Abstract
OBJECTIVE
To determine the relationship between perineural invasion (PNI) on prostate biopsy and radical prostatectomy (RP) outcomes in a contemporary RP series, as there is conflicting evidence on the prognostic significance of PNI in prostate needle biopsy specimens.
PATIENTS AND METHODS
From 2002 to 2007, 1256 men had RP by one surgeon. Multivariable logistic regression and Cox proportional hazards models were used to examine the relationship of PNI with pathological tumour features and biochemical progression, respectively, after adjusting for prostate-specific antigen level, clinical stage and biopsy Gleason score. Additional Cox models were used to examine the relationship between nerve-sparing and biochemical progression among men with PNI.
RESULTS
PNI was found in 188 (15%) patients, and was significantly associated with aggressive pathology and biochemical progression. On multivariate analysis, PNI was significantly associated with extraprostatic extension and seminal vesicle invasion (P < 0.001). Biochemical progression occurred in 10.5% of patients with PNI, vs 3.5% of those without PNI (unadjusted hazard ratio 3.12, 95% confidence interval 1.77–5.52, P < 0.001). However, PNI was not a significant independent predictor of biochemical progression on multivariate analysis. Finally, nerve-sparing did not adversely affect biochemical progression even among men with PNI.
CONCLUSION
PNI is an independent risk factor for aggressive pathology features and a non-independent risk factor for biochemical progression after RP. However, bilateral nerve-sparing surgery did not compromise the oncological outcomes for patients with PNI on biopsy.
Keywords: perineural invasion, prostate biopsy, prostate cancer, radical prostatectomy, outcomes
INTRODUCTION
Prostate cancer has considerable biological heterogeneity, and accordingly, several clinical staging tools have been developed to better characterize the disease for therapeutic decisions and prognostication. Previously published prognostic tools, such as the D’Amico risk groups [1], Han Tables [2], and Kattan nomogram [3] use a combination of PSA level, clinical stage and biopsy Gleason score to predict biochemical progression. The University of California San Francisco Cancer of the Prostate Risk Assessment score [4] is another validated predictive tool which uses the same variables, as well as age and the percentage of positive biopsy cores, to predict the likelihood of biochemical progression. It is likely that the incorporation of additional serum or histopathological variables might further refine pretreatment staging. One potential histopathological marker is perineural invasion (PNI) in the prostate needle-biopsy specimen, defined as the tracking of carcinoma around nerves [5]. Indeed, some studies have shown that PNI predicts a significantly greater risk for aggressive pathology features, as well as a higher risk of progression after radical prostatectomy (RP) or radiotherapy [6]. However, other studies have reported conflicting results. Nevertheless, most of the cited studies were reported earlier within the PSA era or involved relatively few patients [7–9]. Thus, the objective of the present study was to further examine the current prognostic utility of PNI in a large contemporary series of RP.
PATIENTS AND METHODS
From January 2002 to June 2007, 1256 consecutive men had retropubic RP for clinically localized prostate cancer, by one surgeon (P.C.W.). The clinical and pathological features were recorded in a prospective database. All patients provided informed consent and the study protocol received institutional review board approval.
Although many patients were diagnosed with prostate cancer at outside institutions, all or pertinent prostate biopsy material was re-reviewed at our institution before RP. PNI was defined as prostate cancer extension along the perineural sheath. The presence or absence of this finding in the prostate biopsy specimen was recorded in all cases by pathologists at our institution (mandatory reporting). Data on the number of positive biopsy cores and the maximum percentage of biopsy core involvement were available for 1011 (80.5%) and 931 (74%) men, respectively.
After RP, PSA levels were measured and a DRE performed at 3-month intervals for the first year, at 6-month intervals for the second year, and annually thereafter. Biochemical progression was defined as a PSA level of >0.2 ng/mL.
Descriptive statistics were used to examine the demographics and clinical characteristics of men with and without PNI on prostate biopsy. Logistic regression was then used to determine the relationship between PNI with adverse pathological tumour features, adjusting for standard clinical predictors (PSA level, clinical stage, Gleason score). The Kaplan-Meier method was used to calculate progression-free survival rates, which were compared between men with and without PNI on prostate biopsy, using the log-rank test. Finally, we examined the relationship between PNI with biochemical progression using Cox proportional hazards models. Notably, neither the number of positive biopsy cores nor the maximum percentage of core involvement were significant, and therefore these variables were not included in the final model.
RESULTS
The mean age of the 1256 men was 56 years and most were Caucasian (Table 1). The mean preoperative PSA level was 5.7 ng/mL, 76.7% had a Gleason score of ≤6, and 76.1% had clinical stage T1c disease. The mean (SD) number of positive biopsy cores was 2.9 (2.3) and the maximum percentage of biopsy core involvement was 43.2 (29.2)%. At RP, most men (77.7%) had organ-confined disease.
TABLE 1.
The clinical and pathological characteristics of the study population
| Variable | Mean (SD, range) or n (%) |
|---|---|
| Age, years | 56.1 (6.7, 34–75) |
| Race | |
| Caucasian | 1116 (88.9) |
| African-American | 64 (5.1) |
| Other | 63 (5.0) |
| Missing | 13 (1.0) |
| Positive family history (1141 with data) | 461 (40.4) |
| PSA level, ng/mL | 5.7 (3.7, 0.2–43.1) |
| Biopsy Gleason score | |
| ≤6 | 963 (76.7) |
| 7 | 260 (20.7) |
| 8–10 | 33 (2.6) |
| Clinical stage | |
| T1 a/b/c | 958 (76.3) |
| T2a | 222 (17.7) |
| T2b | 67 (5.3) |
| T2c | 6 (0.5) |
| T3 | 2 (0.2) |
| EPE | 274 (21.8) |
| Positive surgical margins | 84 (6.7) |
| Seminal vesicle invasion | 44 (3.5) |
| Lymph node metastases | 29 (2.3) |
Overall, 188 (15%) patients had PNI in the biopsy specimen. A greater proportion of patients with PNI had extraprostatic extension (EPE, 48.9% vs 17.1%), positive surgical margins (11.2% vs 5.9%), seminal vesicle invasion (11.2% vs 2.2%), and lymph node metastases (7.4% vs 1.4%).
Table 2 shows the univariate and multivariate associations between PNI and RP pathology. On univariate analysis, PNI was significantly associated with a higher odds of each adverse pathology feature. After adjusting for PSA level, clinical stage, and biopsy Gleason score, PNI remained independently associated with EPE and seminal vesicle invasion (P ≤ 0.001 for both).
TABLE 2.
Univariable and multivariable logistic regression models for the relationship between biopsy PNI and pathology features
| Odds ratio (95% CI), P |
||
|---|---|---|
| Variable | Unadjusted | Adjusted* |
| EPE | 4.66 (3.36–6.47) <0.001 |
2.99 (2.08–4.30) <0.001 |
| Positive surgical margins | 1.87 (1.12–3.15) 0.017 |
1.54 (0.88–2.70) 0.128 |
| Seminal vesicle invasion | 5.47 (2.98–10.05) <0.001 |
3.24 (1.64–6.40) 0.001 |
| Lymph node metastases | 5.65 (2.68–11.91) <0.001 |
2.29 (0.97–5.40) 0.059 |
For PSA level, clinical stage, and biopsy Gleason score.
At a mean (range) overall follow-up of 2.8 (0–7) years, biochemical progression occurred in 10.5% and 3.5% of men with and without PNI, respectively. Figure 1 shows the Kaplan-Meier progression-free survival curve, stratified by PNI (P < 0.001, log-rank test). On univariate analysis, PNI was associated with a 3.12-fold increased hazard ratio for biochemical progression (95% CI 1.77–5.52, P < 0.001). However, after adjusting for PSA level, clinical stage and biopsy Gleason score, the relationship between PNI with time to progression was no longer statistically significant (Table 3). Additional adjustment for the number or percentage of positive biopsy cores did not change the results (data not shown). Similarly, PNI was not an independent risk factor for biochemical progression in the subgroup with clinical stage T1c disease (adjusted hazard ratio 2.01, 95% CI 0.89–4.52, P = 0.093) adjusting for PSA level and biopsy Gleason score.
FIG. 1.
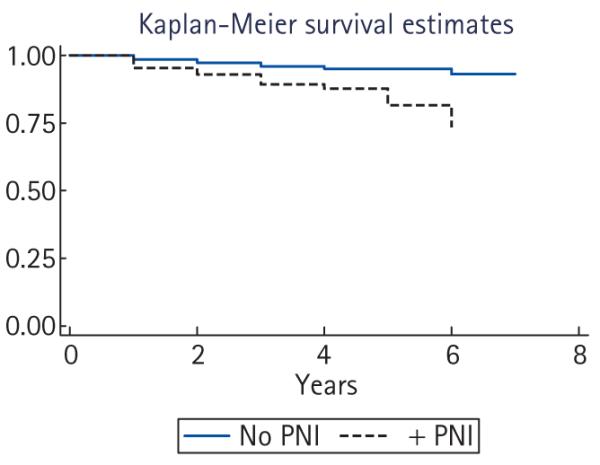
Kaplan-Meier progression-free survival curve, stratified by the presence or absence of PNI on biopsy.
TABLE 3.
Multivariable Cox proportional hazards models for the relationship between biopsy PNI and biochemical progression, adjusting for standard clinical variables, or with additional adjustment for bilateral nerve-sparing technique
| Variable | Hazard ratio (95% CI), P |
|---|---|
| Model 1 | |
| PNI | 1.57 (0.87–2.83), 0.135 |
| PSA level (continuous) | 1.11 (1.07–1.16), <0.001 |
| Clinical stage | 1.28 (0.92–1.78), 0.138 |
| Biopsy Gleason score | 3.82 (2.84–5.14), <0.001 |
| Model 2 | |
| Nerve-sparing | 0.39 (0.19–0.80), 0.010 |
| PNI | 1.56 (0.86–2.82), 0.140 |
| PSA level | 1.12 (1.08–1.17), <0.001 |
| Biopsy Gleason score | 3.23 (2.32–4.49), <0.001 |
| Clinical stage | 1.15 (0.80–1.64), 0.448 |
For the surgical technique, 1069 of 1238 (86.3%) patients with intraoperative data had bilateral nerve-sparing, while unilateral and non-nerve-sparing procedures were performed in the remaining 13.3% and 0.4%, respectively. Bilateral nerve-sparing was performed in 113 (63.5%) men with PNI and 956 (90.2%) without PNI. In men with PNI, bilateral nerve-sparing was not associated with the risk of positive surgical margins (odds ratio 1.28, 95% CI 0.5–3.5, P = 0.633). On univariate analysis, bilateral nerve-sparing had a significant protective association with biochemical progression (hazard ratio 0.19, 95% CI 0.13–0.27, P < 0.001). The significant association between bilateral nerve-sparing technique and biochemical progression was maintained after adjusting for PNI, PSA, clinical stage, and Gleason score (Table 3). Similarly, a separate multivariate Cox proportional hazards model in the subgroup with PNI present on biopsy showed a significantly lower risk of progression with bilateral nerve-sparing (hazard ratio 0.17, 95% CI 0.05–0.60, P = 0.006).
DISCUSSION
Clinical staging is critical to select the appropriate management strategy for prostate cancer, and to guide the optimal application of the chosen treatment. Initially, this can include the decision to undergo active surveillance or immediate definitive therapy. For patients who ultimately select RP, staging can influence the decision to use nerve-sparing. For patients choosing primary radiotherapy, staging helps to determine the need for concomitant hormonal therapy and the optimal radiation field. The impact of PNI on these clinical decisions remains controversial [10].
Numerous studies have examined the association between biopsy PNI and pathological features at RP. For example, Lee et al. [11] reported that biopsy PNI was associated with a significantly higher risk of positive surgical margins and pathological stage T3 disease in a multivariate analysis within each D’Amico risk group.
PNI has traditionally been thought to facilitate extraprostatic tumour spread through the ‘path of least resistance’ by following the course of nerve rootlets extending from the prostate [12]. Furthermore, PNI which is sufficiently extensive to be sampled on needle biopsy might be associated with a greater risk of EPE. More recent studies have also suggested that proximity to nerves might afford a survival advantage for cancer cells in vitro [13].
The relationship between PNI and biochemical progression is more controversial [7–9]. In 2007, Harnden et al. [6] reported a systematic review of 21 articles (through to 2005) on the significance of PNI for predicting progression. Overall, six of 10 surgical studies and five of 11 radiotherapy studies reported a ‘positive’ association between PNI with progression at least on univariate analysis. However, in some studies PNI was only predictive within certain patient subgroups [9,14], or lost its statistical significance after considering standard risk predictors (such as PSA level and Gleason score) in a multivariable model. In addition, PNI correlated with progression in some studies of external beam radiotherapy but not after brachytherapy [15].
Our group previously examined the relationship between PNI and progression after RP in 78 men with PNI compared to 78 matched controls treated by the same surgeon (P.C.W.) during the earlier period from 1984 to 1995 [7]. In that study, there was no difference in the biochemical progression rate based upon PNI at a 7-year mean follow-up. In the present study we expanded upon these findings in a significantly larger contemporary population. Although PNI was associated with a threefold increased hazard ratio for biochemical progression, the relationship was no longer statistically significant after adjusting for PSA level, clinical stage and biopsy Gleason score.
For clinical decision-making, PNI was previously considered a potential contraindication to ipsilateral nerve-sparing due to its association with EPE. For example, Sankin et al. [16] reported that PNI was an independent predictor of side-specific EPE (odds ratio 1.9, 95% CI 0.2–3.2, P = 0.012). By contrast, our group previously examined the association between PNI with EPE in the neurovascular bundle, and found no significant relationship on multivariate analysis [17].
In the present study, the presence of PNI was one of many factors that influenced the decision to use nerve-sparing [18]. Despite a significant relationship between PNI with EPE and other adverse pathological features, there was a significantly lower risk of biochemical progression with bilateral nerve-sparing, even after adjusting for PNI, PSA level, clinical stage and Gleason score. Because bilateral nerve-sparing was used in the overwhelming majority of cases, this probably represents residual confounding. That notwithstanding, our results suggest that bilateral nerve-sparing is feasible for selected men with PNI without compromising oncological outcomes. A limitation of our study is that all patients had RP, and therefore we are unable to determine the effect of PNI on outcomes with other forms of management, such as active surveillance or radiotherapy. Some, but not all, studies have suggested an independent relationship between PNI and worse biochemical outcomes after radiotherapy [14,19]. Further studies are warranted to help to address whether surgical management is preferable for this population, or whether modifications in the radiation treatment (such as combined hormonal therapy or use of a more extended field) lead to an improvement in outcomes.
Another limitation of our study was that detailed biopsy core data were not available for some patients. In particular, we recorded the presence or absence of biopsy PNI in all cases, but did not quantify the extent or laterality of PNI on biopsy. In addition, the mean follow-up was relatively short, and it is possible that greater differences in biochemical progression might emerge with additional follow-up. However, we chose to study this issue in a contemporary cohort to better evaluate the role of PNI in current prostate cancer staging.
A strength of our study is the large sample size of patients treated by one expert surgeon, limiting any influence of variability in surgical technique on the outcomes. In addition, the study population comprised consecutive patients, in which expert pathologists from our institution confirmed the presence or absence of biopsy PNI in all cases.
In conclusion, PNI on prostate needle biopsy is a marker for more aggressive pathology findings at RP, including a significantly higher risk of EPE and seminal vesicle invasion. PNI was also a non-independent risk factor for biochemical progression. Nevertheless, bilateral nerve-sparing surgery was feasible in the vast majority of patients irrespective of PNI, with favourable short-term oncological outcomes.
ACKNOWLEDGEMENTS
We acknowledge funding support for our research from the SPORE grant P50CA58236.
Abbreviations
- PNI
perineural invasion
- EPE
extraprostatic extension
- RP
radical prostatectomy
Footnotes
CONFLICT OF INTEREST None declared.
REFERENCES
- 1.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 2.Han M, Partin AW, Zahurak M, et al. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003;169:517–23. doi: 10.1097/01.ju.0000045749.90353.c7. [DOI] [PubMed] [Google Scholar]
- 3.Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90:766–71. doi: 10.1093/jnci/90.10.766. [DOI] [PubMed] [Google Scholar]
- 4.Cooperberg MR, Pasta DJ, Elkin EP, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005;173:1938–42. doi: 10.1097/01.ju.0000158155.33890.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastacky SI, Walsh PC, Epstein JI. Relationship between perineural tumor invasion on needle biopsy and radical prostatectomy capsular penetration in clinical stage B adenocarcinoma of the prostate. Am J Surg Pathol. 1993;17:336–41. doi: 10.1097/00000478-199304000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Harnden P, Shelley MD, Clements H, et al. The prognostic significance of perineural invasion in prostatic cancer biopsies: a systematic review. Cancer. 2007;109:13–24. doi: 10.1002/cncr.22388. [DOI] [PubMed] [Google Scholar]
- 7.O’Malley KJ, Pound CR, Walsh PC, Epstein JI, Partin AW. Influence of biopsy perineural invasion on long-term biochemical disease-free survival after radical prostatectomy. Urology. 2002;59:85–90. doi: 10.1016/s0090-4295(01)01486-8. [DOI] [PubMed] [Google Scholar]
- 8.de la Taille A, Rubin MA, Bagiella E, et al. Can perineural invasion on prostate needle biopsy predict prostate specific antigen recurrence after radical prostatectomy? J Urol. 1999;162:103–6. doi: 10.1097/00005392-199907000-00025. [DOI] [PubMed] [Google Scholar]
- 9.D’Amico AV, Wu Y, Chen MH, et al. Perineural invasion as a predictor of biochemical outcome following radical prostatectomy for select men with clinically localized prostate cancer. J Urol. 2001;165:126–9. doi: 10.1097/00005392-200101000-00031. [DOI] [PubMed] [Google Scholar]
- 10.Rubin MA, Bismar TA, Curtis S, Montie JE. Prostate needle biopsy reporting: how are the surgical members of the Society of Urologic Oncology using pathology reports to guide treatment of prostate cancer patients? Am J Surg Pathol. 2004;28:946–52. doi: 10.1097/00000478-200407000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Lee IH, Roberts R, Shah RB, et al. Perineural invasion is a marker for pathologically advanced disease in localized prostate cancer. Int J Radiat Oncol Biol Phys. 2007;68:1059–64. doi: 10.1016/j.ijrobp.2007.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassan MO, Maksem J. The prostatic perineural space and its relation to tumor spread: an ultrastructural study. Am J Surg Pathol. 1980;4:143–8. doi: 10.1097/00000478-198004000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Ayala GE, Dai H, Ittmann M, et al. Growth and survival mechanisms associated with perineural invasion in prostate cancer. Cancer Res. 2004;64:6082–90. doi: 10.1158/0008-5472.CAN-04-0838. [DOI] [PubMed] [Google Scholar]
- 14.Bonin SR, Hanlon AL, Lee WR, et al. Evidence of increased failure in the treatment of prostate carcinoma patients who have perineural invasion treated with three-dimensional conformal radiation therapy. Cancer. 1997;79:75–80. doi: 10.1002/(sici)1097-0142(19970101)79:1<75::aid-cncr11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 15.Merrick GS, Butler WM, Wallner KE, et al. Prognostic significance of perineural invasion on biochemical progression-free survival after prostate brachytherapy. Urology. 2005;66:1048–53. doi: 10.1016/j.urology.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Sankin A, Tareen B, Lepor H. Side-specific factors associated with extracapsular extension and seminal vesicular invasion in men undergoing open radical retropubic prostatectomy. Prostate Cancer Prostatic Dis. 2009;12:204–8. doi: 10.1038/pcan.2009.2. [DOI] [PubMed] [Google Scholar]
- 17.Tsuzuki T, Hernandez DJ, Aydin H, et al. Prediction of extraprostatic extension in the neurovascular bundle based on prostate needle biopsy pathology, serum prostate specific antigen and digital rectal examination. J Urol. 2005;173:450–3. doi: 10.1097/01.ju.0000151370.82099.1a. [DOI] [PubMed] [Google Scholar]
- 18.Holmes GF, Walsh PC, Pound CR, Epstein JI. Excision of the neurovascular bundle at radical prostatectomy in cases with perineural invasion on needle biopsy. Urology. 1999;53:752–6. doi: 10.1016/s0090-4295(98)00605-0. [DOI] [PubMed] [Google Scholar]
- 19.Yu HH, Song DY, Tsai YY, et al. Perineural invasion affects biochemical recurrence-free survival in patients with prostate cancer treated with definitive external beam radiotherapy. Urology. 2007;70:111–6. doi: 10.1016/j.urology.2007.03.020. [DOI] [PubMed] [Google Scholar]


