Abstract
Background
The use of alemtuzumab (humanized anti-CD52 monoclonal antibody) has been primarily studied in renal transplantation, and the experience of alemtuzumab induction in pancreas transplantation is still limited. The objective of this study is to analyze the outcome of pancreas transplantation by using a single dose of 30 mg alemtuzumab induction with steroid-free maintenance immunosuppression.
Methods
We performed a total 28 pancreas transplants (17 simultaneous kidney-pancreas transplantation [SPK], 5 pancreas after kidney transplantation [PAK], and 6 pancreas transplant alone [PTA]) between November 2006 and April 2010. Median follow-up was 25 months (range, 8–49 months). Maintenance immunosuppression consists of tacrolimus and mycophenolate. We analyzed patient/graft survival, graft function, and complications.
Results
One-year actuarial patient/graft survival was 100%/100% in SPK, PAK, and PTA. Three-year actuarial patient/pancreas graft survival rates for SPK, PAK, and PTA were 100%/100%, 100%/100%, and 100%/83%, respectively. Excellent pancreas and kidney graft functions were observed. Acute cellular rejection occurred in 42% of patients. Most of the rejection episode occurred approximately 1 or 6 months after transplant. Absolute lymphocyte count remained below preoperative level for 1 year posttransplant and WBC counts were significantly lower for 3 years after transplant compared with pretransplant level. Cytomegalovirus infection and bacterial infection occurred in 28% and 36% of patients, respectively. Eleven percent of patients developed donor-specific antibodies and 7% of patients experienced antibody-mediated rejection.
Conclusion
A single dose of 30 mg alemtuzumab induction with steroid-free maintenance immunosuppression achieved excellent mid-term patient and graft survival for pancreas transplantation with acceptable complication rate.
Keywords: Pancreas transplant, Alemtuzumab, Graft survival
Alemtuzumab is a humanized anti-CD52 monoclonal antibody that is aimed for treatment of chronic lymphocytic leukemia and has now been increasingly used in organ transplantation (1). CD52 is a glycoprotein expressed on approximately 95% of peripheral blood lymphocytes, natural killer cells, monocytes, macrophages, and thymocytes; therefore, almost all mononuclear cells are affected (2). There does not seem to be any effect on plasma cells and similar to other induction agents, alemtuzumab seems to spare memory type cells (3). After binding to its target, alemtuzumab causes cell death through several mechanisms including complement-mediated cytolysis, antibody-mediated cytotoxicity, and apoptosis. Although the plasma elimination half-life of alemtuzumab is approximately 12 days, its clinical effects are far more persistent (1, 4). Lymphocyte depletion of more than 99% can be seen after a single dose with varying rates of cellular recovery depending on the subpopulation of interest (5). The first reports of renal transplantation recipients treated with alemtuzumab induction with low dose cyclosporine monotherapy were described by Calne et al. (6, 7). Subsequently, alemtuzumab has increased in popularity as an induction immunosuppression for organ transplantation (1, 8–11). Alemtuzumab induction has demonstrated its ability of low-dose maintenance immunosuppression without steroid with acceptable risk of early rejection or calcineurin inhibitor and steroid free (12, 13). The majority of the clinical experience with alemtuzumab has been primarily with renal transplantation, and the experience of alemtuzumab induction for pancreas transplantation is still limited. In this report, we describe our experience with a single dose of 30 mg alemtuzumab induction with steroid-free maintenance immunosuppression in pancreas transplantation.
RESULTS
Patient and Graft Survival
One-year actuarial patient/pancreas graft survival was 100%/100% in simultaneous kidney-pancreas transplantation (SPK), pancreas after kidney transplantation (PAK), and pancreas transplant alone (PTA). Three-year actuarial patient/pancreas graft survival rates of SPK, PAK, and PTA were 100%/100%, 100%/100%, and 100%/83%, respectively. One- and 3-year kidney graft survival rates were 100% and 100%, respectively. Only one patient lost pancreas graft due to posttransplant insulin resistance (C-peptide level 5.3 ng/mL).
Graft Function
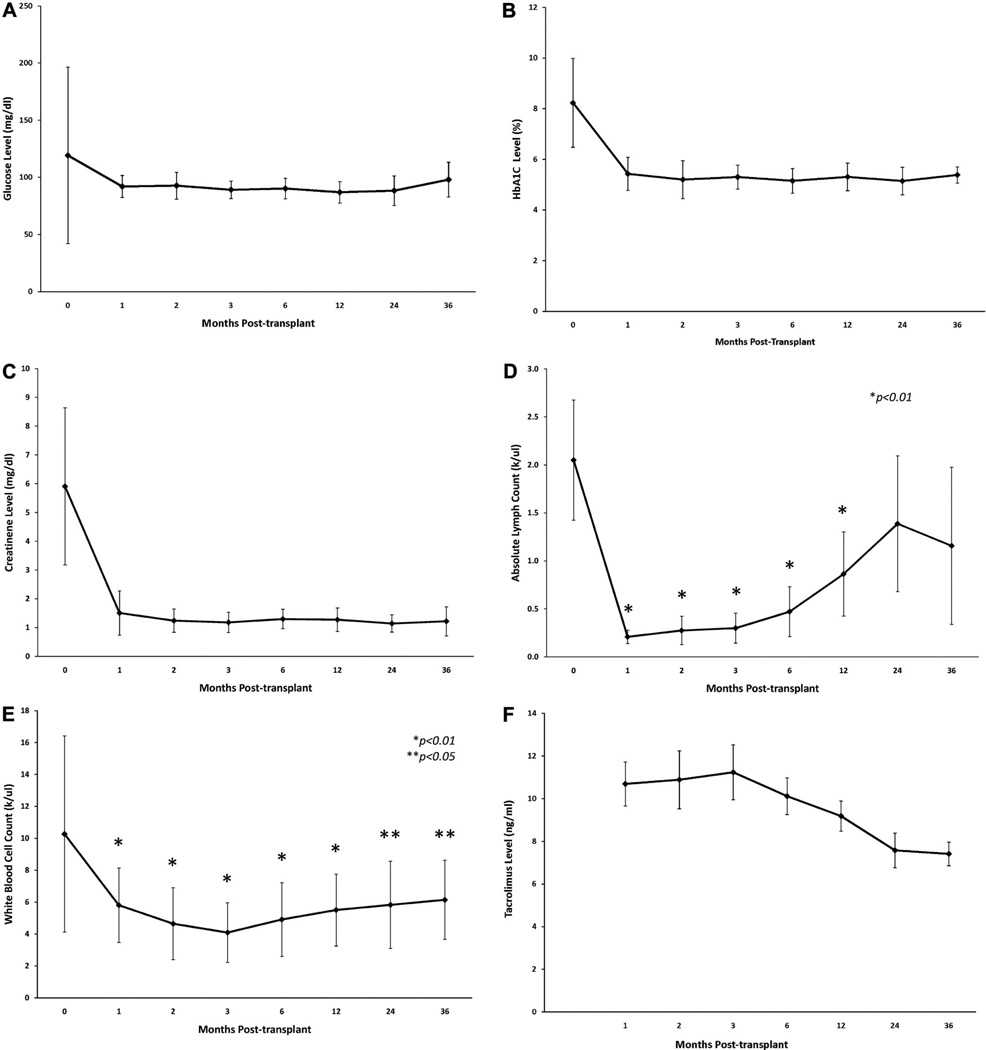
Figure 1(A) shows that the glucose levels were well maintained during the study period. One and 3 years posttransplant, C-peptide level was 2.6±1.4 ng/mL and 3.5±1.2 ng/mL, respectively. Figure 1(B) shows that posttransplant hemoglobin A1C levels also were maintained below 6% during the study period. Figure 1(C) shows that serum creatinine levels in SPK patients were stable during the study period. Creatinine levels at 1 and 3 years posttransplant were 1.51±0.77 mg/dL and 1.22±0.33 mg/dL, respectively.
FIGURE 1.
(A) Mean random glucose level posttransplant. (B) Mean hemoglobin A1C level posttransplant. (C) Mean creatinine level posttransplant. (D) Mean absolute lymphocyte counts level posttransplant *P<0.01 compared with pretransplant level of absolute lymphocyte counts. (E) Mean WBC count level posttransplant. *P<0.01 compared with pretransplant level of WBC, not absolute lymphocyte count **P<0.05 compared with pretransplant level of WBC, not absolute lymphocyte count. (F) Mean trough tacrolimus level posttransplant.
Lymphocyte Depletion and Tacrolimus Trough Level
Figure 1(D,E) shows absolute lymphocyte count and WBC count, respectively. Six patients (21%) required filgrastim (granulocyte colony stimulating factor [G-CSF]) injection for neutropenia. Absolute lymphocyte counts were remarkably decreased at 1 month posttransplant with 0.21±0.07 k/µL, but they gradually increased over the study period (Fig. 1D). Absolute lymphocyte counts were significantly lower than pretransplant levels for 1 year posttransplant (P<0.01) (Fig. 1D). WBC counts were also lower than pretransplant level for the entire study period (Fig. 1E). Tacrolimus trough level is shown in Figure 1(F). The level was maintained above 10 ng/mL for the first 3 months. Thereafter, the level gradually decreased to 7.5±2.7 ng/mL and 7.4±1.8 ng/mL at 2 and 3 years posttransplant, respectively.
Acute Cellular Rejection, Antibody-Mediated Rejection, Posttransplant Anti-Human Leukocyte Antigen Antibodies, and Donor-Specific Antibody
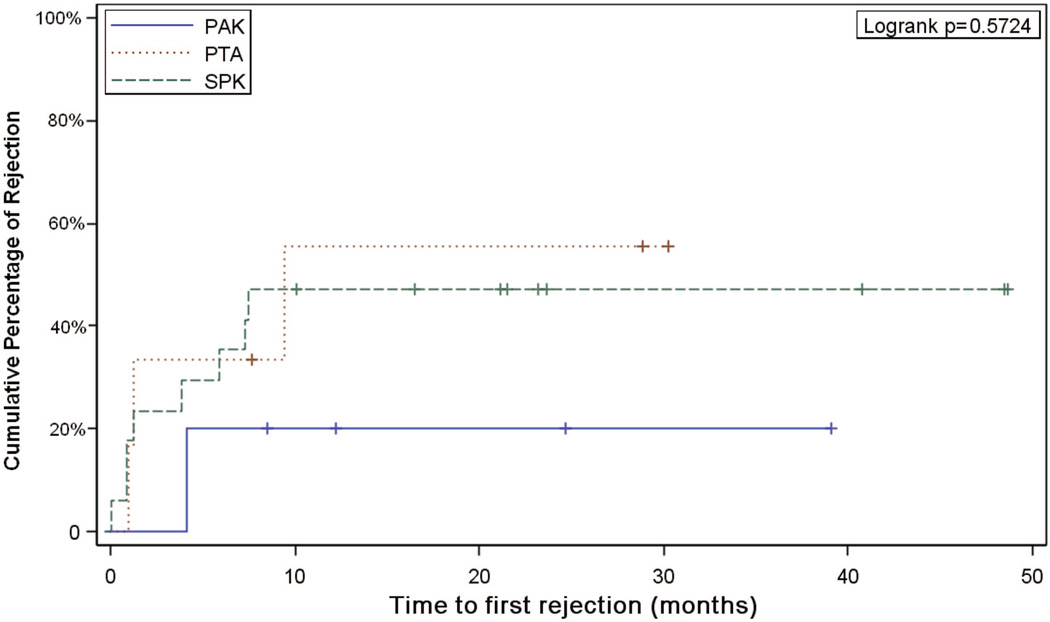
Acute cellular rejection occurred in 12 patients (11 pancreas graft and 7 kidney grafts) (42%) among 28 patients, and of these 12 patients, 8 required antibody treatment (Table 1). Most rejection episodes occurred approximately 1 or 6 months posttransplant, and median time to the first episode of rejection was 87 days (Fig. 2). Six of 12 patients who had acute rejection experienced multiple episodes of rejection. Two patients (7%) experienced antibody-mediated rejection. None of the patients had anti-human leukocyte antigen (HLA) antibody at the time of transplant. Final crossmatch was negative in all patients (complement-dependent cytotoxicity crossmatch, n = 13; T- and B-cell flow cytometry crossmatch, n = 15). During posttransplant monitoring, posttransplant panel reactive antibody was maintained at 0% in most patients except in four patients (3 donor-specific antibody [DSA] and 1 non-DSA). One patient developed DSA (anti-DQ5) at 8 months posttransplant (non-adherent with medications), and persisted despite treatments including plasma exchange. The other three patients developed anti-HLA antibodies transiently at 1 to 20 months posttransplant, but disappeared with or without intervention.
TABLE 1.
Incidence of posttransplant complication
| Complication | SPK (n=17), n (%) |
PAK (n=5), n (%) |
PTA (n=6), n (%) |
Total (n=28), n (%) |
|---|---|---|---|---|
| Acute cellular rejection | 8 (47) | 1 (20) | 3 (50) | 12 (42) |
| Antibody-mediated rejection | 2 (11) | 0 | 0 | 2 (7) |
| Donor-specific antibodies | 3 (17) | 0 | 0 | 3 (10) |
| CMV | 7 (41) | 1 (20) | 0 | 8 (28) |
| BK nephropathy | 1 (6) | 0 | n/a | 1 (5) |
| Bacterial infection | 7 (41) | 1 (20) | 2 (33) | 10 (36) |
| Neutropenia required G-CSF | 5 (29) | 0 | 1 (16) | 6 (21) |
| PTLD | 0 | 0 | 0 | 0 |
SPK, simultaneous kidney-pancreas transplantation; PAK, pancreas after kidney transplantation; PTA, pancreas transplant alone; CMV, cytomegalovirus; PTLD, posttransplant lymphoproliferative disorder.
FIGURE 2.
Actuarial incidence of biopsy-proven rejection. SPK, simultaneous kidney-pancreas transplantation; PAK, pancreas after kidney transplantation; PTA, pancreas transplant alone.
Infectious Complication
Eight patients had cytomegalovirus (CMV) infection and one patient had BK nephropathy (Table 1). Bacterial and fungal infections were diagnosed as follows: five urinary tract infections, four bacteremia, three intestinal infections, and one pneumonia. A total 10 patients (36%) experienced at least one infection during this study period. Median days to the first infection were 30 days. Most of the infectious complications were not severe except for two patients: one patient required ICU care and one patient required surgical intervention. None of the patients manifested posttransplant lymphoproliferative disorder.
DISCUSSION
There are several retrospective studies published on alemtuzumab induction on pancreas transplantation. Gruessner et al. (12) reported a nonrandomized study of 75 pancreas-kidney and solitary pancreas transplants. They received four doses of induction (30 mg administered intravenously [IV] each dose) and up to 12 doses within the first year with mycophenolate mofetil (MMF) monotherapy (2 g≥day). In their study, graft and patient survivals were not statistically different from historical control group of thymoglobulin induction with tacrolimus maintenance. However, the incidence of rejection was significantly higher in alemtuzumab with MMF monotherapy group. Kaufman et al. (10) reported alemtuzumab induction with a steroid-free protocol in simultaneous pancreas and kidney transplantation compared with thymoglobulin induction. They observed no difference in 1- and 3-year actuarial patient, pancreas or renal graft survival or function between the two groups. There is only one randomized trial report published of alemtuzumab versus thymoglobulin induction for renal and pancreas transplantation (14). In their study, alemtuzumab induction achieved similar graft survival thymoglobulin induction and less biopsy-proven acute rejection.
Corticosteroid avoidance protocol is gaining acceptance in the field of renal transplantation because of favorable long-term safety and efficacy outcome (13, 15, 16). There are limited data on the long-term outcome of steroid avoidance protocol for pancreas transplantation. Clinical monitoring of pancreas allograft status has proven to be difficult. Monitoring of serum amylase and lipase (or urinary amylase in bladder-drained pancreas allografts) is used, but these markers of pancreatic acinar injury and inflammation are inadequately sensitive and specific (17, 18). In addition, historically, pancreas allograft biopsies have been avoided because of technical difficulty and risks of complications such as bleeding and leakage of exocrine secretions (19). These difficulties of monitoring rejection may result in hesitance of steroid avoidance protocol for pancreas transplantation. Including our study, six studies of alemtuzumab induction with steroid-free protocol for pancreas transplants have been reported so far (Table 2). The graft survival of alemtuzumab induction with steroid-free protocol was similar with thymoglobulin or basiliximab induction steroid maintenance groups and rejection rate was reported 8% to 41% during the study period (Table 2). The number of doses of alemtuzumab used for induction varied from 1 to 4 doses in those reports. In this study, we used a single 30 mg dose of alemtuzumab. Most of the reports concluded that there was no difference in infectious rate compared with the other induction agents, but several studies showed higher infectious complication in alemtuzumab induction group (20, 21). Indeed, Magliocca et al. (20) altered their induction protocol to a single 30 mg dose of alemtuzumab instead of two doses due to a higher incidence of CMV infection. In this study, we also experienced 41% of CMV infection in SPK patients. CMV infections have been linked to increased risk of new onset posttransplantation diabetes (22). It is important to follow these patients in the long term.
TABLE 2.
Alemtuzumab induction with steroid-free protocol
| Reference | Study design | Organ | N | Maintenance | Steroid free | Induction and dose |
Major endpoints | Results | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Gruessner et al. (12) |
Prospective observational with historical control |
SPK/PAK/PTA | 75 | MMF | Yes | Alemtuzumab and Thymoglobulin 30 mg×4 and 1.25 mg/kg×1 vs. NR |
Patient survival Graft survival Rejection Serious infection Creatinine clearance |
No difference No difference 41% vs 9% in SPK (P > 0.0003) No difference No difference |
6 mo |
| Kaufman et al. (10) |
Retrospective review with historical control |
SPK | 50 | Tac, sirolimus | Yes | Alemtuzumab vs. Thymoglobulin 30 mg×1 vs. 1 mg/ kg×6 |
Patient survival Kidney graft survival Pancreas graft survival Rejection Viral infection |
91% vs. 92% at 3 yr 91% vs. 86% at 3 yr 92% vs. 97% at 3 yr 8% vs. 5% in 2 yr 10% vs. 40% in 3 yr |
22–41 mo |
| Thai et al. (38) |
Retrospective review |
SPK/PAK/PTA | 60 | Tac | Yes | Alemtuzumab vs. none 30 mg×1 vs. none |
Patient survival Kidney graft survival Pancreas graft survival Rejection CMV infection |
95% at 1 yr 90% at 1 yr 93% at 1 yr 30% in 18 mo 12% in study period |
17–33 mo |
| Clatworthy et al. (39) |
Retrospective review |
SPK | 54 | Tac, MMF | Yes | Alemtuzumab subQ vs. Alemtuzumab IV 30 mg subQ×2 vs. 20 mg IV×2 |
Patient survival Kidney graft survival Pancreas graft survival Rejection Adverse reaction |
100% subQ group at 1 yr 100% subQ group at 1 yr 95% subQ group at 1 yr 14% subQ group in study period No reaction in subQ group |
2 yr |
| Magliocca et al. (20) |
Retrospective review with historical control |
SPK | 105 | Tac, MMF, Pred | No | Alemtuzumab vs. Basiliximab 30 mg×2 vs. 20 mg×2 |
Patient survival Kidney graft survival Pancreas graft survival Renal rejection Pancreas rejection CMV infection |
99% vs. 95% at 2 yr 93% vs. 90% at 2 yr 91% vs. 85% at 2 yr 20% vs. 31% in 2 yr (P = 0.09) 27% vs. 18% in 2 yr (P = 0.12) 29% vs. 16% at 2 yr (P = 0.002) |
2 yr |
| Muthusamy et al. (32) |
Retrospective review with historical control |
SPK/PAK/PTA | 102 | Tac, MMF | Yes | Alemtuzumab vs. none 30 mg×2 vs. none |
Patient survival Kidney graft survival Pancreas graft survival Rejection CMV infection BK nephropathy |
97% at 1 yr 89% at 1 yr 94% at 1 yr 25% in study period 6% in study period 4% in study period |
8–41 mo |
| Pascual et al. (28) |
Retrospective review with historical control |
SPK | 97 | Tac, MMF, Pred | No | Alemtuzumab vs. Basiliximab 30 mg×2 vs. 20 mg×2 |
Same as Magliocca et al. (20) C4d negative ACR AMR |
3% vs. 15% in 2 yr (P = 0.017) 18% vs. 14% in 2 yr (P = 0.6) |
2 yr |
| Farney et al. (14) |
Randomized prospective study |
SPK/PAK | 42 | Tac, MMF, pred early steroid withdrawal or rapid steroid taper |
No | Alemtuzumab vs. Thymoglobulin 30 mg×1 vs. 1.5 mg/kg×3–7 |
Patient survival Kidney graft survival Pancreas graft survival Rejection |
88% vs. 86% 89% vs. 93% 26% vs. 36% in 3 yr (P = 0.29) |
Median 2 yr |
SPK, simultaneous kidney-pancreas transplantation; PAK, pancreas after kidney transplantation; PTA, pancreas transplant alone; CMV, cytomegalovirus; Tac, tacrolimus; MMF, mycophenolate mofetil; Pred, prednisone; ACR, acute cellular rejection; AMR, antibody-mediated rejection.
Furthermore, in this study, the total absolute lymphocyte count gradually increased after 1 month; however, it still remained below preoperative level for the first year posttransplant. We also have experienced neutropenia in as many as 21% of patients who required G-CSF injection. Neutropenia is a common complication after organ transplantation and Hartmann et al. (23) reported that leukopenia was significantly related to alemtuzumab induction with steroid-free protocol in kidney and pancreas transplantation compared with anti-thymocyte globulin induction.
Neutropenic patients seem to experience more bacterial or CMV infection compared with non-neutropenic counterparts (24). Recent analysis of United States Renal Data System also showed neutropenia was increased risk of allgraft loss and death after kidney transplantation (25). In this study, we used G-CSF in addition to reduction or temporal discontinuation of MMF or valganciclovir, because G-CSF seems to accelerate recovery of neutropenia and does not increase risk of graft loss or rejection (24–26). Considering higher rate of infection and neutropenia, we conclude that a single 30 mg dose of alemtuzumab should be sufficient dose for pancreas or simultaneous pancreas-kidney transplantation.
In this study, excellent 3-year patient and pancreas graft survival (100% and 96%) was achieved compared with the US national average of 3-year pancreas graft survival (79% in SPK, 60% in PTA, 65% in PAK) (27). Glucose control and C-peptide level were also well maintained. Furthermore, posttransplant panel reactive antibody (anti-HLA antibodies for class I and II) remained at 0% in 24 of 28 patients, although three patients of 28 patients developed DSA (one patient was noncompliant with medication). Our data show that alemtuzumab induction with steroid-free maintenance suppressed posttransplant antibody production. Our data are supported by a recent publication concluding that the cumulative incidence of antibody-mediated rejection was not different between alemtuzumab and basiliximab induction for simultaneous pancreas-kidney transplantation (14.4% vs. 18% in 2 years, respectively) (28).
A recent publication based on UNOS registry showed that alemtuzumab is more effective at preventing acute rejection during the initial hospitalization, but this benefit disappears by 6 months posttransplantation in deceased donor kidney transplantation (29). In this study, half of rejection episodes occurred approximately 1 month posttransplantation, and the other half of the rejection episodes developed approximately 6 months posttransplantation (Fig. 2). These results and the recent UNOS registry data for deceased donor kidney transplant using alemtuzumab suggest that maintenance immunosuppression plays an important role in preventing rejection after 6 months posttransplantation in patients who received alemtuzumab induction (29).
Table 2 is a summary of current publications about alemtuzumab induction for simultaneous pancreas-kidney transplantation and pancreas transplantation. All protocol showed satisfactory patient and graft survival, rejection rate, and infection rate. Five protocols were steroid-free maintenance immunosuppression and two protocols were a single dose of alemtuzumab with steroid-free maintenance immunosuppression. The rejection rate varies from 8% to 41%, whereas we experienced more than 40% acute cellular rejection. It is not clear why we had more rejection episodes compared with the other studies; one possible explanation is that we had a low threshold of routinely performing biopsies whenever creatinine or pancreatic enzymes were elevated. Needless to say, it is important to closely follow-up in the long term because acute rejection has impact on graft survival (30). Interestingly, PAK group experienced few incidence of rejection compared with SPK or PTA groups. The immune status of PAK groups may be prepared for pancreas transplantation because these patients have already been immunosuppressed for kidney transplantation.
We recognize that there are limitations to this study due to lack of the control groups because we exclusively used alemtuzumab for the study period. However, we experienced an excellent graft survival with alemtuzumab induction comparing with the US national average (27).
In summary, alemtuzumab is a reasonable induction strategy for pancreas transplantation with short- and medium-term graft and patient survival comparable with that seen with other induction agents. The optimal dose and ideal combination of maintenance immunosuppression strategies using alemtuzumab have not been determined. However, our results of a single dose of 30 mg alemtuzumab induction with steroid-free maintenance immunosuppression achieved excellent mid-term patient and graft survival for simultaneous pancreas-kidney transplantation and pancreas transplantation. This is an uncontrolled study in a relatively small sized patient group with medium-term follow-up, a long-term follow-up is necessary in a larger controlled population.
MATERIALS AND METHODS
This study included all patients who underwent pancreas transplantation, as in SPK, PAK, or PTA between November 2006 and April 2010. A total of 28 patients (17 SPK, 5 PAK, and 6 PTA) were included at the Penn State Milton S. Hershey Medical Center during this period. Recipient and donor demographics are shown in Table 3. The median follow-up period was 25 months (range, 8–49 months). All pancreas grafts were implanted intraperitoneally with enteric exocrine drainage and systemic venous drainage. Kidney and pancreas grafts in SPK were implanted in the same side as described previously (31). Pancreas graft failure was defined as requirement of exogenous insulin (10, 32), and kidney graft failure was defined as requirement of regular dialysis. Patient and donor data were obtained from the medical center transplant database. This study was approved by the Pennsylvania State University, College of Medicine Institutional Review Board (No. 34420).
TABLE 3.
Recipient and donor demographics
| Recipient characteristics | ||||
| Gender | 19 Males | 9 Females | ||
| Race | 27 White | 1 African American | ||
| Age (yr) | Mean 40.2 | Median 41.0 (range 28–57) | ||
| Follow-up (days) | Mean 850 | Median 755 (Range 240–1475) | ||
| PRA>20% | 0 patients | |||
| Donor characteristics | ||||
| Gender | 17 Males | 11 Females | ||
| Race | 19 White | 3 African American | 2 Hispanic | 4 Unknown |
| Age (Yr) | Mean 24 | Median 24 (range 9–37) | ||
| CIT (min) | Mean 493 | Median 483 (range 200–720) |
PRA, panel reactive antibody; CIT, cold ischemia time.
Immunosuppression
All patients received 1000 mg of methylprednisolone IV perioperatively as premedication for alemtuzumab induction. One dose of alemtuzumab 30 mg IV (Campath-1H) was administered over 2 hr at 1 hr after methylprednisolone. Maintenance immunosuppression consisted of tacrolimus and MMF 1 g/d. No steroids were given routinely after the transplantation. Target trough level of tacrolimus was between 10 and 12 ng/mL for the first year of transplant and between 8 and 10 ng/mL thereafter.
Infection Prophylaxis
Antibiotic prophylaxis consisted of trimethoprim/sulfamathoxasole (1 year) and nystatin (3 months). Valganciclovir prophylaxis (450 mg orally daily) was maintained for 6 months. Quantitative CMV polymerase chain reaction was performed weekly for the first 3 months, and monthly thereafter. For SPK patients, quantitative BK virus polymerase chain reaction in recipient serum was also measured weekly for the first 3 months, and monthly thereafter.
HLA Typing and Antibody Assays
All patients were typed for HLA-A, B, Cw, DR, and DQ by serologically and sequence-specific primer methods. Pretransplant serum was tested by complement-dependent cytotoxicity crossmatch or T- and B-cell flow cytometric crossmatch (three-color assay) (33). HLA antibody was screened by LABScreen panel reactive antibody (One Lambda, CA) in pre- and posttransplant serum samples. Antibody specificity was identified by LABScreen Single Antigen (One Lambda). DSA was evaluated using mean fluorescence intensity of LABScreen beads corresponding to donor HLA antigens.
Rejection and Treatment
Postoperative serum amylase and lipase were routinely monitored. Elevation of these pancreatic enzymes, usually twice the baseline, underwent an ultrasound-guided pancreas biopsy. For SPK patients, a 20% increase in serum creatinine above baseline prompted a renal biopsy. Pancreas allograft biopsies were evaluated by Banff scheme (34), and renal allograft biopsies were evaluated by routine histology, and immunofluorescence using guidelines provided by the Banff ‘97 schema of renal allograft pathology (35–37). BK nephropathy was diagnosed by positive viral inclusion bodies staining for SV40 on immunohistochemistry. Mild acute rejection (grade I) of pancreas allografts was treated with steroid bolus and moderate and severe rejection (grade II and III) and steroid-resistant rejection were treated with thymoglobulin 6–9 mg/kg over 4 to 6 days. The patients who required thymoglobulin for rejection remained on maintenance steroid for 3 to 6 months after treatment. Borderline, 1A and 1B acute rejections of kidney allografts were treated with steroid bolus. Acute rejection 2A, 2B, and steroid resistance rejection were treated with thymoglobulin 6 to 9 mg/kg over 4 to 6 days with maintenance steroid for 3 to 6 months posttreatment. The starting dose of 20 mg prednisone was used for maintenance steroid and weaned off over 3 to 6 months. Patients with antibody-mediated rejection received five doses of plasmapheresis followed by a dose of 2 g/kg of intravenous immunoglobulin and 375 mg/m2 of body surface area with rituximab.
Statistical Analysis
The data are presented as mean±SD. Comparisons of continuous variables between two groups were made using Student’s t test. Absolute lymphocyte count and WBC count at each time point (1, 2, 3, and 6 months; 1, 2, and 3 years) were compared with mean pretransplant value. Significance level was set at 0.05.
Acknowledgments
Dr. Nasrollah Ghahramani is supported by Award Number K23DK084300 from National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Footnotes
All other authors declare no funding or conflicts of interest.
T.U. participated in research design, performance of the research, research design, data analysis, and writing of the manuscript. V.R. participated in performance of data analysis. K.M., H.S., T.V., O.K., N.G., R.S., U.F., A.K., and Z.K. participated in the performance of the research.
REFERENCES
- 1.Magliocca JF, Knechtle SJ. The evolving role of alemtuzumab (Campath-1H) for immunosuppressive therapy in organ transplantation. Transpl Int. 2006;19:705. doi: 10.1111/j.1432-2277.2006.00343.x. [DOI] [PubMed] [Google Scholar]
- 2.Hale G, Xia MQ, Tighe HP, et al. The CAMPATH-1 antigen (CDw52) Tissue Antigens. 1990;35:118. doi: 10.1111/j.1399-0039.1990.tb01767.x. [DOI] [PubMed] [Google Scholar]
- 3.Kirk AD, Hale DA, Mannon RB, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H) Transplantation. 2003;76:120. doi: 10.1097/01.TP.0000071362.99021.D9. [DOI] [PubMed] [Google Scholar]
- 4.Flynn JM, Byrd JC. Campath-1H monoclonal antibody therapy. Curr Opin Oncol. 2000;12:574. doi: 10.1097/00001622-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Ciancio G, Burke GW, Gaynor JJ, et al. The use of Campath-1H as induction therapy in renal transplantation: Preliminary results. Transplantation. 2004;78:426. doi: 10.1097/01.tp.0000128625.29654.eb. [DOI] [PubMed] [Google Scholar]
- 6.Calne R, Friend P, Moffatt S, et al. Prope tolerance, perioperative cam-path 1H, and low-dose cyclosporin monotherapy in renal allograft recipients. Lancet. 1998;351:1701. doi: 10.1016/S0140-6736(05)77739-4. [DOI] [PubMed] [Google Scholar]
- 7.Calne R, Moffatt SD, Friend PJ, et al. Campath IH allows low-dose cyclosporine monotherapy in 31 cadaveric renal allograft recipients. Transplantation. 1999;68:1613. doi: 10.1097/00007890-199911270-00032. [DOI] [PubMed] [Google Scholar]
- 8.Kato T, Gaynor JJ, Selvaggi G, et al. Intestinal transplantation in children: A summary of clinical outcomes and prognostic factors in 108 patients from a single center. J Gastrointest Surg. 2005;9:75. doi: 10.1016/j.gassur.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Ciancio G, Burke GW., III Alemtuzumab (Campath-1H) in kidney transplantation. Am J Transplant. 2008;8:15. doi: 10.1111/j.1600-6143.2007.02053.x. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman DB, Leventhal JR, Gallon LG, et al. Alemtuzumab induction and prednisone-free maintenance immunotherapy in simultaneous pancreas-kidney transplantation comparison with rabbit antithymocyte globulin induction—Long-term results. Am J Transplant. 2006;6:331. doi: 10.1111/j.1600-6143.2005.01166.x. [DOI] [PubMed] [Google Scholar]
- 11.Tzakis AG, Tryphonopoulos P, Kato T, et al. Preliminary experience with alemtuzumab (Campath-1H) and low-dose tacrolimus immunosuppression in adult liver transplantation. Transplantation. 2004;77:1209. doi: 10.1097/01.tp.0000116562.15920.43. [DOI] [PubMed] [Google Scholar]
- 12.Gruessner RW, Kandaswamy R, Humar A, et al. Calcineurin inhibitor- and steroid-free immunosuppression in pancreas-kidney and solitary pancreas transplantation. Transplantation. 2005;79:1184. doi: 10.1097/01.tp.0000161221.17627.8a. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman DB, Leventhal JR, Axelrod D, et al. Alemtuzumab induction and prednisone-free maintenance immunotherapy in kidney transplantation: Comparison with basiliximab induction—Long-term results. Am J Transplant. 2005;5:2539. doi: 10.1111/j.1600-6143.2005.01067.x. [DOI] [PubMed] [Google Scholar]
- 14.Farney AC, Doares W, Rogers J, et al. A randomized trial of alemtuzumab versus antithymocyte globulin induction in renal and pancreas transplantation. Transplantation. 2009;88:810. doi: 10.1097/TP.0b013e3181b4acfb. [DOI] [PubMed] [Google Scholar]
- 15.Matas AJ, Kandaswamy R, Humar A, et al. Long-term immunosuppression, without maintenance prednisone, after kidney transplantation. Ann Surg. 2004;240:510. doi: 10.1097/01.sla.0000137140.79206.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knight SR, Morris PJ. Steroid avoidance or withdrawal after renal transplantation increases the risk of acute rejection but decreases cardiovascular risk. A meta-analysis. Transplantation. 2010;89:1. doi: 10.1097/TP.0b013e3181c518cc. [DOI] [PubMed] [Google Scholar]
- 17.Papadimitriou JC, Drachenberg CB, Wiland A, et al. Histologic grading of acute allograft rejection in pancreas needle biopsy: Correlation to serum enzymes, glycemia, and response to immunosuppressive treatment. Transplantation. 1998;66:1741. doi: 10.1097/00007890-199812270-00030. [DOI] [PubMed] [Google Scholar]
- 18.Cashion A, Sabek O, Driscoll C, et al. Correlation of genetic markers of rejection with biopsy findings following human pancreas transplant. Clin Transplant. 2006;20:106. doi: 10.1111/j.1399-0012.2005.00450.x. [DOI] [PubMed] [Google Scholar]
- 19.Troxell ML, Koslin DB, Norman D, et al. Pancreas allograft rejection: Analysis of concurrent renal allograft biopsies and posttherapy follow-up biopsies. Transplantation. 2010;90:75. doi: 10.1097/TP.0b013e3181dda17e. [DOI] [PubMed] [Google Scholar]
- 20.Magliocca JF, Odorico JS, Pirsch JD, et al. A comparison of alemtuzumab with basiliximab induction in simultaneous pancreas-kidney transplantation. Am J Transplant. 2008;8:1702. doi: 10.1111/j.1600-6143.2008.02299.x. [DOI] [PubMed] [Google Scholar]
- 21.Park SH, Choi SM, Lee DG, et al. Infectious complications associated with alemtuzumab use for allogeneic hematopoietic stem cell transplantation: Comparison with anti-thymocyte globulin. Transpl Infect Dis. 2009;11:413. doi: 10.1111/j.1399-3062.2009.00414.x. [DOI] [PubMed] [Google Scholar]
- 22.Zanone MM, Favaro E, Quadri R, et al. Association of cytomegalovirus infections with recurrence of humoral and cellular autoimmunity to islet autoantigens and of type 1 diabetes in a pancreas transplanted patient. Transpl Int. 2010;23:333. doi: 10.1111/j.1432-2277.2009.00994.x. [DOI] [PubMed] [Google Scholar]
- 23.Hartmann EL, Gatesman M, Roskopf-Somerville J, et al. Management of leukopenia in kidney and pancreas transplant recipients. Clin Transplant. 2008;22:822. doi: 10.1111/j.1399-0012.2008.00893.x. [DOI] [PubMed] [Google Scholar]
- 24.Zafrani L, Truffaut L, Kreis H, et al. Incidence, risk factors and clinical consequences of neutropenia following kidney transplantation: A retrospective study. Am J Transplant. 2009;9:1816. doi: 10.1111/j.1600-6143.2009.02699.x. [DOI] [PubMed] [Google Scholar]
- 25.Hurst FP, Belur P, Nee R, et al. Poor outcomes associated with neutropenia after kidney transplantation: Analysis of United States Renal Data System. Transplantation. 2010;92:36. doi: 10.1097/TP.0b013e31821c1e70. [DOI] [PubMed] [Google Scholar]
- 26.Peddi VR, Hariharan S, Schroeder TJ, et al. Role of granulocyte colony stimulating factor (G-CSF) in reversing neutropenia in renal allograft recipients. Clin Transplant. 1996;10(1 Pt 1):20. [PubMed] [Google Scholar]
- 27.Axelrod DA, McCullough KP, Brewer ED, et al. Kidney and pancreas transplantation in the United States: 1999–2008: The changing face of living donation. Am J Transplant. 2010;10(4 Pt 2):987. doi: 10.1111/j.1600-6143.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- 28.Pascual J, Pirsch JD, Odorico JS, et al. Alemtuzumab induction and antibody-mediated kidney rejection after simultaneous pancreas-kidney transplantation. Transplantation. 2009;87:125. doi: 10.1097/TP.0b013e31818c6db0. [DOI] [PubMed] [Google Scholar]
- 29.Huang E, Cho YW, Hayashi R, et al. Alemtuzumab induction in deceased donor kidney transplantation. Transplantation. 2007;84:821. doi: 10.1097/01.tp.0000281942.97406.89. [DOI] [PubMed] [Google Scholar]
- 30.de Kort H, Munivenkatappa RB, Berger SP, et al. Pancreas allograft biopsies with positive c4d staining and anti-donor antibodies related to worse outcome for patients. Am J Transplant. 2010;10:1660. doi: 10.1111/j.1600-6143.2010.03079.x. [DOI] [PubMed] [Google Scholar]
- 31.Fridell JA, Shah A, Milgrom ML, et al. Ipsilateral placement of simultaneous pancreas and kidney allografts. Transplantation. 2004;78:1074. doi: 10.1097/01.tp.0000135461.16794.4d. [DOI] [PubMed] [Google Scholar]
- 32.Muthusamy AS, Vaidya AC, Sinha S, et al. Alemtuzumab induction and steroid-free maintenance immunosuppression in pancreas transplantation. Am J Transplant. 2008;8:2126. doi: 10.1111/j.1600-6143.2008.02373.x. [DOI] [PubMed] [Google Scholar]
- 33.Bray RA, Lebeck LK, Gebel HM. The flow cytometric crossmatch. Dual-color analysis of T cell and B cell reactivities. Transplantation. 1989;48:834. [PubMed] [Google Scholar]
- 34.Drachenberg CB, Odorico J, Demetris AJ, et al. Banff schema for grading pancreas allograft rejection: Working proposal by a multidisciplinary international consensus panel. Am J Transplant. 2008;8:1237. doi: 10.1111/j.1600-6143.2008.02212.x. [DOI] [PubMed] [Google Scholar]
- 35.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 36.Racusen LC, Colvin RB, Solez K, et al. Antibody-mediated rejection criteria—An addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3:708. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 37.Sis B, Mengel M, Haas M, et al. Banff ’09 meeting report: Antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010;10:464. doi: 10.1111/j.1600-6143.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- 38.Thai NL, Khan A, Tom K, et al. Alemtuzumab induction and tacrolimus monotherapy in pancreas transplantation: One- and two-year outcomes. Transplantation. 2006;82:1621. doi: 10.1097/01.tp.0000250712.12389.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clatworthy MR, Sivaprakasam R, Butler AJ, et al. Subcutaneous administration of alemtuzumab in simultaneous pancreas-kidney transplantation. Transplantation. 2007;84:1563. doi: 10.1097/01.tp.0000295718.55669.3a. [DOI] [PubMed] [Google Scholar]