Abstract
Adaptive evolution is currently accepted as playing a significant role in biological invasions. Adaptations relevant to invasions are typically thought to occur either recently within the introduced range, as an evolutionary response to novel selection regimes, or within the native range, because of long-term adaptation to the local environment. We propose that recent adaptation within the native range, in particular adaptations to human-altered habitat, could also contribute to the evolution of invasive populations. Populations adapted to human-altered habitats in the native range are likely to increase in abundance within areas frequented by humans and associated with human transport mechanisms, thus enhancing the likelihood of transport to a novel range. Given that habitats are altered by humans in similar ways worldwide, as evidenced by global environmental homogenization, propagules from populations adapted to human-altered habitats in the native range should perform well within similarly human-altered habitats in the novel range. We label this scenario ‘Anthropogenically Induced Adaptation to Invade’. We illustrate how it differs from other evolutionary processes that may occur during invasions, and how it can help explain accelerating rates of invasions.
Keywords: adaptation, agriculture, contemporary evolution, evolutionary theory, habitat degradation, invasive species
Introduction
The increasing rate at which species are invading new ranges is fundamentally linked to the expansion of international trade (Carlton and Geller 1993; Cohen and Carlton 1998; Levine and D'Antonio 2003; Pysek et al. 2010). Policies have been implemented to minimize new introductions via trade (McAusland and Costello 2004; Olson and Roy 2010), yet rates of invasion continue to increase, suggesting that other additional processes might play a role. We propose that human alteration of habitats within the native range induce evolutionary changes that could promote invasion into novel ranges. We employ a broad definition of invasion, encompassing successful establishment and spread in a new range with or without particular environmental or economic impacts.
The facilitating role of evolution in invasions, particularly rapid adaptive evolution during invasions, has recently become a major subject of research (e.g., Carroll and Dingle 1996; Reznick and Ghalambor 2001; Lee 2002; Lambrinos 2004; Wares et al. 2005; Facon et al. 2006; Prentis et al. 2008). Much of this research has a temporal and geographic focus on evolutionary shifts that occur following introduction into a (usually remote) new location, rather than a focus on evolution within the native range. This perspective is logical, given that introduction into a new environment is likely to impose a novel selection regime, making rapid evolution probable. Striking examples of evolution following introduction include reduced size at reproduction in fish (Bohn et al. 2004), increased size or reproductive capacity of invasive plants (e.g., Blair and Wolfe 2004), rapid evolution of physiologic tolerance to fresh water (Lee et al. 2003, 2011), and increased dispersal distance in toads (Phillips et al. 2006; Phillips et al. 2010).
However, evolution within the native range, prior to introduction to a remote and novel range, can also promote biological invasions (Di Castri 1989; Lee and Gelembiuk 2008). This process has been referred to as ‘preadaptation’, in the invasion literature. However, the term preadaptation already has a widely recognized and well-established meaning in the evolutionary literature, that of exaptation (Bock 1959; Gould and Vrba 1982). In Box 1, we discuss the two different meanings of preadaptation and how they each might contribute to invasion. To differentiate between the two meanings, we use the terms ‘exaptation’ and ‘prior adaptation’ (Box 1), where prior adaptation is simply evolution of traits in the native range, prior to introduction to a new range, that enhance success of introduced populations without a change in function. Both exaptation and prior adaptation have the potential to promote successful invasion, but prior adaptation is likely to be more important.
Box 1: Preadaptation: exapatation and prior adaptation.
Evolution within the native range can lead to traits that confer higher fitness, i.e., are adaptive, within a novel habitat. Generally, this has been called ‘preadaptation’. The term encompasses two distinct processes, however: exaptation and what we call here prior adaptation. Exaptation occurs when a trait that has evolved under one selection regime is co-opted by chance for a different function (Bock 1959; Gould and Vrba 1982; see also Grant 1977; Futuyma 2005). This is the original meaning of the term preadaptation, from the evolutionary biology literature. The classic example of exaptation is feathers in dinosaurs. Their original function is thought to have been thermoregulation, and then they were co-opted for use in movement and eventual flight. While we know of no clear example from invasion biology whereby a trait acquired a truly new adaptive function that enhanced its invasiveness in the new range, theoretically it is possible. Thus, exaptation constitutes one mechanism by which traits could evolve in the native range that would facilitate invasion of a novel environment.
To avoid confusion with exaptation, we prefer to distinguish this second meaning of preadaptation as ‘prior adaptation’. Prior adaptation denotes the case in which adaptation to one or more facets of the environment within the native range facilitates invasions to similar environments in the novel range (Parker and Gilbert 2004; Dietz and Edwards 2006; Bossdorf et al. 2008; Fausch 2008; Treier et al. 2009). Thus, with prior adaptation, there is not a change in function as there is with exaptation. Prior adaptations can be associated with an evolutionary history in fluctuating environments in the native range (Lee and Gelembiuk 2008), which might select for organismal flexibility or evolvability, both of which could facilitate invasion into a wide range of habitats. This appears to be the case in the copepod Eurytemora affinisLee et al. (2003).
Alternatively, prior adaptation can occur via local adaptation, which can facilitate the founding and spread of new populations if those populations happen to be introduced to a region with a similar environment, and thus, traits that conferred high fitness in the native environment do so in the novel environment as well (Sax and Brown 2000; Blumenthal 2006; Dietz and Edwards 2006). One example of this mechanism appears to be found in Senecio inaequidens (Bossdorf et al. 2008; Lachmuth et al. 2010). Within its native southern African range, it is able to use a variety of habitats, while in parts of the introduced range in Europe this species invades rocky railroad tracks and motorways. Common garden comparisons reveal that invasive populations are phenotypically most similar to native populations originating from rocky slopes and dry riverbeds of mountainous regions in Southern Africa. Bossdorf et al. (2008) thus hypothesize that populations in the native range found on (and presumably adapted to) rocky slopes are the source of the populations in Europe invading, which have prior adaptations to similarly disturbed and open environments of the introduced range.
We introduce here another mechanism leading to prior adaptation called Anthropogenically Induced Adaptation to Invade (AIAI), which we detail in the main text. Fundamentally, AIAI begins with local adaptation, but rather than adaptation being to the native habitat, it is to new human-altered habitat. Thus, other forms of prior adaptation facilitate invasion when traits that are adaptive in the native range are, essentially by chance, adaptive in the introduced range as well. In contrast, because AIAI starts with adaptation to human-altered environments, it directly facilitates invasions into human-altered environments. As such, it may contribute to ever increasing rates of invasion.
Either local adaptation to a stable environment or particular disturbance regime and an evolutionary history in fluctuating environments (Lee and Gelembiuk 2008) can lead to prior adaptation to a novel environment (Box 1). The evolutionary processes leading to local adaptation to native environments can span many generations and often act over the long-term evolutionary history of the species within its native range and continue up to the present day. These longstanding evolutionary processes in the native range cannot fully help us understand the ever-increasing rate of biological invasions, because contemporary invasive populations are increasingly facing anthropogenic change within their native ranges, often marked by sudden, dramatic, and episodic impacts. Such impacts impose selection for new adaptative states that may create populations within the native ranges that are primed to become invasive. Thus, we argue here that anthropogenic change introduces a unique set of circumstances that warrants a separate category.
We propose that contemporary adaptation to human-altered environments within the native range is a central means by which prior adaptations to invasion evolve. Because the rate at which humans alter environments is increasing, the process we outline might aid in understanding mechanisms underlying the ever-increasing rate of invasions. We call this process Anthopogenically Induced Adaptation to Invade (AIAI). AIAI is summarized in Fig. 1. Briefly, species are exposed to human-altered habitats in their native range, and commonly become adapted to those habitats. This process leads to an increase in abundance within close proximity to human transportation systems, increasing the likelihood that they will be transported to a new range. Furthermore, the very adaptations that confer advantages within the human-altered habitat in the native range will also confer advantages in remote, similarly-altered habitats, facilitating successful establishment of new populations and subsequent invasions.
Figure 1.
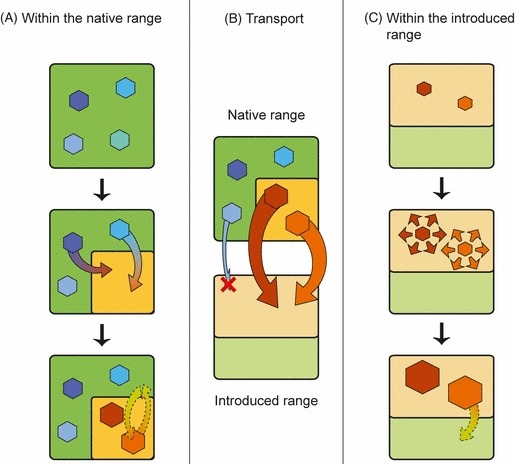
Schematic representation of the AIAI scenario. (A) Within the natural habitats (in green) of the native range, local populations (blue hexagons) are exposed to human-altered habitat (in light orange). Some populations adapt to this new type of habitat becoming either generalists able to use both habitat types, or specialists on the human-altered habitat (orange and brown hexagon, respectively; see Box 2). Generalist populations are more likely to have substantial flow of movement and genes across habitat boundaries (dashed arrow). (B) Most long-distance transport happens between two human-altered habitats (hence the large arrows). The presence of adapted populations in the human-altered habitat of the native range results in increased transport probability and a diminished need for further adaptation in the human-altered habitat of the introduction range. In contrast, populations from natural habitats of the native range are expected to suffer both from rare introduction events and lack of necessary adaptations to start a population in the introduced range (red cross). C. The introduced populations are expected to invade rapidly, because of previous adaptation to a similar habitat. Generalist phenotypes may also further cross human-altered habitat boundaries (dashed arrow) without the additional adaptations needed by specialist phenotypes.
Accounting for the effects of anthropogenic change on invasive success is critical because anthropogenic changes can fundamentally alter the rate of invasions and the type of species that are likely to invade. AIAI represents a unique series of ecological and evolutionary processes in the native and then introduced range. First, the adaptation is to human-altered habitats in particular, rather than to natural environments. Second, anthropogenic change fundamentally alters the landscape nearly instantaneously, such that the evolutionary response that enhances subsequent invasion is strictly concurrent with the anthropogenic change rather than a longstanding response occurring over historic and contemporary time scales. Third, an increase in prevalence of human-altered habitat increases the likelihood of adaptation and transport to a new range. As such, we argue that the conceptual framework of AIAI is important to distinguish from other types of evolutionary change in the native range because it fills in an important gap in our understanding of factors that promote invasive success and helps us recognize and understand the rapidity and increasing pace of biological invasions. It also highlights that contemporary adaptation facilitating invasions is not restricted to the introduced ranges of invasive species.
In support of our ideas, it has long been noted that adaptation to human-altered habitats occurs (Wet and Harlan 1975). Additionally, Crosby (1986) and Di Castri (1989) suggested that the dominance of European species among invaders might be due to their longstanding association with human disturbances. Jeschke and Strayer (2006) found that affiliation with humans per se (not just the increased propagule pressure that comes with such an affiliation) is associated with increased success of invasion. Additionally, in a recent study, Foucaud et al. (2009) introduced a process similar to AIAI, calling it a ‘two-step’ invasion, without detailing the evolutionary factors at play, the expected phenotypic outcomes and the general implications for biological invasions. To fill in these gaps and develop this idea more fully, we (i) describe the AIAI process in detail and review some basic evolutionary principles underlying it, (ii) outline the evidence necessary to illustrate it and present several systems that are candidates for AIAI, and (iii) conclude by further discussing its importance.
Anthropogenically induced adaptation to invade
As is well documented, humans are altering the environment at an increasing pace, driven by many factors (Sala et al. 2000; Daily et al. 2001; Pereira et al. 2004; Scharlemann et al. 2004; Jetz et al. 2007). Indeed, the most common and widespread environmental perturbations today are those caused by humans. Similar types of alterations can be found on different continents. Many of the same agricultural crops grown in the same fundamental ways are found essentially worldwide. For example, maize culture and other cropping systems offer a relatively homogeneous habitat throughout the world, from Africa to Asia, North and South America and Europe (Anonymous 1993). Likewise, forests are harvested, forest edges are created, roadsides are mown, and nutrients and other pollutants are added to terrestrial and aquatic ecosystems in a similar manner on different continents. Indeed, there is general agreement that habitats and biota are becoming more homogeneous worldwide (McKinney and Lockwood 1999; Tilman et al. 2001; Olden et al. 2004).
These newly altered habitats represent novel environments that impose strongly altered selection regimes. They might select for a wide variety of traits including an increased or altered host range of parasites (sensu Price 1980), increased tolerance to physiologic stressors (such as reduced humidity associated with edge effects) or a faster ‘r-selected’ life history. The potential for adaptive evolution in response to the novel selection regime is likely to be high within the native range because of greater effective population sizes, genetic variation, and propagule pressure than might be expected in the introduced range. Box 2 provides additional theoretic background for these processes. In particular, it details when an outcome of adaptation to human-altered habitats within the native range is likely, and under what conditions local adaptation to only the novel human-altered habitat is expected (the evolution of habitat specialists), and under what conditions adaptation of high performance in both the natural and the human-altered habitat is expected (the evolution of habitat generalists). Populations that adapt, either as specialists or generalists, to human-altered habitats within the native range, may increase in size or become more abundant in those habitats (Kawecki 2008). Human alteration of habitat is typically associated with human transportation systems. Thus, when a species becomes abundant in human-altered habitats, the likelihood that propagules will be taken up by various modes of long-distance transportation will increase (Lockwood et al. 2007). This will favor the species reaching a new range in numbers substantial enough to establish a new (invasive) population. It is well known that many introduced species are associated with agriculture (including both cropping systems and rangeland) and urbanization, both because they increase resource availability (or fluctuation in resource ability; Davis et al. 2000) and are associated with high propagule pressure (Lockwood et al. 2007). We argue that, in addition, many of these species may have adapted to anthropogenic modifications in the native range prior to introduction to a novel range. Given the global nature of human–habitat alterations, the likelihood that similar human-altered habitat will be found in the region of introduction is increased and the adaptations from the human-altered native range should be advantageous in similarly altered habitats in the introduced range. These newly introduced species themselves will then contribute to the increased homogenization of habitats worldwide (Mack et al. 2000), which can then further facilitate additional invasions (Simberloff and Von Holle 1999).
Box 2: Factors affecting the potential for adaptation to human-altered habitats within the native range and outcomes expected in term of life-history strategies.
Anthropogenically induced adaptation to invade occurs in a transition zone between natural and human-altered habitats within the native range; thus, ample genetic variation can be maintained more easily than in populations introduced to a new range. In such a setting, adaptation to human-altered habitats is not always possible. When adaptation does occur, it can lead to habitat generalists or habitat specialists (or both, Abrams 2006). Here, we provide readers with the fundamental theory underlying which outcome is expected.
From a management perspective, whether a generalist or specialist evolves can be important. Generalists may be slow to establish initially, but might readily invade habitats within their new range that are not human-altered. Specialists on the human-altered habitat, in contrasts, may immediately exhibit high population growth rates upon introduction to comparable human-altered habitats in a new range, but their spread from those habitats may be constrained.
Adaptation to human-altered habitats not possible
A population might fail to adapt to the newly available human-altered habitat for three main reasons. The first reason is lack of adequate genetic variation. For instance, habitat alteration might be so drastic that the variation required to adapt is simply not available. Second, adaptation may not occur if there is too much migration from the natural habitat to the new human-altered one, hindering adaptation to the new conditions (underlying this pattern are gene swamping, as well as hard selection leading to differences in the number of individuals produced and subsequent migrational meltdown; Dempster 1955; Kawecki 2000; Ronce and Kirkpatrick 2001; Lenormand 2002; Travis et al. 2005; Bridle and Vines 2007; Kawecki 2008; Ravigné et al. 2009).
The third reason for failure to adapt to human-altered habitat is because of selective processes, that lead the population to remain ‘trapped’ around the source optimum (i.e., that of the natural habitat). This can happen if the traits under selection evolve through small mutation steps (as expected when such traits are determined by many loci of small effect) and the trade-off between adaptation to both habitats is very strong (Holt and Gaines 1992; Kawecki 2000; Ronce and Kirkpatrick 2001; Rueffler et al. 2004; Ravigné et al. 2009). Technically, the trade-off curve is said to be convex (Fig. 1A). In this case, the adaptive valley that separates both optima is so steep that intermediate evolutionary steps are strongly selected against.
Adaptation to human-altered habitats possible producing either generalists or specialists
Generalist phenotype
A generalist phenotype is expected to evolve when the trade-off between adaptation to both habitats is weak, so that the fitness of the intermediate phenotype is greater than the mean fitness of any mixture of specialists (e.g., Levins 1968; Brown 1990; van Tienderen 1991, 1997; Wilson and Yoshimura 1994; Egas et al. 2004; Rueffler et al. 2004; Ravigné et al. 2009). Technically, the trade-off curve is said to be concave (Fig. 1B). This outcome is favored by a combination of high migration rate and small mutational effects, two conditions that tend to hamper the differentiation between habitats (Kawecki 2000, 2004, 2008; Ronce and Kirkpatrick 2001). In many instances, generalist species, although less fit than habitat specialists within their preferred habitats, may be fit enough to be invasive [e.g., potentially exhibiting ‘jack-of-all-trades’ phenotypic plasticity sensu Richards et al. (2006)]. This is expected when trade-offs are weak or when habitats are subject to important temporal variability under which a generalist strategy may be advantageous.
Specialist phenotype
A true specialist of the human-altered habitat, with high fitness (and thus particularly high potential for invasiveness upon introduction to similarly human-altered habitats) will tend to emerge more readily when migration is low and the traits under selection evolve through large mutation steps (few loci with large effects) (e.g., Levene 1953; Dempster 1955; Maynard Smith 1966; Hedrick 1990; Kawecki 2008). In contrast, if the traits under selection evolve through small mutation steps (many loci with small effects), then adapting to the human-altered habitat additionally requires a moderate trade-off in adaptation to both habitats (slightly convex trade-off curve) as well as some independent density-regulation in both habitats (i.e., soft selection; e.g., van Tienderen 1997; Kisdi and Geritz 1999; Ronce and Kirkpatrick 2001; Ravigné et al. 2009).
Complex theory and missing data
From a theoretical perspective, the effects of all factors cited earlier on the outcome of adaptation to a new habitat are now widely documented in a vast number of models, only a small subset of which was cited here. It has now become quite clear that no single factor by itself can determine the outcome. For instance, a weak trade-off may select for either specialization or generalization depending on the level of migration and the genetic architecture of traits underlying adaptation. Understanding these factors as deeply as possible will aid in predicting the risk that invasive populations emerge through adaptation to human-altered habitats. Although some factors may be very tricky to document (e.g., trade-off strength or the mode of density-regulation), others, though not trivial, may be feasible to estimate (e.g., habitat frequencies, the existence of a strong dissymmetry in habitat productivities, sexual vs. asexual mating system, whether adaptation is likely to evolve through small or large mutation steps). To improve our ability to forecast and to prevent biological invasions, better integration of empirical and theoretical research is hence much needed. Future models should explore more thoroughly the relative importance of the various factors at play, and how they interact (e.g., Kawecki 1994, 1996 for another view on trade-offs), while empirical studies should explicitly measure those factors already agreed to be critical in determining outcomes.
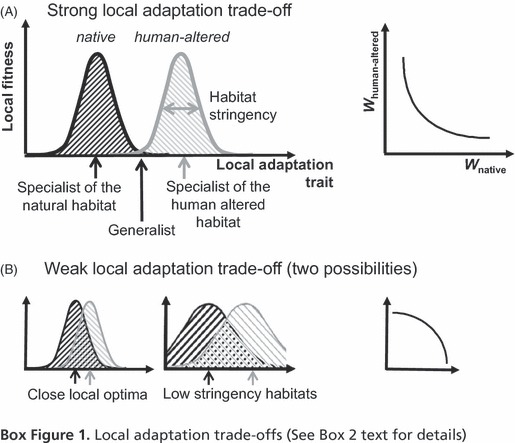 |
Trade-offs in the ability to locally adapt are central to the theory of adaptation to heterogeneous environments. Trade-offs are constraints on the set of possible fitness values (also called fitness sets, Levins 1968) such that when at the optimum in the natural habitat, better adaptation to human-altered conditions translates to a loss of adaptation to natural conditions. Figure 1 of Box 2 presents the local fitness sets for the natural (black curves) and human-altered (gray curves) habitats. Narrow fitness curves lead to highly stringent habitats (gray horizontal arrow) in which only a narrow range of trait values produces individuals with high relative fitness. Wide fitness curves produce less stringent habitats in which a wide range of trait values can produce individuals with high relative fitness. (i) Strong trade-offs in the ability to locally adapt. The generalist phenotype, which is intermediate between both specialist phenotypes, has low fitness in both habitats. A strong trade-off (also called a convex trade-off) tends to hamper adaptation to the conditions in human-altered habitats if mutation effects are small. In contrast, moderately strong trade-offs may favor the emergence of specialists. (ii) Weak trade-offs with the ability to locally adapt. Weak trade-offs (also called concave trade-offs) may exist if the optimum trait values for both natural and human-altered habitat are close or if habitat stringencies are low (or any combination of the two). Weak trade-offs enable the generalist phenotype to achieve good fitness in both habitats. Weak trade-offs may favor the evolution of a generalist population when migration is high or specialists when migration is low enough for differentiation to occur.
Thus, the combination of high evolutionary potential and strong selection in human-altered habitats within the native range is likely to lead to rapid adaptation prior to introduction elsewhere, and furthermore to increase the probability of successful introduction into a novel location. Upon introduction to a new location, such altered populations are indeed likely to perform well, particularly when introduced to habitats affected by the environmental homogenization occurring globally. It is worth pointing out that the AIAI scenario is evolutionarily parsimonious because rather than requiring that rapid adaptive evolution occur multiple times when organisms are introduced to multiple different places around the globe, the critical adaptations need to evolve only once. At the same time, however, given that human-altered habitats are often created in multiple places within one species’ range, there is the opportunity for different populations of a species to adapt separately to those habitats. Finally, the AIAI process by no means precludes either continued adaptive evolution in the new range (e.g., Blair and Wolfe 2004) or a role for hybridization and outcrossing in the new range (Kolbe et al. 2004; Lavergne and Molofsky 2007; Facon et al. 2008).
The necessary evidence
Documenting the AIAI scenario is not a trivial task and requires both ecological and genetic approaches. Briefly, it should be demonstrated that contemporary adaptive evolution has occurred within the native range, and that it leads to native populations with higher fitness in a new, human-altered habitat relative to a naïve native population that has not experienced that habitat. This adaptive evolution would also need to confer higher fitness than a naïve native population would have upon introduction to the new range. Additionally, data from neutral genetic markers, appropriately analyzed (Keller and Taylor 2008; Estoup and Guillemaud 2010), should provide evidence that the populations from human-altered habitat within the native range were the actual source for populations found in human-altered habitat in the introduced range. Table 1 outlines in detail evidence required for unambiguous support for this process.
Table 1.
Evidence required to support conclusively the anthropogenically induced adaptation to invade scenario
| Evidence needed | |
|---|---|
| Native range | |
| Habitat | Documentation that the species is in a habitat that is human-altered relative to historical habitat of species |
| Altered habitat presents a known and measurable challenge (e.g., change in salinity) | |
| Organism | Quantitative genetic evidence that the population within the altered habitat has adapted in response to anthropogenic change |
| Population genetic structure | Populations are structured at neutral loci within the native range, making it possible to identify areas of origin of the invasive populations |
| Introduced range | |
| Habitat | Habitat documented to be similar to the altered habitat within the native range (e.g. comparable salinity) |
| Organism | Evidence that introduced populations grew to large size in the human-altered habitat similar to native-range human-altered habitat. |
| Quantitative genetic evidence that the introduced populations show similar adaptations to those found for native populations within the altered habitat. | |
| Population genetic structure | Evidence that introduced populations originated directly (primary introduction) or indirectly (secondary introduction) from population(s) located in the human altered habitat within the native range. |
Candidate systems
We are not aware of any study system for which robust and complete data sets supporting each separate point from Table 1 are available. At this point in time, some of the most clear-cut examples of adaptation to human-altered habitat occurring prior to invasion come from crop pests. One likely example is Leptinotarsa decemlineata, the Colorado potato beetle, which is a pest of many solanaceous crops. Its original geographic distribution includes Mexico and parts of the western and central USA, and its original host range is thought to span only three species of the genus Solanum, Solanum rostratum, S. angustifolium, and S. elaeagnifolium (Forister et al. 2007). The human alteration of habitat comes in the form of potato farming. The potato, Solanum tuberosum, was introduced from South America into North America and Europe for intensive cropping in the 18th century (Glendinning 1983) and the beetle L. decemlineata started to use it as a host in the 1830s and 1840s within its native range (the central US). Subsequently, the beetle spread throughout both North America and Europe as a major pest. Evidence suggests that adaptation was involved in the use of potato as a host plant. Studies illustrate that potato, rather than the native hosts, is the most suitable host for US pest populations of Leptinotarsa decelimneata (Hare 1990). In contrast, potato elicits only weak oviposition and feeding in populations associated with the original hosts S. angustifolium and S. elaeagnifolium (Hsiao 1978, 1985; Harrison and Mitchell 1988; Lu and Logan 1994a,b,c). Furthermore, Forister et al. (2007) conducted quantitative genetic experiments showing genetic variation in many traits associated with host use, suggesting that a dietary shift itself might have evolved as a distinct trait in L. decelimneata. After the inclusion of potato in its diet, the beetle was hence able to spread far beyond its original geographic range, both to contiguous areas and to other continents where potatoes are grown, notably Europe, via human-aided long-distance dispersal.
As biological invasions are generally considered a contemporary phenomenon, most candidate systems have spread recently (e.g., neophytes and neozoa introduced to a new range within the last 2000 years). However, there were human-altered habitats much earlier than that, and thus, older examples also may fit this pattern. One possibility is the ascomycete Mycosphaerella graminicola, one of the most damaging fungal pathogens of wheat. Phylogeographic studies located at the center of origin of M. graminicola in the Middle East (Banke et al. 2004). In addition, using Bayesian inference on DNA sequence data, Stukenbrock et al. (2007) have provided evidence that the divergence between M. graminicola and its congeners (sampled on noncultivated grasses in the Middle East) occurred approximately 10 500 years ago, coincident with the beginning of agriculture and the domestication of wild grasses in the Fertile Crescent. The timing of divergence strongly suggests that M. graminicola originated from populations of pathogens associated with wild grasses that then adapted to wheat during its domestication. The invasive populations of M. graminicola are specific to wheat (Eyal et al. 1973, 1985; van Ginkel and Scharen 1987; Saadaoui 1987), and following their divergence, spread throughout the world on cultivated wheat crops.
The AIAI process is not restricted to agricultural pests. The little fire ant, Wasmannia auropunctata, is a species originating from Central and South America that has been successfully spreading over the World tropics and parts of the Mediterranean zone since the beginning of the last century (Wetterer and Porter 2003; Vonshak et al. 2009). As yet, the precise geographic origin of the introduced populations within the native range is still unknown, but one possibility is that introductions occurred in association with food products shipped from plantations to markets worldwide. In natural areas of its native range (primary forests), low density, mostly sexually reproducing populations are found. The human-altered habitat consists of forest edges and plantations. In these areas, the little fire ant occurs at high density and has become ecologically dominant (Orivel et al. 2009). A clear shift toward clonal reproduction is associated with the human-altered habitat (Foucaud et al. 2009), and that shift appears to be genetically based (Foucaud et al. 2010a). Additionally, populations in the human-altered habitat exhibit greater tolerance to stressful temperature and humidity conditions than populations from the natural forest habitats (J. Foucaud, O. Rey, A. Estoup, B. Facon, unpublished data). These life-history and physiologic changes appear likely to be adaptations to the human-altered habitat within the native range. Populations in the introduced range are most common in human-altered habitats, and are most similar with respect to life-history and physiology traits to populations in human-altered habitats in the native range; that is to say, they are clonal, and characterized by a high tolerance of stressful temperature and humidity conditions. Mikheyev and Mueller (2007) and Foucaud et al. (2010b) show that the main vector of W. auropunctata long-distance dispersal is human trade.
Animal and human diseases may also follow an AIAI scenario. Take, for example, AIDS, one of the most fatal infectious diseases facing humankind. Human immunodeficiency virus-type 1 (HIV-1) group M is responsible for the great majority of all HIV infections in humans and has infected more than 50 million individuals worldwide (Hahn et al. 2000). Current evidence indicates that HIV-1 moved to human hosts in west equatorial Africa, and arose via transmission from a simian lentivirus (SIVcpz) infecting chimpanzees (Pan troglodytes troglodytes) (Keele et al. 2006). A fundamental part of most human-altered habitats is an increased density of humans themselves, which represents a large unused pool of potential hosts. Increased human population density may also have increased opportunities for contact with the original host and hence the likelihood of the initial transmission event. Molecular data show that the introduction of SIVcpz into humans, giving rise to HIV-1 group M, most likely occurred in the early part of the 20th century. It is thought that differences in selection pressures in the two hosts have led to differentiation of the viruses (Hahn et al. 2000), and that viral adaptation to the new human host contributed to the outbreak of AIDS as an epidemic by the 1980s (Worobey et al. 2008). In association with dense and highly mobile human populations (e.g., migrant workers) transmission rates would be high, favoring further adaptation leading to the evolution of increased virulence.
Conclusion and implications
We describe a mechanism promoting biological invasion that we label as AIAI. We argue that the combination of high evolutionary potential provided by high effective population size and strong novel selection imposed by human-altered habitats within the native range is likely to lead to rapid adaptation prior to introduction elsewhere, and simultaneously increased probability of introduction. Upon introduction to a new location, propagules adapted to human-altered habitats are likely to perform well, particularly when they are introduced into habitats that have been modified in a manner similar to that of their native range. This phenomenon is likely, given the environmental homogenization that is occurring globally. We argue that AIAI is fundamentally distinct from other mechanisms leading to prior adaptation primarily because the evolution is strictly contemporary rather than longstanding, and because the AIAI scenario emphasizes the central role of humans in imposing selective pressures within the native range and in enhancing dispersal via global trade.
Anthropogenically induced adaptation to invade is thus a contemporary phenomenon that is occurring at accelerated rates and is homogenizing the globe. As such, the AIAI paradigm sheds new insights into causes of biological invasions, as well as their ever-increasing pace. As noted, this scenario is evolutionarily parsimonious because rather than requiring rapid adaptive evolution with each introduction into a new location, the critical adaptations need only evolve once. With the AIAI scenario, the adaptive challenges are shifted to the native range where populations are less likely to have passed through bottlenecks, and variation in traits under selection is less likely to be limiting.
It is worth emphasizing, however, that once a population's invasion is facilitated by adaptation to human-altered habitats in the native range, including establishment within similar habitats in the novel range, continued ecological and evolutionary change might enable it to invade further into environments that are not strongly human-altered. As Box 2 illustrates, the outcome of evolutionary processes in the native range can either lead to habitat specialists or to habitat generalists. Upon introduction to a new range, specialists could evolve to use different habitats in the introduced range (either through evolution of a new specialist phenotype or a generalist phenotype). However, if a generalist phenotype invades, then it is likely to be immediately able to colonize further into the introduced range in environments that are not strongly human-altered, provided that there is not strong resistance from locally adapted species.
The AIAI scenario improves our understanding of some fundamental issues in invasion biology. First, it supports the idea that populations with ‘invasive’ behaviors (e.g. high densities or reproductive rates) can be found within native ranges (Valery et al. 2009) when they evolve to ‘invade’ human-altered habitats. Thus, exceptions to a strictly geographic (i.e. native/introduced) (Wilson et al. 2009) understanding of invasions may exist.
Second, AIAI may further elucidate the degree to which the Imperialist Dogma, the idea that there is a European bias to invasions (Crosby 1986; Di Castri 1989), might be true and when and why it might not be (Jeschke and Strayer 2006; Fridley 2008).
Third, the AIAI scenario provides yet an additional argument against the presumed paradox of invasion (Sax and Brown 2000; Frankham 2005; Hufbauer 2008) which suggests that adaptive evolution during invasion is constrained by low genetic variation, inbreeding and inbreeding depression. While bottlenecks can constrain evolution (e.g., Pujol and Pannell 2008), the opposite can also occur. It is now clear that reduction of variation at putatively neutral loci may not reflect variance available in quantitative genetic traits, and thus, sufficient variation may be available for adaptation even following bottlenecks (Van Buskirk and Willi 2006; Olivieri 2009). Also, even if individual groups of propagules have passed through bottlenecks, often multiple introductions can occur, which can maintain, or even increase, genetic variability in the introduced populations (Kolbe et al. 2004; Lavergne and Molofsky 2007; Roman and Darling 2007; Dlugosch and Hays 2008; Dlugosch and Parker 2008; Facon et al. 2008; Hufbauer 2008, Olivieri 2009). Bottlenecks may even be of a size that can actually purge genetic load leading to inbreeding depression (Facon et al. 2011). With the AIAI scenario, the adaptive challenges are shifted to the native range where populations are less likely to have passed through bottlenecks, and variation in traits under selection is less likely to limit the rate and magnitude of adaptive evolution. Thus, the AIAI contributes to further resolving the initial paradox of biological invasion.
Finally, given the increase in human alteration of habitats worldwide, the AIAI scenario also may help explain why it is that rates of invasion continue to increase despite intensive efforts to prevent them. The ever-increasing alteration of natural habitats by human activities, which leads to contemporary adaptation of native populations to such altered habitats, should increase the likelihood both of being transported, and of being able to establish into similar human-altered habitats within a new geographic range.
Many species appear to conform to the AIAI scenario, requiring only a little additional evidence for verification. We hope that bringing this explicitly evolutionary perspective to how species adapt to human-altered environments in their native ranges, and how that can help us understand the increasing rates of invasion into human-altered environments in their introduced ranges, will motivate further studies to test for it.
Acknowledgments
We thank Dana Blumenthal, the Norton and Hufbauer laboratory groups members of the NSF Global Invasions Network RCN (DEB-0541673) and Thomas Guillemaud for fruitful discussions. R.A.H. received support from Fulbright-France, INRA, NSF DEB-0541673 and the Colorado Agricultural Experiment Station during this work. The work was supported by the French Agropolis Fondation (RTRA – Montpellier, BIOFIS project number 1001-001). V. R. received support from Agropolis Foundation and RNSC (project ModPEA, Covenant support number 0902-013), INRA (call for proposal ‘Gestion durable des résistances aux bio-agresseurs’, project Metacarpe, contract number 394576).
Literature cited
- Abrams PA. The prerequisites for and likelihood of generalist-specialist coexistence. American Naturalist. 2006;167:329–342. doi: 10.1086/499382. [DOI] [PubMed] [Google Scholar]
- Anonymous. Maize in Human Nutrition. Rome: Food and Agriculture Organization of the United Nations; 1993. [Google Scholar]
- Banke S, Peschon A, McDonald BA. Phylogenetic analysis of globally distributed Mycosphaerella graminicola populations based on three DNA sequence loci. Fungal Genetics and Biology. 2004;41:226–238. doi: 10.1016/j.fgb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Blair AC, Wolfe LM. The evolution of an invasive plant: an experimental study with Silene latifolia. Ecology. 2004;85:3035–3042. [Google Scholar]
- Blumenthal D. Interactions between resource availability and enemy release in plant invasions. Ecology Letters. 2006;9:887–895. doi: 10.1111/j.1461-0248.2006.00934.x. [DOI] [PubMed] [Google Scholar]
- Bock WJ. Preadaptation and multiple evolutionary pathways. Evolution. 1959;13:194–211. [Google Scholar]
- Bohn T, Sandlund OT, Amundsen PA, Primicerio R. Rapidly changing life history during invasion. Oikos. 2004;106:138–150. [Google Scholar]
- Bossdorf O, Lipowsky A, Prati D. Selection of preadapted populations allowed Senecio inaequidens to invade Central Europe. Diversity and Distributions. 2008;14:676–685. [Google Scholar]
- Bridle JR, Vines TH. Limits to evolution at range margins: when and why does adaptation fail? Trends in Ecology & Evolution. 2007;22:140–147. doi: 10.1016/j.tree.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Brown JS. Habitat selection as an evolutionary game. Evolution. 1990;44:732–746. doi: 10.1111/j.1558-5646.1990.tb05951.x. [DOI] [PubMed] [Google Scholar]
- Carlton JT, Geller JB. Ecological roulette: the global transpport of nonindigenous marine organisms. Science. 1993;261:78–82. doi: 10.1126/science.261.5117.78. [DOI] [PubMed] [Google Scholar]
- Carroll SP, Dingle H. The biology of post-invasion events. Biological Conservation. 1996;78:207–214. [Google Scholar]
- Cohen AN, Carlton JT. Accelerating invasion rate in a highly invaded estuary. Science. 1998;279:555–558. doi: 10.1126/science.279.5350.555. [DOI] [PubMed] [Google Scholar]
- Crosby AW. Ecological Imperialism: The Biological Expansion of Europe, 900-1900. Cambridge, MA: Cambridge University Press; 1986. [Google Scholar]
- Daily GC, Ehrlich PR, Sanchez-Azofeifa GA. Countryside biogeography: use of human-dominated habitats by the avifauna of southern Costa Rica. Ecological Applications. 2001;11:1–13. [Google Scholar]
- Davis MA, Grime JP, Thompson K. Fluctuating resources in plant communities: a general theory of invisibility. Journal of Ecology. 2000;88:528–534. [Google Scholar]
- De Wet JMJ, Harlan JR. Weeds and domesticates: evolution in the mad-made habitat. Economic Botany. 1975;29:99–108. [Google Scholar]
- Dempster ER. Maintenance of genetic heterogeneity. Cold Spring Harbor Symposia on Quantitative Biology. 1955;20:25–32. doi: 10.1101/sqb.1955.020.01.005. [DOI] [PubMed] [Google Scholar]
- Di Castri F. History of biological invasions with special emphasis on the Old World. In: Drake JA, et al., editors. Biological Invasions: A Global Perspective. Chichester: Wiley; 1989. pp. 1–29. [Google Scholar]
- Dietz H, Edwards PJ. Recognition that causal processes change during plant invasion helps explain conflicts in evidence. Ecology. 2006;87:1359–1367. doi: 10.1890/0012-9658(2006)87[1359:rtcpcd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Dlugosch KM, Hays CG. Genotypes on the move: some things old and some things new shape the genetics of colonization during species invasions. Molecular Ecology. 2008;17:4583–4585. doi: 10.1111/j.1365-294X.2008.03932.x. [DOI] [PubMed] [Google Scholar]
- Dlugosch KM, Parker IM. Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Molecular Ecology. 2008;17:431–449. doi: 10.1111/j.1365-294X.2007.03538.x. [DOI] [PubMed] [Google Scholar]
- Egas M, Dieckmann U, Sabelis MW. Evolution restricts the coexistence of specialists and generalists: the role of trade-off structure. American Naturalist. 2004;163:518–531. doi: 10.1086/382599. [DOI] [PubMed] [Google Scholar]
- Estoup A, Guillemaud T. Reconstructing routes of invasion using genetic data: why, how and so what? Molecular Ecology. 2010;19:4113–4130. doi: 10.1111/j.1365-294X.2010.04773.x. [DOI] [PubMed] [Google Scholar]
- Eyal Z, Amiri Z, Wahl I. Physiologic specialization of Septoria tritici. Phytopathology. 1973;63:1087–1091. [Google Scholar]
- Eyal Z, Scharen AL, Huffman MD, Prescott JM. Global insights into virulence frequencies of Mycosphaerella graminicola. Phytopathology. 1985;75:1456–1462. [Google Scholar]
- Facon B, Genton BJ, Shykoff J, Jarne P, Estoup A, David P. A general eco-evolutionary framework for understanding bioinvasions. Trends in Ecology and Evolution. 2006;21:130–135. doi: 10.1016/j.tree.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Facon B, Pointier JP, Jarne P, Sarda V, David P. High genetic variance in life-history strategies within invasive populations by way of multiple introductions. Current Biology. 2008;18:363–367. doi: 10.1016/j.cub.2008.01.063. [DOI] [PubMed] [Google Scholar]
- Facon B, Hufbauer RA, Tayeh A, Loiseau A, Lombaert E, Vitalis R, Guillemaud T, et al. Inbreeding depression is purged in the invasive insect Harmonia axyridis. Current Biology. 2011;21:424–427. doi: 10.1016/j.cub.2011.01.068. [DOI] [PubMed] [Google Scholar]
- Fausch KD. A paradox of trout invasions in North America. Biological Invasions. 2008;10:685–701. [Google Scholar]
- Forister ML, Ehmer AG, Futuyma DJ. The genetic architecture of a niche: variation and covariation in host use traits in the Colorado potato beetle. Journal of Evolutionary Biology. 2007;20:985–996. doi: 10.1111/j.1420-9101.2007.01310.x. [DOI] [PubMed] [Google Scholar]
- Foucaud J, Orivel J, Fournier D, Delabie JHC, Loiseau A, Le Breton J, Cerdan P, et al. Reproductive system, social organization, human disturbance and ecological dominance in native populations of the little fire ant, Wasmannia auropunctata. Molecular Ecology. 2009;18:5059–5073. doi: 10.1111/j.1365-294X.2009.04440.x. [DOI] [PubMed] [Google Scholar]
- Foucaud J, Estoup A, Loiseau A, Rey O, Orivel J. Thelytokous parthenogenesis, male clonality and genetic caste determination in the little fire ant: new evidence and insights from the lab. Heredity. 2010a;105:205–212. doi: 10.1038/hdy.2009.169. [DOI] [PubMed] [Google Scholar]
- Foucaud J, Orivel J, Loiseau A, Delabie JHC, Jourdan H, Konghouleux D, Vonshak M, et al. Worldwide invasion by the little fire ant: routes of introduction and eco-evolutionary pathways. Evolutionary Applications. 2010b;3:363–374. doi: 10.1111/j.1752-4571.2010.00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankham R. Invasion biology – resolving the genetic paradox in invasive species. Heredity. 2005;94:385. doi: 10.1038/sj.hdy.6800634. [DOI] [PubMed] [Google Scholar]
- Fridley JD. Of Asian forests and European fields: Eastern U.S. plant invasions in a global floristic cotext. PLoS ONE. 2008;3:1–8. doi: 10.1371/journal.pone.0003630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futuyma DJ. Evolution. Sunderland, MA, USA: Sinauer Associates; 2005. p. 543. [Google Scholar]
- van Ginkel M, Scharen AL. Generation mean analysis and heritabilities of resistance to Septoria tritici in durum wheat. Phytopathology. 1987;77:1629–1633. [Google Scholar]
- Glendinning DR. Potato introductions and breeding up to the early 20th century. New Phytologist. 1983;94:479–505. [Google Scholar]
- Gould SJ, Vrba E. Exaptation: a missing term in the science of form. Paleobiology. 1982;8:4–15. [Google Scholar]
- Grant V. Organismic Evolution. San Francisco, CA: W. H. Freeman and Co Lts; 1977. p. 418. [Google Scholar]
- Hahn BH, Shaw GM, De Cock KM, Sharp PM. AIDS – AIDS as a zoonosis: scientific and public health implications. Science. 2000;287:607–614. doi: 10.1126/science.287.5453.607. [DOI] [PubMed] [Google Scholar]
- Hare JD. Ecology and mangement of the Colorado potato beetle. Annual Review of Entomology. 1990;35:81–100. [Google Scholar]
- Harrison GD, Mitchell BK. Host-plant acceptance by geographic populations of the Colorado potato beetle, Leptinotarsa decelimneata: role of solanaceous alkaloids as sensory deterrents. Journal of Chemical Ecology. 1988;14:777–788. doi: 10.1007/BF01018772. [DOI] [PubMed] [Google Scholar]
- Hedrick PW. Theoretical analysis of habitat selection and maintenance of genetic variation. In: Barker JSF, Starmer WT, MacIntyre RJ, editors. Ecological and Evolutionary Genetics of Drosophila. New York: Plenum Press; 1990. pp. 209–227. [Google Scholar]
- Holt RD, Gaines MS. Analysis of adaptation in heterogeneous landscapes: implications for the evolution of fundamental niches. Evolutionary Ecology. 1992;6:433–447. [Google Scholar]
- Hsiao TH. Host plant adaptations among geographical populations of the Colorado potato beetle. Entomologia Experimentalis Et Applicata. 1978;24:437–447. [Google Scholar]
- Hsiao TH. Ecophysiological adaptations among geographic populations of the Colorado potato beetle in North America. In: Ferro DN, Voss RH, editors. Amherst, MA: Agricultural Experiment Station; 1981. pp. 69–85. Proceedings of the Symposium on the Colorado Potato Beetles, 18th International Congress of Entomology, Research Bulletin 704. [Google Scholar]
- Hufbauer RA. Biological invasions: paradox lost and paradise gained. Current Biology. 2008;18:R246–R247. doi: 10.1016/j.cub.2008.01.038. [DOI] [PubMed] [Google Scholar]
- Jeschke JM, Strayer DL. Determinants of vertebrate invasion success in Europe and North America. Global Change Biology. 2006;12:1608–1619. [Google Scholar]
- Jetz W, Wilcove DS, Dobson AP. Projected impacts of climate and land-use change on the global diversity of birds. PLoS Biology. 2007;5:1211–1219. doi: 10.1371/journal.pbio.0050157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawecki TJ. Accumulation of deleterious mutations and the evolutionary cost of being a generalist. American Naturalist. 1994;144:833–838. [Google Scholar]
- Kawecki TJ. Sympatric speciation driven by beneficial mutations. Proceedings of the Royal Society of London Series B-Biological Sciences. 1996;263:1515–1520. [Google Scholar]
- Kawecki TJ. Adaptation to marginal habitats: contrasting influence of the dispersal rate on the fate of alleles with small and large effects. Proceedings of the Royal Society of London Series B-Biological Sciences. 2000;267:1315–1320. doi: 10.1098/rspb.2000.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawecki TJ. Ecological and evolutionary consequences of source-sink population dynamics. In: Hanski I, Gaggiotti OE, editors. Ecology, Genetics, and Evolution of Metapopulations. Oxford, UK: Oxford Academic; 2004. pp. 387–414. [Google Scholar]
- Kawecki TJ. Adaptation to marginal habitats. Annual Review of Ecology, Evolution and Systematics. 2008;39:321–342. [Google Scholar]
- Keele BF, Van Heuverswyn F, Li YY, Bailes E, Takehisa J, Santiago ML, Bibollet-Ruche F, et al. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science. 2006;313:523–526. doi: 10.1126/science.1126531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SR, Taylor DR. History, chance and adaptation during biological invasions: separating stochastic phenotypic evolution from response to selection. Ecology Letters. 2008;11:852–866. doi: 10.1111/j.1461-0248.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- Kisdi E, Geritz SAH. Adaptive dynamics in allele space: evolution of genetic polymorphism by small mutations in a heterogeneous environment. Evolution. 1999;53:993–1008. doi: 10.1111/j.1558-5646.1999.tb04515.x. [DOI] [PubMed] [Google Scholar]
- Kolbe JJ, Glor RE, Schettino LRG, Lara AC, Larson A, Losos JB. Genetic variation increases during biological invasion by a Cuban lizard. Nature. 2004;431:177–181. doi: 10.1038/nature02807. [DOI] [PubMed] [Google Scholar]
- Lachmuth SW, Durka W, Schurr FM. The making of a rapid plant invader: genetic diversity and differentiation in the native and invaded range of Senecio inaequidens. Molecular Ecology. 2010;19:3952–3967. doi: 10.1111/j.1365-294X.2010.04797.x. [DOI] [PubMed] [Google Scholar]
- Lambrinos JG. How interactions between ecology and evolution influence contemporary invasion dynamics. Ecology. 2004;85:2061–2070. [Google Scholar]
- Lavergne S, Molofsky J. Increased genetic variation and evolutionary potential drive the success of an invasive grass. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3883–3888. doi: 10.1073/pnas.0607324104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CE. Evolutionary genetics of invasive species. Trends in Ecology and Evolution. 2002;17:386–391. [Google Scholar]
- Lee CE, Gelembiuk GW. Evolutionary origins of invasive populations. Evolutionary Applications. 2008;1:427–448. doi: 10.1111/j.1752-4571.2008.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CE, Remfert JL, Gelembiuk GW. Evolution of physiological tolerance and performance during freshwater invasions. Integrative and Comparative Biology. 2003;43:439–449. doi: 10.1093/icb/43.3.439. [DOI] [PubMed] [Google Scholar]
- Lee CE, Kiergaard M, Eads BD, Gelembiuk GW, Posavi M. Pumping ions: rapid parallel evolution of ionic regulation following habitat invasions. Evolution. 2011;65:2229–2244. doi: 10.1111/j.1558-5646.2011.01308.x. [DOI] [PubMed] [Google Scholar]
- Lenormand T. Gene flow and the limits to natural selection. Trends in Ecology and Evolution. 2002;17:183–189. [Google Scholar]
- Levene H. Genetic equilibrium when more than one ecological niche is available. American Naturalist. 1953;87:331–333. [Google Scholar]
- Levine JM, D'Antonio CM. Forecasting biological invasions with increasing international trade. Conservation Biology. 2003;17:322–326. [Google Scholar]
- Levins R. Evolution in Changing Environments. Princeton, NJ: Princeton University Press; 1968. [Google Scholar]
- Lockwood JL, Hoopes MF, Marchetti MP. Invasion Ecology. Oxford: Blackwell Publishing; 2007. [Google Scholar]
- Lu WH, Logan P. Effects of potato association on oviposition behavior of Mexican Leptinotarsa decelimneata (Coleoptera, Chrysomelidae) Environmental Entomology. 1994a;23:85–90. [Google Scholar]
- Lu WH, Logan P. Genetic variation in oviposition between and within populations of Leptinotarsa decelimneata (Coleoptera, Chrysomelidae) Annals of the Entomological Society of America. 1994b;87:634–640. [Google Scholar]
- Lu WH, Logan P. Geographic variation in larval feeding acceptance and performance of Leptinotarsa decelimneata (Coleoptera, Chrysomelidae) Annals of the Entomological Society of America. 1994c;87:460–469. [Google Scholar]
- Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA. Biotic invasions: causes, epidemiology, global consequences, and control. Ecological Applications. 2000;10:689–710. [Google Scholar]
- Maynard Smith J. Sympatric speciation. American Naturalist. 1966;100:637–650. [Google Scholar]
- McAusland C, Costello C. Avoiding invasives: trade-related policies for controlling unintentional exotic species introductions. Journal of Environmental Economics and Management. 2004;48:954–977. [Google Scholar]
- McKinney ML, Lockwood JL. Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends in Ecology and Evolution. 1999;14:450–453. doi: 10.1016/s0169-5347(99)01679-1. [DOI] [PubMed] [Google Scholar]
- Mikheyev AS, Mueller UG. Genetic relationships between native and introduced populations of the little fire ant Wasmannia auropunctata. Diversity and Distributions. 2007;13:573–579. [Google Scholar]
- Olden JD, Poff NL, Douglas MR, Douglas ME, Fausch KD. Ecological and evolutionary consequences of biotic homogenization. Trends in Ecology and Evolution. 2004;19:18–24. doi: 10.1016/j.tree.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Olivieri I. Alternative mechanisms of range expansion are associated with different changes of evolutionary potential. Trends in Ecology and Evolution. 2009;24:289–292. doi: 10.1016/j.tree.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Olson LJ, Roy S. Dynamic sanitary and phytosanitary trade policy. Journal of Environmental Economics and Management. 2010;60:21–30. [Google Scholar]
- Orivel J, Grangier J, Foucaud J, Le Breton J, Andres FX, Jourdan H, Delabie JHC, et al. Ecologically heterogeneous populations of the invasive ant Wasmannia auropunctata within its native and introduced ranges. Ecological Entomology. 2009;34:504–512. [Google Scholar]
- Parker IM, Gilbert GS. The evolutionary ecology of novel plant–pathogen interactions. Annual Review of Ecology, Evolution and Systematics. 2004;35:675–700. [Google Scholar]
- Pereira HM, Daily GC, Roughgarden J. A framework for assessing the relative vulnerability of species to land-use change. Ecological Applications. 2004;14:730–742. [Google Scholar]
- Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecological Modeling. 2006;190:231–259. [Google Scholar]
- Phillips BL, Brown GP, Shine R. Evolutionarily accelerated invasions: the rate of dispersal evolves upwards during the range advance of cane toads. Journal of Evolutionary Biology. 2010;23:2595–2601. doi: 10.1111/j.1420-9101.2010.02118.x. [DOI] [PubMed] [Google Scholar]
- Prentis PJ, Wilson JRU, Dormontt EE, Richardson DM, Lowe AJ. Adaptive evolution in invasive species. Trends in Plant Science. 2008;13:288–294. doi: 10.1016/j.tplants.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Price PW. Evolutionary Biology of Parasites. Princeton NJ, USA: Princeton University Press; 1980. p. 237. [Google Scholar]
- Pujol B, Pannell JR. Reduced responses to selection after species range expansion. Science. 2008;321:96. doi: 10.1126/science.1157570. [DOI] [PubMed] [Google Scholar]
- Pysek P, Jarosik V, Hulme PE, Kuhn I, Wild J, Arianoutsou M, Bacher S, et al. Disentangling the role of environmental and human pressures on biological invasions across Europe. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12157–12162. doi: 10.1073/pnas.1002314107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravigné V, Dieckmann U, Olivieri I. Live where you thrive: joint evolution of habitat choice and local adaptation facilitates specialization and promotes diversity. American Naturalist. 2009;174:E141–E169. doi: 10.1086/605369. [DOI] [PubMed] [Google Scholar]
- Reznick DN, Ghalambor CK. The population ecology of contemporary adaptations: what empirical studies reveal about the conditions that promote adaptive evolution. Genetica. 2001;112:183–198. [PubMed] [Google Scholar]
- Richards CL, Bossdorf O, Muth NZ, Gurevitch J, Pigliucci M. Jack of all trades, master of some? On the role of phenotypic plasticity in plat invasions. Ecology Letters. 2006;9:981–993. doi: 10.1111/j.1461-0248.2006.00950.x. [DOI] [PubMed] [Google Scholar]
- Roman J, Darling JA. Paradox lost: genetic diversity and the success of aquatic invasions. Trends in Ecology and Evolution. 2007;22:454–464. doi: 10.1016/j.tree.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Ronce O, Kirkpatrick M. When sources become sinks: migrational meltdown in heterogeneous habitats. Evolution. 2001;55:1520–1531. doi: 10.1111/j.0014-3820.2001.tb00672.x. [DOI] [PubMed] [Google Scholar]
- Rueffler C, Van Dooren TJM, Metz JAJ. Adaptive walks on changing landscapes: Levins’ approach extended. Theoretical Population Biology. 2004;65:165–178. doi: 10.1016/j.tpb.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Saadaoui EM. Physiologic specialization of Septoria tritici in Morocco. Plant Disease. 1987;71:153–155. [Google Scholar]
- Sala OE, Chapin FS, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, et al. Biodiversity – global biodiversity scenarios for the year 2100. Science. 2000;287:1770–1774. doi: 10.1126/science.287.5459.1770. [DOI] [PubMed] [Google Scholar]
- Sax DF, Brown JH. The paradox of invasion. Global Ecology and Biogeography. 2000;9:363–371. [Google Scholar]
- Scharlemann JPW, Green RE, Balmford A. Land-use trends in endemic bird areas: global expansion of agriculture in areas of high conservation value. Global Change Biology. 2004;10:2046–2051. [Google Scholar]
- Simberloff D, Von Holle B. Positive interactions of nonindigenous species: invasional meltdown? Biological Invasions. 1999;1:21–32. [Google Scholar]
- Stukenbrock EH, Banke S, Javan-Nikkhah M, McDonald BA. Origin and domestication of the fungal wheat pathogen Mycosphaerella graminicola via sympatric speciation. Molecular Biology and Evolution. 2007;24:398–411. doi: 10.1093/molbev/msl169. [DOI] [PubMed] [Google Scholar]
- van Tienderen PH. Evolution of generalists and specialists in spatially heterogeneous environments. Evolution. 1991;45:1317–1331. doi: 10.1111/j.1558-5646.1991.tb02638.x. [DOI] [PubMed] [Google Scholar]
- van Tienderen PH. Generalists, specialists, and the evolution of phenotypic plasticity in sympatric populations of distinct species. Evolution. 1997;51:1372–1380. doi: 10.1111/j.1558-5646.1997.tb01460.x. [DOI] [PubMed] [Google Scholar]
- Tilman D, Fargione J, Wolff B, D'Antonio C, Dobson A, Howarth R, Schindler D, et al. Forecasting agriculturally driven global environmental change. Science. 2001;292:281–284. doi: 10.1126/science.1057544. [DOI] [PubMed] [Google Scholar]
- Travis JMJ, Hammershøj M, Stephenson C. Adaptation and propagule pressure determine invasion dynamics: insights from a spatially explicit model for sexually reproducing species. Evolutionary Ecology Research. 2005;7:37–51. [Google Scholar]
- Treier UA, Broennimann O, Normand S, Guisan A, Schaffner U, Steinger T, Müller-Schärer H. Shift in cytotype frequency and niche space in the invasive plant Centaurea maculosa. Ecology. 2009;90:1366–1377. doi: 10.1890/08-0420.1. [DOI] [PubMed] [Google Scholar]
- Valery L, Fritz H, Lefeuvre JC, Simberloff D. Invasive species can also be native. Trends in Ecology and Evolution. 2009;24:585. doi: 10.1016/j.tree.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Van Buskirk J, Willi Y. The change in quantitative genetic variation with inbreeding. Evolution. 2006;60:2428–2434. [PubMed] [Google Scholar]
- Vonshak M, Dayan T, Foucaud J, Estoup A, Hefetz A. The interplay between genetic and environmental effects on colony insularity in the clonal invasive little fire ant Wasmannia auropunctata. Behavioral Ecology and Sociobiology. 2009;63:1667–1677. [Google Scholar]
- Wares JP, Hughes AR, Grosberg RK. Mechanisms that drive evolutionary change: insights from species introductions and invasions. In: Sax D, Stachowicz J, Gaines SD, editors. Species Invasions: Boon or Bane for Ecology and Evolution? Sunderland, MA, USA: Sinauer Publishing; 2005. pp. 229–257. [Google Scholar]
- Wetterer JK, Porter SD. The little fire ant, Wasmannia auropunctata: distribution, impact, and control. Sociobiology. 2003;42:1–41. [Google Scholar]
- Wilson DS, Yoshimura J. On the coexistence of specialists and generalists. American Naturalist. 1994;144:692–707. [Google Scholar]
- Wilson JRU, Dormontt EE, Prentis PJ, Lowe AJ, Richardson DM. Biogeographic concepts define invasion biology. Trends in Ecology and Evolution. 2009;24:586. doi: 10.1016/j.tree.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Worobey M, Gemmel M, Teuwen DE, Haselkorn T, Kunstman K, Bunce M, Muyembe JJ, et al. Direct evidence of extensive diversity of HIV-1 in Kinshasa by 1960. Nature. 2008;455:661–664. doi: 10.1038/nature07390. [DOI] [PMC free article] [PubMed] [Google Scholar]


