Abstract
Background
Prader-Willi syndrome (PWS) is a genetic, neurodevelopmental disorder characterized by intellectual disabilities, growth hormone dysregulation, hyperphagia, increased risks of morbid obesity, compulsive behaviors, and irritability. As aberrant serotonergic functioning is strongly implicated in PWS, we examined associations between the PWS phenotype and polymorphisms in tryptophan hydroxylase 2 (TPH2), the rate-limiting enzyme in the biosynthesis of serotonin in the brain.
Methods
92 individuals with PWS aged 4 to 50 years (M = 21.97) were genotyped for the TPH2 G703-T polymorphism. IQ testing was conducted in offspring, and parents completed questionnaires that tapped their child’s compulsivity, hyperphagia, and other behavior problems.
Results
As expected, the frequency of G/T or T/T polymorphisms in participants with PWS (39%) was similar to rates found in the general population (38%). Compared to those with a homozygous (G/G) genotype, individuals with a T allele had significantly higher hyperphagic behavior, drive, and severity scores, and they also had a younger age of onset of hyperphagia. Those with a T allele also had higher IQ scores than their counterparts. Females with a T allele had significantly higher internalizing symptoms, primarily anxiety and depression, than all others.
Conclusions
TPH2 G/T polymorphisms, and presumed loss of enzyme function, were associated with specific aspects of the PWS phenotype. Aberrant serotonergic functioning is strongly implicated in hyperphagia in PWS, and females with TPH2 T alleles may be at higher risk for affective or mood disorders. Findings hold promise for examining other serotonin-altering genes in PWS, and for future serotonin-altering treatment trials.
Prader-Willi syndrome (PWS) is a relatively rare genetic disorder well-known for its genetic and phenotypic complexities. Seen in approximately 1 in 15,000 births, PWS is characterized by mild to moderate levels of intellectual disability, growth hormone dysregulation, hyperphagia, and increased risks of morbid obesity (Cassidy & Driscoll, 2009). The PWS behavioral phenotype includes high rate of repetitive, compulsive behaviors, as well as irritability, negative and labile moods, impulsivity, and tantrums (Dykens, Summar, & Roof, 2005). These problems, coupled with hyperphagia, necessitate that affected individuals receive lifelong supervision and supports.
PWS is caused by a lack of paternally-derived imprinted material on chromosome 15q11-q13. In approximately 70% of cases, PWS is due to a paternal deletion at 15q11-q13, and deletions can be further characterized according to size, with Type I deletions being about 500 kb larger than Type II deletions. Approximately 25% of PWS cases are due to maternal uniparental disomy (mUPD), or when both copies of chromosome 15 are inherited from the mother, and 5% to translocations or imprinting center mutations.
Genetic causes of PWS aside, a growing literature on biomarkers suggests that genetic variation outside of the PWS critical region may greatly influence phenotypic expression in PWS. Based on converging clinical and genetic findings, for example, aberrant serotonergic functioning is strongly implicated in PWS. Individuals with PWS are prone to disorders or symptoms regulated by serotonin, including aberrant appetite and sleep, affective illness and mood disorders, and compulsive behaviors that are similar to those seen in autism spectrum disorders or obsessive-compulsive disorder (Clarke et al., 2002; Dykens, Cassidy, & Leckman, 1996). More severe symptoms or disorders (e.g., psychosis) are typically manifest in those with mUPD (e.g., Soni et al., 2007; Vogels et al., 2003). Treatment with selective serotonin reuptake inhibitors (SSRI’s) is widely used and in some people, reduces irritability, moodiness, outbursts, and compulsions (Dykens & Shah, 2003).
The PWS critical region is also implicated in the production of serotonin. A small nucleolar RNA (snoRNA), HBII-52 (SNORD115), located in the 15q11-q13 region, has been shown to regulate the processing of the mRNA of the serotonin 2C receptor, located elsewhere on the genome (Kishore & Stamm, 2006). Several mouse models of PWS exist, including mice deleted for HBII-52 (Ding et al., 2008), or with altered 2C receptor mRNA functioning (Morabito et al., 2010). As with other PWS models, these mice are hyperphagic but not obese. Sahoo et al. (2008) reported a boy with features consistent with the diagnosis of PWS who had a small deletion of only the snoRNA HBII-85 (SNORD116) and part of HBII-52 (SNORD115) region, strongly implicating these clusters in the PWS phenotype. Lack of HBII-52 expression in PWS may lead to altered processing of serotonin 2C receptor mRNA, which may impact the efficacy of SSRIs in this population. Treatments with SSRI’s are not consistently beneficial in PWS, with some persons showing a worsening of target symptoms (Dykens & Shah, 2003). This variability in treatment response suggests that the PWS phenotype may also be influenced by genes that are known to alter serotonin production among people in general.
Although many genes are involved in the production of serotonin, we selected tryptophan hydroxylase (TPH2) for initial study, as it is the first-step and rate-limiting enzyme in the biosynthesis of serotonin in the brain. While TPH1 is expressed mostly in cells of the gut, TPH2 is expressed predominantly in the brainstem and is responsible for the production of serotonin in the brain (Walther & Bader, 2003). Polymorphisms in TPH2 are implicated in depression, anxiety, and aggression, as well as in the regulation of mood, attention, appetite, and sleep; all of these processes are disrupted in PWS (Cassidy & Driscoll, 2009).
Associations have been reported between sequence variants (single nucleotide polymorphisms, SNPs) in the TPH2 promoter (G-703T; dbSNP accession number rs4570625) and children with OCD (Mosser et al., 2006), autism (Coon et al., 2005), and ADHD (Walitza et al., 2005), and in adult depression or bi-polar disorder (Harvey et al., 2004; Zhang et al., 2005). Other investigators have examined the effects of the TPH2, G-703T polymorphisms on broader personality traits or emotional reactivity. Compared to those homozygous for the G allele, Brown et al. (2005) identified increased amygdala activation in response to angry or fearful faces in healthy adults with the T allele. Canli et al. (2005) subsequently demonstrated increased amygdala activation in participants with the T allele versus G/G allele in response to faces depicting both negative and positive affect. The TPH2 -703 T allele may thus modulate emotional arousal in general, and is implicated in disorders involving emotional dysregulation.
This study identified TPH2 G/T allele status in a large cohort of 92 participants with genetically confirmed PWS, and compared salient features of PWS across those with or without polymorphisms in this allele. We predicted that the frequency of polymorphisms in the TPH2 G/T allele in PWS would be similar to those found in the general population. We also hypothesized that, compared to participants who are homozygous for the G/G allele, those with a loss of TPH2 enzyme function, or with a G/T or T/T allele, would show significantly more behavioral, mood, or emotional problems. Finally, although gender differences are rarely reported in PWS, gender by genotype differences may exist as females in the general population are more prone to mood disorders (Kessler et al., 2003). Significant gender effects of the TPH2 T/G allele have been identified in the emotional startle response (Ambruster et al., 2010), susceptibility to depression (Utge et al., 2010), and panic disorder (Maron et al., 2007). As such, we reasoned that females with PWS may be more vulnerable to the effects of the T polymorphism and reduced serotonin production.
Methods
Participants
The sample included 92 individuals (47 males, 45 females) with PWS aged 4 to 51 years (M age = 21.97, SD = 11.66) and their parents. Based on Center for Disease Control (CDC) guidelines, most adults (62%) with PWS were classified as obese (see Table 1), and the mean BMI for adults aged 21 years and older was 34.66 (SD = 9.24). The mean BMI among children and adolescents aged 4 to 20 years was 27.52 (SD = 8.04), with 67% classified as obese using CDC age-and gender-percentiles.
Table 1.
Characteristics of 92 participants with PWS.
| M | SD | |
|---|---|---|
| Age | 22.66 | (11.66) |
| K-BIT-II IQ | 65.01 | (12.52) |
| BMI Adults (n = 47) | 34.66 | (9.24) |
| % Normal | 8% | |
| % Overweight | 30% | |
| % Obese | 62% | |
| BMI Children (n = 45) | 27.52 | (8.04) |
| % Normal | 21% | |
| % Overweight | 12% | |
| % Obese | 67% | |
| Genetic Subtypes | ||
| Deletion | 58% (21% Type I, 32% Type II, 5% unknown) | |
| mUPD | 38% | |
| Other | 4% | |
Families were recruited and gave their written, informed consent either as part of an ongoing longitudinal study on behavior in PWS, or through a survey on medications and interventions commonly seen in PWS (e.g., SSRI’s, growth hormone treatment, food restrictions). Survey participants (60% of sample) were somewhat older than those followed longitudinally (M ages = 24.30 versus 19.85 years, t (91) = −1.93, p = .07), but did not significantly differ on any of the behavioral or cognitive measures.
Participants had genetic testing that confirmed their PWS diagnosis and genetic subtype (see Table 1). The study included slightly more cases of mUPD (38%) than typically reported in the literature (25%) because the longitudinal study deliberately oversampled persons with this genetic subtype.
Consistent with previous literature, the IQ scores of participants with PWS ranged from 40 to 88, with a mean IQ of 65.03 (SD = 12.62). IQ scores were derived in 42% of the sample from direct testing with the Kaufman Brief Intelligence Test-2 (K-BIT-2; Kaufman & Kaufman, 2004), and in remaining participants, from records of previous cognitive testing obtained from parents. IQ scores across these two sources were highly correlated (r = .86, p < .001) and remarkably similar (K-BIT M = 65.30; previous IQ tests M = 64.75). Approximately 32% of participants, primarily children, were on growth hormone treatment, and the majority (71%) were taking psychotropic medications; of these 48% were on SSRIs.
Mothers served as informants, and their average age was 52 years. Mothers were generally well-educated: 36% had high school degrees, 35% completed 2 or 4 years of college, and 29% had graduate or professional school training.
TPH2 Genotyping
Participants with PWS provided a saliva sample by chewing on a cotton swab, and DNA was extracted from saliva using Vanderbilt Institute for Clinical and Translational Research core facilities. TPH2 genotyping was conducted by PCR amplification of an 1154 bp fragment of the TPH2 gene that included the −703 G/T SNP (rs4570625) using the following primers: forward 5′ ACTCTGCATAGAGGCATCACAGGA 3′; reverse 5′ GGAGAAATTTGAGGTGTGCGTGCT 3′. Sequencing of the fragment was performed using an ABI Genetic Analyzer 3100 (Applied Biosystems, Foster City, CA) and standard protocols.
Procedures
Mothers completed behavioral measures either during their research visits to the lab, or at their convenience at home. The packet included the following measures:
Demographics
These responses identified family composition and parental educational and employment status, as well as specific information about offspring with PWS, including: previous diagnostic and genetic evaluations, current and previous medications, growth hormone treatment, sleep and health habits, the onset of hyperphagia, and dietary and other interventions.
Child Behavior Checklist (CBCL, Achenbach, 2001)
The widely-used CBCL asks parents to rate 112 problem behaviors on a three-point scale (not true to very true or often true.) The CBCL contains an Internalizing Domain (Anxiety/depression; Withdrawal, Somatic Complaints), Externalizing Domain (Aggression, Rule-Breaking) and other clinical domains (Social, Thought, and Attentional problems). The CBCL has been successfully used to identify problems in children or adults with developmental disabilities. Data analyses used CBCL Raw Scores.
Hyperphagia Questionnaire
This 13-item questionnaire (Dykens et al., 2007) probes symptoms of hyperphagia in PWS. Previous factor analyses identified three robust factors: Hyperphagic Drive (e.g., how persistent in asking for food; how easy to direct away from food); Hyperphagic Behaviors (e.g., how fast or clever in obtaining food, how often steal food), and Hyperphagic Severity (time spent talking about food; extent that food interferes with everyday functioning). Items are rated by care providers on a 5-point scale (1 = not a problem to 5 = a severe and/or frequent problem). Raw scores for each factor were used in data analyses, and the three domains were also summed for an overall summary index of hyperphagia.
Yale–Brown Obsessive Compulsive Scale (Y-BOCS; Goodman et al., 1998)
The informant version of the Y-BOCS consists of 30 symptoms that are rated as occurring ever or in the last week. Informants also rate time spent engaged in compulsive behaviors, and the degree of distress and adaptive impairment associated with symptoms (0=none to 5=extreme). The Y-BOCS has been used in previous studies in PWS, and analyses used the sum of lifetime obsessions, compulsions, and current symptom severity (Clarke et al., 2002; Dykens et al., 1996)
Results
Genotyping
Of the 92 PWS cases examined, 36 had the G/T (n = 32) or T/T (n = 4) polymorphism. The homozygous genotype, G/G, was seen in 57 participants. As expected, the rate of the G/T or T/T polymorphisms in our PWS sample (39%) is similar to that reported for the general population (38%; Brown et al., 2005).
TPH2 and Participant Characteristics
We used t-test or chi-square analyses to assess if TPH2 allele status differed across such participant characteristics as medication status, age, gender, or genetic subtype of PWS. No significant differences were found. The G/G group (64% males) averaged 23.82 years (SD = 12.53) and the mean age in the T allele group (45% males) was 20.82 years (SD = 10.04). Of the PWS deletion cases, 64% had a G/G genotype, and 36% a T allele, a rate that was similar to mUPD cases, with 65% in the G/G group, and 35% with a T allele. Thus, the frequencies of TPH2 polymorphisms were distributed across those with PWS regardless of their medication status, age, gender, or genetic subtype.
TPH2 and PWS Phenotype
We used 2 × 2 ANOVAS (TPH2 normal G/G versus loss G/T or T/T by gender) to determine if TPH2 genotype had a significant effect on salient phenotypic features of PWS. These features included IQ, compulsivity, maladaptive behaviors, BMI, food-related behaviors, and hyperphagia. In order to conduct meaningful statistical analyses, the 4 participants with the T/T allele were grouped with those with a G/T allele, and the T polymorphism group was compared to those with the G/G genotype. Although this practice is consistent with existent TPH2 literature, we followed up significant group differences with Neuman-Keuls post-hoc tests to ensure that group differences were not solely driven by the T/T group.
As shown in Table 2, no significant main effects or interactions for TPH2 status or gender were found for Y-BOCS compulsivity, BMI, or CBCL externalizing behavior problems. Significant main effects for TPH2 group status emerged for IQ and for all three domains of the Hyperphagia Questionnaire, such that the T allele group had higher IQ and hyperphagia scores. The T group also had a significantly younger age of onset of hyperphagia; on average, their heightened interests in food began a full year before their counterparts in the G/G group. Regarding dietary interventions, virtually all participants were on reduced calorie diets, were supervised around food, and exercised an average of 4.28 days a week, for 38 minutes. No group or gender differences were seen in their diets or exercise patterns. Those with a T polymorphism, however, slept significantly longer each night (see Table 2). Locking food sources was also more common in those with the T polymorphism (83%) than the G/G group (63%; X2 (1) = 7.81, p < .01).
Table 2.
Means and SDs for behavioral measures by TPH2 group and gender, and F and p values.

|

|
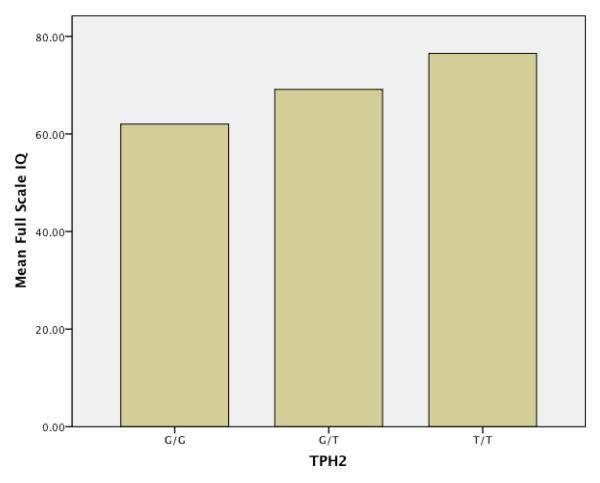
Post-hoc analyses of significant Hyperphagia Questionnaire and IQ findings revealed significant differences between the G/G and G/T groups, even when the 4 participants with T/T alleles were removed from analyses. Although the small size of the T/T allele group prohibited formal statistical analyses, these 4 individuals had substantially higher mean IQ and hyperphagia scores than the G/T group. Figure 1 demonstrates this relationship for IQ, and Figure 2, for the total score of the Hyperphagia Questionnaire.
Figure 1.
Mean IQ across TPH2 allele status
Figure 2.
Mean total Hyperphagia Questionnaire scores across TPH2 allele status
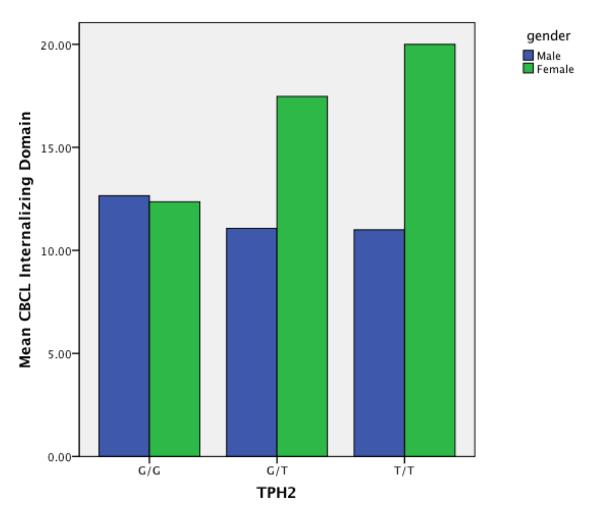
A significant interaction was found between TPH2 status and gender for CBCL internalizing problems. Table 2 shows that females with the T polymorphism had significantly higher internalizing scores than all remaining participants. Figure 3 also depicts this interaction. Follow-up analyses of the subdomains that comprise the internalizing domain revealed that females with the T polymorphism specifically had significantly higher depression/anxiety subdomain scores that their counterparts.
Figure 3.
Gender interaction with CBCL internalizing domain and TPH2 allele status
Discussion
Aberrant serotonergic functioning has long been implicated in the phenotypic expression of PWS. This study examined the rate and phenotypic effects of an important serotonin-altering gene, TPH2, in a relatively large cohort of 92 well-characterized individuals with PWS. As expected, the frequency of the T polymorphism in our sample of 92 individuals (39%) was remarkably similar to the rate found in the general population (38%, Brown et al., 2005). Within the PWS sample, however, the presence of the T polymorphism had significant, circumscribed effects on specific aspects of the PWS phenotype.
Relative to those with a G/G allele status, participants with the T polymorphism had significantly higher scores on the Hyperphagia Questionnaire, including the hyperphagic behavior, drive, and severity domains. The T allele group also had, on average, a significantly earlier age of onset of hyperphagia in childhood, and higher parental ratings of being overweight. Participants with the homozygous G/G genotype, showed the lowest hyperphagia scores. Significantly higher hyperphagia scores were manifest by those with the G/T allele, and although less frequent, the highest hyperphagia scores were manifest by those with the homozygous T/T genotype. On the surface these findings seem logical, as reduced expression related to the T allele of TPH2 is associated with reduced neural synthesis of serotonin, and lower peripheral levels of serotonin are associated with increased appetite in the general population (e.g., Halford et al., 2007). Conversely, treatment with selective SSRIs increase synaptic availability of serotonin, and is associated with decreased appetite and weight loss.
On the other hand, findings are striking in relation to PWS. Phenotypic effects of TPH2 were targeted and circumscribed, and converged on a salient, life-threatening feature of PWS. Further, while other biomarkers of appetite regulation are aberrant in PWS, it is unclear how they relate to the drive for food and eating behaviors in affected individuals (Butler & Bittel, 2007; Goldstone, 2006). Plasma levels of ghrelin, for example, are markedly higher in PWS, but do not relate to food seeking behaviors, nor do ghrelin blockers lead to reduced appetite or food intake (De Waela, 2009). Although additional work with other serotonin-altering genes is necessary, TPH2 findings implicate altered serotonergic functioning as a key player in the regulation of hyperphagia in PWS.
Relations between serotonin and appetite are well-established (Halford et al., 2007), yet few studies have specifically examined the impact of TPH2 polymorphisms on food intake or appetite. Schott et al. (2010) reported a rare case of a child with hypothalamic syndrome and altered serotoninergic functioning related to TPH2 impairment. The child had early onset hyperphagia, obesity, sleep difficulties, precocious puberty, hypoventilation, and behavioral problems. To our knowledge, however, studies have yet to examine associations between TPH2 polymorphisms, obesity, and the drive for food among people in general.
While TPH2 allele status was associated with hyperphagia, it did not predict the degree of obesity (BMI) in affected individuals. The lack of association between TPH2 status and BMI likely reflects the strict environmental dietary and food controls that are universally applied to those with PWS. Regardless of their age, gender, or PWS genetic subtype, virtually all participants in this study were closely supervised around food, on reduced calorie diets, and they also engaged in regular exercise. Beyond these standards of care in PWS, locking food sources is also often recommended, a practice that can reduce anticipatory worry about meals or food sneaking. Locking food sources was more likely to occur in the homes of PWS participants with a T allele, which likely reflects their heightened hyperphagia.
Compared to those in the G/G group, participants with TPH2 T polymorphisms also had significantly higher IQ scores. These IQ findings are puzzling, as several studies suggest impaired cognitive and executive control performance in people with G/T or T/T alleles. Although IQ has not been examined in this work, healthy adults with the T allele committed more errors on a continuous performance task (Strobel et al. (2007), and showed diminished inhibitory control on Stroop tasks (Osinksy et al., 2009). Further, individuals who undergo tryptophan depletion, which involves a transient reduction in serotonin transmission, have impaired memory consolidation (Mendelsohn, Riedel & Sambeth, 2009). These same tryptophan-depleted individuals, however, have improved focused attention, especially on tasks requiring effortful control (Evers et al., 2006). Similarly, Reuter et al. (2008) found no differences in working memory task performance between G/G versus T carrier adults. Using fMRI, however, they report increased activation in adults with T alleles in brain regions associated with working memory, suggesting that this group uses compensatory processes to complete complex cognitive tasks. Such compensatory processes and focused attention may also be called into play during cognitive testing, leading to improved IQ test performance in PWS participants with T polymorphisms.
Higher IQs in the T group may also be associated with their longer duration of sleep each night. Compelling evidence from both clinical and typical populations link sleep duration and quality to specific aspects of memory and cognition (see Durmer & Dinges, 2005 for a review). Disrupted sleep is associated with behavior problems in people with intellectual disabilities (e.g., Malow et al., 2006; Wiggs & Stores, 1996), but few studies have examined relations between sleep and cognition in PWS or other disability groups. Persons with PWS are prone to obstructive sleep apnea, a known risk-factor for cognitive deficits, which further highlights the need for research on cognition, sleep, and the tryptophan-serotonin-melatonin pathway in this syndrome.
Despite the fact that aberrant serotonergic functioning is implicated in a variety of psychiatric disorders, we did not find uniformly elevated emotional or problem behaviors in PWS participants with the T allele. Instead, a significant interaction emerged with gender, such that females with a T allele had significantly higher internalizing problems than remaining participants. Females with T alleles had particularly elevated scores on the CBCL’s depression/anxiety domain, which may reflect the increased risk of mood disorders among females versus males in general (Kessler et al., 2003). Maron et al. (2007) found that women with (versus without) panic disorders were more apt to have a TPH2 A/G polymorphism. Utge and colleagues (2010) recently completed a Finnish population-based association study of 14 candidate genes related to depression, with or without co-occurring fatigue or sleep disturbance. TPH2 was strongly and uniquely associated with depression and co-occurring fatigue, but only in women. Consistent with these population-based findings, females with PWS and TPH2 polymorphisms may be similarly vulnerable to depression and fatigue.
No associations were found in the present study between TPH2 polymorphisms and repetitive, compulsive behaviors. These behaviors are salient in PWS and are also a central feature of people with autism spectrum disorders. Previous work examining TPH2 and autism risk, as well as the effects of TPH2 on the autism phenotype, have failed to find relations between TPH2 status and repetitive, compulsive behaviors (Ramoz et al., 2006; Sacco et al., 2007). Our PWS findings are consistent with these autism studies, which collectively do not implicate TPH2 G/T polymorphisms in compulsivity in these two developmental disorders.
This study had several limitations. First, we used a screener of behavioral and emotional problems instead of psychiatric diagnoses. Although the CBCL is widely used in people with and without developmental disabilities, it is not a substitute for careful psychiatric phenotyping, and such data would have been helpful in shedding light on gender effects in this study. Second, we obtained IQ scores using different methods - direct testing with the KBIT-2 and reports of previous testing. Although scores were similar these sources, participants did not receive the same IQ test, which adds a note of caution in interpreting IQ findings. Third, while we identified TPH2 genetic polymorphisms that are associated with altered expression of TPH2 and thus the synthesis of serotonin, we did not measure expression of TPH2 or peripheral plasma levels of serotonin. Future studies need to do so in order to better describe how alterations in serotonergic pathways relate to the PWS phenotype. Finally, this study examined just one of many genes that are known to alter serotonergic functioning. Future studies are needed on how the PWS phenotype is also impacted by genes and proteins that regulate serotonin receptors, uptake, storage, transport, and metabolism.
Despite these weaknesses, this study highlights the promise of using phenotypically relevant genes or SNPs to shed new light on behavioral phenotypes in genetic syndromes. This study is the first to demonstrate how serotonin-altering genes outside of the PWS 15q11-q13 critical region impact salient aspects of the PWS behavioral phenotype. Persons with PWS and TPH2 -703 T polymorphism had significantly higher IQs, increased hyperphagic behavior, drive, and severity, an earlier age at onset of hyperphagia, and on average, they also slept more each night. Relative to all others in the sample, females with TPH2 T polymorphisms had increased internalizing problems, including symptoms of depression and anxiety. These findings hold promise for future studies that examine other genes involved in the production of serotonin in people with PWS. Such work can help identify important biomarkers associated with high or low levels of hyperphagia or mood problems in PWS, and eventually to more personalized interventions that reflect these relative risks.
Acknowledgements
The authors are grateful to the families and individuals with Prader-Willi syndrome who participated in the study. This work was supported by NICHD Rare Disease Consortium Grant U54 HD061222 (Dykens and Butler), the Prader-Willi Syndrome Associations (USA) (Dykens and Butler), the Vanderbilt CTSA (Vanderbilt Institute for Clinical and Translational Research), and NICHD Grants R01HD035684 (Dykens) and P30HD015052 (Dykens).
References
- Achenbach TM. Manual for the Child Behavior Checklist/4-18 and 2001 profile. University of Vermont, Department of Psychiatry; Burlington, VT: 2001. [Google Scholar]
- Armbruster D, Mueller A, Strobel A, Kirschbaum C, Lesch K-P, Brocke B. Influence of functional tryptophan hydroxylase 2 gene variaion and sex on the startle response in children, young adults, and older adults. Biological Psychiatry. doi: 10.1016/j.biopsycho.2009.12.010. (in press) [DOI] [PubMed] [Google Scholar]
- Brown SM, Peet E, Williamson DE/, Dahl RE, Harin AR. A regulatory variant of the human tryptophan hydroxylase-2 gene biases amydala reactivity. Molecular Psychiatry. 2005;10:884–888. doi: 10.1038/sj.mp.4001716. [DOI] [PubMed] [Google Scholar]
- Butler MG, Bittel D. Plasma obestatin and ghrelin levels in subjects with Prader-Willi syndrome. American Journal of Medical Genetics. 2007;143A:415–421. doi: 10.1002/ajmg.a.31687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Congdon E, Gutknecht L, Constable RT, Lesch KP. Amydala responsiveness is modulated by tryptophan hydroxylase-2 gene variation. Journal of Neural Transmission. 2005;112:1479–1485. doi: 10.1007/s00702-005-0391-4. [DOI] [PubMed] [Google Scholar]
- Cassidy SB, Driscoll DJ. Prader-Willi syndrome. European Journal of Human Genetics. 2009;17:3–13. doi: 10.1038/ejhg.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke D, Boer H, Whittington J, Holland A, Butler J, Webb T. Prader-Willi syndrome, compulsive and ritualistic behaviors: the first population-based study. British Journal of Psychology. 2002;180:358–362. doi: 10.1192/bjp.180.4.358. [DOI] [PubMed] [Google Scholar]
- Coon H, Dunn D, Lainhart J, Miller J, Hamil C, Battaglia A, Tancredi R, Leppert MF, Weiss R, McMahon W. Possible association between autism and variants in the brain-expressed tryptophan hydroxylase gene (TPH2) American Journal of Medical Genetics, Part B. 2005;135B:42–46. doi: 10.1002/ajmg.b.30168. [DOI] [PubMed] [Google Scholar]
- De Waela K, Ishkanian SL, Bogarin R, Miranda CA, Ghatel MA, Bloom SR, Pacaid D, Charoine JD. Long-acting octreotide treatment caused a sustained decrease in ghrelin concentrations but does not affect weight, behavior, and appetite in subjects with Prader-Willi syndrome. European Journal of Endocrinology. 2008;159:381–388. doi: 10.1530/EJE-08-0462. [DOI] [PubMed] [Google Scholar]
- Ding F, Hua H, Zhang S, Solomon NM, Camper S, Pinchas C, Francke U. SnoRNA Snord116 (Pwcr1/MBII-85) deletion causes growth deficiency and hyperphagia in mice. PLoS ONE. 2008;3(3):e1709. doi: 10.1371/journal.pone.0001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Seminar in Neurology. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Leckman JF, Cassidy SB. Obsessions and compulsions in Prader-Willi syndrome. Journal of Child Psychology and Psychology. 1996;37:995–1002. doi: 10.1111/j.1469-7610.1996.tb01496.x. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Maxwell MA, Pantino E, Kossler R, Roof E. Assessment of hyperphagia in Prader-Willi syndrome. Obesity. 2007;15:1816–1826. doi: 10.1038/oby.2007.216. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Shah B. Psychiatric disorders in Prader-Willi syndrome: Epidemiology and treatment. CNS Drugs. 2003;17:167–178. doi: 10.2165/00023210-200317030-00003. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Summar K, Roof E. Adults with Prader-Willi syndrome. In: Reynolds C, Goldstein S, editors. Handbook of Neurodevelopmental and Genetic Disorders in Adults. Guilford; New York: 2005. pp. 439–457. [Google Scholar]
- Evers EAT, van der Veen FM, Julles J, Deutz NEP, Schmitt JAJ. Acute tryptophan depletion improves performance and modulates BOLD response during a Stroop task in healthy females. Neuroimage. 2006;32:248–255. doi: 10.1016/j.neuroimage.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Goldstone AP. The hypothalamus, hormones, and hunger: Alterations in human obesity and illness. Progress in Brain Research. 2006;153:57–73. doi: 10.1016/S0079-6123(06)53003-1. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger G, Charney D. Yale-Brown Obsessive-Compulsive Scale: Development, use, and reliability. Archives of General Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Halford JC, Harrold JA, Boyland EJ, Lawton CL, Blundell JE. Serotonergic drugs: Effects on appetite expression and use for the treatment of obesity. Drugs. 2007;67:27–55. doi: 10.2165/00003495-200767010-00004. [DOI] [PubMed] [Google Scholar]
- Harvey M, Shink E, Tremblay M, Gagne B, Raymond C, Labbe M, et al. Support for the involvement of TPH2 gene in affective disorders. Molecular Psychiatry. 2004;9:980–981. doi: 10.1038/sj.mp.4001557. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test-2 manual. Pearson; Minneapolis, MN: 2004. [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merinkangas K, Rush A, Walters EE, Wang PS. Epidemiology of major depressive disorder: Results from the National CoMorbidity Survey Replication (NCS-R) Journal of the American Medical Association. 2003;18:3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kishore S, Stamm S. The snoRNA HBII-52 regulates alternative splicing of the serotonin receptor 2C. Science. 2006;311:230–232. doi: 10.1126/science.1118265. [DOI] [PubMed] [Google Scholar]
- Malow BA, Marzec ML, McGrew SG, Wang L, Henderson LM, Stone WL. Characterizing sleep in children with autism spectrum disorders. Sleep. 2006;29:1563–1571. doi: 10.1093/sleep/29.12.1563. [DOI] [PubMed] [Google Scholar]
- Morabito M, Abbas A, Hood J, Kesterson R, Jacobs M, Kump D, Hachey D, Roth B, Emeson R. Mice with altered serotonin 2C receptor RNA editing display characteristics of Prader–Willi syndrome. Neurobiology of Disease. 2010;39:169–180. doi: 10.1016/j.nbd.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron E, Toru I, Must A, Tasa G, Toover E, Vasar V, Lang A, Shlik J. Association study of tryptophan hydroxylase 2 gene polymorphisms in panic disorder. Neuroscience Letters. 2007;411:180–184. doi: 10.1016/j.neulet.2006.09.060. [DOI] [PubMed] [Google Scholar]
- Mossner R, Walitza S, Geller F, Scherag A, Gutknecht L, Jacob C, Bogusch L, Remschmidt H, Simons M, et al. Transmission disequilibrium of polymorphic variants in the TPH2 gene in children and adolescnts with obsessive-compulsive disorder. International Journal of Neuropsychopharmacology. 2006;9:437–442. doi: 10.1017/S1461145705005997. [DOI] [PubMed] [Google Scholar]
- Osinsky R, Schmitz A, Alexander N, Kuepper Y, Kozyra E, Hennig J. TPH2 gene variation and conflict processing in a cognitive and an emotional Stroop task. Behavioural Brain Research. 2009;198:404–410. doi: 10.1016/j.bbr.2008.11.022. [DOI] [PubMed] [Google Scholar]
- Ramoz N, Cai G, Reichert J, Corwin TE, Kryzak LA, Smith CJ, Silverman JM, Hollander E, Buxbaum JD. Family-based associations study of TPH1 and TH2 polymorphisms in autism. American Journal of Medical Genetics, Part B. 2006;141B:861–867. doi: 10.1002/ajmg.b.30356. [DOI] [PubMed] [Google Scholar]
- Reuter M, Esslinger C, Montag C, Lis S, Gallhofer B, Kirsch P. A functional variant of the tryptophan hydroxylase 2 gene impacts working memory: A genetic imaging study. Biological Psychiatry. 2008;79:111–117. doi: 10.1016/j.biopsycho.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Sacco R, Papeleo V, Hager J, Rousseau F, Moessner R, Militerni R, Bravaccio C, Trillo S, Schnieder C, Melmed R, Elia M, Curatolo P, Manzi B, Pascucci T, Allegra S, Reichelt K, Perisco A. Case control and family-based association studies of candidate genes in autistic disorder and its endophenotypes: TPH2 and GLO1. BMC Medical Genetics. 2007;8:11. doi: 10.1186/1471-2350-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo T, del Gaudio D, German J, Shinawi M, Peters SU, Person RE, Garnica A, Cheung SW, Beaudet AL. Prader-Willi phenotype caused by paternal deficiency for the HBIII-85C/D box small nucleolar RNA cluster. Nature Genetics. 2008;40:719–721. doi: 10.1038/ng.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni S, Whittington J, Holland AJ, Webb T, Maina E, Boer H, Clarke D. The course and outcome of psychiatric illness in people with Prader-Willi syndrome: Implications for management and treatment. Journal of Intellectual Disability Research. 2007;51:32–42. doi: 10.1111/j.1365-2788.2006.00895.x. [DOI] [PubMed] [Google Scholar]
- Strobel A, Driesbach G, Muller J, Goschke T, Brocke B, Lesch K. Genetic variation of serotonin function and cognitive control. Journal of Cognitive Neuroscience. 2007;19:1923–1931. doi: 10.1162/jocn.2007.19.12.1923. [DOI] [PubMed] [Google Scholar]
- Schotta DA, Nicolaib J, de Vriesc JE, Keulartsc ML, Rubio-Gozalboa ME, Gervera cWJM. Disorder in the serotonergic system due to tryptophan hydroxylation impairment: A cause of hypothalamic syndrome? Hormone Research in Paediatrics. 2010;73:68–73. doi: 10.1159/000271918. [DOI] [PubMed] [Google Scholar]
- Utge S, Soronen P, Partonen T, Loukola A, Kronholm E, Pirkola S, Nyman E, Porkka-Heiskanen T, Paunio T. A population-based association study of candidate genes for depression and sleep disturbance. American Journal of Medical Genetics, Part B. 2010;153B:468–476. doi: 10.1002/ajmg.b.31002. [DOI] [PubMed] [Google Scholar]
- Vogels A, Matthijs G, Legius E, Devriendt K, Fryns J. Chromosome 15 maternal unipaternal disomy and psychosis in Prader-Willi syndrome. Journal of Medical Genetics. 2003;40:72–73. doi: 10.1136/jmg.40.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walitza S, Renner TJ, Dempfle A, Konrad K, Wewetzer C, Halbach A, Herpertz-Dahlmann B, Remschmidt H, Smidt J, Linder M, Flierl L, Knolker U, Friedel S, Schafer H, Gross C, Hebebrand J, Warnke A, Lesch KP. Transmission disequilibrium of polymorphic variants in the tryptophan hydroxylase-2 gene in attention-deficit hyperactivity disorder. Molecular Psychiatry. 2005;10:1126–1132. doi: 10.1038/sj.mp.4001734. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochemical Pharmacology. 2003;66:1673–1680. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- Wiggs L, Stores G. Severe sleep disturbance and daytime challenging behaviour in children with severe learning disabilities. Journal of Intellectual Disability Research. 1996;40:518–528. doi: 10.1046/j.1365-2788.1996.799799.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gainetdinov RR, Neaulieu JM, Sotnikova TD, Burch LH, Wiliams RB, Schwartz DA, Krishnan KR, Caron MG. Loss of function mutation in tryptophan hydroxalyse-2 identified in unipolar major depression. Neuron. 2005;45:11–16. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]