide. Prostate. 2006;66:413–420. doi: 10.1002/pros.20365. [DOI] [PubMed] [Google Scholar]
- Huang S, Sinicrope FA. Sorafenib inhibits STAT3 activation to enhance TRAIL-mediated apoptosis in human pancreatic cancer cells. Molecular Cancer Therapeutics. 2010;9:742–750. doi: 10.1158/1535-7163.MCT-09-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Chen XQ, Huang Y, Chen N, Zeng H. The multikinase inhibitor sorafenib induces caspase-dependent apoptosis in PC-3 prostate cancer cells. Asian Journal of Andrology. 2010;12:527–534. doi: 10.1038/aja.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: A Cancer Journal for Clinicians. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Kane RC, Farrell AT, Saber H, Tang S, Williams G, Jee JM, Liang C, Booth B, Chidambaram N, Morse D, et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clinical Cancer Research. 2006;12:7271–7278. doi: 10.1158/1078-0432.CCR-06-1249. [DOI] [PubMed] [Google Scholar]
- Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. PNAS. 1993;90:3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewska M, Krajewski S, Epstein JI, Shabaik A, Sauvageot J, Song K, Kitada S, Reed JC. Immunohistochemical analysis of bcl-2, bax, bcl-X, and mcl-1 expression in prostate cancers. American Journal of Pathology. 1996;148:1567–1576. [PMC free article] [PubMed] [Google Scholar]
- Kreisberg JI, Malik SN, Prihoda TJ, Bedolla RG, Troyer DA, Kreisberg S, Ghosh PM. Phosphorylation of Akt (Ser473) is an excellent predictor of poor clinical outcome in prostate cancer. Cancer Research. 2004;64:5232–5236. doi: 10.1158/0008-5472.CAN-04-0272. [DOI] [PubMed] [Google Scholar]
- Lang L. FDA approves sorafenib for patients with inoperable liver cancer. Gastroenterology. 2008;134:379. doi: 10.1053/j.gastro.2007.12.037. [DOI] [PubMed] [Google Scholar]
- Lian J, Ni Z, Dai X, Su C, Smith AR, Xu L, He F. Sorafenib sensitizes (−)-gossypol-induced growth suppression in androgen-independent prostate cancer cells via Mcl-1 inhibition and Bak activation. Molecular Cancer Therapeutics. 2012;11:416–426. doi: 10.1158/1535-7163.MCT-11-0559. [DOI] [PubMed] [Google Scholar]
- Madan RA, Pal SK, Sartor O, Dahut WL. Overcoming chemotherapy resistance in prostate cancer. Clinical Cancer Research. 2011;17:3892–3902. doi: 10.1158/1078-0432.CCR-10-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Molecular Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Mellinghoff IK, Vivanco I, Kwon A, Tran C, Wongwipat J, Sawyers CL. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6:517–527. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Nesterov A, Lu X, Johnson M, Miller GJ, Ivashchenko Y, Kraft AS. Elevated AKT activity protects the prostate cancer cell line LNCaP from TRAIL-induced apoptosis. Journal of Biological Chemistry. 2001;276:10767–10774. doi: 10.1074/jbc.M005196200. [DOI] [PubMed] [Google Scholar]
- Patterson SG, Wei S, Chen X, Sallman DA, Gilvary DL, Zhong B, Pow-Sang J, Yeatman T, Djeu JY. Novel role of Stat1 in the development of docetaxel resistance in prostate tumor cells. Oncogene. 2006;25:6113–6122. doi: 10.1038/sj.onc.1209632. [DOI] [PubMed] [Google Scholar]
- Ploussard G, Terry S, Maille P, Allory Y, Sirab N, Kheuang L, Soyeux P, Nicolaiew N, Coppolani E, Paule B, et al. Class III beta-tubulin expression predicts prostate tumor aggressiveness and patient response to docetaxel-based chemotherapy. Cancer Research. 2010;70:9253–9264. doi: 10.1158/0008-5472.CAN-10-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhr M, Santer FR, Neuwirt H, Marcias G, Hobisch A, Culig Z. SOCS-3 antagonises the proliferative and migratory effects of fibroblast growth factor-2 in prostate cancer by inhibition of p44/p42 MAPK signalling. Endocrine-Related Cancer. 2010;17:525–538. doi: 10.1677/ERC-10-0007. [DOI] [PubMed] [Google Scholar]
- Qian DZ, Rademacher BL, Pittsenbarger J, Huang CY, Myrthue A, Higano CS, Garzotto M, Nelson PS, Beer TM. CCL2 is induced by chemotherapy and protects prostate cancer cells from docetaxel-induced cytotoxicity. Prostate. 2010;70:433–442. doi: 10.1002/pros.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani M, Davis EM, Bauer C, Dent P, Grant S. Apoptosis induced by the kinase inhibitor BAY 43-9006 in human leukemia cells involves down-regulation of Mcl-1 through inhibition of translation. Journal of Biological Chemistry. 2005;280:35217–35227. doi: 10.1074/jbc.M506551200. [DOI] [PubMed] [Google Scholar]
- Santer FR, Höschele PP, Oh SJ, Erb HH, Bouchal J, Cavarretta IT, Parson W, Meyers DJ, Cole PA, Culig Z. Inhibition of the acetyltransferases p300 and CBP reveals a targetable function for p300 in the survival and invasion pathways of prostate cancer cell lines. Molecular Cancer Therapeutics. 2011;10:1644–1645. doi: 10.1158/1535-7163.MCT-11-0182. [DOI] [PubMed] [Google Scholar]
- Sircar K, Yoshimoto M, Monzon FA, Koumakpayi IH, Katz RL, Khanna A, Alvarez K, Chen G, Darnel AD, Aprikian AG, et al. PTEN genomic deletion is associated with p-Akt and AR signalling in poorer outcome, hormone refractory prostate cancer. Journal of Pathology. 2009;218:505–513. doi: 10.1002/path.2559. [DOI] [PubMed] [Google Scholar]
- Sonpavde G, Hutson TE, Berry WR, Boehm KA, Asmar L. Phase II trial of sunitinib for the therapy of progressive metastatic castration-refractory prostate cancer after previous docetaxel chemotherapy. Clinical Genitourinary Cancer. 2008;6:134–137. doi: 10.3816/CGC.2008.n.023. [DOI] [PubMed] [Google Scholar]
- Steinbild S, Mross K, Frost A, Morant R, Gillessen S, Dittrich C, Strumberg D, Hochhaus A, Hanauske AR, Edler L, et al. A clinical phase II study with sorafenib in patients with progressive hormone-refractory prostate cancer: a study of the CESAR Central European Society for Anticancer Drug Research – EWIV. British Journal of Cancer. 2007;97:1480–1485. doi: 10.1038/sj.bjc.6604064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. New England Journal of Medicine. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- Tepper CG, Boucher DL, Ryan PE, Ma AH, Xia L, Lee LF, Pretlow TG, Kung HJ. Characterization of a novel androgen receptor mutation in a relapsed CWR22 prostate cancer xenograft and cell line. Cancer Research. 2002;62:6606–6614. [PubMed] [Google Scholar]
- Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullen A, Farnebo M, Thyrell L, Mahmoudi S, Kharaziha P, Lennartsson L, Grander D, Panaretakis T, Nilsson S. Sorafenib induces apoptosis and autophagy in prostate cancer cells in vitro. International Journal of Oncology. 2010;37:15–20. doi: 10.3892/ijo_00000648. [DOI] [PubMed] [Google Scholar]
- Ulivi P, Arienti C, Zoli W, Scarsella M, Carloni S, Fabbri F, Tesei A, Chiadini E, Orlandi A, Passeri D, et al. In vitro and in vivo antitumor efficacy of docetaxel and sorafenib combination in human pancreatic cancer cells. Current Cancer Drug Targets. 2010;10:600–610. doi: 10.2174/156800910791859489. [DOI] [PubMed] [Google Scholar]
- Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinanen R, Palmberg C, Palotie A, Tammela T, Isola J, Kallioniemi OP. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nature Genetics. 1995;9:401–406. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- Vlietstra RJ, van Alewijk DC, Hermans KG, van Steenbrugge GJ, Trapman J. Frequent inactivation of PTEN in prostate cancer cell lines and xenografts. Cancer Research. 1998;58:2720–2723. [PubMed] [Google Scholar]
- Wei G, Wang M, Hyslop T, Wang Z, Carr BI. Vitamin K enhancement of sorafenib-mediated HCC cell growth inhibition in vitro and in vivo. International Journal of Cancer. 2010;127:2949–2958. doi: 10.1002/ijc.25498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Research. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- Yadav A, Kumar B, Teknos TN, Kumar P. Sorafenib enhances the antitumor effects of chemoradiation treatment by downregulating ERCC-1 and XRCC-1 DNA repair proteins. Molecular Cancer Therapeutics. 2011;10:1241–1251. doi: 10.1158/1535-7163.MCT-11-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Bruzek A, Meng XW, Gores GJ, Carter CA, Kaufmann SH, Adjei AA. The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43-9006. Oncogene. 2005;24:6861–6869. doi: 10.1038/sj.onc.1208841. [DOI] [PubMed] [Google Scholar]
- Zemskova M, Sahakian E, Bashkirova S, Lilly M. The PIM1 kinase is a critical component of a survival pathway activated by docetaxel and promotes survival of docetaxel-treated prostate cancer cells. Journal of Biological Chemistry. 2008;283:20635–20644. doi: 10.1074/jbc.M709479200. [DOI] [PMC free article] [PubMed] [Google Scholar]