Abstract
Functional reorganization of the motor system occurs in response to both aging and Parkinson's disease (PD). Since PD typically develops in older adults, disease progression and the effects of treatment may interact with normal aging. Using event‐related functional magnetic resonance imaging, we studied patients “on” and “off” their normal dopaminergic medication, age‐matched controls and younger adults on tasks of action and action selection. For manual movements, aging increased activity in bilateral motor, premotor and cingulate cortex. Activation in the premotor regions of “on” patients was higher relative to age‐matched controls. However, in contrast to controls and “off” patients, the activations for patients when “on” decreased with age. Voluntary selection of actions was associated with activation in a bilateral network of fronto‐parietal cortex. Within this network, advancing severity of PD was associated with decreased activity particularly in premotor and ventrolateral prefrontal cortex. Together, these results reveal very different patterns of age‐related changes in health and PD. Younger patients are able to exert greater compensatory activity in premotor cortex than older patients, even after correction for disease severity. This effect is dopamine dependant, and may in part explain the clinical observation of reduced dopamine responsiveness in older patients with PD. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: disease severity, fMRI, levodopa, motor, prefrontal cortex
INTRODUCTION
We often take it for granted that we can choose and carry out actions, expressing our volition as part of normal human behavior. However, the processes underlying the selection and execution of responses change with healthy aging [Ward, 2006] and neurodegenerative diseases like Parkinson's disease (PD) [Ballanger et al., 2008; Buhmann et al., 2003; Eckert et al., 2006; Haslinger et al., 2001; Rascol et al., 1994, 1997, 1998; Rowe et al., 2002; Sabatini et al., 2000; Yu et al., 2007]. Since PD is an archetypal movement disorder, and typically develops in older adults, it is necessary to understand better the independent and interacting effects of healthy aging and PD [Levy, 2007; Levy et al., 2005].
Our primary focus is the effect of healthy aging and PD on the motor systems that support voluntary action. Neuroimaging with functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) have been used for many years to study the neural basis of voluntary actions in PD. Many studies have used ∼8 patients with mild to moderate PD in a relative “on” or “off” state, and compared regional brain activity with healthy control subjects, on a variety of “simple” motor tasks including sequential finger thumb opposition, button presses, or joystick movements. The different statistical methods and thresholds of these studies reflect the evolution of neuroimaging methodologies over 20 years, but most studies still make a categorical distinction between groups, and do not explore the effects of disease severity or age.
Despite these potential limitations, several patterns have emerged. For simple actions, patients with PD in the “off” state are shown to have impaired activity in medial frontal cortex (including SMA) and prefrontal areas [Sabatini et al., 2000; Samuel et al., 1997; Yu et al., 2007], and more so for self‐initiated compared to externally cued movements [Ballanger et al., 2008; Jahanshahi et al., 1995; Playford et al., 1992]. Additional increases in activity in “off” patients are reported in lateral premotor cortex [Haslinger et al., 2001; Sabatini et al., 2000; Samuel et al., 1997], motor cortex [Haslinger et al., 2001; Yu et al., 2007], and cerebellum [Rascol et al., 1997; Yu et al., 2007]. The cortical shift from medial to lateral premotor cortex [Samuel et al., 1997] and subcortical shift from basal ganglia to cerebellum [Yu et al., 2007] are proposed to be compensatory. Dopaminergic medications can partially restore SMA dysfunction [Buhmann et al., 2003; Haslinger et al., 2001; Jenkins et al., 1992; Rascol et al., 1994, 1997], although the activation of rostral SMA may remain abnormal [Haslinger et al., 2001]. However, these studies do not distinguish age‐related changes that may arise across the typical 50–75 years age range of the PD cohorts.
How then does healthy aging affect the neural basis of simple motor actions? Even for simple tasks, motor functions are reported to change significantly with age [Ward, 2006] including increases in neural activity in the motor, premotor cortex, and SMA [Heuninckx et al., 2005; Hutchinson et al., 2002; Mattay et al., 2002; Naccarato et al., 2006; Ward and Frackowiak, 2003]. However reduced activation of the motor cortex has also been reported [D'Esposito et al., 1999; Hesselmann et al., 2001; Tekes et al., 2005]. Further to increased activity in the motor networks, there is evidence of reorganization with recruitment of additional brain areas including ipsilateral motor and premotor cortex [Naccarato et al., 2006; Ward and Frackowiak, 2003], which may result from changes in transcallosal inhibitory connections [Naccarato et al., 2006; Rowe et al., 2006].
Reduced lateralization may be part of a generic “hemispheric asymmetry reduction in older age,” described by the HAROLD model of aging cognition. The HAROLD model suggests that increased bilateral activation may compensate for aging [Dolcos et al., 2002]. Evidence that this reorganization is compensatory comes from negative correlations of reactions times with activations in the motor areas [Heuninckx et al., 2008; Mattay et al., 2002], which imply that additional activation is required by older subjects to maintain good performance. There are also similarities between age‐related changes in regional activation and changes observed after stroke: post‐stroke patients show widespread ncreases in task‐related activity relative to controls, which normalizes with recovery [Calautti et al., 2007; Ward et al., 2003a, b].
These data suggest clear differences between aging and PD on the motor and premotor cortical activations associated with movement. Whereas PD shifts activity from medial to more lateral motor networks, aging increases bilateral motor/premotor cortical activity. Our first aim was therefore to compare the effects of aging and PD on the neural mechanisms of action, with particular reference to the medial (SMA) and lateral premotor cortex. However, the relationship between age and disease may be complicated by the heterogeneity of PD and medication state. Thus, our second aim was to estimate the effects of disease severity and dopaminergic medication on motor processes rather than assume an average effect of PD over all patients. This has been shown to be relevant for cognitive and motor tasks [Rowe et al., 2008b]. Additionally PD patients have greater difficulty with voluntary actions relative to externally cued responses [Cunnington et al., 1995], so our third aim was to examine the interaction of aging and PD on self‐selected responses compared to externally specified responses using a task known to activate prefrontal regions [Rowe et al., 2005, 2008b].
METHODS
Subjects
Sixteen patients (50–80) with idiopathic PD were recruited from the Cambridge Centre for Brain Repair's PD research clinic, using the UK PD Brain Bank clinical diagnostic criteria. Patients were tested once on their usual dopaminergic medication and once after dopaminergic mediation had been withdrawn (minimum 12 h for short acting preparations, 24 h for long acting preparations, such as cabergoline or modified/slow release preparations of Madopar and Sinemet). The order of testing was counterbalanced so that half patients were “off” on their first visit and half were “on.” Patients were examined on both occasions using the UPDRS‐III motor rating scale and classified with the Hoehn and Yahr [ 1967] and Schwab and England [ 1969] scales. Fifteen healthy older adults (50–80) and twenty‐eight healthy younger adults, (18–48) were recruited from the same PD research clinic database and the volunteer panel of the MRC Cognition and Brain Sciences Unit. Subject details are summarized in Table I.
Table I.
Demographic and drug details of PD patients and controls
| UPDRS | Other l‐Dopa | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Sex | Age | Schwab | H + Y (on) | Duration | On | Off | Ldopa | Ldopa equivalenta | A | C | R | E | Otherb |
| 1 | f | 65 | 90 | 2.5 | 4.6 | 30 | 38 | 600 | 1,560 | 24 | ||||
| 2 | m | 61 | 70 | 2 | 15.4 | 17 | 53 | 2,200 | 2,585 | 300 | T 300 | |||
| 3 | m | 59 | 80 | 2 | 5.7 | 29 | 36 | 500 | 1,140 | 200 | 4 | |||
| 4 | f | 53 | 80 | 2 | 8.7 | 14 | 24 | 900 | 2,200 | 100 | 21 | |||
| 5 | m | 78 | 80 | 3 | 13.5 | 30 | 50 | 700 | 512 | O 120 | ||||
| 6 | f | 59 | 70 | 2 | 5.5 | 31 | 34 | 300 | 1,260 | 6 | ||||
| 7 | m | 68 | 80 | 2 | 9.0 | 20 | 32 | 250 | 915 | 4 | S 5 | |||
| 8 | m | 66 | 80 | 2 | 6.9 | 25 | 33 | 800 | 1,009 | 200 | 1 | 600 | ||
| 9 | m | 65 | 70 | 2.5 | 13.5 | 19 | 36 | 700 | 1,196 | 800 | S 10 | |||
| 10 | f | 65 | 100 | 2 | 5.0 | 7 | 12 | 250 | 1,090 | 21 | O 50, D 30 | |||
| 11 | f | 49 | 90 | 2 | 9.5 | 15 | 34 | 300 | 1,200 | 200 | 15 | |||
| 12 | f | 78 | 90 | 1 | 5.7 | 1 | 14 | 600 | 600 | |||||
| 13 | f | 62 | 90 | 1 | 5.4 | 10 | 26 | 500 | 1,020 | 18 | 600 | |||
| 14 | m | 64 | 90 | 2 | 3.0 | 17 | 20 | 400 | 1,290 | 100 | P 2.1 | |||
| 15 | m | 62 | 100 | 2 | 5.2 | 19 | 30 | 800 | 1,640 | 100 | P 1 | |||
| 16 | m | 69 | 80 | 2 | 4.8 | 18 | 29 | 400 | 1,120 | 4 | S 1.25 | |||
| 9m, 7f | Mean | 63.9 | 83.8 | 2.0 | 7.6 | 18.9 | 31.3 | 637.5 | 1271.1 | |||||
| Sd | 7.5 | 9.6 | 0.5 | 3.7 | 8.6 | 11.0 | 465.7 | 527.8 | ||||||
| Older Controlsc | ||||||||||||||
| 7m, 8f | Mean | 66.5 | ||||||||||||
| Sd | 5.9 | |||||||||||||
| Younger Controls | ||||||||||||||
| 17m, 11f | Mean | 31.0 | ||||||||||||
| Sd | 10.6 | |||||||||||||
Equivalent levodopa dose = [levodopa (× 1.2 if COMT inhibitor) (× 1.2 if 10 mg of S or × 1.1 if 5 mg of S)] + [P × 400] + [R × 40] + [C × 160] + [pergolide × 200] + [bromocriptine × 10] + [lisuride × 160]; all doses are in milligrams. (Williams Gray C. (2007) J Neurosci 27 (18) 4832–4838).
Other drugs as mg/day are: A = amantadine, C = Cabergoline, R = Ropinirole, E = entacapone, T = tolcapone, S = selegiline, P = pramipexole, O = orphenadrine, D = domperidone.
Medications taken by four older controls were: 1 × fenofibrate, 2 × antihypertensives, 1 × ibuprofen, co‐codamol, zopiclone, tamsulosin hydrochloride and etodolac.
Schwab, Schwab and England; H+Y, Hoehn and Yahr scale; UPDRS, Unified Parkinson's Disease Rating Scale motor subscale III; MMSE, Folstein Mini‐Mental State Examination; Duration, Duration of disease from diagnosis in years.
All subjects were right handed and none had current depressive illness, and no known dementia based on prior cognitive assessment. No subjects in the healthy older and younger groups had a history of significant neurological, rheumatological, or psychiatric illness, nor had any cognitive complaints. Medication is specified in Table I. One additional patient was recruited but later excluded due to severe dyskinesia during MRI. Two additional older healthy subjects were recruited and scanned but then removed from the analysis because of extensive idiopathic calcification of the basal ganglia and hydrocephalus, respectively. The study was given a favorable opinion by the local Research Ethics Committee and participants gave written informed consent according to the 1991 Declaration of Helsinki.
Task
The “finger‐tapping” task is a visually paced right hand button press task. Subjects were presented with a picture of a right hand and pressed a button with one of their four right hand fingers in response to a cue. The cue was either a “specified” cue in which a single opaque circle indicated which finger to press, or “chosen” cue in which all circles appeared opaque indicating subjects could choose any one finger to press (see Fig. 1). Subjects were asked to “make a fresh selection on each trial.” They were specifically not asked to make “random” selections as this may paradoxically exaggerate monitoring of sequential responses and imply unspecified rule constraints on choices [cf. Baddeley et al., 1998]. The task comprised 40 specified trials (10 for each finger) and 40 action‐selection trials, interspersed with 40 null events in which no cue was presented. Cues were presented for 1 s with a stimulus onset asynchrony of 2.5 s, and were randomly intermixed excluding four or more responses of the same type (action‐selection, specified or null) in a row. The presentation of data was controlled using Cogent 2000 software (http://www.vislab.ucl.ac.uk/Cogent2000) using Matlab 7.1 (http://www.mathworks.com) in Windows XP (http://www.microsoft.com). Mean reaction time and response accuracy were analyzed in SPSS 11.0 (SPSS, Chicago) using repeated measures analysis of variance and using Greenhouse‐Geisser correction for nonsphericity where appropriate. Randomness of responses for the action‐selection condition was analyzed using Simpson's Equitability Index [Simpson, 1949]. Response frequency was calculated for three different patterns for selecting a finger in the action‐selection condition: (1) repeating the same finger as previously chosen (e.g., repeating the index finger), (2) selecting the immediate neighboring finger of the one previously chosen (e.g., index then middle), (3) selecting a more remote finger (e.g., index then ring finger). Frequencies were adjusted for the number of possible selections available (i.e., when repeating a finger there is only one available option, whereas a neighbor has two options for the middle and ring finger, etc).
Figure 1.
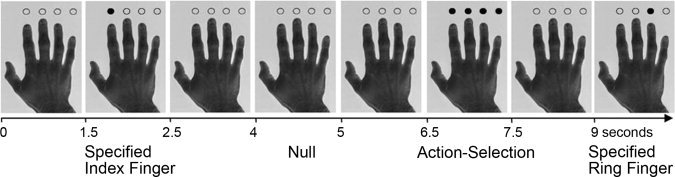
An example of a sequence of trials. Cues to which finger to press are followed by null events in which no cue is presented and the hand remains on the screen for 1.5 s. Examples shown include a specified index finger cue, an action‐selection cue, and a specified ring finger cue.
MRI Data Acquisition and Analysis
A Siemens Tim Trio 3 Tesla scanner was used to acquire BOLD sensitive T2* weighted EPI images (TR 2000 ms, TE 30 ms, FA 78 degrees) with 32 slices, 3.0 mm thick, in‐plane resolution 3 × 3 mm, with slice separation 0.75 mm, in sequential descending order. One hundred fifty six volumes were acquired, the first six of which were discarded to allow for steady‐state magnetization. An MPRAGE T1‐weighted structural image was also acquired for each subject (TR 2250 ms, TE 2.99 ms, FA 9 degrees, IT 900 ms, 256 × 256 × 192 isotropic 1‐mm voxels) and single volume TSE (TR 5,060 ms, TE 102 ms, FA 140, 28 × 4 mm slices) for the purposes of normalization of images, localization of activations on individual and group brains, and assurance of structural normality.
Data preprocessing and analysis used SPM5 (http://www.fil.ion.ucl.ac.uk/spm) in Matlab 7 environment (R14, Mathworks, CA). fMRI data were converted from DICOM to NIFTII format, spatially realigned to the first image and sinc interpolated in time to the middle slice to correct for acquisition delay. The mean fMRI volume and MPRAGE were coregistered using mutual information, and the MPRAGE segmented and normalized to the Montreal Neurological Institute T1 template in SPM by linear and nonlinear deformations. The normalization parameters were then applied to all spatiotemporally realigned functional images, the mean image and structural images, prior to spatial smoothing of fMRI data with an isotropic Gaussian kernel FWHM 10 mm.
First level Statistical Parametric Modeling for each subject used a general linear model with one regressor representing the presentation of a trial (of any type, excluding null trials). This was subject to parametric modulation according to the two conditions “specified” and “action‐selection,” and also to reaction time. Two second level models (random effects) for aging effects and for “on/off” effects were made for each contrast of interest using an ANOVA of the contrast images from each subject's analysis at the first level.
Model 1: Aging and disease. Each second level ANOVA had a similar design for each contrast of interest, and included four regressors specifying: All task activity, mean corrected age, group: PD vs. age‐matched controls (but not younger subjects), interaction between group and age, and mean corrected UPDRS score. Model 2: Dopaminergic effects. Each second level ANOVA had eight regressors specifying: All task activity, controls vs. patients, “on” vs. “off,” mean corrected age in three separate columns for control subjects, “on” and “off” patients, mean corrected UPDRS score when “on,” and mean corrected UPDRS score when “off.” For both models SPM(t) maps were generated using t‐contrasts for each effect of interest, thresholded such that false discovery rate (FDR) was 0.05 for whole brain comparisons. In addition, some results of specific interest at alternative uncorrected thresholds are also included, either for comparison with other reports or where a negative result would be of particular relevance.
RESULTS
Behavioral Analysis
A repeated measures ANOVA was conducted on the mean RT of each condition (action‐selection and specified) with age as a covariate and disease as a between subjects factor (all controls vs. PD patients). The analysis revealed no significant effect of condition, RTs were similar for both action‐selection and specified trials (action‐selection: mean = 765.8 ms, SD = 172; specified: mean = 675.5 ms, SD = 138; F(1,56) = 1.6, P > 0.05). There was a significant effect of age on RT, with RTs being slower in older age (F(1,56) = 14.5, P < 0.001) but no effect of disease (F(1,56) = 2.3, P > 0.05) and no interaction of condition with age or disease.
A repeated measures ANOVA on mean RT for each condition between patients “on” versus “off” dopaminergic medication showed an overall difference between the two conditions (F(1,29) = 15.51, P < 0.05), but no overall effect on RT of taking dopaminergic medication (F(1,56) = 1.3, P > 0.05) and no interaction of dopaminergic medication and condition (F(1,29) = 0.1, P > 0.05). There was also no overall effect of disease severity (as measured by UPDRS) on RT, but severity did interact with condition (F(1,29) = 9.6, P > 0.05); patients who were worse took longer to respond in the specified condition. Figure 2A,B show the reaction times of all the subjects for both conditions. The age‐related increase in RT accords with previous studies of healthy aging.
Figure 2.

Mean RTs for (A) specified and (B) action‐selection conditions for the controls (grey squares) and PD groups (On = black triangles, Off = white triangles) across the age range. (C) Frequency of choices for the action‐selection condition, including repeating the previous selection, choosing the immediate neighboring finger to the previous selection or selecting a remote finger. A value of one indicates a true random selection, above one indicates a preference for that strategy, and below one indicates a tendency to suppress that strategy. The control subjects show a preference to not repeat the same finger choosing instead another finger, whereas patients in the “off” state show no preference but when in the “on” state they show a tendency to perseverate. (D) Frequency of repeats correlated with UPDRS score for patients when “on” and “off.” There is a significant trend of increasing perseveration with increasing disease severity.
The degree of randomness in the action‐selection condition was analyzed in one‐way ANOVAs of Simpsons equitability, but showed no difference between groups (Younger, Older and PD “on,” and PD “on” vs. “off”); however, there was a difference in the frequency of responses between the younger and older controls and the patients. The patients showed a tendency to repeat the same finger, whereas the controls tended to choose a neighboring finger and suppress repetitions (Repeat: F(2,58) = 5.05, P < 0.05; Neighbor F(2,58) = 3.1, P < 0.05, Remote: F(1,58) = 2.2, P > 0.05, see Fig. 2C). An ANOVA of dopaminergic effects also showed a significant difference in strategy when “on” and “off” dopaminergic medication (F(2,58) = 5.6, P < 0.01) with “on” patients showing more perseverative behavior than “off” patients. This effect might be influenced by the type of dopaminergic medication and a posthoc analysis of treatment type was motivated by recent evidence that behavioral choices may not be equally affected by dopamine agonists and l‐dopa [Van Eimeren et al., 2009]. An ANOVA of repetitive responses by treatment state (“on” vs. “off”) and medication type (levodopa vs. dopamine agonists) revealed a trend between the medication types (F(1,13) = 3.3, P < 0.1) when covarying the UPDRS difference between “on” and “off” states. This suggests that the increase in perseveration when “on” compared to when “off” is greater for the 12 subjects on dopamine agonists, although this effect is small and underpowered.
Disease severity also had a significant effect on strategy (F(2,58) = 6.2, P < 0.01): patients with more severe disease were more perseverative. These results show that patients used more inflexible strategies when selecting a response, which became more marked with disease progression and dopaminergic therapy (Fig. 2D).
NEUROIMAGING RESULTS
Model 1: Aging and Disease Effects on Task‐Related Activity
Overall task performance (combining both action‐selection and specified trials compared to baseline, FDR P < 0.05) showed activity in a wide bilateral cortical and subcortical network. Aging was associated with increased activity in bilateral motor cortex, right premotor cortex and medial cingulate cortex, and decreasing activity in the right anterior cingulate cortex. The increased activity in the right premotor cortex is particularly interesting since the younger subjects show deactivation of this area (Fig. 3A and Table II). Furthermore, mean RT correlated negatively with peak activity for older subjects in the right premotor and cingulate cortex (R = −0.36, P < 0.05 and R = −0.4, P < 0.05, respectively; controlling for PD). These negative correlations suggest that amongst older subjects, greater activation supports relatively faster responses. For the younger group, there was no significant correlation between activity in anterior cingulate and mean RT (anterior cingulate: R = −0.16, P > 0.05).
Figure 3.
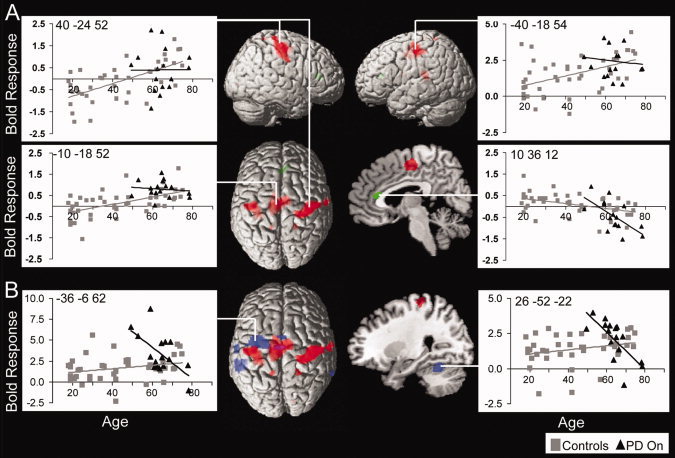
Age and PD effects on task activity for both conditions (specified and action‐selection trials) versus baseline displayed on the MNI reference brain corrected for multiple comparisons (FDR P < 0.05). (A) Regions of increasing activity with age are shown in red, including bilateral motor cortex, right premotor cortex and mid cingulate cortex; The green cluster shows decreasing activity with age in right anterior cingulate cortex. (B) Increased activity for patients when “on” versus age‐matched control subjects are shown in blue including left premotor cortex, caudal SMA, left middle and superior temporal gyrus, right middle temporal gyrus, and right cerebellum. Images are masked by activity related to the task for all subjects at FDR P < 0.05. Peak activity for each cluster is plotted against age showing linear trends of activity (control subjects are in gray squares and the PD group in black triangles). The aging effects (in red) are overlaid on the PD effects (blue) to show the clear differences between aging and PD. Disease effects are more rostral and left, whereas healthy aging is more bilateral.
Table II.
Regions of significant activation from Model 1: Task‐related activity and interactions with age and disease
| Region | X | Y | Z | t | |
|---|---|---|---|---|---|
| Aging (positive correlation) | |||||
| Cingulate | L | −10 | −18 | 52 | 6.04 |
| R | 8 | −12 | 52 | 4.81 | |
| Primary Motor Cortex | R | 40 | −24 | 52 | 5.03 |
| PreMotor Cortex | R | 26 | −30 | 68 | 3.86 |
| R | 22 | −28 | 68 | 3.73 | |
| Primary Motor Cortex | L | −40 | −18 | 54 | 4.07 |
| L | −44 | −20 | 54 | 4.0 | |
| L | −36 | −20 | 52 | 3.97 | |
| L | −30 | −30 | 50 | 3.97 | |
| Somatosensory Cortex | R | 62 | −14 | 40 | 4.43 |
| R | 20 | −46 | 68 | 3.52 | |
| Precuneus/Somatosensory | L | −14 | −48 | 70 | 3.49 |
| Inferior Frontal Gyrus | L | −30 | −34 | 12 | 4.28 |
| Aging (negative correlation) | |||||
| Anterior Cingulate | R | 10 | 36 | 12 | 5.18 |
| PD vs. Controls | |||||
| PreMotor Cortex | L | −36 | −6 | 62 | 5.32 |
| L | −20 | −12 | 72 | 3.94 | |
| L | −44 | −6 | 56 | 3.82 | |
| SMA | L | −8 | −14 | 48 | 4 |
| L | −2 | 0 | 58 | 3.78 | |
| R | 2 | 0 | 54 | 3.74 | |
| Middle Temporal | L | −58 | −38 | 12 | 5.03 |
| R | 66 | −46 | −2 | 3.6 | |
| Superior Temporal Gyrus | L | −64 | −14 | 14 | 4.35 |
| Cerebellum | R | 26 | −52 | −22 | 4.36 |
R, right; L, left; XYZ, coordinates of maximal activated voxel in standard anatomic space using the MNI template; t, Maximum t level at this voxel. P < 0.05 FDR.
To examine the effects of PD, the patients “on” dopaminergic medication were compared to age‐matched controls within an inclusive mask of task activity vs. baseline (FDR P < 0.05). Patients showed increased activity in the left premotor cortex, caudal SMA, left middle and superior temporal gyrus and right middle temporal gyrus and right cerebellum (Fig. 3B and Table II). These disease effects are more rostral and left lateralized than the effects of healthy aging and all were negatively related with age: younger patients showed greater activity for all regions (P < 0.001 uncorrected). However, there were no significant correlations between peak activity and mean RT. There was also no effect of disease severity on task activity, suggesting that (younger) patients activate a wider network of activity independent of the stage of disease.
Action Selection
When selecting actions (compared to making specified responses) subjects activated a broad bilateral network that has been described in previous studies [Rowe et al., 2005, 2008a]. This included dorsal and ventrolateral prefrontal cortex, middle and inferior frontal gyrus, SMA and premotor cortex, inferior parietal cortex, right supramarginal gyrus, right hippocampus, fusiform gyrus, thalamus, and cerebellum (Fig. 4 and Table III). This contrast was used as an inclusive mask for analyses of age and disease effects.
Figure 4.
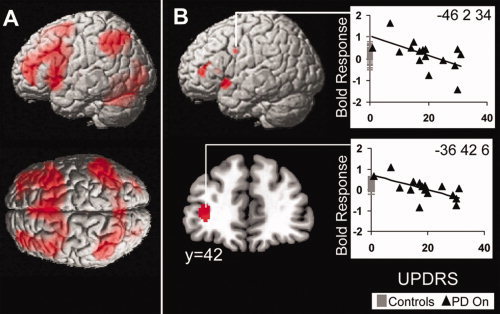
Activity involved in action‐selection versus specified trials corrected for multiple comparisons (FDR < 0.05). (A) Increased activity for action‐selection in wide bilateral network. including: dorsal and ventro ‐lateral prefrontal cortex, middle and inferior frontal gyrus, SMA and premotor cortex, inferior parietal cortex, right supramarginal gyrus, fusiform gyrus, and cerebellum. (B) In the patient group action‐selection activity was modulated by UPDRS in left lateral prefrontal cortex, bilateral inferior premotor cortex and left insula when “on” (Masked by Action‐selection vs. Specified contrast at FDR P < 0.05). Plots of peak activity across UPDRS scores show how BOLD response declines with more severe disease, age‐matched control subjects are also presented (gray lines) to show normal range.
Table III.
Regions of significant activation from Model 1: Activity related to action selection and its correlation with disease severity
| Region | X | Y | Z | t | |
|---|---|---|---|---|---|
| Action‐Selection vs. Specified | |||||
| Dorsolateral PFC | L | −40 | 52 | 16 | 6.57 |
| R | 44 | 38 | 30 | 4.94 | |
| Ventrolateral PFC | L | −40 | 54 | −2 | 6.40 |
| Medial Frontal Cortex | L | −46 | 22 | 36 | 7.05 |
| L | −38 | 4 | 32 | 6.53 | |
| R | 38 | 20 | 44 | 6.89 | |
| R | 40 | 6 | 30 | 5.11 | |
| Inferior Frontal Cortex | L | −48 | 16 | 8 | 6.92 |
| L | −30 | 18 | −6 | 5.80 | |
| R | 36 | 22 | −10 | 4.52 | |
| SMA | 0 | 18 | 52 | 7.13 | |
| Pre motor cortex | L | −26 | 2 | 60 | 5.00 |
| R | 14 | 14 | 62 | 6.90 | |
| Inferior Parietal | R | 42 | −58 | 50 | 7.31 |
| R | 12 | −62 | 48 | 4.95 | |
| L | −40 | −60 | 52 | 6.46 | |
| L | −4 | −66 | 44 | 5.28 | |
| Supramarginal gyrus | R | 52 | −38 | 48 | 5.35 |
| ParaHippocampal gyrus | L | −12 | −10 | −12 | 3.08 |
| Hippocampus | R | 16 | −36 | 16 | 2.82 |
| Fusiform | L | −32 | −76 | −6 | 5.38 |
| R | 30 | −84 | −6 | 5.44 | |
| Thalamus | L | −12 | −10 | 4 | 3.20 |
| L | −14 | −30 | 14 | 2.99 | |
| Cerebellum | L | −34 | −54 | −32 | 5.35 |
| L | −20 | −72 | −26 | 4.50 | |
| R | 30 | −64 | −28 | 4.40 | |
| R | 40 | −52 | −30 | 4.31 | |
| Disease Severity (negative correlation) | |||||
| Ventrolateral PFC | L | −36 | 42 | 6 | 4.54 |
| Premotor cortex | L | −46 | 2 | 34 | 3.90 |
| R | 38 | 4 | 34 | 4.41 | |
| Insula | L | −32 | 14 | −10 | 5.3 |
| L | −26 | 24 | 8 | 4.0 | |
R, right; L, left; XYZ, coordinates of maximal activated voxel in standard anatomic space using the MNI template; t, Maximum t level at this voxel; P < 0.05 FDR.
There were no significant effects of age on action‐selection related activations. There were no overall effects of PD compared to controls, and no interaction between PD and age on action‐selection related activations.
There were significant effects of the stage of disease, as measured by the UPDRS. Activity in left ventrolateral prefrontal cortex, bilateral inferior premotor cortex, and left insula decreased as disease severity increased (Fig. 4B). Thus although there were no categorical differences between the controls and the patients for action‐selection, there were changes within the patient group related to disease severity. It is interesting to note from Figure 4B that the mildest patients showed activation in prefrontal cortex above the range of healthy controls, whereas more severe patients' activations were at or below the normal range.
Model 2: Effects of PD and Dopaminergic Therapy
This model included older controls and patients, both scanned on two occasions. There were no significant categorical differences in task‐related activity between patients “on” and “off” dopaminergic medications, when corrected for multiple comparisons. Earlier articles have reported differences between patients particularly within the SMA and pre‐SMA at more liberal thresholds, for example, P < 0.05 uncorrected [Jenkins et al., 1992], P < 0.005 [Mallol et al., 2007], or P < 0.001 uncorrected [Buhmann et al., 2003; Haslinger et al., 2001; Samuel et al., 1997]. Exploring our data at these more liberal thresholds, we also find greater activity in pre‐SMA (xyz = −12 24 54, t = 3.06, P = 0.003) for patients “on” compared to the “off” state (Table IV).
Table IV.
Regions of significant activation from Model 2: Activation peaks related to task (combining selection and specified conditions) and action‐selection (selection versus specified trials), in relation to age, disease severity, and medication status
| Region | X | Y | Z | t | ||
|---|---|---|---|---|---|---|
| Task vs. Rest | ||||||
| Controls Aging vs. PDOn Aging | Lateral Prefrontal Cortex | R | 52 | 34 | 10 | 3.97 |
| SMA | L | −4 | 0 | 56 | 3.96 | |
| Pre‐motor Cortex | L | −34 | −6 | 64 | 4.49 | |
| Thalamus | R | 16 | −6 | 8 | 4.01 | |
| Cerebellum | R | 30 | −52 | −26 | 5.07 | |
| Controls Aging vs. PDOff Aging* | Cerebellum | R | 30 | −52 | −26 | 3.45 |
| PDOff Aging vs. PDOn Aging | Superior Orbital Gyrus | R | 20 | 50 | −6 | 4.84 |
| Anterior Cingulate | L | −4 | 26 | 24 | 4.63 | |
| Insula | R | 36 | −22 | 8 | 3.92 | |
| Middle Temporal Gyrus | L | −48 | −20 | −16 | 4.49 | |
| Thalamus | R | 10 | −18 | −4 | 4.16 | |
| PDOn vs. PDOff** | PreSMA | L | −12 | 24 | 54 | 3.06 |
| Action‐Selection vs. Specified | ||||||
| PDOn and PDOff: Disease Severity (negative correlation) | Insula | L | −42 | 10 | −8 | 5.1 |
| Superior Parietal | L | −30 | −52 | 64 | 4.86 | |
| PDOff vs. PDOn | Ventral Premotor Cortex | R | 54 | −4 | 22 | 4.95 |
| R | 30 | −22 | 22 | 4.87 | ||
| Insula | L | −42 | −24 | 14 | 4.27 | |
| Hippocampus | R | 12 | −40 | 16 | 4.21 | |
R, right; L, left; XYZ, coordinates of maximal activated voxel in standard anatomic space using the MNI template; t, Maximum t level at this voxel. P < 0.05 FDR, except.
P < 0.001 uncorrected.
P < 0.05 uncorrected.
There was a significant interaction between aging and PD, and a significant interaction between aging and “on” vs. “off” states. In contrast to control subjects, younger “on” patients showed an increase in task related activity in lateral prefrontal cortex, SMA and premotor cortex, thalamus, and right superior cerebellum that decreased with age (Fig. 5A and Table IV). In contrast, “off” patients compared with controls did not show an increase in cortical regions nor a decline in activation with age, except right cerebellar activation, which was increased in young “off” patients and decreased with age (Table IV).
Figure 5.
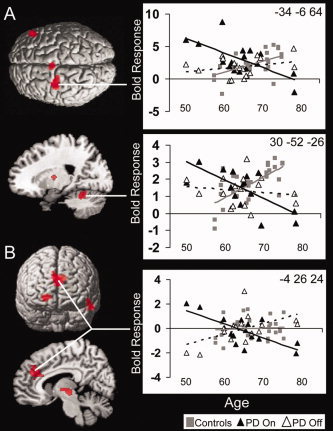
Dopaminergic dependent interactions with aging on task activity (for both action‐selection and specified conditions). (A) Patients taking dopaminergic medication, compared to control subjects, have an early increase in activity in right inferior frontal cortex, SMA and premotor cortex, thalamus, and right superior cerebellum which decreases with age. In contrast, with healthy aging there is a gradual increase in activity in these regions (P < 0.05 FDR). (B) When “off” medications compared to the “on” state, patients have increasing activity in right superior orbital gyrus, anterior cingulate, insula, thalamus and left temporal cortex, with age (P < 0.05 FDR), whilst when “on” medication activity in these regions declines with age.
There was an effect of dopaminergic therapy on aging related activations. In the “on” state compared to the “off” state, there was increased activation in younger patients in right superior orbital frontal gyrus, anterior medial cingulate cortex, insula, left temporal gyrus, and thalamus. These activations decreased with age. In contrast, in the “off” state a gradual increase in activity with age was observed in these areas (see Fig. 5B and Table IV).
There were no effects of disease severity on activations related to task performance, combining specified and selected responses. Thus, both “off” patients and older healthy subjects show increasing cortical activations with progressive aging, patients in the “on” state show the opposite pattern: cortical activation is greater in younger patients and declines with age, independent of disease severity.
Action Selection
For activity related to action selection, there were no categorical differences between “on” and “off” patients. Action‐selection related activations were again relatively stable across the age range (no difference at FDR P < 0.05 or P < 0.001 uncorrected).
Both groups of patients showed an effect of disease severity on action‐selection related activations, but these effects depended on dopaminergic medication state. For both “on” and “off” patients, there was less activity in left anterior insula (xyz = −42 10 −8, t = 5.1, P < 0.05) and superior parietal (xyz = −30 −52 64, t = 4.86, P < 0.05) with more advanced disease. For patients “off” compared to the “on” state, there was an increase in right inferior frontal/ventral premotor cortex, insula, and hippocampal activity with advancing severity whilst when “on,” patients showed a decrease in activity in these areas with disease severity (see Table IV).
DISCUSSION
The key results of this study are that (1) with healthy aging, activity increased in motor cingulate cortex and in bilateral motor and premotor cortex. (2) Aging differentially affected PD patients and control subjects and interacted with dopaminergic medication. When “off,” task‐related activity in PD increased with age similarly to control subjects, with the exception of the right cerebellum. In contrast to controls, “on” younger patients had elevated activity in right lateral prefrontal cortex, SMA, left premotor cortex, right thalamus and cerebellum, which decreased with age. In contrast to the “off” state, younger patients when “on” also had higher levels of activity that again decreased with age in superior orbital gyrus, anterior cingulate, insula, temporal cortex, and thalamus. (3) Advancing disease severity, for both “on” and “off” states reduced activity related to action‐selection in insula and superior parietal cortex, and additionally in ventrolateral prefrontal and premotor cortex for “on” patients. (4) Dopaminergic medication made the pattern of sequential responses in the action‐selection condition more perseverative.
The bilateral increase in motor areas with age is consistent with previous reports of spreading motor activation with aging [Rowe et al., 2006; Ward and Frackowiak, 2003] and a generalized loss of lateralization with aging [Cabeza, 2002]. Our data go beyond these previous reports by showing that the increase in extent of motor activations occurs gradually across the adult lifespan and is not just a feature of older adults. Several mechanisms may contribute to this motor reorganization. There may be changes in transcallosal inhibition [Naccarato et al., 2006] or a compensatory response to maintain good performance [Heuninckx et al., 2008, Mattay et al., 2002]. Our data support the latter mechanism, in terms of the correlations between behavioral performance (RT) and peak activity. However, the magnitude of the reorganization may depend on the degree of age‐related impairments in connectivity among distant motor‐related cortical areas [Rowe et al., 2006].
In contrast to healthy aging, PD was associated with a different set of changes in activation of the cortical and subcortical motor network, and these changes interacted with age and medication. There was an increase in motor network activity for younger PD patients, specifically when “on” medications. These effects were age dependent and not due to differences in disease severity that were factored out in the analysis. This increase in activity in younger “on” patients suggests that dopamine enables compensatory changes in younger patients that cannot be exploited by older patients and may even be detrimental in terms of task‐related activity. The worsening motor response to levodopa with increasing age has previously been attributed to the likelihood of lesions in nondopaminergic systems in older patients [Blin et al., 1991; Durso et al., 1993; Gomez Arevalo et al., 1997; Levy, 2007; Levy et al., 2005]. One possibility is an increase in the frequency of gait disturbance and bradykinesia related to pedunculopontine dysfunction rather than striatal dopamine depletion [Ballanger et al., 2009]. However, our data also suggest that younger patients can access a dopamine‐dependant mechanism for action mediated by lateral premotor cortex.
We observed significantly increased lateral premotor cortex activation in younger “on” patients, and a weak trend toward reduced activation of SMA when “off.” Previous reports discuss this effect as a shift from medial (SMA) to lateral premotor cortex [Mallol et al., 2007; Samuel et al., 1997] occurring despite, or because of, a mild hyperdopaminergic state in medial frontal cortex in mild to moderate PD [Rakshi et al., 1999]. This reorganization in response to PD in younger patients may be a compensatory mechanism for deficient inputs to medial premotor systems from the basal ganglia [Buhmann et al., 2003] or from direct pathology that occurs in the mesocortex before lateral frontal cortex [Braak et al., 2006]. For activation related to manual movements, STN‐DBS does normalize the underactivity of SMA and over activity or lateral premotor cortex [Grafton et al., 2006], suggesting that the cortical changes in PD are at least in part secondary to subcortical changes. However, interpretation is complicated by the effects of STN‐DBS on resting state blood flow, with reductions in both in both premotor cortex and SMA [Geday et al., 2009; Karimi et al., 2008].
In our study, the categorical difference between medication states was not large although levodopa is often reported to enhance pre‐SMA activity in PD. For our visuomotor task, activity (averaging both conditions) in the “off” state was reduced in the pre‐SMA compared to when “on,” only at liberal thresholds as used in some previous studies [Buhmann et al., 2003; Haslinger et al., 2001; Jenkins et al., 1992; Mallol et al., 2007; Samuel et al., 1997]. Despite the liberal threshold, the replication of the effects of PD and levodopa therapy in the SMA and pre‐SMA across so many studies increases confidence in the result [Haslinger et al., 2001; Jahanshahi et al., 1995; Jenkins et al., 1992; Rascol et al., 1997; Samuel et al., 1997; Yu et al., 2007]. Task differences may also affect the size of the deficit in pre‐SMA of patients in the present study. For example, many studies use repetitive or sequential movements that are cued by a pacing tone [Haslinger et al., 2001; Jenkins et al., 1992; Samuel et al., 1997; Yu et al., 2007] rather than visually cued movements. It is also possible that the relative hyperdopaminergic state in early PD [Rakshi et al., 1999] or greater neuroplasticity in younger patients contribute to the variability of effects in pre‐SMA across studies.
Interactions between age, disease, severity, and dopaminergic therapy may also influence the pre‐SMA effect. In early untreated PD, impairments in the pre‐SMA are sometimes evident [Buhmann et al., 2003], but not always [Eckert et al., 2006; Martin et al., 2008]. Patients with more advanced disease show more consistent impairments [Camicioli et al., 2007; Yu et al., 2007]. It may be that the early stage younger patients even have an elevated pre‐SMA response for some motor contrasts [Catalan et al., 1999; Eckert et al., 2006; Rowe et al., 2002; Sabatini et al., 2000]. This inconsistency may depend on the subtleties between patient groups or the motor and cognitive details of the tasks in question, but it may also result from a dysregulation of SMA, for example, by deafferentation from its prefrontal cortical influences [Rowe et al., 2000]. We now turn to one of these functions associated with prefrontal inputs to premotor regions, namely the selection between alternative actions.
Action‐Selection
We examined the effects of aging and disease on action selection by comparing selected responses with specified responses. Selected responses resulted in greater activity in characteristic bilateral fronto‐parietal network that is robust across many studies of selected manual and nonmanual tasks making it well suited to the study of aging and PD [Deiber et al., 1991; Forstmann et al., 2008; Hyder et al., 1997; Jueptner et al., 1997; Rowe et al., 2008a; Weeks et al., 2001].
Normal responses on this paradigm are not wholly random. We did not ask subjects to generate random responses, since this paradoxically increases deviations from random behaviors [Baddeley et al., 1998]. Rather, we asked subjects to “make a fresh choice on each trial” and intermixed choice trials with null trials and specified trials. This successfully reduced the rate of redundancy in sequential chosen responses in control subjects [cf. Baddeley et al., 1998 or Jahanshahi and Dirnberger, 1999], in which responses were asked to be random. Nonetheless, healthy subjects of all ages tend to avoid repetitions of responses. This avoidance of repetition was absent for patients in the “off” state, and reversed when “on.” That is to say, that dopaminergic treatment did not normalize response patterns, but made them even more abnormal with greater perseveration. A posthoc comparison suggested that this effect may be greater for dopamine agonists than l‐dopa. This trend is consistent with recent evidence of differential effects on response strategy [e.g., Van Eimeren et al., 2009] leading to more perseveration with dopamine agonists, although we note that our study was not designed or powered to examine this difference definitively.
The fMRI data showed a stable effect of age on choice‐related activations in prefrontal and parietal cortex. In addition, there were no categorical differences between patients and controls, and between medication states in the patients. However, PD is not a unitary phenomenon, and we identified effects of disease severity (as measured by the motor UPDRS), which in some regions interacted with “on/off” state. When “on,” patients with less severe disease showed greater activation in left ventrolateral prefrontal cortex and premotor cortex, together with greater tendency to maintain a varied sequence of responses without perseveration. With advanced disease, there was both reduced activation (in prefrontal and premotor cortex) and a greater tendency to perseverate on chosen responses. We interpret this as evidence for prefrontal‐premotor interactions enabling flexible response selection in health and early PD.
The decrease in frontal activation accords with the increasingly perseverative behavior in sequential choices with more advancing disease (Fig. 2D). Perseveration can arise from impaired switching to new responses or response strategies. PD patients are impaired at set shifting in cued set shift paradigms with feedback, being relatively stuck in set [Cools et al., 2001a; Downes et al., 1989; Owen et al., 1992; Rowe et al., 2008b] especially when competing information is present [Cools et al., 2001a]. In such task‐set paradigms, dopaminergic therapies may improve cognitive flexibility [Cools et al., 2001a] but do so in proportion to baseline performance and baseline striatal dopamine function in health and PD [Cools et al., 2009; Cropley et al., 2008].
Interestingly, perseveration of chosen responses was worsened by treatment with dopaminergic medication. Why do our patients show more abnormal response patterns on dopaminergic treatment, with more perseveration? Dopamine supports striatum mediated stimulus‐response habits [Everitt and Robbins, 2005], so one possibility is that with our medication withdrawal regimen the formation of within‐session stimulus response habits is impaired. These habits could lead to repetitive response choices over successive “choice” trials. However, our task includes no formal instrumental learning phase or differential rewards, which would be expected if habits were to emerge from instrumental behaviors.
An alternative explanation lies in the difference between the two processes underlying the nonrandomness of normal response selection—to remember the previous moves and to switch to an alternative. Dopaminergic medication therapy may increase the representation of recent moves, because of its essential role in stabilizing neuronal representations in working memory [Cohen et al., 2002; Durstewitz et al., 1999, 2000]. Responses can then be more constrained by this response history. If there remains a partial deficit in response switching despite dopaminergic medication [Cools et al., 2001b; Cropley et al., 2008], then the enhanced memory for prior moves would increase repetition. However, it is difficult to dissociate a failure of working memory from a failure of response control when both lead to perseverative responses [Collins et al., 1998]. With sequential responses on a self‐ordered task, cytotoxic lesions but not dopamine depletion of prefrontal cortex increased perseveration of responses [Collins et al., 1998]. A nondopaminergic mechanism is therefore implied for the failure to inhibit repetitious moves or to switch to alternative moves. Candidates include the serotonergic and noradrenergic projections to prefrontal cortex, which modulate inhibition and set‐shifting [Clarke et al., 2005, 2007; McGaughy et al., 2008; Mehta et al., 2004] and which are impaired in PD [Scatton et al., 1983].
Limitations
Despite the inclusion of “on” and “off” states and repeat scanning of control subjects in a pseudofactorial design, there are clearly limitations to our study. There may be biased sampling of the population inherent in our recruitment procedures, which may confound the effects of aging and PD, through educational, medical, or genetic cofactors. We believe these effects are likely to be small, not only because of similarity of age, sex and NART estimates of premorbid IQ between patients and older controls, but also the simplicity of the motor task itself. Disease heterogeneity also precludes a unitary measure of disease severity. Like many, we have used the UPDRS‐III motor subscale, but this has only a partial correlation with nonmotor aspects of PD, and pathological or neurochemical disease progression. It would be interesting, for example, to use radioligand‐PET estimates of the integrity of dopamine and nondopamine projections as objective measures of disease progression.
The effects of drugs and aging on the neurovascular coupling must also be considered. We do not have multimodality imaging on our participants to definitively address this issue, but as suggested by Iannetti and Wise regarding pharmacological MRI [Iannetti and Wise, 2007] studied tasks that engage relevant psychological processes; used standardized unbiased preprocessing and data preparation methods including motion artifacts; and focused on regionally specific effects with clear hypothesis testing. Such condition‐specific and regionally specific effects of aging, disease and medication, are less likely to be due to generic differences in neurovascular coupling. It is also possible that differences in movement artefacts may lead to differences of within‐subject variance between groups. We sought to reduce this by selecting patients without severe dyskinesia or tremor and using second level models dominated by between‐subject variance, and a systematic approach to modeling movement variance.
CONCLUSION
Our data show differential effects of healthy aging and PD on simple motor responses. Younger patients exhibit a compensatory dopamine dependant increase in left dorsal premotor cortex, independent of disease severity. For action‐selection, both disease severity and dopaminergic therapy increase perseverative responses, while normal activations in prefrontal and ventral premotor cortex declined with advancing disease, independent of age. These interactions between disease, age, and severity may have implications for the management of PD, particularly with respect to older and more severe patients.
Acknowledgements
The authors thank the patients for taking part in this research and especially for withdrawing from their medication.
REFERENCES
- Baddeley A, Emslie H, Kolodny J, Duncan J ( 1998): Random generation and the executive control of working memory. Q J Exp Psychol A 51: 819–852. [DOI] [PubMed] [Google Scholar]
- Ballanger B, Baraduc P, Broussolle E, Le Bars D, Desmurget M, Thobois S ( 2008): Motor urgency is mediated by the contralateral cerebellum in Parkinson's disease. J Neurol Neurosurg Psychiatry 79: 1110–1116. [DOI] [PubMed] [Google Scholar]
- Ballanger B, Lozano AM, Moro E, van Eimeren T, Hamani C, Chen R, Cilia R, Houle S, Poon YY, Lang AE, Strafella AP ( 2009): Cerebral blood flow changes induced by pedunculopontine nucleus stimulation in patients with advanced Parkinson's disease: A [(15)O] H(2)O PET study. Hum Brain Mapp 30: 3901–3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin J, Dubois B, Bonnet AM, Vidailhet M, Brandabur M, Agid Y ( 1991): Does ageing aggravate parkinsonian disability? J Neurol Neurosurg Psychiatry 54: 780–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Muller CM, Rub U, Ackermann H, Bratzke H, de Vos RA, Del Tredici K ( 2006): Pathology associated with sporadic Parkinson's disease—Where does it end? J Neural Transm Suppl 70: 89–97. [DOI] [PubMed] [Google Scholar]
- Buhmann C, Glauche V, Sturenburg HJ, Oechsner M, Weiller C, Buchel C ( 2003): Pharmacologically modulated fMRI—Cortical responsiveness to levodopa in drug‐naive hemiparkinsonian patients. Brain 126 ( Part 2): 451–461. [DOI] [PubMed] [Google Scholar]
- Cabeza R ( 2002): Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging 17: 85–100. [DOI] [PubMed] [Google Scholar]
- Calautti C, Naccarato M, Jones PS, Sharma N, Day DD, Carpenter AT, Bullmore ET, Warburton EA, Baron JC ( 2007): The relationship between motor deficit and hemisphere activation balance after stroke: A 3T fMRI study. Neuroimage 34: 322–331. [DOI] [PubMed] [Google Scholar]
- Camicioli RM, Hanstock CC, Bouchard TP, Gee M, Fisher NJ, Martin WR ( 2007): Magnetic resonance spectroscopic evidence for presupplementary motor area neuronal dysfunction in Parkinson's disease. Mov Disord 22: 382–386. [DOI] [PubMed] [Google Scholar]
- Catalan MJ, Ishii K, Honda M, Samii A, Hallett M ( 1999): A PET study of sequential finger movements of varying length in patients with Parkinson's disease. Brain 122 ( Part 3): 483–495. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Crofts HS, Dalley JW, Robbins TW, Roberts AC ( 2005): Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J Neurosci 25: 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC ( 2007): Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex 17: 18–27. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Braver TS, Brown JW ( 2002): Computational perspectives on dopamine function in prefrontal cortex. Curr Opin Neurobiol 12: 223–229. [DOI] [PubMed] [Google Scholar]
- Collins P, Roberts AC, Dias R, Everitt BJ, Robbins TW ( 1998): Perseveration and strategy in a novel spatial self‐ordered sequencing task for nonhuman primates: Effects of excitotoxic lesions and dopamine depletions of the prefrontal cortex. J Cogn Neurosci 10: 332–354. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW ( 2001a): Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cereb Cortex 11: 1136–1143. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW ( 2001b): Mechanisms of cognitive set flexibility in Parkinson's disease. Brain 124 ( Part 12): 2503–2512. [DOI] [PubMed] [Google Scholar]
- Cools R, Frank MJ, Gibbs SE, Miyakawa A, Jagust W, D'Esposito M ( 2009): Striatal dopamine predicts outcome‐specific reversal learning and its sensitivity to dopaminergic drug administration. J Neurosci 29: 1538–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropley VL, Fujita M, Bara‐Jimenez W, Brown AK, Zhang XY, Sangare J, Herscovitch P, Pike VW, Hallett M, Nathan PJ, Innis RB ( 2008): Pre‐ and post‐synaptic dopamine imaging and its relation with frontostriatal cognitive function in Parkinson disease: PET studies with [11C]NNC 112 and [18F]FDOPA. Psychiatry Res 163: 171–182. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Iansek R, Bradshaw JL, Phillips JG ( 1995): Movement‐related potentials in Parkinson's disease. Presence and predictability of temporal and spatial cues. Brain 118 ( Part 4): 935–950. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Zarahn E, Aguirre GK, Rypma B ( 1999): The effect of normal aging on the coupling of neural activity to the bold hemodynamic response. Neuroimage 10: 6–14. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Passingham RE, Colebatch JG, Friston KJ, Nixon PD, Frackowiak RS ( 1991): Cortical areas and the selection of movement: A study with positron emission tomography. Exp Brain Res 84: 393–402. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Rice HJ, Cabeza R ( 2002): Hemispheric asymmetry and aging: Right hemisphere decline or asymmetry reduction. Neurosci Biobehav Rev 26: 819–825. [DOI] [PubMed] [Google Scholar]
- Downes JJ, Roberts AC, Sahakian BJ, Evenden JL, Morris RG, Robbins TW ( 1989): Impaired extra‐dimensional shift performance in medicated and unmedicated Parkinson's disease: Evidence for a specific attentional dysfunction. Neuropsychologia 27: 1329–1343. [DOI] [PubMed] [Google Scholar]
- Durso R, Isaac K, Perry L, Saint‐Hilaire M, Feldman RG ( 1993): Age influences magnitude but not duration of response to levodopa. J Neurol Neurosurg Psychiatry 56: 65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durstewitz D, Kelc M, Gunturkun O ( 1999): A neurocomputational theory of the dopaminergic modulation of working memory functions. J Neurosci 19: 2807–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ ( 2000): Dopamine‐mediated stabilization of delay‐period activity in a network model of prefrontal cortex. J Neurophysiol 83: 1733–1750. [DOI] [PubMed] [Google Scholar]
- Eckert T, Peschel T, Heinze HJ, Rotte M ( 2006): Increased pre‐SMA activation in early PD patients during simple self‐initiated hand movements. J Neurol 253: 199–207. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW ( 2005): Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat Neurosci 8: 1481–1489. [DOI] [PubMed] [Google Scholar]
- Forstmann BU, Wolfensteller U, Derrfuss J, Neumann J, Brass M, Ridderinkhof KR, von Cramon DY ( 2008): When the choice is ours: Context and agency modulate the neural bases of decision‐making. PLoS One 3: e1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geday J, Ostergaard K, Johnsen E, Gjedde A ( 2009): STN‐stimulation in Parkinson's disease restores striatal inhibition of thalamocortical projection. Hum Brain Mapp 30: 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez Arevalo G, Jorge R, Garcia S, Scipioni O, Gershanik O ( 1997): Clinical and pharmacological differences in early‐ versus late‐onset Parkinson's disease. Mov Disord 12: 277–284. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Turner RS, Desmurget M, Bakay R, Delong M, Vitek J, Crutcher M ( 2006): Normalizing motor‐related brain activity: Subthalamic nucleus stimulation in Parkinson disease. Neurology 66: 1192–1199. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Kampfe N, Boecker H, Rummeny E, Schwaiger M, Conrad B, Ceballos‐Baumann AO ( 2001): Event‐related functional magnetic resonance imaging in Parkinson's disease before and after levodopa. Brain 124 ( Part 3): 558–570. [DOI] [PubMed] [Google Scholar]
- Hesselmann V, Zaro Weber O, Wedekind C, Krings T, Schulte O, Kugel H, Krug B, Klug N, Lackner KJ ( 2001): Age related signal decrease in functional magnetic resonance imaging during motor stimulation in humans. Neurosci Lett 308: 141–144. [DOI] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Debaere F, Peeters R, Swinnen SP ( 2005): Neural basis of aging: The penetration of cognition into action control. J Neurosci 25: 6787–6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Swinnen SP ( 2008): Systems neuroplasticity in the aging brain: Recruiting additional neural resources for successful motor performance in elderly persons. J Neurosci 28: 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD ( 1967): Parkinsonism: Onset, progression and mortality. Neurology 17: 427–442. [DOI] [PubMed] [Google Scholar]
- Hutchinson S, Kobayashi M, Horkan CM, Pascual‐Leone A, Alexander MP, Schlaug G ( 2002): Age‐related differences in movement representation. Neuroimage 17: 1720–1728. [DOI] [PubMed] [Google Scholar]
- Hyder F, Phelps EA, Wiggins CJ, Labar KS, Blamire AM, Shulman RG ( 1997): “Willed action”: A functional MRI study of the human prefrontal cortex during a sensorimotor task. Proc Natl Acad Sci USA 94: 6989–6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannetti GD, Wise RG ( 2007): BOLD functional MRI in disease and pharmacological studies: Room for improvement? Magn Reson Imaging 25: 978–988. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Dirnberger G ( 1999): The left dorsolateral prefrontal cortex and random generation of responses: Studies with transcranial magnetic stimulation. Neuropsychologia 37: 181–190. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ ( 1995): Self‐initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement‐related potentials in normal and Parkinson's disease subjects. Brain 118 ( Part 4): 913–933. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Fernandez W, Playford ED, Lees AJ, Frackowiak RS, Passingham RE, Brooks DJ ( 1992): Impaired activation of the supplementary motor area in Parkinson's disease is reversed when akinesia is treated with apomorphine. Ann Neurol 32: 749–757. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE ( 1997): Anatomy of motor learning. II. Subcortical structures and learning by trial and error. J Neurophysiol 77: 1325–1337. [DOI] [PubMed] [Google Scholar]
- Karimi M, Golchin N, Tabbal SD, Hershey T, Videen TO, Wu J, Usche JW, Revilla FJ, Hartlein JM, Wernle AR, Mink JW, Perlmutter JS ( 2008): Subthalamic nucleus stimulation‐induced regional blood flow responses correlate with improvement of motor signs in Parkinson disease. Brain 131 ( Part 10): 2710–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy G ( 2007): The relationship of Parkinson disease with aging. Arch Neurol 64: 1242–1246. [DOI] [PubMed] [Google Scholar]
- Levy G, Louis ED, Cote L, Perez M, Mejia‐Santana H, Andrews H, Harris J, Waters C, Ford B, Frucht S, Fahn S, Marder K ( 2005): Contribution of aging to the severity of different motor signs in Parkinson disease. Arch Neurol 62: 467–472. [DOI] [PubMed] [Google Scholar]
- Mallol R, Barros‐Loscertales A, Lopez M, Belloch V, Parcet MA, Avila C ( 2007): Compensatory cortical mechanisms in Parkinson's disease evidenced with fMRI during the performance of pre‐learned sequential movements. Brain Res 1147: 265–271. [DOI] [PubMed] [Google Scholar]
- Martin WR, Wieler M, Gee M, Hanstock CC, Camicioli RM ( 2008): Intact presupplementary motor area function in early, untreated Parkinson's disease. Mov Disord 23: 1756–1759. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR ( 2002): Neurophysiological correlates of age‐related changes in human motor function. Neurology 58: 630–635. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Ross RS, Eichenbaum H ( 2008): Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set‐shifting. Neuroscience 153: 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Goodyer IM, Sahakian BJ ( 2004): Methylphenidate improves working memory and set‐shifting in AD/HD: Relationships to baseline memory capacity. J Child Psychol Psychiatry 45: 293–305. [DOI] [PubMed] [Google Scholar]
- Naccarato M, Calautti C, Jones PS, Day DJ, Carpenter TA, Baron JC ( 2006): Does healthy aging affect the hemispheric activation balance during paced index‐to‐thumb opposition task? An fMRI study. Neuroimage 32: 1250–1256. [DOI] [PubMed] [Google Scholar]
- Owen AM, James M, Leigh PN, Summers BA, Marsden CD, Quinn NP, Lange KW, Robbins TW ( 1992): Fronto‐striatal cognitive deficits at different stages of Parkinson's disease. Brain 115: 1727–1751. [DOI] [PubMed] [Google Scholar]
- Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RS, Brooks DJ ( 1992): Impaired mesial frontal and putamen activation in Parkinson's disease: A positron emission tomography study. Ann Neurol 32: 151–161. [DOI] [PubMed] [Google Scholar]
- Rakshi JS, Uema T, Ito K, Bailey DL, Morrish PK, Ashburner J, Dagher A, Jenkins IH, Friston KJ, Brooks DJ ( 1999): Frontal, midbrain and striatal dopaminergic function in early and advanced Parkinson's disease. A 3D [(18)F]dopa‐PET study. Brain 122 ( Part 9): 1637–1650. [DOI] [PubMed] [Google Scholar]
- Rascol O, Sabatini U, Chollet F, Fabre N, Senard JM, Montastruc JL, Celsis P, Marc‐Vergnes JP, Rascol A ( 1994): Normal activation of the supplementary motor area in patients with Parkinson's disease undergoing long‐term treatment with levodopa. J Neurol Neurosurg Psychiatry 57: 567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascol O, Sabatini U, Fabre N, Brefel C, Loubinoux I, Celsis P, Senard JM, Montastruc JL, Chollet F ( 1997): The ipsilateral cerebellar hemisphere is overactive during hand movements in akinetic parkinsonian patients. Brain 120 ( Part 1): 103–110. [DOI] [PubMed] [Google Scholar]
- Rascol O, Sabatini U, Brefel C, Fabre N, Rai S, Senard JM, Celsis P, Viallard G, Montastruc JL, Chollet F ( 1998): Cortical motor overactivation in parkinsonian patients with l‐dopa‐induced peak‐dose dyskinesia. Brain 121 ( Part 3): 527–533. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE ( 2000): The prefrontal cortex: Response selection or maintenance within working memory? Science 288: 1656–1660. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Stephan KE, Friston K, Frackowiak R, Lees A, Passingham R ( 2002): Attention to action in Parkinson's disease: Impaired effective connectivity among frontal cortical regions. Brain 125 ( Part 2): 276–289. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Stephan KE, Friston K, Frackowiak RS, Passingham RE ( 2005): The prefrontal cortex shows context‐specific changes in effective connectivity to motor or visual cortex during the selection of action or colour. Cereb Cortex 15: 85–95. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Siebner H, Filipovic SR, Cordivari C, Gerschlager W, Rothwell J, Frackowiak R ( 2006): Aging is associated with contrasting changes in local and distant cortical connectivity in the human motor system. Neuroimage 32: 747–760. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Hughes L, Eckstein D, Owen AM ( 2008a): Rule‐selection and action‐selection have a shared neuroanatomical basis in the human prefrontal and parietal cortex. Cereb Cortex 18: 2275–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Hughes L, Ghosh BC, Eckstein D, Williams‐Gray CH, Fallon S, Barker RA, Owen AM ( 2008b): Parkinson's disease and dopaminergic therapy—Differential effects on movement, reward and cognition. Brain 131 ( Part 8): 2094–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini U, Boulanouar K, Fabre N, Martin F, Carel C, Colonnese C, Bozzao L, Berry I, Montastruc JL, Chollet F, Rascol O ( 2000): Cortical motor reorganization in akinetic patients with Parkinson's disease: A functional MRI study. Brain 123 ( Part 2): 394–403. [DOI] [PubMed] [Google Scholar]
- Samuel M, Ceballos‐Baumann AO, Blin J, Uema T, Boecker H, Passingham RE, Brooks DJ ( 1997): Evidence for lateral premotor and parietal overactivity in Parkinson's disease during sequential and bimanual movements. A PET study. Brain 120 ( Part 6): 963–976. [DOI] [PubMed] [Google Scholar]
- Scatton B, Javoy‐Agid F, Rouquier L, Dubois B, Agid Y ( 1983): Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson's disease. Brain Res 275: 321–328. [DOI] [PubMed] [Google Scholar]
- Schwab R, England A ( 1969): Projection Techniques for Evaluating Surgery in Parkinson's Disease. Royal College of Surgeons in Edinburgh: E. & S. Livingstone Ltd. [Google Scholar]
- Simpson E ( 1949): Measurement of Diversity. Nature 163: 688. [Google Scholar]
- Tekes A, Mohamed MA, Browner NM, Calhoun VD, Yousem DM ( 2005): Effect of age on visuomotor functional MR imaging. Acad Radiol 12: 739–745. [DOI] [PubMed] [Google Scholar]
- Van Eimeren T, Ballanger B, Pellecchia G, Miyasaki J, Lang A, Strafella A ( 2009): Dopamine agonists diminish value sensitivity of the orbitofrontal cortex: A trigger for pathological gambling in Parkinson's Disease? Neuropsychopharmacology 34: 2758–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS ( 2006): Compensatory mechanisms in the aging motor system. Ageing Res Rev 5: 239–254. [DOI] [PubMed] [Google Scholar]
- Ward NS, Frackowiak RS ( 2003): Age‐related changes in the neural correlates of motor performance. Brain 126 ( Part 4): 873–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS ( 2003a): Neural correlates of motor recovery after stroke: A longitudinal fMRI study. Brain 126 ( Part 11): 2476–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS ( 2003b): Neural correlates of outcome after stroke: A cross‐sectional fMRI study. Brain 126 ( Part 6): 1430–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks RA, Honda M, Catalan MJ, Hallett M ( 2001): Comparison of auditory, somatosensory, and visually instructed and internally generated finger movements: A PET study. Neuroimage 14 ( 1 Part 1): 219–230. [DOI] [PubMed] [Google Scholar]
- Yu H, Sternad D, Corcos DM, Vaillancourt DE ( 2007): Role of hyperactive cerebellum and motor cortex in Parkinson's disease. Neuroimage 35: 222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]


