Abstract
Preclinical Alzheimer’s disease (pAD) reflects neuropathological findings of AD in cognitively normal subjects. The present study represents an effort to determine if differences could be identified in the longitudinal patterns of cognitive performance in persons classified as pAD compared to those who did not meet criteria for AD at autopsy. We included 121 subjects who were cognitively normal from baseline through their last assessment before death and who underwent autopsy. Participants were classified into two groups: pathologically normal (PN; NIA-Reagan low or no-likelihood of AD, n=89) and preclinical AD (pAD; NIA-Reagan criteria of intermediate or high-likelihood of AD in the absence of clinical dementia symptoms, n=32) followed for a mean 7.5 years prior to death. Longitudinal rates and patterns of change in scores on a standard cognitive battery were compared between these two groups. While cognitive results at baseline and last evaluations revealed no clear cross sectional group differences after adjustment for age, APOE status, education, and gender, statistically significant differences between the pAD and PN groups in slope of decline were seen on a composite score of cognitive function. Further analyses showed three components of this score reached significance: constructional praxis, delayed recall of a word list, and category verbal fluency. Despite being clinically viewed as normal at enrollment and at the final exam, there are significant differences in rates of cognitive decline in participants classified as pAD compared to those without this pathology. Longitudinal changes in slope of decline in specific cognitive test measures can serve as non-invasive methods for the detection of pAD.
Keywords: Alzheimer’s disease, cognition, normal, preclinical
INTRODUCTION
The finding of significant Alzheimer’s disease (AD) pathology (amyloid plaques and neurofibrillary tangles) in the brains of cognitively normal persons coming to autopsy, termed “preclinical AD” (pAD), has been reported by several groups using semi-quantitative rating scales [1–10] and more recently, using quantitative histological measures by our group and others [3, 8, 10]. It has been postulated that the antemortem identification of pAD may be possible based on changes in longitudinal cognitive performance in specific standard cognitive test instruments and through the use of antemortem biomarkers such as cerebrospinal fluid measures, FDG-PET, amyloid-ligand binding PET studies, volumetric MRI, and fMRI studies [3, 7, 8, 11–24]. The potential for these biomarkers to serve diagnostic purposes in the antemortem detection of pAD has been highlighted in the recently developed proposed diagnostic criteria for pAD from an NIH/NIA working group (http://www.alz.org/research/diagnostic_criteria/preclinical_recommendations.pdf).
The debate on the diagnostic accuracy of antemortem biomarkers in pAD will require resolution through prospective clinical-pathological investigation. Currently, several published studies suggest that patterns of longitudinal cognitive decline in neuropathologically confirmed pAD, detected by specific cognitive tests can accurately detect pAD via non-invasive methodologies [5, 8, 11, 12, 16, 22–27]. Further investigations in this area using longitudinal cohort data, in well-characterized subjects that have come to autopsy and have pathological verification are needed to better guide the development of sensitive and specific non-invasive cognitive test batteries to detect preclinical AD.
The University of Kentucky Alzheimer’s Disease Center (UK-ADC) follows a large group of cognitively normal subjects that agree to undergo extensive longitudinal annual clinical evaluations and consent to brain autopsy at death [3, 8, 28]. Analysis of pAD in this cohort may provide further insights that can help shape our development of diagnostic algorithms for the detection of pAD. The present analyses focus on two groups whose cognitive performance remained unimpaired throughout life and for whom neuropathological data are available: 1) those classified as having pAD, defined by NIA-Reagan intermediate or high-likelihood of AD despite the absence of clinical symptoms and 2) those who were clinically and pathologically normal (NIA-Reagan low or no-likelihood of AD) [29].
METHODS
Subjects
Research subjects were from the UK-ADC normal control clinic. Inclusion criteria were a minimum age of 65 years; cognitive and neurological normality by enrollment examination; designated informant for structured interviews; willingness to undergo annual cognitive testing, and physical and neurological examinations; and brain donation to the UK-ADC at death. Excluded at enrollment are individuals with a history of substance abuse (including alcohol); major head injury; major psychiatric and neurological illness; medical illnesses that are nonstable, impairing, or that have an effect on the CNS; chronic infectious diseases; stroke or TIA; encephalitis; meningitis; or epilepsy. Annual standardized assessment includes extensive medical, cognitive, social, and functional evaluations as previously described [28]. All subjects were contacted at six-month intervals had detailed cognitive function testing annually and had neurologic and physical examinations biannually or annually. Since 2005 we have used the standard test battery required by the National Alzheimer’s Coordinating Center for all NIA-funded Alzheimer’s Disease Centers [30, 31]. Annual diagnostic classification as normal was based on consensus between the examining neurologist and neuropsychologist after reviewing cognitive test scores, medical history, medication usage, physical and neurological examination. Neither examiner was blinded to previous examination scores or diagnostic criteria. Subjects meeting criteria for mild cognitive impairment (MCI) or dementia were excluded from the study[32–37].
Neuropathological evaluation
Fresh brain weights were determined at the time of autopsy. Specimens for histologic evaluation included at least 23 different brain regions. Sections were taken from the left cerebral hemisphere at the time of autopsy from the following regions and fixed in 4% formaldehyde: frontal pole (Brodmann area 10), middle frontal gyrus (area 9), gyrus rectus (area 11), temporal pole (area 38), superior and middle temporal gyri (areas 21, 22), inferior parietal lobule (areas 39, 40), occipital lobe (areas 17, 18), anterior cingulate gyrus (area 24), posterior cingulate gyrus (area 23), and insular cortex (areas 7,9). Specimens were also taken from the hippocampus at the level of the lateral geniculate nucleus, entorhinal cortex, amygdala, ambient gyrus, basal ganglia, nucleus basalis of Meynert, thalamus, midbrain, pons, medulla, and cerebellum. Similar sections were taken from the right hemisphere of most cases following fixation of the brain. Sections were cut at 8 micron thickness, stained with hematoxylin and eosin and the modified Bielschowsky method. Gallyas stain was used for sections of the entorhinal cortex, hippocampus, and amygdala. Sections of the neocortex were stained with 10D-5 or the amyloid-β antibody (Novacaster, Newcastle, United Kingdom).
Amyloid plaques were separated into DPs (plaques without neurites) and NPs (plaques with neurites) in each region as described previously [38]. Neurofibrillary tangles(NFTs), DPs, and NPs were counted using Bielschowsky-stained sections of middle frontal gyrus, middle temporal gyrus (area 21), inferior parietal lobule), occipital lobe including primary visual area. Gallyas stained sections were used to quantify NFTs in hippocampal CA1, subiculum, amygdala, and entorhinal cortex. An arithmetic mean was calculated from the count of the 5 most involved fields for DPs (number of DPs per 2.35 mm2), NPs (number of NPs per 2.35 mm2), and NFTs (number of NFTs per 0.586 mm2) for each region. Mean medial temporal lobe counts represent an average derived from the entorhinal cortex, amygdala, CA1, and subiculum. Mean neocortical counts represent an average derived from the four cortical areas described above. Braak staging [39] and CERAD plaque scores [40] were used to determine NIA-Reagan diagnosis of pathological AD [29]. The presence of clinical dementia required for a CERAD and subsequent NIA-Reagan diagnosis was waived in the present study, as all subjects by virtue of inclusion criteria were cognitively normal at death.
For assessment of Lewy body pathology (LBP), the α-synuclein mouse monoclonal antibody (Novacaster, Newcastle, United Kingdom) immunohistochemistry was used as described in detail elsewhere [41, 42].
Size, location, and histologic age of large and small vessel infarcts were recorded. Microinfarcts were considered potentially significant if 3 or more were found in the reviewed H&E sections. Criteria for grading amyloid angiopathy include: grade 0-no amyloid present; grade 1-minimal amyloid present in leptomeningeal vessels only; grade 2- mild to moderate amyloid present in meningeal and small parenchymal vessels; grade 3- severe amyloid present in meningeal and small as well as larger parenchymal vessels; grade 4- severe amyloid angiopathy with micro-hemorrhage.
All pathological diagnoses were made blinded to clinical information.
Cognitive Evaluations
Cognitive test variables used in the present analyses include the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) standard battery comprising: verbal fluency (animal naming), word list memory, constructional praxis, and short versions of the Boston Naming Test (BNT; 15- and 30-item versions) [43–45]. The CERAD battery was supplemented with Logical Memory (immediate recall)[46] and the Trail Making Test (Part A)[47]. The verbal fluency procedure measures verbal production, semantic memory, and language. It requires the subject to name as many examples of the category “animal” as possible in 1 minute. The BNT measures visual naming and presents 15 or 30 line drawings of common objects. These items are stratified into three groups, representing objects of high (easy to name), medium, and low (hard to name) frequency of occurrence in the English language. The maximum score is 15 or 30, depending on the version used.
The word list memory test is a free recall memory test that assesses learning ability for new verbal information. Participants are presented 10 unrelated items to remember on printed cards and are instructed to read aloud each word as it is presented. Immediately following presentation of the 10 words, the participant is asked to recall as many items as possible. On each of the three learning trials, the 10 words are presented in a different order. The maximum score on each trial is 10. The maximum total score is 30. The delayed word list recall measure assesses the ability to recall, after 15 minutes, the 10 words given in the word list memory test. The maximum number of correct responses is 10. Word list recognition counts the number of 10 words presented in the word list memory task correctly recognized (Rec-yes). These words are presented among 10 distractor words. The number of distractor words correctly identified (Rec-no) is also counted. The maximum score for each is 10.
The constructional praxis task measures visuospatial and constructional abilities and requires the subject to copy four line drawings presented in order of increasing complexity (circle, diamond, overlapping rectangles, and cube). The total possible score is 11.
The CERAD T- score, a composite measure comprised of scores derived from a subset of these tests was the primary measure used in analyses of change over time in the pAD and normal groups [48].
Statistical analysis
Standard descriptive and comparative analyses were performed and included mean, standard deviations, mode, Student’s t-test, Mann-Whitney U-test, Fisher exact test, and Chi-square tests where appropriate for analysis of demographic, genetic and pathological variables. Adjusted analyses were performed using generalized linear regression techniques for cognitive variables. Covariates for adjusted analyses included age, ApoE carrier status, and education, although these latter two variables did not differ between groups. Adjustment for ApoE carrier status and education did not change the results or conclusions (data not shown). Accordingly the analyses presented include only the adjustment based on age as a covariate in the regression modeling.
For each cognitive instrument and the CERAD-T score a linear mixed model (LMM) was constructed to compare the decline in cognition between the two groups, as nonlinear analysis did not improve the models fit for any of the variables studied. In this model it was assumed that scores for each individual subject declined linearly over time on study with a separate intercept and slope per individual (random effects) and that the intercept was influenced by the covariates age at first visit, gender, and educational level (coded as ≤ 12 years, 13–15, 16, or > 16). The purpose of the analysis was to compare the average slopes between the two groups. Statistical significance was set at p < 0.05 throughout for this exploratory analysis.
RESULTS
The present analyses include 121 cognitively normal subjects who came to autopsy for whom detailed and quantitative clinical and pathological data were available. Subjects were classified into two groups: those with pre-clinical Alzheimer’s Disease (pAD), defined as having met criteria for NIA-Reagan intermediate or high-likelihood of AD despite the absence of clinical symptoms (n= 32; 26%) and those who were pathologically normal (PN), defined as having met criteria for NIA-Reagan low or no-likelihood of AD ( n=89; 74%). The cause of death varied (stroke, myocardial infarction, congestive heart failure, trauma, pneumonia, cancer, renal failure, etc.), but none were considered related to underlying degenerative neurological disease state nor did they differ between group.
The pAD and normal groups were comparable on mean number of visits, mean time between the last visit and death, education, APOE status, and gender (Table 1). However, the pAD group was on average 5 years older than the normal group at the first visit (79.8 ± 7.8 versus 74.8 ± 6.7, p = 0.0009). Cognitive test performance did not differ on any variable on entry to the study (Table 1).
Table 1.
Comparison of pre-clinical Alzheimer’s disease (pAD) and pathologically normal subjects on demographic variables. (n.s., p>0.05)
| pAD (n = 32) | Normal (n = 89) | p value | |
|---|---|---|---|
| Age at first visit | 79.8 ± 7.8 | 74.8 ± 6.7 | 0.0009 |
| Number of annual visits | 7.5 ± 3.4 | 7.2 ± 4.2 | n.s |
| Interval: last visit to death (years) | 0.80 ± 0.58 | 0.68 ± 0.57 | n.s |
| Male | 50.0% | 38.2% | n.s |
| Education | n.s | ||
| ≤ 12 years | 12.5% | 12.4% | |
| 13–15 | 15.6% | 18.0% | |
| 16 years | 46.9% | 31.5% | |
| > 16 | 25.0% | 38.2% | |
| ApoE status | n.s | ||
| ε2/ε3 | 17.9% | 12.5% | |
| ε2/ε4 | 0.0% | 3.1% | |
| ε3/ε3 | 64.3% | 59.4% | |
| ε3/ε4 | 17.9% | 21.9% | |
| ε4/ε4 | 0.0% | 3.1% | |
| MMSE | 28.7 ± 0.3 | 29.1 ± 0.1 | n.s |
| Boston Naming Test (% Correct) | 0.96 ± .01 | 0.98 ± .004 | n.s |
| Verbal Fluency (animal naming) | 18.1 ± 1.1 | 18.6 ± 0.5 | n.s |
| Trail Making Test A | 49.0 ± 4.8 | 44.2 ± 1.9 | n.s |
| Logical Memory Immediate | 13.5 ± 0.6 | 13.7 ± 0.4 | n.s |
| CERAD Word List Trials 1 – 3 Total | 19.7 ± 0.07 | 20.7 ± 0.04 | n.s |
| CERAD Word List Recognition True Positives | 9.6 ± 0.2 | 9.6 ± 0.1 | n.s |
| CERAD Word List Recognition True Negatives | 9.94 ± 0.04 | 9.95 ± 0.02 | n.s |
| CERAD Word List Delayed Recall | 6.8 ± 0.3 | 6.9 ± 0.2 | n.s |
| Constructional Praxis | 9.6 ± 0.9 | 9.6 ± 1.1 | n.s |
| CERAD T-Score | 60.3 ± 1.2 | 60.5 ± 0.9 | n.s |
Data on associated pathological features are presented in Table 2. Preclinical LBP was seen in 6.7% normal and 9.2% pAD cases, and although the distribution of LBP varied in these cases from brainstem predominant, to limbic, to neocortical, the neuroanatomical distributions of LBP did not differ between normal and pAD cases. Large vessel infarcts, lacunar infarcts, microinfarcts, hemorrhagic infarcts, amyloid angiopathy, and atherosclerosis were frequent pathologic findings in this series. No significant between-group differences were seen for gross brain weight or coexistent vascular or LBP, with the exception of amyloid angiopathy, which was higher grade in pAD compared to pathologically normal cases (Table 2).
Table 2.
Co-existing, non-Alzheimer’s neuropathological features.
| Parameter | Preclinical Alzheimer’s disease (pAD) (n = 32) |
Normal (n = 89) | p |
|---|---|---|---|
| Brain Weight (mean g ± SD) | 1190.8±119.9 | 1244.3±124.7 | 0.039† |
| Brain Weight* (mean g ± SE) | 1205.9±11.5 | 1254.7±11.5 | 0.033 |
| LBD (% cases) | 3.1 | 3.4 | 0.947‡ |
| Macro infarct (% cases) | 31.3 | 30.3 | 0.923‡ |
| Lacunar infarct (% cases) | 6.3 | 3.4 | 0.483‡ |
| Microinfarct (% cases) | 59.4 | 41.6 | 0.083‡ |
| Hemorrhagic infarct (% cases) | 12.5 | 2.5 | 0.022‡ |
| Amyloid angiopathy (% cases) | 84.4 | 46.7 | <0.0001‡ |
| Atherosclerosis (% cases) | 96.7 | 78.6 | 0.022‡ |
adjusted for age and gender;
t-test
Chi-square test
Cognitive test results from the last evaluation before death between pAD and pathologically normal groups are presented in Table 3. These test data proximal to death and autopsy demonstrated significant differences in MMSE scores, wordlist total, and category fluency (animal naming) between pAD and pathologically normal cases in unadjusted analyses. These differences were diminished below the level of significance after including age at baseline, gender, and education as covariates in this study. These cross-sectional findings prompted the investigation of possible group differences in longitudinal changes in slope of decline.
Table 3.
Cross-sectional cognitive data at last evaluation before death in preclinical AD (pAD) and pathologically normal subjects. Unadjusted and adjusted means with standard errors. Adjusted means account for the effects of age, education, gender, and time from test date to death.
| Instrument | pAD | Normal | p | |||
|---|---|---|---|---|---|---|
| (Mean±SE) | N | (Mean±SE) | N | |||
| CERAD T-Score | Unadj. | 52.5±2.7 | 28 | 57.1±1.3 | 79 | 0.088 |
| Adj. | 54.5±2.5 | 28 | 56.1±1.4 | 75 | 0.596 | |
| MMSE | Unadj. | 27.2±0.5 | 32 | 28.5±0.2 | 88 | 0.019 |
| Adj. | 27.5±0.3 | 32 | 28.3±0.2 | 82 | 0.050 | |
| Logical Memory Imm. | Unadj. | 14.4±0.8 | 28 | 14.9±0.5 | 83 | 0.575 |
| Adj. | 15.0±0.8 | 28 | 14.7±0.5 | 79 | 0.712 | |
| Word List Delayed Recall | Unadj. | 5.6±0.4 | 28 | 6.6±0.2 | 78 | 0.040 |
| Adj. | 6.2±0.4 | 28 | 6.3±0.2 | 74 | 0.887 | |
| Trails A | Unadj. | 52.3±4.6 | 22 | 55.2±3.6 | 78 | 0.629 |
| Adj. | 46.5±6.2 | 22 | 56.0±3.4 | 73 | 0.194 | |
| Word List Total | Unadj. | 18.2±0.8 | 28 | 20.7±0.5 | 79 | 0.013 |
| Adj. | 19.1±0.9 | 28 | 20.3±0.5 | 75 | 0.246 | |
| Verbal Fluency | Unadj. | 13.7±0.8 | 32 | 16.0±0.6 | 87 | 0.034 |
| Adj. | 14.9±0.9 | 32 | 15.5±0.6 | 82 | 0.586 | |
| Constructional Praxis | Unadj. | 8.9±0.2 | 24 | 9.2±0.1 | 72 | 0.262 |
| Adj. | 8.9±0.2 | 24 | 9.2±0.1 | 69 | 0.435 | |
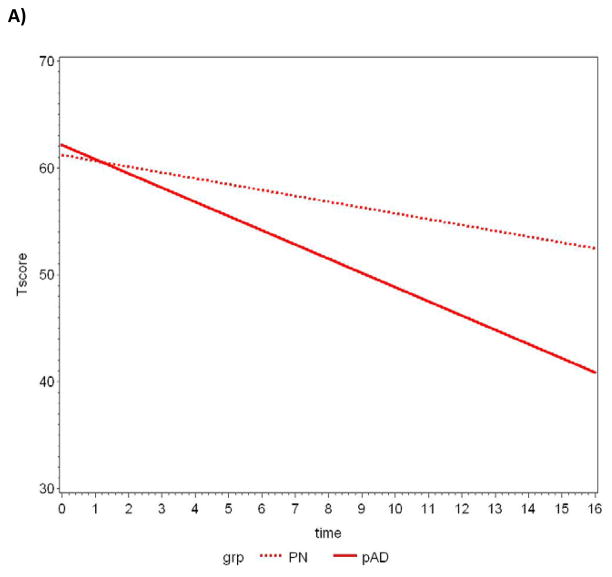
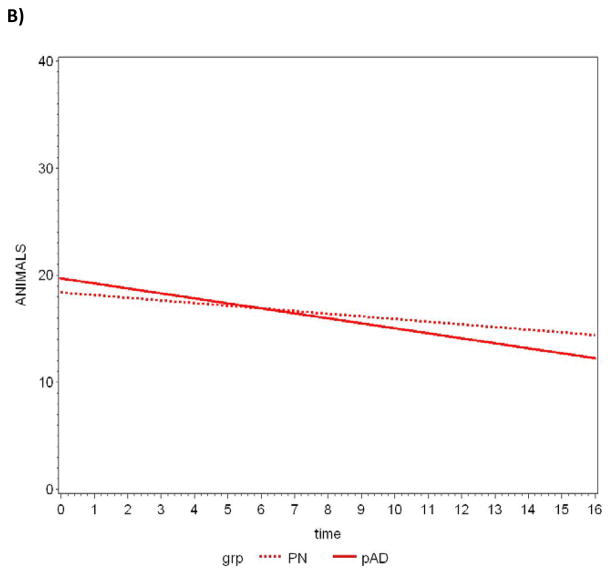
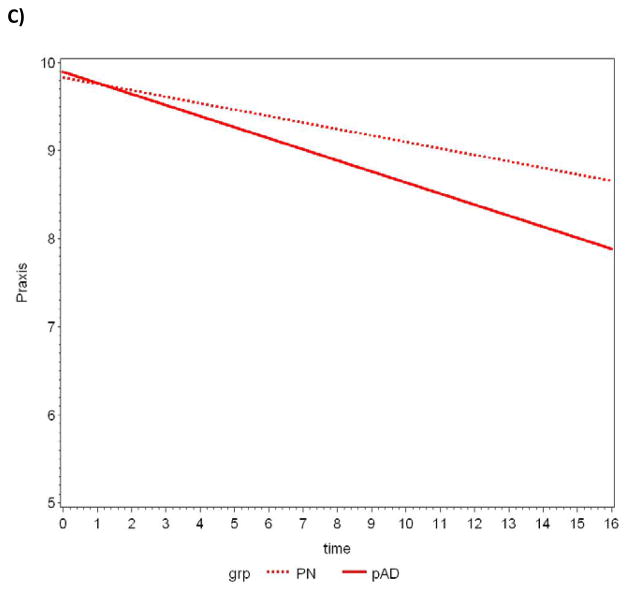
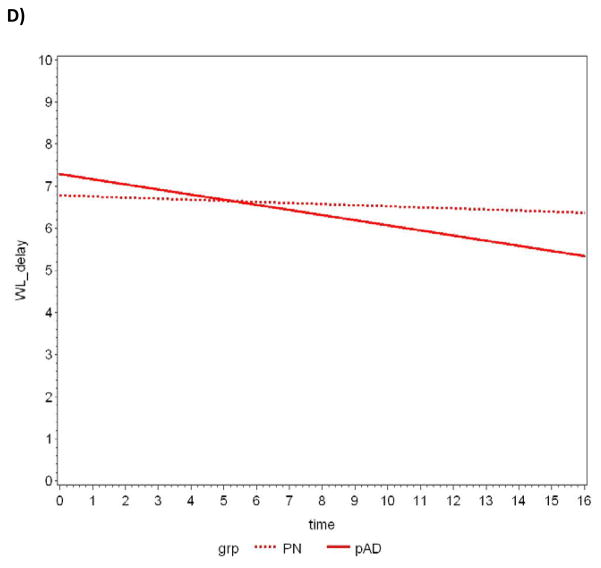
Between-group comparisons of the average slopes and intercepts are summarized in Table 4. The CERAD T-score achieved statistical significance when comparing average slopes, with pAD subjects declining at a faster rate. Further analyses revealed three components of this composite measure also reached statistical significance: constructional praxis, word list delay, and verbal fluency (animal naming). A plot of the data and the lines fitted by the LMM for each measure appears in Figure 1a–d.
Table 4.
Between-group comparisons of cognitive slopes of change for pAD and pathologically normal groups
| Instrument | pAD | Normal | p slope | ||||
|---|---|---|---|---|---|---|---|
| n | slope | SE | n | slope | SE | ||
| CERAD T score | 31 | −1.3298* | 0.2985 | 83 | −0.5453* | 0.1881 | 0.027* |
| MMSE | 31 | −0.1698* | 0.0370 | 85 | −0.0943* | 0.0225 | 0.082 |
| Logical Memory Imm. | 30 | 0.0803 | 0.0765 | 84 | 0.1722* | 0.0456 | 0.303 |
| Word List Delayed Recall | 31 | −0.1213* | 0.0359 | 83 | −0.0259 | 0.0213 | 0.023* |
| Trails A | 28 | 2.5326* | 0.7371 | 84 | 1.7005* | 0.4401 | 0.333 |
| Word List Total | 31 | −0.1541* | 0.0718 | 83 | −0.0119 | 0.0427 | 0.089 |
| Verbal Fluency | 31 | −0.4666* | 0.0929 | 85 | −0.2470* | 0.0549 | 0.043* |
| Constructional Praxis | 29 | −0.1256* | 0.0199 | 82 | −0.0734* | 0.0115 | 0.024* |
Significant at p < 0.05
Figure 1.
Slopes of decline over time in CERAD T-scores for pathologically normal (PN) and pAD controls determined by random effects linear mixed modeling. A) CERAD T-Score, B) Verbal fluency-animal naming, C) Constructional praxis, D) Delayed word recall.
Repeat analyses, censoring for subjects who had test scores that were clinically below normal (more than 1.5SD below the mean for the group) at baseline, resulted in the same pattern of findings as seen when the full samples of subjects were included (results not shown). In addition, analyses that eliminated the last cognitive exam were performed in order to examine possible effects of terminal decline in cognitive function [25, 49]. None of the results were significantly altered in this set of analyses (results not shown).
DISCUSSION
This study demonstrates that that while standard cross-sectional cognitive test measures proximal to death were not able to reliably detect the presence or absence of significant AD pathology in cognitively normal individuals, significant acceleration in slopes of decline over time in pAD can be detected using these measures. An important caveat is that this data should not be interpreted to suggest that all cognitive test measures are unreliable in the determination of early cognitive decline in AD, but rather should suggest that further refinement of existing test measures and development of novel test measures are imperative for progress in the field as we move the diagnosis of AD steadily backwards along the cognitive continuum. Such progress may allow the future identification of pAD subjects to be accomplished in a non-invasive manner, and has been highlighted as an important area of development in the recently proposed new diagnostic criteria for pAD.
By definition, the pAD cases in this autopsy series had sufficient AD pathology to meet NIA-Reagan Intermediate or High-likelihood diagnostic criteria for AD based on semi-quantitative pathological findings. Among our participants, pAD represents a pathologically milder form of AD than seen in clinically evident cases meeting criteria for dementia, with few having Braak stage V or greater [2–4, 6–9, 29, 38, 40, 50–53]. The significant degree of AD pathology in the brains of cognitively normal persons supports the concept of cognitive reserve [54], even though the mechanisms underlying reserve are not yet completely understood. The participants classified as having preclinical AD in this study were able to reach the end of their lives without showing clinically impaired cognition as measured by the CERAD battery of cognitive tests. The mechanisms that provide for such compensation are likely to be due at least to some degree to younger age, higher education, and female gender [54]. However, while test scores at first and last examinations were unable to differentiate between the pAD and normal groups, our results demonstrate that the pAD group declined at a more rapid rate than that seen for pathologically normal controls on a composite measure of cognitive function and three of the subtests included in that measure. These findings may, in part, reflect a relative insensitivity of the CERAD measures to subtle changes in cognition or the need for multiple measures of memory [55, 56], or the need for alternative methods to analyze change in performance [57, 58], that may detect change reflecting early AD pathology. They may also reflect the need for AD pathology to reach a threshold before clinical change is detected.
Previous studies investigating longitudinal decline in cognitively intact subjects who progress to dementia or a cognitively impaired state have shown similar results [5, 8, 11, 12, 16, 22, 23, 25]. Such studies have suggested that the Clinical Dementia Rating, verbal fluency tasks such as animal naming, Trail Making Test Part B scores, and delayed recall measures can predict the future development of AD by many years. A key component for analysis of AD pathological evolution may be within subject change[8, 23, 25, 26, 59–64]. Several of these studies have included autopsy validation. Each of these studies has contributed to our overall understanding of longitudinal cognitive test decline in persons who will go on to meet criteria for MCI or dementia. Our present study, using a well-characterized sample that included autopsy-confirmed pathologic diagnoses, contributes to the emerging literature that supports the use of longitudinal decline on standard neuropsychological test measures in the detection of preclinical AD.
While the data are presented in terms of linear decline, investigation of nonlinear decline is important for accurate interpretation of the results as nonlinear decline has been noted in previous studies of longitudinal cognitive decline in patients who already reached the Alzheimer diagnosis[60, 61, 63]. Additional analyses included assessment of nonlinear decline were performed and found only modest evidence of nonlinearity. Refitting the linear mixed model described in Table 4 with a quadratic (nonlinear) trend identified only two variables that showed a lack of linearity (i.e., had significant quadratic trends). One of these latter variables was logical memory, where decline was quadratic in time within both groups but not different between groups. The other variable was constructional praxis which had a significant quadratic trend in the normal group only. As these findings do not change the conclusions drawn from Table 4, we have chosen to present the data in terms of linear decline only.
The disease process in AD has been shown to begin years before clinical symptoms appear [1–9, 65–68], raising several important implications of such observations. Our data reflect the likelihood that a substantial proportion of so-called “normal” older control subjects actually meet neuropathologic criteria for AD. The assertion that pAD can only be detected using biological measures would neglect our extensive understanding of longitudinal decline across the cognitive continuum. While biological measures of disease state in the detection of pAD represent a vital field of emerging research, the importance of cognitive measures in the identification of pre-clinical Alzheimer’s disease continue to serve a critical role in this area [3, 7, 8, 11–24]. The recent NIH/NIA working group on diagnostic criteria for pAD (http://www.alz.org/research/diagnostic_criteria/preclinical_recommendations.pdf) recognized this issue and emphasized the importance of increasing our further understanding of subtle cognitive decline in such “preclinical” disease states. The development of more sensitive and specific tests for the detection of pAD can be informed by the results of the current study, along with previous reports, and warrant further study [5, 8, 11, 12, 16, 22–24].
One limitation of this study is the potential challenge of generalizing the findings to racial and socioeconomic groups that are more varied than ours, which is exclusively Caucasian and well educated. The strengths of this study include the large number of clinically well-characterized, cognitively normal subjects studied within a year of autopsy and the detailed, quantitative neuropathologic analysis allowing the characterization of pAD and pathologically normal participants. Another major caveat is the issue of circularity in that the tests used to confirm intact cognition, are also the measures used to analyze longitudinal change in this study. Confusion as to the “dependency” of the independent variables used in the study needs to be carefully evaluated. The dependent variable in the present study is slope of change over time, not the absolute scores on a test variable which serve as the independent variables for the study. The conclusions drawn illustrate the utility of longitudinal change rather than the robustness of the individual measures used. While some degree of circularity is inherent in such analyses, the present data supports the use of analysis of longitudinal change on standard cognitive measures for the detection of pAD until such time as more accurate diagnostic markers are developed and proven in their utility for diagnosing pAD.
In summary, our data affirm previous observations that a significant proportion of “cognitively normal control” subjects included in many studies of AD and aging may in fact have at least moderate levels of AD neuropathology. This is true when test scores most proximal to death are evaluated [2, 8, 23, 62, 64, 66, 69, 70]. Our data are also consistent with recent studies suggesting that longitudinal studies may be able to differentiate cognitively normal individuals who have pAD from those who do not by examining the rate of decline in certain cognitive tests [5, 8, 12, 22, 23, 25, 26, 59, 62, 64, 71–73]. In our data, these measures included the composite CERAD T-score, as well as verbal fluency, delayed word list recall and constructional praxis. Our data suggest that evidence of decline in tests of episodic memory as well as measures of semantic memory and visuospatial construction may signal the presence of AD pathology in persons who are still performing within normal limits on cognitive test batteries. The detection of such changes in research participants who are classified as having “normal” cognition may warrant a separate classification for these individuals in longitudinal studies. The existence of pAD also has implications for early therapeutic intervention with disease modifying agents that are currently under development [13, 14]. It is possible that by examining the trajectories of decline in living individuals, those who are likely to develop clinically significant levels of AD pathology may be identified and treated well in advance of the emergence of symptoms of clinically-significant cognitive impairment.
Acknowledgments
This study was approved by the University of Kentucky IRB and supported by NIH/NIA 1P30 AG028383, 2R01 AG019241, and 1R01 AG027219. The authors acknowledge substantial contributions in the acquisition of data, neuropathological analysis, concept, and study design to the late Dr. William R. Markesbery.
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=816).
References
- 1.Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology. 1992;42:1681–1688. doi: 10.1212/wnl.42.9.1681. [DOI] [PubMed] [Google Scholar]
- 2.Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 3.Davis DG, Schmitt FA, Wekstein DR, Markesbery WR. Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol. 1999;58:376–388. doi: 10.1097/00005072-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Haroutunian V, Perl DP, Purohit DP, Marin D, Khan K, Lantz M, Davis KL, Mohs RC. Regional distribution of neuritic plaques in the nondemented elderly and subjects with very mild Alzheimer disease. Arch Neurol. 1998;55:1185–1191. doi: 10.1001/archneur.55.9.1185. [DOI] [PubMed] [Google Scholar]
- 5.Hulette CM, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, McIntyre LM. Neuropathological and neuropsychological changes in “normal” aging: evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol. 1998;57:1168–1174. doi: 10.1097/00005072-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, Smith GE, Dickson DW, Johnson KA, Petersen LE, McDonald WC, Braak H, Petersen RC. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62:1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- 7.Morris JC, Price AL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer’s disease. J Mol Neurosci. 2001;17:101–118. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- 8.Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. “Preclinical” AD revisited: neuropathology of cognitively normal older adults. Neurology. 2000;55:370–376. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- 9.Troncoso JC, Martin LJ, Dal Forno G, Kawas CH. Neuropathology in controls and demented subjects from the Baltimore Longitudinal Study of Aging. Neurobiol Aging. 1996;17:365–371. doi: 10.1016/0197-4580(96)00028-0. [DOI] [PubMed] [Google Scholar]
- 10.Price JL, McKeel DW, Jr, Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, Smith CD, Davis DG, Schmitt FA, Markesbery WR, Kaye J, Kurlan R, Hulette C, Kurland BF, Higdon R, Kukull W, Morris JC. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30:1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc. 2001;7:631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- 12.Chong MS, Sahadevan S. Preclinical Alzheimer’s disease: diagnosis and prediction of progression. Lancet Neurol. 2005;4:576–579. doi: 10.1016/S1474-4422(05)70168-X. [DOI] [PubMed] [Google Scholar]
- 13.de Leon MJ, Mosconi L, Blennow K, DeSanti S, Zinkowski R, Mehta PD, Pratico D, Tsui W, Saint Louis LA, Sobanska L, Brys M, Li Y, Rich K, Rinne J, Rusinek H. Imaging and CSF studies in the preclinical diagnosis of Alzheimer’s disease. Ann N Y Acad Sci. 2007;1097:114–145. doi: 10.1196/annals.1379.012. [DOI] [PubMed] [Google Scholar]
- 14.Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O’Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 15.Gron G, Riepe MW. Neural basis for the cognitive continuum in episodic memory from health to Alzheimer disease. Am J Geriatr Psychiatry. 2004;12:648–652. doi: 10.1176/appi.ajgp.12.6.648. [DOI] [PubMed] [Google Scholar]
- 16.Jobst KA, Barnetson LP, Shepstone BJ. Accurate prediction of histologically confirmed Alzheimer’s disease and the differential diagnosis of dementia: the use of NINCDS-ADRDA and DSM-III-R criteria, SPECT, X-ray CT, and APO E4 medial temporal lobe dementias. The Oxford Project to Investigate Memory and Aging. Int Psychogeriatr. 1997;9(Suppl 1):191–222. discussion 247–152. [PubMed] [Google Scholar]
- 17.Johnson KA, Jones K, Holman BL, Becker JA, Spiers PA, Satlin A, Albert MS. Preclinical prediction of Alzheimer’s disease using SPECT. Neurology. 1998;50:1563–1571. doi: 10.1212/wnl.50.6.1563. [DOI] [PubMed] [Google Scholar]
- 18.Korf ES, Wahlund LO, Visser PJ, Scheltens P. Medial temporal lobe atrophy on MRI predicts dementia in patients with mild cognitive impairment. Neurology. 2004;63:94–100. doi: 10.1212/01.wnl.0000133114.92694.93. [DOI] [PubMed] [Google Scholar]
- 19.Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 20.Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Cerebral white matter lesions and the risk of dementia. Arch Neurol. 2004;61:1531–1534. doi: 10.1001/archneur.61.10.1531. [DOI] [PubMed] [Google Scholar]
- 21.Riley KP, Snowdon DA, Markesbery WR. Alzheimer’s neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol. 2002;51:567–577. doi: 10.1002/ana.10161. [DOI] [PubMed] [Google Scholar]
- 22.Tierney MC, Szalai JP, Snow WG, Fisher RH, Nores A, Nadon G, Dunn E, St George-Hyslop PH. Prediction of probable Alzheimer’s disease in memory-impaired patients: A prospective longitudinal study. Neurology. 1996;46:661–665. doi: 10.1212/wnl.46.3.661. [DOI] [PubMed] [Google Scholar]
- 23.Tierney MC, Yao C, Kiss A, McDowell I. Neuropsychological tests accurately predict incident Alzheimer disease after 5 and 10 years. Neurology. 2005;64:1853–1859. doi: 10.1212/01.WNL.0000163773.21794.0B. [DOI] [PubMed] [Google Scholar]
- 24.Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, Fishel MA, Kulstad JJ, Green PS, Cook DG, Kahn SE, Keeling ML, Craft S. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry. 2005;13:950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- 25.Grober E, Hall CB, Lipton RB, Zonderman AB, Resnick SM, Kawas C. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer’s disease. J Int Neuropsychol Soc. 2008;14:266–278. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall CB, Lipton RB, Sliwinski M, Stewart WF. A change point model for estimating the onset of cognitive decline in preclinical Alzheimer’s disease. Stat Med. 2000;19:1555–1566. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1555::aid-sim445>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Johnson DK, Storandt M, Morris JC, Langford ZD, Galvin JE. Cognitive profiles in dementia: Alzheimer disease vs healthy brain aging. Neurology. 2008;71:1783–1789. doi: 10.1212/01.wnl.0000335972.35970.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmitt FA, Wetherby MM, Wekstein DR, Dearth CM, Markesbery WR. Brain donation in normal aging: procedures, motivations, and donor characteristics from the Biologically Resilient Adults in Neurological Studies (BRAiNS) Project. Gerontologist. 2001;41:716–722. doi: 10.1093/geront/41.6.716. [DOI] [PubMed] [Google Scholar]
- 29.Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol Aging. 1997;18:S1–2. [PubMed] [Google Scholar]
- 30.Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 31.Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, Peskind E, Dietrich W, Beekly DL, Kukull WA, Morris JC. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khachaturian ZS. Diagnosis of Alzheimer’s disease. Arch Neurol. 1985;42:1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- 33.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 34.Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; Washington, D.C: 1994. [Google Scholar]
- 35.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 36.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 37.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 38.Nelson PT, Jicha GA, Schmitt FA, Liu H, Davis DG, Mendiondo MS, Abner EL, Markesbery WR. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol. 2007;66:1136–1146. doi: 10.1097/nen.0b013e31815c5efb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 40.Gearing M, Mirra SS, Hedreen JC, Sumi SM, Hansen LA, Heyman A. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part X. Neuropathology confirmation of the clinical diagnosis of Alzheimer’s disease. Neurology. 1995;45:461–466. doi: 10.1212/wnl.45.3.461. [DOI] [PubMed] [Google Scholar]
- 41.Jicha GA, Schmitt FA, Abner E, Nelson PT, Cooper GE, Smith CD, Markesbery WR. Prodromal clinical manifestations of neuropathologically confirmed Lewy body disease. Neurobiol Aging. 31:1805–1813. doi: 10.1016/j.neurobiolaging.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Markesbery WR, Jicha GA, Liu H, Schmitt FA. Lewy body pathology in normal elderly subjects. J Neuropathol Exp Neurol. 2009;68:816–822. doi: 10.1097/NEN.0b013e3181ac10a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heyman A, Fillenbaum GG, Mirra SS. Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): clinical, neuropsychological, and neuropathological components. Aging (Milano) 1990;2:415–424. doi: 10.1007/BF03323962. [DOI] [PubMed] [Google Scholar]
- 44.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 45.Morris JC, Mohs RC, Rogers H, Fillenbaum G, Heyman A. Consortium to establish a registry for Alzheimer’s disease (CERAD) clinical and neuropsychological assessment of Alzheimer’s disease. Psychopharmacol Bull. 1988;24:641–652. [PubMed] [Google Scholar]
- 46.Wechsler D. WAIS: Wechsler Adult Intelligence Scale - Revised. American Psychological Corporation; San Antonio, Tx: 1981. [Google Scholar]
- 47.Reitan R. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical and Interpretation. Neuropsychology Press; Tuscon, AZ: 1985. [Google Scholar]
- 48.Chandler MJ, Lacritz LH, Hynan LS, Barnard HD, Allen G, Deschner M, Weiner MF, Cullum CM. A total score for the CERAD neuropsychological battery. Neurology. 2005;65:102–106. doi: 10.1212/01.wnl.0000167607.63000.38. [DOI] [PubMed] [Google Scholar]
- 49.Hassing LB, Johansson B, Berg S, Nilsson SE, Pedersen NL, Hofer SM, McClearn G. Terminal decline and markers of cerebro- and cardiovascular disease: findings from a longitudinal study of the oldest old. J Gerontol B Psychol Sci Soc Sci. 2002;57:P268–276. doi: 10.1093/geronb/57.3.p268. [DOI] [PubMed] [Google Scholar]
- 50.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 51.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 52.Petersen RC, Parisi JE, Dickson DW, Johnson KA, Knopman DS, Boeve BF, Jicha GA, Ivnik RJ, Smith GE, Tangalos EG, Braak H, Kokmen E. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 2006;63:665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- 53.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 54.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- 55.Loewenstein DA, Acevedo A, Potter E, Schinka JA, Raj A, Greig MT, Agron J, Barker WW, Wu Y, Small B, Schofield E, Duara R. Severity of medial temporal atrophy and amnestic mild cognitive impairment: selecting type and number of memory tests. Am J Geriatr Psychiatry. 2009;17:1050–1058. doi: 10.1097/JGP.0b013e3181b7ef42. [DOI] [PubMed] [Google Scholar]
- 56.Loewenstein DA, Acevedo A, Small BJ, Agron J, Crocco E, Duara R. Stability of different subtypes of mild cognitive impairment among the elderly over a 2- to 3-year follow-up period. Dement Geriatr Cogn Disord. 2009;27:418–423. doi: 10.1159/000211803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 58.Shankle WR, Romney AK, Hara J, Fortier D, Dick MB, Chen JM, Chan T, Sun X. Methods to improve the detection of mild cognitive impairment. Proc Natl Acad Sci U S A. 2005;102:4919–4924. doi: 10.1073/pnas.0501157102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adak S, Illouz K, Gorman W, Tandon R, Zimmerman EA, Guariglia R, Moore MM, Kaye JA. Predicting the rate of cognitive decline in aging and early Alzheimer disease. Neurology. 2004;63:108–114. doi: 10.1212/01.wnl.0000132520.69612.ab. [DOI] [PubMed] [Google Scholar]
- 60.Ashford JW, Schmitt FA. Modeling the time-course of Alzheimer dementia. Curr Psychiatry Rep. 2001;3:20–28. doi: 10.1007/s11920-001-0067-1. [DOI] [PubMed] [Google Scholar]
- 61.Ashford JW, Shan M, Butler S, Rajasekar A, Schmitt FA. Temporal quantification of Alzheimer’s disease severity: ‘time index’ model. Dementia. 1995;6:269–280. doi: 10.1159/000106958. [DOI] [PubMed] [Google Scholar]
- 62.Galvin JE, Powlishta KK, Wilkins K, McKeel DW, Jr, Xiong C, Grant E, Storandt M, Morris JC. Predictors of preclinical Alzheimer disease and dementia: a clinicopathologic study. Arch Neurol. 2005;62:758–765. doi: 10.1001/archneur.62.5.758. [DOI] [PubMed] [Google Scholar]
- 63.Mendiondo MS, Ashford JW, Kryscio RJ, Schmitt FA. Modelling mini mental state examination changes in Alzheimer’s disease. Stat Med. 2000;19:1607–1616. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1607::aid-sim449>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 64.Saxton J, Lopez OL, Ratcliff G, Dulberg C, Fried LP, Carlson MC, Newman AB, Kuller L. Preclinical Alzheimer disease: neuropsychological test performance 1.5 to 8 years prior to onset. Neurology. 2004;63:2341–2347. doi: 10.1212/01.wnl.0000147470.58328.50. [DOI] [PubMed] [Google Scholar]
- 65.Baars MA, van Boxtel MP, Dijkstra JB, Visser PJ, van den Akker M, Verhey FR, Jolles J. Predictive value of mild cognitive impairment for dementia. The influence of case definition and age. Dement Geriatr Cogn Disord. 2009;27:173–181. doi: 10.1159/000200465. [DOI] [PubMed] [Google Scholar]
- 66.Riley KP, Snowdon DA, Desrosiers MF, Markesbery WR. Early life linguistic ability, late life cognitive function, and neuropathology: findings from the Nun Study. Neurobiol Aging. 2005;26:341–347. doi: 10.1016/j.neurobiolaging.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 67.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. Jama. 1997;277:813–817. [PubMed] [Google Scholar]
- 68.Braskie MN, Klunder AD, Hayashi KM, Protas H, Kepe V, Miller KJ, Huang SC, Barrio JR, Ercoli LM, Siddarth P, Satyamurthy N, Liu J, Toga AW, Bookheimer SY, Small GW, Thompson PM. Plaque and tangle imaging and cognition in normal aging and Alzheimer’s disease. Neurobiol Aging. 2010;31:1669–1678. doi: 10.1016/j.neurobiolaging.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bunce D, Fratiglioni L, Small BJ, Winblad B, Backman L. APOE and cognitive decline in preclinical Alzheimer disease and non-demented aging. Neurology. 2004;63:816–821. doi: 10.1212/01.wnl.0000137041.86153.42. [DOI] [PubMed] [Google Scholar]
- 70.Markesbery WR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR. Neuropathologic substrate of mild cognitive impairment. Arch Neurol. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- 71.Backman L. Memory and cognition in preclinical dementia: what we know and what we do not know. Can J Psychiatry. 2008;53:354–360. doi: 10.1177/070674370805300604. [DOI] [PubMed] [Google Scholar]
- 72.Kawas CH, Corrada MM, Brookmeyer R, Morrison A, Resnick SM, Zonderman AB, Arenberg D. Visual memory predicts Alzheimer’s disease more than a decade before diagnosis. Neurology. 2003;60:1089–1093. doi: 10.1212/01.wnl.0000055813.36504.bf. [DOI] [PubMed] [Google Scholar]
- 73.Lange KL, Bondi MW, Salmon DP, Galasko D, Delis DC, Thomas RG, Thal LJ. Decline in verbal memory during preclinical Alzheimer’s disease: examination of the effect of APOE genotype. J Int Neuropsychol Soc. 2002;8:943–955. doi: 10.1017/s1355617702870096. [DOI] [PMC free article] [PubMed] [Google Scholar]