Abstract
Human-induced trait change has been documented in freshwater, marine, and terrestrial ecosystems worldwide. These trait changes are driven by phenotypic plasticity and contemporary evolution. While efforts to manage human-induced trait change are beginning to receive some attention, managing its ecological consequences has received virtually none. Recent work suggests that contemporary trait change can have important effects on the dynamics of populations, communities, and ecosystems. Therefore, trait changes caused by human activity may be shaping ecological dynamics on a global scale. We present evidence for important ecological effects associated with human-induced trait change in a variety of study systems. These effects can occur over large spatial scales and impact system-wide processes such as trophic cascades. Importantly, the magnitude of these effects can be on par with those of traditional ecological drivers such as species presence. However, phenotypic change is not always an agent of ecological change; it can also buffer ecosystems against change. Determining the conditions under which phenotypic change may promote vs prevent ecological change should be a top research priority.
Keywords: conservation, contemporary evolution, eco-evolutionary dynamics, evolutionary impact assessment, fisheries-induced evolution, habitat fragmentation, harvest, phenotypic plasticity
Introduction
Human activity is causing rapid changes in the phenotypic traits of wild populations. Such trait changes occur via phenotypic plasticity and contemporary evolution (i.e., evolution occurring over less than a few hundred years). Phenotypic plasticity in response to rapid environmental change has been recognized for some time (Bradshaw 1965; Stearns 1989). While early examples of human-induced evolution provide some textbook examples of microevolution (e.g., industrial melansim in the peppered moth, Kettlewell 1958), the general acceptance that humans are driving contemporary evolution in wild populations is more recent (Palumbi 2001; Stockwell et al. 2003).
Now, there exists an abundance of examples of human-induced trait change in wild populations (reviewed in Bradshaw and Holzapfel 2006; Carroll et al. 2007; Hendry et al. 2008; Allendorf and Hard 2009; Darimont et al. 2009). As a result, human activity can now be considered a global driver of trait change in the wild. Human-induced trait change has been documented worldwide in freshwater, marine, and terrestrial ecosystems on every continent except Antarctica (Fig. 1). Most studies to date have not parsed the relative contributions of plasticity and evolution to human-induced trait change, although the available evidence suggests important contributions from both sources (Hendry et al. 2011). Alongside mounting evidence for human effects on traits, there is increasing evidence that contemporary trait changes can have important impacts on ecological processes. Such effects can occur via phenotypic plasticity and contemporary evolution (Ellner et al. 2011; Yamamichi et al. 2011). As described in detail below, we consider both genetic and plasticity effects to fall under the general purview of eco-evolutionary dynamics, as these effects operate together to shape traits and ecological responses.
Figure 1.
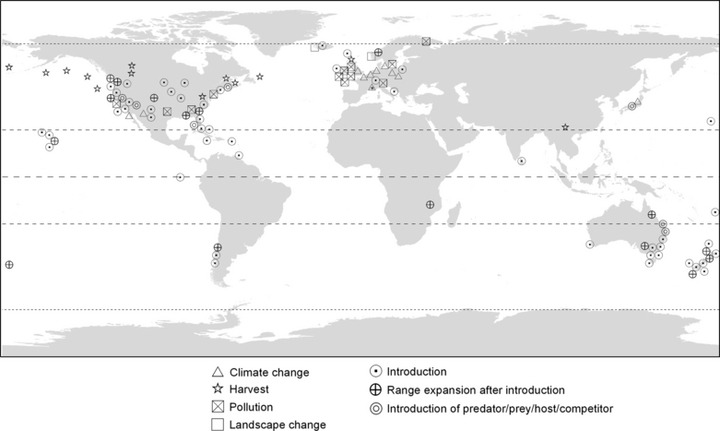
Global distribution of study systems documenting anthropogenic trait change in wild populations, with symbols representing documented drivers of change. The triangle with an asterisk inside (in Europe) represents migration timing for 65 bird species reported in Jenni and Kery (2003).
If contemporary trait change can have important ecological effects, then human-driven trait change may be impacting ecological processes worldwide. But how prevalent are such effects? And should we be concerned outside of a few special cases? To address these questions, we reverse the traditional chain of causality. Instead of considering trait change as the consequence of ecological change, we consider it as the driver. This perspective is reflective of the emerging paradigm that eco-evolutionary dynamics are inherently bidirectional (Pelletier et al. 2009; Post and Palkovacs 2009; Schoener 2011). Therefore, human-induced trait change may be more than just the outcome of human-driven ecological change or the ‘canary in the coal mine,’ indicating that dramatic ecological change is eminent. In some cases, anthropogenic trait change itself may be the cause of ecological change.
Here, we discuss phenotypic plasticity and contemporary evolution as important sources of human-induced trait change. We briefly review the scope of human-induced trait change in wild populations. We do not consider cases from medicine and agriculture, as these contexts have received their own recent detailed attention (reviewed in Nesse and Stearns 2008; Gluckman et al. 2011; Thrall et al. 2011). We then discuss emerging evidence that human-induced trait change may be a key driver of ecological dynamics. We propose that understanding the ecological consequences of human-induced trait change should be an urgent priority requiring the collaboration of eco-evolutionary researchers and natural resource managers.
Human-induced trait change
Ecological processes are influenced by trait change owing to evolution and phenotypic plasticity (Ellner et al. 2011; Yamamichi et al. 2011). In our consideration of the eco-evolutionary consequences of human activity, we include both mechanisms (Table 1). We take this approach for three reasons. Our first two are theoretical. First, it is phenotypes, shaped by the joint effects of genetic change and plasticity, which interact with the biotic and abiotic environment. Therefore, when it comes to predicting ecological outcomes, what likely matters most is the rate and pattern of overall phenotypic change. Most cases of contemporary trait change involve both genetic and plastic components, and isolating one mechanism of trait change and ecological effects does not itself preclude the other (Hendry et al. 2011). Second, plasticity is the product of past evolution and can influence the trajectory of future evolution (Ghalambor et al. 2007). Simply stated, plasticity evolves and can do so in response to human disturbance (Crispo et al. 2010). Such an effect has been shown for Daphnia, where the resurrection of resting eggs has been used to demonstrate the evolution of plasticity in response to fish stocking (Cousyn et al. 2001) and eutrophication (Hairston et al. 2001). Therefore, excluding phenotypic plasticity from eco-evolutionary dynamics excludes an important component of evolution.
Table 1.
Selected case studies of human-induced trait change, mechanisms tested, and demonstrated (D) or hypothetical (H) ecological effects. ‘Phenotypic’ studies employed wild organisms, whereas ‘genetic’ studies utilized common garden experiments or statistical methods to show a heritable basis for trait change. ‘Plastic’ studies subjected wild organisms to differing environmental conditions. The ‘evolution of plasticity’ has been demonstrated using the resurrection of resting eggs
| Case | Traits | Mechanism | Ecological effects | References |
|---|---|---|---|---|
| Habitat fragmentation on birds | Wing shape | Phenotypic | Metapopulation dynamics (H) | Desrochers (2010) |
| Supplemental feeding on European blackcap | Wing shape Beak shape | Phenotypic | Niche diversification (H) | Rolshausen et al. (2009) |
| Migratory direction | Genetic | Reproductive isolation (D) | Berthold et al. (1992) | |
| Dam construction on stream fishes | Body shape | Phenotypic Genetic | Trophic interactions (H) | Haas et al. (2010) Franssen et al. (2011) |
| Dam construction on alewife | Migratory behavior Gape size Gill raker spacing Prey selectivity | Phenotypic | Trophic cascade (D) Zooplankton community (D) Nutrient subsidies (D) | Post et al. (2008), Palkovacs and Post (2009), Walters et al. (2009) |
| Commercial fishing on marine top predators | Fish body size | Phenotypic | Trophic cascade (D) | Shackell et al. (2010) |
| Recreational fishing on largemouth bass | Metabolic rate Growth rate | Genetic | Social behavior (D) Trophic interactions (H) Nutrient excretion (H) | Cooke et al. (2007), Philipp et al. (2009), Redpath et al. (2009, 2010) |
| Trophy hunting on bighorn sheep | Horn size Body size | Genetic | Population growth (H) | Coltman et al. (2003) |
| Urbanization on seed dispersal | Dispersal structure on fruits | Genetic | Metapopulation dynamics (H) | Cheptou et al. (2008) |
| Fish introduction on Daphnia | Predator avoidance behavior | Evolution of plasticity | Trophic cascades (H) | Cousyn et al. (2001) |
| Eutrophication on Daphnia | Resistance to toxic cyanobacteria | Evolution of plasticity | Consumer-resource dynamics (H) | Hairston et al. (2001) |
| Elevated CO2 on plants | Leaf nitrogen composition | Plastic | Herbivore density (D) Herbivore feeding behavior (D) | Stiling et al. (2003) |
| Trout introduction on mayflies | Predator avoidance behavior | Plastic | Trophic cascades (D) | Peckarsky and McIntosh (1998) |
Our third reason for considering both plasticity and contemporary evolution is practical. Most of the studies of contemporary trait change in wild populations have not tested the mechanisms underlying phenotypic change (Hendry et al. 2008), and fewer still have convincingly isolated the genetic component of trait change underlying subsequent ecological effects (see Table 1). Common garden experiments are used to isolate the genetic contribution to overall trait change, but such experiments are difficult to perform in wild populations of wide-ranging, large-bodied species, limiting their utility for many species of interest. Alternate statistical methods have been developed for such circumstances, but some of these methods have come under recent scrutiny (see Hadfield et al. 2010; Kinnison et al. 2011; Uusi-Heikkila et al. 2011), making a strict categorization of studies somewhat subjective in practice.
Hendry et al. (2008) classified cases of contemporary trait change to ascertain the relative effects of humans compared to more natural contexts. That study identified three primary contexts in which humans drive trait changes: in situ anthropogenic disturbance, introduction of populations to new habitats, and introductions of new interacting species (e.g., hosts, pathogens, and predators) within the existing range of a species. The first of these, in situ disturbance, includes a large diversity of subcontexts, including cases of point source pollution, acidification, harvest, and a subset of instances of climate change. The authors labeled cases where populations diverged from one another within an introduced range to be natural, but clearly humans also played a role in the opportunity for evolutionary divergence. Human activity can also have the opposite effect, facilitating introgression (Hendry et al. 2006; Seehausen 2006). When published cases of contemporary trait change are considered in sum, human activity is involved in 162 of the 198 total study systems in which contemporary trait change has been documented in the wild. This tally represents the 61 study systems examined by Hendry et al. (2008) plus 137 study systems added to this database, using the same methodology, since that study was published. Overall, trait change driven by humans appears to happen roughly twice as fast as trait changes associated with nonanthropogenic drivers (Hendry et al. 2008). Harvest is particularly potent. Trait change associated with the harvest of wild populations averages three times faster than nonanthropogenic rates and occurs even faster than most other anthropogenic contexts (Darimont et al. 2009). This result suggests that, if contemporary trait change is generally important for ecological dynamics (Pelletier et al. 2009; Post and Palkovacs 2009; Schoener 2011), then changes linked to human activity, especially harvest, might be even more so.
As mentioned, most studies of contemporary trait change in the wild do not distinguish between heritable trait change and phenotypic plasticity. Anthropogenic contexts that favor changes in selective conditions are also likely to involve changes to environmental conditions that could directly influence the expression of phenotypes through plasticity (Hendry et al. 2011). Indeed, the analysis of Hendry et al. (2008) found that differences between anthropogenic and natural contexts were only statistically apparent when assessing ‘phenotypic’ studies of evolution – i.e., those studies that did not use common garden rearing or statistical approaches to isolate genetic effects. Significant differences were not found for the set of ‘genetic’ studies that did isolate heritable effects. This finding was interpreted as potential evidence that phenotypic plasticity is particularly important to contemporary trait change. This may be true; however, that inference must be tempered with the fact that genetic studies are less common (reduced statistical power) and that phenotypic and genetic studies tend to include different types of organisms and different agents of trait change. This latter limitation is particularly apparent when one considers that studies of trait change owing to harvest represented a large proportion of ‘phenotypic’ studies, but such studies are rare among ‘genetic’ studies. Hence, if harvest really does drive the fastest rates of evolution (Darimont et al. 2009), then phenotypic rate comparisons of natural and anthropogenic contexts would be expected to show greater differences than genetic comparisons simply due to bias in the available data.
Although there is no contesting the value of exploring the relative roles of evolution and plasticity to contemporary trait change, such a distinction can be difficult to achieve in practice and may distract from the broader goal of linking trait change (regardless of the cause) to ecological dynamics. As we have described, human-induced trait change is pervasive and potent. Discerning the genetic or plastic contributions to trait change is undoubtedly important for understanding some important eco-evolutionary dynamics (e.g., feedback effects; Post and Palkovacs 2009). However, many management contexts would benefit most immediately from obtaining a basic understanding of the ecological impacts of overall phenotypic change. As discussed below, such ecological impacts can be substantial.
Ecological consequences
The prevalence and scale of anthropogenic trait change, combined with emerging evidence for ecological effects of trait change, lead to the prediction that anthropogenic trait change may have important ecological consequences. To date, however, such effects have not been widely investigated. Here, we highlight the potential for ecological consequences of anthropogenic trait change. Although such effects are likely across many contexts, we focus on two: habitat fragmentation and the harvesting of wild populations. We emphasize many examples from fishes, as this represents our area of expertise, although effects on other taxa are likely just as important.
Habitat fragmentation can impact traits related to migration, movement, and habitat selection. In North American songbirds, wing shape appears to have evolved over the past 100 years in response to changes in forest cover, with increased fragmentation appearing to select for increased dispersal ability (Desrochers 2010). In the European black cap (Sylvia atricapilla), supplemental feeding has led to a novel migratory pathway, shaped wing and beak morphology, and even led to reproductive isolation (Berthold et al. 1992; Rolshausen et al. 2009). In stream fishes, body shape changes have occurred in response to the construction of dams, which disrupt gene flow and create pond-like habitats (Haas et al. 2010; Franssen 2011). The disruption of hydrologic connectivity can also lead to the evolution on nonmigratory populations of normally migratory fishes (McDowall 1988). Such a transition does not require the complete blockage of a river; hydrological changes that increases the energetic or survival costs of migration relative to the growth and fecundity benefits can select for increased residency (Hendry et al. 2004).
The ecological consequences of changes in movement can be dramatic. In fishes, the transition from an anadromous (sea-going) to freshwater resident life history can be important because many anadromous fishes, most notably Pacific salmon (Oncorhynchus spp.), provide important subsidies of marine-derived nutrients to rivers, lakes, and streams (reviewed in Schindler et al. 2003; Quinn 2005; Janetski et al. 2009). When freshwater resident populations evolve from anadromous ancestors, these nutrient subsidies are severed. The loss of these subsidies can impact important ecological processes such as rates of primary production, decomposition, and energy flow through food webs (Flecker et al. 2010).
In addition to modifying nutrient translocation, phenotypic responses to altered landscape connectivity can have the effect of changing food web interactions by modifying trophic traits and foraging behavior. In the planktivorous alewife (Alosa pseudoharengus), landlocked (freshwater resident) populations have evolved recently from anadromous ancestors, perhaps in response to the construction of dams by European settlers (Palkovacs et al. 2008). Changes in the trophic traits of these recently landlocked populations have caused major changes to the ecology of lake ecosystems, including changes in zooplankton biomass and community structure (Post et al. 2008; Palkovacs and Post 2009). These community-level changes in zooplankton modify the strength of trophic cascades, thereby driving differences in phytoplankton abundance (Post et al. 2008). Importantly, trait divergence between anadromous and landlocked populations can create larger magnitude ecological effects than alewife presence or absence (Fig. 2). Thus, contemporary trait change may be as important for shaping ecological dynamics as are traditional ecological variables such as species presence – a result not addressed by traditional ecological theory but increasingly supported by studies of eco-evolutionary dynamics (Hairston et al. 2005; Ezard et al. 2009; Palkovacs et al. 2009; Bassar et al. 2010; Ellner et al. 2011). The ecological effects of human-driven extinctions have long received attention from ecologists (reviewed in Hooper et al. 2005). The above result suggests that human-driven trait changes can have equally large effects on ecological dynamics.
Figure 2.
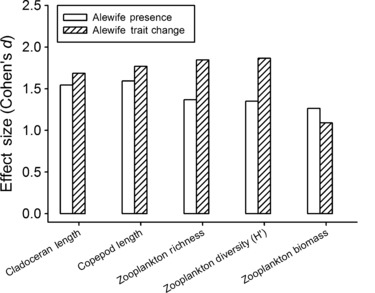
Effect sizes for alewife presence and trait change (divergence between anadromous and landlocked forms) from the experiment reported in Palkovacs and Post (2009). Alewife presence had larger effects on zooplankton biomass, whereas trait change had larger effects on mean zooplankton length for cladocerans and copepods, zooplankton species richness, and zooplankton diversity. These results suggest that anthropogenic trait change can have ecological effects on par with those of traditional ecological drivers, such as species presence and diversity.
In addition to direct effects on ecological processes, human-induced trait change can have cascading evolutionary consequences for other species, which can themselves translate into important ecological effects. The evolution of the aforementioned alewife populations has shaped the evolution one of its prey species, Daphnia ambigua (Walsh and Post 2011). Because Daphnia is a dominant zooplankton grazer, its evolution can have even further ecological effects, especially on trophic cascades (M. R. Walsh and D. M. Post, unpublished data). Thus, anthropogenic disturbances can set off cascades of eco-evolutionary effects that can ripple through ecosystems.
We now turn to harvest, one of the most potent agents of anthropogenic trait change (Allendorf and Hard 2009; Darimont et al. 2009; Stenseth and Dunlop 2009), which has potentially far-reaching ecological consequences. In a classic study, Coltman et al. (2003) showed that trophy hunting in a population of bighorn sheep (Ovis canadensis) drove reductions in horn and body size and removed high breeding value males from the population, thereby potentially influencing population growth. These changes were reported by Coltman et al. (2003) to be genetic, but the methodology used to derive this conclusion has been recently questioned (Hadfield et al. 2010). Fisheries have repeatedly been shown to drive earlier age and smaller size at maturation, and probabilistic maturation reaction norms have frequently been used as a statistical approach to infer the genetic basis of such changes (reviewed in Jørgensen et al. 2007; Kuparinen and Merila 2007; Hard et al. 2008; Hutchings and Fraser 2008; Dunlop et al. 2009; Sharpe and Hendry 2009). While it is almost certain that a large degree of this trait change does indeed have a genetic basis, probabilistic maturation reaction norms have also been found to have limitations with respect to isolating the various genetic contributions to overall trait change (Kinnison et al. 2011; Uusi-Heikkila et al. 2011).
Fisheries-induced trait changes are predicted have dramatic effects on trophic interactions. The food webs of freshwater and marine ecosystems are highly size-structured, and fishes often occupy the upper and middle trophic levels in such ecosystems. Therefore, changes in fish body size, especially at the top trophic levels, can have major impacts on food web interactions and trophic cascades (Fig. 3). Indeed, there is strong evidence that declines in body size, but not total biomass, of heavily harvested top fish predators on the Western Scotian Shelf underlie observed increases in biomass at lower trophic levels (Shackell et al. 2010). Thus, anthropogenic trait change may have important impacts on trophic cascades that act independently (or in concert with) of changes in top predator density and biomass.
Figure 3.
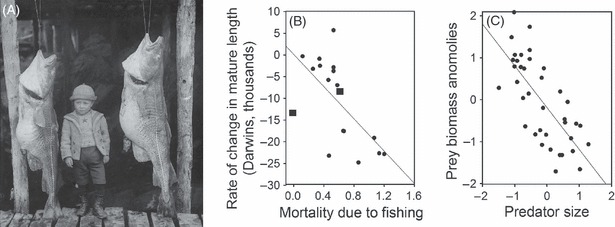
Effects of fisheries-induced trait change on the Western North Atlantic ecosystem. (A) The photograph Big cod fish from the trap, Battle Harbour, Labrador/Robert Edwards Holloway [1901] shows the size of Atlantic cod (Gadus morhua) harvested off Newfoundland and Labrador, Canada, at the turn of the last century. The plate noted: ‘The larger fish measured 5 ft. 5 in., and weighed 60 lbs’. (B) Intense mortality owing to fishing has driven rapid declines in mature fish length for 18 commercially exploited fish stocks (panel modified from Sharpe and Hendry 2009). Cod stocks in the Western North Atlantic are highlighted as squares in this panel. Note that the rate of decrease in size and the intensity of fishing pressure for cod are average for the stocks included in this study, suggesting that the dramatic and well-publicized declines seen in cod are typical (not exceptional) for intensively harvested fish stocks. (C) Decreases in the body size of cod and other top predators on the Western Scotian Shelf have resulted in a 300% increase in the biomass of prey species (zooplankton, small planktivorous, and detritivorous fishes), despite no change in total predator biomass (panel modified from Shackell et al. 2010). The analysis of Shackell et al. (2010) found anomalies from the mean prey biomass over 38 years of surveys were strongly and negatively related to a standardized index of top predator size. Taken together, these data provide evidence that harvest-induced changes in predator body size can cause trophic cascades that can impact the functioning of entire marine ecosystems.
Perhaps even less appreciated than the food web effects of fisheries-induced trait change are its potential consequences for nutrient cycling in aquatic ecosystems. Fish excretion is an important source of nutrients in aquatic ecosystems (Vanni 2002). Small fish excrete nutrients at higher rates and at lower N/P than large fish; therefore, harvest-induced trait changes may have important impacts on nutrient availability (Hall et al. 2007). Assuming a compensatory increase in the biomass of small individuals (as shown by Shackell et al. 2010), harvested populations dominated by small, early maturing individuals are predicted to recycle nutrients at higher rates and at lower N/P compared to unharvested populations (Hall et al. 2007).
The effects of excretion changes have not been directly examined in any harvested ecosystems, but relevant studies in Trinidadian guppies (Poecilia reticulata) suggest that such effects could be substantial. Trinidadian guppies are a model system for the study of fisheries-induced evolution because life history traits are known to evolve in response to natural predators in a manner consistent with the evolutionary effects of human harvest (Reznick and Ghalambor 2005). Palkovacs et al. (2009) found that guppy populations from streams with predators (high mortality) excreted nutrients at higher rates compared to populations from streams lacking top predators (low mortality). This finding is consistent with the expectation that, at an equal biomass, exploited populations that have evolved smaller body size should excrete nutrients at higher rates than unexploited populations. In mesocosms, heightened excretion increased nutrient availability and was associated with greater algal production (Palkovacs et al. 2009). Bassar et al. (2010) confirmed excretion differences at low guppy density but found no differences in population-level excretion rates at high guppy density. Both studies suggested that differences in guppy consumption play an important role in causing differences in algal production, an effect that could be driven by the evolution of heightened consumption rates in low-mortality, high-competition environments (Palkovacs et al. 2011).
These experiments in a model system suggest that harvest-induced trait change may have important effects on nutrient dynamics and ecosystem processes. But such effects have not been tested in a wild, harvested ecosystem. We predict that such effects may be most important in ecosystems where a single harvested species serves as the dominant control for nutrient dynamics and ecosystem processes. In South American rivers, the flannelmouth characin (Prochilodus mariae) may be just such a species (Flecker 1996; Taylor et al. 2006). Declines in body size for this species are being driven by size-selective harvest (Taylor et al. 2006) and may have significant consequences for the ecosystem processes of harvested rivers (Taylor et al. 2006; McIntyre et al. 2007). This and similar systems, including Pacific salmon and Atlantic cod (Gadus morhua), may be excellent candidates for testing the effects of fisheries-induced trait change on freshwater and marine ecosystem dynamics.
In addition to causing changes in life history traits, some fishing methods, such as hook and line angling, may have the added effect of selectively removing the most active individuals, with the highest basal metabolic rates. In largemouth bass (Micropterus salmoides), the removal of these individuals selects for slowed growth, lowered aggression and territoriality, reduced parental care, and less active foraging behavior (Cooke et al. 2007; Philipp et al. 2009; Redpath et al. 2009, 2010). Decreased parental care and territorial behavior may, in theory, lead to changes in population dynamics. Reduced foraging activity and lowered consumption rates may have important effects on prey communities and trophic interactions. Lowered metabolic rates may impact excretion rates, with important effects on nutrient availability and primary production. In recreational fisheries, such effects may occur alongside life history changes (Matsumura et al. 2011) to impact the ecological dynamics of harvested ecosystems.
We have described cases where human-induced trait change causes, or is likely to cause, major changes to ecological processes. But what about cases where trait change buffers populations, communities, and ecosystems against change? Several studies have shown that such effects can increase the stability and resilience of ecosystems. Reusch et al. (2005) showed that higher levels of genetic diversity in seagrass (Zostera marina) can help maintain ecosystem function in the face of extreme climate events. Lennon and Martiny (2008), working in microbial chemostats, showed that the evolution of resistance to a virus buffered ecosystems against changes in nutrient cycling. More recently, Ellner et al. (2011) applied a method for parsing the nonheritable, genetic, and environmental drivers of ecological change to a diverse set of study systems. Their analysis showed that contemporary evolution may be most important when it acts to oppose environmental change on traits – what they describe as a temporal analogue to countergradient selection. They conclude that contemporary evolution may be key to the persistence of species and communities (see also Kinnison and Hairston 2007; Bell and Gonzalez 2011) and critical for maintaining ecosystem function in the face of environmental change. But more work is needed to determine the general conditions under which trait change promotes vs prevents ecological change. We consider this to be one of the greatest challenges facing the nascent study of eco-evolutionary dynamics, and a critical question to answer in the face of a rapidly changing, and increasingly human dominated world. We encourage researchers and resource managers to work together to apply emerging eco-evolutionary principles to real-world management scenarios, for both the benefit of management and the knowledge to be gained in the process.
Acknowledgments
We thank Diana Sharpe for providing data on exploited fish stocks. Joachim Mergeay and two anonymous reviewers provided comments that helped improve the paper. The Rooms Provincial Archives Division of Newfoundland and Labrador kindly provided a digital copy and permission to publish the photograph Big cod fish from the trap, Battle Harbour, Labrador/Robert Edwards Holloway [1901].
Literature cited
- Allendorf FW, Hard JJ. Human-induced evolution caused by unnatural selection through harvest of wild animals. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9987–9994. doi: 10.1073/pnas.0901069106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassar RD, Marshall MC, López-Sepulcre A, Zandonà E, Auer S, Travis J, Pringle CM, et al. Local adaptation in Trinidadian guppies alters ecosystem processes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3616–3621. doi: 10.1073/pnas.0908023107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G, Gonzalez A. Adaptation and evolutionary rescue in metapopulations experiencing environmental deterioration. Science. 2011;332:1327–1330. doi: 10.1126/science.1203105. [DOI] [PubMed] [Google Scholar]
- Berthold P, Helbig AJ, Mohr G, Querner U. Rapid microevolution of migratory behavior in a wild bird species. Nature. 1992;360:668–670. [Google Scholar]
- Bradshaw AD. Evolutionary significance of phenotypic plasticity in plants. Advances in Genetics. 1965;13:115–155. [Google Scholar]
- Bradshaw WE, Holzapfel CM. Evolutionary response to rapid climate change. Science. 2006;312:1477–1478. doi: 10.1126/science.1127000. [DOI] [PubMed] [Google Scholar]
- Carroll SP, Hendry AP, Reznick DN, Fox CW. Evolution on ecological time-scales. Functional Ecology. 2007;21:387–393. [Google Scholar]
- Cheptou PO, Carrue O, Rouifed S, Cantarel A. Rapid evolution of seed dispersal in an urban environment in the weed Crepis sancta. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3796–3799. doi: 10.1073/pnas.0708446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltman DW, O'Donoghue P, Jorgenson JT, Hogg JT, Strobeck C, Festa-Bianchet M. Undesirable evolutionary consequences of trophy hunting. Nature. 2003;426:655–658. doi: 10.1038/nature02177. [DOI] [PubMed] [Google Scholar]
- Cooke SJ, Suski CD, Ostrand KG, Wahl DH, Philipp DP. Physiological and behavioral consequences of long-term artificial selection for vulnerability to recreational angling in a teleost fish. Physiological and Biochemical Zoology. 2007;80:480–490. doi: 10.1086/520618. [DOI] [PubMed] [Google Scholar]
- Cousyn C, De Meester L, Colbourne JK, Brendonck L, Verschuren D, Volckaert F. Rapid, local adaptation of zooplankton behavior to changes in predation pressure in the absence of neutral genetic changes. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6256–6260. doi: 10.1073/pnas.111606798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispo E, DiBattista JD, Correa C, Thibert-Plante X, McKellar AE, Schwartz AK, Berner D, et al. The evolution of phenotypic plasticity in response to anthropogenic disturbance. Evolutionary Ecology Research. 2010;12:47–66. [Google Scholar]
- Darimont CT, Carlson SM, Kinnison MT, Paquet PC, Reimchen TE, Wilmers CC. Human predators outpace other agents of trait change in the wild. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:952–954. doi: 10.1073/pnas.0809235106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrochers A. Morphological response of songbirds to 100 years of landscape change in North America. Ecology. 2010;91:1577–1582. doi: 10.1890/09-2202.1. [DOI] [PubMed] [Google Scholar]
- Dunlop ES, Enberg K, Jørgensen C, Heino M. Toward Darwinian fisheries management. Evolutionary Applications. 2009;2:246–259. doi: 10.1111/j.1752-4571.2009.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellner SP, Geber MA, Hairston NG. Does rapid evolution matter? Measuring the rate of contemporary evolution and its impacts on ecological dynamics. Ecology Letters. 2011;14:603–614. doi: 10.1111/j.1461-0248.2011.01616.x. [DOI] [PubMed] [Google Scholar]
- Ezard THG, Cote SD, Pelletier F. Eco-evolutionary dynamics: disentangling phenotypic, environmental and population fluctuations. Philosophical Transactions of the Royal Society B-Biological Sciences. 2009;364:1491–1498. doi: 10.1098/rstb.2009.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flecker AS. Ecosystem engineering by a dominant detritivore in a diverse tropical stream. Ecology. 1996;77:1845–1854. [Google Scholar]
- Flecker AS, McIntyre PB, Moore JW, Anderson JT, Taylor BW, Hall RO. Migratory fishes as material and process subsidies in riverine ecosystems. In: Gido KB, Jackson D, editors. Community Ecology of Stream Fishes: Concepts, Approaches, and Techniques. Bethesda, Maryland: American Fisheries Society Symposium; 2010. pp. 559–592. [Google Scholar]
- Franssen NR. Anthropogenic habitat alteration induces rapid morphological divergence in a native stream fish. Evolutionary Applications. 2011 doi: 10.1111/j.1752-4571.2011.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghalambor CK, McKay JK, Carroll SP, Reznick DN. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Functional Ecology. 2007;21:394–407. [Google Scholar]
- Gluckman PD, Low FM, Buklijas T, Hanson MA, Beedle AS. How evolutionary principles improve the understanding of human health and disease. Evolutionary Applications. 2011;4:249–263. doi: 10.1111/j.1752-4571.2010.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas TC, Blum MJ, Heins DC. Morphological responses of a stream fish to water impoundment. Biology Letters. 2010;6:803–806. doi: 10.1098/rsbl.2010.0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfield JD, Wilson AJ, Garant D, Sheldon BC, Kruuk LEB. The misuse of BLUP in ecology and evolution. American Naturalist. 2010;175:116–125. doi: 10.1086/648604. [DOI] [PubMed] [Google Scholar]
- Hairston NG, Jr, Holtmeier CL, Lampert W, Weider LJ, Post DM, Fischer JM, Caceres CE, et al. Natural selection for grazer resistance to toxic cyanobacteria: evolution of phenotypic plasticity? Evolution. 2001;55:2203–2214. doi: 10.1111/j.0014-3820.2001.tb00736.x. [DOI] [PubMed] [Google Scholar]
- Hairston NG, Ellner SP, Geber MA, Yoshida T, Fox JA. Rapid evolution and the convergence of ecological and evolutionary time. Ecology Letters. 2005;8:1114–1127. [Google Scholar]
- Hall RO, Koch BJ, Marshall MC, Taylor BW, Tronstad LM. How body size mediates the role of animals in nutrient cycling in aquatic ecosystems. In: Hildrew A, Raffaelli D, Edmonds-Brown R, editors. Body Size: The Structure and Function of Aquatic Ecosystems. Cambridge: Cambridge University Press; 2007. pp. 286–305. [Google Scholar]
- Hard JJ, Gross MR, Heino M, Hilborn R, Kope RG, Law R, Reynolds JD. Evolutionary consequences of fishing and their implications for salmon. Evolutionary Applications. 2008;1:388–408. doi: 10.1111/j.1752-4571.2008.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry AP, Bohlin T, Jonsson B, Berg OK. To sea or not to sea? Anadromy versus non-anadromy in Salmonids. In: Hendry AP, Stearns SC, editors. Evolution Illuminated Salmon and their Relatives. Oxford: Oxford University Press; 2004. pp. 92–125. [Google Scholar]
- Hendry AP, Grant PR, Grant BR, Ford HA, Brewer MJ, Podos J. Possible human impacts on adaptive radiation: beak size bimodality in Darwin's finches. Proceedings of the Royal Society B-Biological Sciences. 2006;273:1887–1894. doi: 10.1098/rspb.2006.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry AP, Farrugia TJ, Kinnison MT. Human influences on rates of phenotypic change in wild animal populations. Molecular Ecology. 2008;17:20–29. doi: 10.1111/j.1365-294X.2007.03428.x. [DOI] [PubMed] [Google Scholar]
- Hendry AP, Kinnison MT, Heino M, Day T, Smith TB, Fitt G, Bergstrom CT, et al. Evolutionary principles and their practical application. Evolutionary Applications. 2011;4:159–183. doi: 10.1111/j.1752-4571.2010.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper DU, Chapin FS, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, et al. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecological Monographs. 2005;75:3–35. [Google Scholar]
- Hutchings JA, Fraser DJ. The nature of fisheries- and farming-induced evolution. Molecular Ecology. 2008;17:294–313. doi: 10.1111/j.1365-294X.2007.03485.x. [DOI] [PubMed] [Google Scholar]
- Janetski DJ, Chaloner DT, Tiegs SD, Lamberti GA. Pacific salmon effects on stream ecosystems: a quantitative synthesis. Oecologia. 2009;159:583–595. doi: 10.1007/s00442-008-1249-x. [DOI] [PubMed] [Google Scholar]
- Jenni L, Kery M. Timing of autumn bird migration under climate change: advances in long-distance migrants, delays in short-distance migrants. Proceedings of the Royal Society B-Biological Sciences. 2003;270:1467–1471. doi: 10.1098/rspb.2003.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen C, Enberg K, Dunlop ES, Arlinghaus R, Boukal DS, Brander K, Ernande B, et al. Managing evolving fish stocks. Science. 2007;318:1247–1248. doi: 10.1126/science.1148089. [DOI] [PubMed] [Google Scholar]
- Kettlewell HBN. A survey of the frequencies of Biston betularia (L) (Lep.) and its melanic forms in Great Britain. Heredity. 1958;12:51–72. [Google Scholar]
- Kinnison MT, Hairston NG. Eco-evolutionary conservation biology: contemporary evolution and the dynamics of persistence. Functional Ecology. 2007;21:444–454. [Google Scholar]
- Kinnison MT, Quinn TP, Unwin MJ. Correlated contemporary evolution of life history traits in New Zealand Chinook salmon, Oncorhynchus tshawytscha. Heredity. 2011;106:448–459. doi: 10.1038/hdy.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuparinen A, Merila J. Detecting and managing fisheries-induced evolution. Trends in Ecology & Evolution. 2007;22:652–659. doi: 10.1016/j.tree.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Lennon JT, Martiny JBH. Rapid evolution buffers ecosystem impacts of viruses in a microbial food web. Ecology Letters. 2008;11:1178–1188. doi: 10.1111/j.1461-0248.2008.01225.x. [DOI] [PubMed] [Google Scholar]
- Matsumura S, Arlinghaus R, Dieckmann U. Assessing evolutionary consequences of size-selective recreational fishing on multiple life-history traits, with an application to northern pike (Esox lucius. Evolutionary Ecology. 2011;25:711–735. [Google Scholar]
- McDowall RM. Diadromy in Fishes Migrations between Freshwater and Marine Environments. London: Croom Helm; 1988. [Google Scholar]
- McIntyre PB, Jones LE, Flecker AS, Vanni MJ. Fish extinctions alter nutrient recycling in tropical freshwaters. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4461–4466. doi: 10.1073/pnas.0608148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesse RM, Stearns SC. The great opportunity: evolutionary applications to medicine and public health. Evolutionary Applications. 2008;1:28–48. doi: 10.1111/j.1752-4571.2007.00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palkovacs EP, Post DM. Experimental evidence that phenotypic divergence in predators drives community divergence in prey. Ecology. 2009;90:300–305. doi: 10.1890/08-1673.1. [DOI] [PubMed] [Google Scholar]
- Palkovacs EP, Dion KB, Post DM, Caccone A. Independent evolutionary origins of landlocked alewife populations and rapid parallel evolution of phenotypic traits. Molecular Ecology. 2008;17:582–597. doi: 10.1111/j.1365-294X.2007.03593.x. [DOI] [PubMed] [Google Scholar]
- Palkovacs EP, Marshall MC, Lamphere BA, Lynch BR, Weese DJ, Fraser DF, Reznick DN, et al. Experimental evaluation of evolution and coevolution as agents of ecosystem change in Trinidadian streams. Philosophical Transactions of the Royal Society B-Biological Sciences. 2009;364:1617–1628. doi: 10.1098/rstb.2009.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palkovacs EP, Wasserman BA, Kinnison MT. Eco-evolutionary trophic dynamics: loss of top predators drives trophic evolution and ecology of prey. PLoS ONE. 2011;6:e18879. doi: 10.1371/journal.pone.0018879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbi SR. Humans as the world's greatest evolutionary force. Science. 2001;293:1786–1790. doi: 10.1126/science.293.5536.1786. [DOI] [PubMed] [Google Scholar]
- Peckarsky BL, McIntosh AR. Fitness and community consequences of avoiding multiple predators. Oecologia. 1998;113:565–576. doi: 10.1007/s004420050410. [DOI] [PubMed] [Google Scholar]
- Pelletier F, Garant D, Hendry AP. Eco-evolutionary dynamics. Philosophical Transactions of the Royal Society B-Biological Sciences. 2009;364:1483–1489. doi: 10.1098/rstb.2009.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp DP, Cooke SJ, Claussen JE, Koppelman JB, Suski CD, Burkett DP. Selection for vulnerability to angling in largemouth bass. Transactions of the American Fisheries Society. 2009;138:189–199. [Google Scholar]
- Post DM, Palkovacs EP. Eco-evolutionary feedbacks in community and ecosystem ecology: interactions between the ecological theatre and the evolutionary play. Philosophical Transactions of the Royal Society B-Biological Sciences. 2009;364:1629–1640. doi: 10.1098/rstb.2009.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post DM, Palkovacs EP, Schielke EG, Dodson SI. Intraspecific variation in a predator affects community structure and cascading trophic interactions. Ecology. 2008;89:2019–2032. doi: 10.1890/07-1216.1. [DOI] [PubMed] [Google Scholar]
- Quinn TP. The Behavior and Ecology of Pacific Salmon and Trout. Seattle: University of Washington Press; 2005. [Google Scholar]
- Redpath TD, Cooke SJ, Arlinghaus R, Wahl DH, Philipp DP. Life-history traits and energetic status in relation to vulnerability to angling in an experimentally selected teleost fish. Evolutionary Applications. 2009;2:312–323. doi: 10.1111/j.1752-4571.2009.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redpath TD, Cooke SJ, Suski CD, Arlinghaus R, Couture P, Wahl DH, Philipp DP. The metabolic and biochemical basis of vulnerability to recreational angling after three generations of angling-induced selection in a teleost fish. Canadian Journal of Fisheries and Aquatic Sciences. 2010;67:1983–1992. [Google Scholar]
- Reusch TBH, Ehlers A, Hammerli A, Worm B. Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2826–2831. doi: 10.1073/pnas.0500008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick DN, Ghalambor CK. Can commercial fishing cause evolution? Answers from guppies (Poecilia reticulata. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:791–801. [Google Scholar]
- Rolshausen G, Segelbacher G, Hobson KA, Schaefer HM. Contemporary evolution of reproductive isolation and phenotypic divergence in sympatry along a migratory divide. Current Biology. 2009;19:2097–2101. doi: 10.1016/j.cub.2009.10.061. [DOI] [PubMed] [Google Scholar]
- Schindler DE, Scheuerell MD, Moore JW, Gende SM, Francis TB, Palen WJ. Pacific salmon and the ecology of coastal ecosystems. Frontiers in Ecology and the Environment. 2003;1:31–37. [Google Scholar]
- Schoener TW. The newest synthesis: understanding the interplay of evolutionary and ecological dynamics. Science. 2011;331:426–429. doi: 10.1126/science.1193954. [DOI] [PubMed] [Google Scholar]
- Seehausen O. Conservation: losing biodiversity by reverse speciation. Current Biology. 2006;16:R334–R337. doi: 10.1016/j.cub.2006.03.080. [DOI] [PubMed] [Google Scholar]
- Shackell NL, Frank KT, Fisher JAD, Petrie B, Leggett WC. Decline in top predator body size and changing climate alter trophic structure in an oceanic ecosystem. Proceedings of the Royal Society B-Biological Sciences. 2010;277:1353–1360. doi: 10.1098/rspb.2009.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe DMT, Hendry AP. Life history change in commercially exploited fish stocks: an analysis of trends across studies. Evolutionary Applications. 2009;2:260–275. doi: 10.1111/j.1752-4571.2009.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns SC. The evolutionary significance of phenotypic plasticity. BioScience. 1989;39:436–445. [Google Scholar]
- Stenseth NC, Dunlop ES. Evolution: unnatural selection. Nature. 2009;457:803–804. doi: 10.1038/457803a. [DOI] [PubMed] [Google Scholar]
- Stiling P, Moon DC, Hunter MD, Colson J, Rossi AM, Hymus GJ, Drake BG. Elevated CO2 lowers relative and absolute herbivore density across all species of a scrub-oak forest. Oecologia. 2003;134:82–87. doi: 10.1007/s00442-002-1075-5. [DOI] [PubMed] [Google Scholar]
- Stockwell CA, Hendry AP, Kinnison MT. Contemporary evolution meets conservation biology. Trends in Ecology & Evolution. 2003;18:94–101. [Google Scholar]
- Taylor BW, Flecker AS, Hall RO. Loss of a harvested fish species disrupts carbon flow in a diverse tropical river. Science. 2006;313:833–836. doi: 10.1126/science.1128223. [DOI] [PubMed] [Google Scholar]
- Thrall PH, Oakeshott JG, Fitt G, Southerton S, Burdon JJ, Sheppard A, Russell RJ, et al. Evolution in agriculture: the application of evolutionary approaches to the management of biotic interactions in agro-ecosystems. Evolutionary Applications. 2011;4:200–215. doi: 10.1111/j.1752-4571.2010.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusi-Heikkila S, Kuparinen A, Wolter C, Meinelt T, O'Toole AC, Arlinghaus R. Experimental assessment of the probabilistic maturation reaction norm: condition matters. Proceedings of the Royal Society B-Biological Sciences. 2011;278:709–717. doi: 10.1098/rspb.2010.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanni MJ. Nutrient cycling by animals in freshwater ecosystems. Annual Review of Ecology and Systematics. 2002;33:341–370. [Google Scholar]
- Walsh MR, Post DM. Interpopulation variation in a fish predator drives evolutionary divergence in prey in lakes. Proceedings of the Royal Society B-Biological Sciences. 2011;278:2628–2637. doi: 10.1098/rspb.2010.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters AW, Barnes RT, Post DM. Anadromous alewives (Alosa pseudoharengus) contribute marine-derived nutrients to coastal stream food webs. Canadian Journal of Fisheries and Aquatic Sciences. 2009;66:439–448. [Google Scholar]
- Yamamichi M, Yoshida T, Sasaki A. Comparing the effects of rapid evolution and phenotypic plasticity on predator-prey dynamics. American Naturalist. 2011;178:287–304. doi: 10.1086/661241. [DOI] [PubMed] [Google Scholar]


