Abstract
Toxic algal blooms are an important problem worldwide. The literature on toxic cyanobacteria blooms in inland waters reports widely divergent results on whether zooplankton can control cyanobacteria blooms or cyanobacteria suppress zooplankton by their toxins. Here we test whether this may be due to genotype × genotype interactions, in which interactions between the large-bodied and efficient grazer Daphnia and the widespread cyanobacterium Microcystis are not only dependent on Microcystis strain or Daphnia genotype but are specific to genotype × genotype combinations. We show that genotype × genotype interactions are important in explaining mortality in short-time exposures of Daphnia to Microcystis. These genotype × genotype interactions may result in local coadaptation and a geographic mosaic of coevolution. Genotype × genotype interactions can explain why the literature on zooplankton–cyanobacteria interactions is seemingly inconsistent, and provide hope that zooplankton can contribute to the suppression of cyanobacteria blooms in restoration projects.
Keywords: cyanobacteria toxins, eco-evolutionary dynamics, genotype × genotype interactions, geographic mosaic of coevolution, harmful algal blooms, top–down control
Introduction
Cyanobacteria are an ancient group of autotrophic bacteria that are found in both freshwater and marine environments and are an important component of the primary producers (Huisman et al. 2005). Cyanobacteria dominate at high nutrient concentrations and high temperatures, and in inland standing waters throughout the world, there is an increasing incidence of dense cyanobacteria blooms fuelled by eutrophication and climate change (Kardinaal and Visser 2005; Zurawell et al. 2005; Jöhnk et al. 2008; Paerl and Huisman 2008; Kosten et al. 2011). Many cyanobacteria species produce a diverse range of toxic metabolites and bioactive compounds such as hepato-, neuro-, cyto- and endotoxins (Sivonen and Jones 1999; Codd et al. 2005) that are hazardous to both human and livestock health (Kuiper-Goodman et al. 1999; Codd et al. 2005). Cyanobacteria blooms can cause major problems both in terms of ecological structure and functioning of aquatic systems (Ghadouani et al. 2003; Dao et al. 2010) as well as public health, livestock health and recreation (Bell and Codd 1994; Jochimsen et al. 1998; Kuiper-Goodman et al. 1999; Ouellette and Wilhelm 2003; Zimba et al. 2006; Stewart et al. 2008; Martínez Hernandez et al. 2009). Much effort is therefore invested in preventing or controlling cyanobacteria blooms (Chorus and Mur 1999; Codd 2000; Paerl et al. 2001). The most effective management is to avoid cyanobacteria blooms by reducing nutrient loads and restoring water quality (Chorus and Mur 1999; Paerl et al. 2001; Anderson et al. 2002). Hence, most applications with respect to the control of nuisance cyanobacteria blooms take a bottom-up approach. They often involve profound interference with the physical or chemical structure of water bodies, such as artificial mixing or flushing (e.g. Huisman et al. 2004, 2005; Maier et al. 2004) or precipitation and fixation of phosphorus in the sediments with La- or Al-rich clays (Douglas et al. 1999; Robb et al. 2003; van Oosterhout and Lürling 2011). A reduction of nutrient loads is, however, sometimes hard to achieve, especially when sources of nutrient input are diffuse or when nutrient enrichment is partly because of atmospheric deposition. And even when successful, most of these approaches are expensive and work only in relatively small water bodies and in systems for which a heavy investment is counterbalanced by strong added value, such as public swimming waters. Another much advocated strategy to improve the ecological quality of nutrient-enriched water bodies and prevent the occurrence of cyanobacterial blooms is to combine control of external nutrient inputs with food-web manipulation (Moss et al. 1996; Madgwick 1999; Jeppesen et al. 2007; Kasprzak et al. 2007). Several recent studies have focused on potential agents of biological control for the prevention of cyanobacterial blooms, using bacteria, viruses and unicellular grazers (e.g. Sigee et al. 1999; Nishibe et al. 2004; Choi et al. 2005; Tucker and Pollard 2005; Honjo et al. 2006; Zhang et al. 2008; Van Wichelen et al. 2010), exploiting allelopathic interactions (Wu et al. 2011) or manipulating fish stocks (Madgwick 1999; Jeppesen et al. 2007; Kasprzak et al. 2007). An important asset of biological control of cyanobacteria blooms resides in the fact that the controlling agent through its population growth may exert its impact throughout larger water bodies and for longer periods of time. Top–down control may also be more powerful in shallow water bodies where internal eutrophication through resuspension of sediments reduces the strength of bottom-up control (Moss 2010). There are many known cases of successful biomanipulation, including a few cases in which bloom-forming cyanobacteria were kept under control by zooplankton grazing (Peretyatko et al. 2010).
Zooplankton–cyanobacteria interactions have been discussed extensively over the years, yet the literature yields a highly inconsistent picture (see also Zurawell et al. 2005). Several studies indicate that Daphnia may control the development of Microcystis blooms depending on initial conditions and history (e.g. Christoffersen et al. 1993; Matveev et al. 1994; Sarnelle 2007; Dejenie et al. 2009; Peretyatko et al. 2010). Other studies, however, reported that toxic Microcystis could not be controlled by zooplankton grazing, as Microcystis suppressed Daphnia population growth and resulted in a decrease in zooplankton biomass and a shift in zooplankton community structure towards smaller species and individuals (e.g. Ghadouani et al. 2003). In line with these observations, there are several cases where biomanipulation failed when cyanobacteria were present (Gliwicz 1990; Gulati et al. 2008). Large-bodied zooplankton species are claimed to be particularly vulnerable as they can ingest the cyanobacteria and thus get intoxicated (Gliwicz and Siedlar 1980). Microcystis, a commonly occurring cyanobacterium genus, can suppress zooplankton in several ways. First, through the formation of colonies, they may reduce ingestion and interfere with filtering activity in Daphnia (Lampert 1981, 1982). Secondly, cyanobacteria tend to be poor food. They feature low levels of highly unsaturated fatty acids and low sterol contents (Brett and Müller-Navarra 1997; von Elert et al. 2003), and their membrane and mucilage layers are not readily digestible (Kurmayer and Jüttner 1999), which renders them nutritionally unfavourable for zooplankton compared with, for example, green algae. Thirdly, Microcystis strains produce a wide range of secondary metabolites. Examples of cyanotoxins that are deleterious to Daphnia are microcystins (Chen et al. 2005), protease inhibitors (Schwarzenberger et al. 2010), microviridin peptides (Kaebernick et al. 2001), and the polyunsaturated fatty acid gamma-linolenic acid (Reinikainen et al. 2001), among others (Nizan et al. 1986; Jungmann and Benndorf 1994). Other studies, however, did not find any deleterious effect of cyanobacteria on Daphnia (De Bernardi et al. 1981; Matveev et al. 1994).
An important finding in the debate on Microcystis–zooplankton interactions is the observation that there are genetic differences both in the grazer and the prey in their mutual responses (Kurmayer et al. 2001; Wilson et al. 2005). For example, the ability to form colonies in the presence of grazers (e.g. van Gremberghe et al. 2009a), the fatty acid composition (e.g. Martin-Creuzburg et al. 2008), and secondary metabolites differs among Microcystis strains, thus potentially inducing a very diverse response in zooplankton (Jungmann 1992; Czarnecki et al. 2006). Likewise, differences in responses of Daphnia when exposed to cyanobacteria have been reported (Hietala et al. 1995; Hairston et al. 2001; Schwarzenberger et al. 2010). In recent years, evidence has accumulated that Daphnia may develop tolerance against toxic cyanobacteria (Gustafsson and Hansson 2004; Sarnelle and Wilson 2005; Blom et al. 2006; Wilson et al. 2006; Sarnelle et al. 2010) and may genetically adapt to better cope with cyanotoxins (Hairston et al. 1999, 2001; Gustafsson et al. 2005). Sarnelle and Wilson (2005) and Blom et al. (2006) compared Daphnia clones isolated from lakes with low and high prevalence of bloom-forming cyanobacteria and concluded that populations exposed to high cyanobacterial levels over long periods of time can genetically adapt to being more tolerant to toxic cyanobacteria in the diet. Hairston et al. (1999, 2001) similarly showed genetic adaptation of Daphnia in Lake Constance to increased abundances of cyanobacteria over time using a resurrection ecology approach, hatching Daphnia clones from different time periods corresponding to different eutrophication periods of the dormant egg bank. Gustafsson and Hansson (2004) and Gustafsson et al. (2005) demonstrated induced and maternally transferred tolerance in Daphnia when pre-exposed to Microcystis. They observed a higher survival probability, accelerated maturation and early first reproduction as well as a higher number of offspring when comparing animals born from Microcystis-exposed mothers compared to naive Daphnia.Sarnelle et al. (2010) observed that Daphnia populations with prior experience with toxic cyanobacteria may show positive population growth even at high concentrations of cyanobacterial toxins. Acclimation and genetic adaptation likely play a significant role in determining Microcystis–Daphnia interactions.
Microcystis and Daphnia may strongly interact with each other, as Microcystis may intoxicate Daphnia, whereas Daphnia may feed on Microcystis. The high amount of genetic variation in defence mechanisms in Microcystis strains and in resistance to Microcystis toxins in Daphnia then raises the question to what extent populations of both species may coevolve in response to each other, leading to local coadaptation (Thompson 2005). The occurrence of genotype × genotype interactions is a prerequisite for the development of local adaptation in a dynamic, geographic mosaic of coevolution (Thompson 2005). As a first test of this idea, we designed an experiment to quantify to what extent susceptibility to Microcystis in Daphnia is not only dependent on Daphnia genotype and Microcystis strain, but also on genotype × genotype interactions, similar as in, for example, host–parasite interactions (e.g. Carius et al. 2001). Genotype × genotype interactions would explain why in some studies Daphnia seem to be able to control Microcystis, whereas in other systems, Microcystis seem to control Daphnia. Using a meta-analysis approach, Wilson et al. (2006) also concluded that toxicity induced by cyanobacteria on growth rate and survival is strongly dependent on the cyanobacterium and zooplankton strains used, and not as much on the presence or absence of microcystins, as is generally accepted. Here, we experimentally test for genotype × genotype interactions in a systematic way by confronting 10 different genotypes of the water flea Daphnia with 10 different strains of the cyanobacterium Microcystis in a full factorial design. Getting a better grip on genotype × genotype interactions and potential coadaptation between daphnia and toxic cyanobacteria might help to develop successful strategies for top–down control of toxic blooms by zooplankton grazers.
Material and methods
Experimental organisms
We worked with 10 Microcystis strains (Table 1) isolated from three different populations in Belgium: strains ML76, ML50, ML49, ML14 were isolated from a 7-ha lake in the natural reserve of Leeuwenhof at Drongen (Ghent, September 2004), strains MT50, MT45, MT38, MT6 were isolated from a pond in the natural reserve of Tiens Broek at Tienen (August 2005), and strains MW24 and MW31 were isolated from a pond in Westveld Park at Sint-Amandsberg (Ghent, MW24 in July 2007, MW31 in July 2008). All strains have been genotyped using 16S and 23S rDNA internal transcribed spacer sequences (Janse et al. 2004). They all belong to the species Microcystis aeruginosa, except for MW24, which belongs to Microcystis viridis (I. van Gremberghe personal observation; Van Wichelen et al. 2010). All strains have also been analysed for their microcystin content using ELISA (Enzyme Linked Immuno-Sorbent Assay; van Gremberghe et al. 2009a; Van Wichelen et al. 2010). ELISA revealed the presence of microcystin in strains ML76, ML50, MT50, MT45, MW24 and MW31, but not in the remaining four strains. These strains are known not to contain the mcy genes A and E (van Gremberghe et al. 2009a). All strains were cultured in WC-medium (Guillard and Lorenzen 1972 without pH adjustment) in 750-mL tissue culture flasks with filter caps. They were incubated in a light-chamber with light intensity of ca. 35 μmol photons m−2 s−1, temperature of 20 ± 1°C, and a light/dark cycle of 16 h:8 h. The batch cultures were harvested every 2 weeks by centrifugation of the cultures at 1812 g, 20°C for 10 min, discarding the supernatant, and resuspending the cells with fresh autoclaved WC-medium.
Table 1.
Characterization of the Microcystis strains used in the experiment
| Name | Origin | Isolation date | Microcystin concentration (in pg per ng C) | Growth rate | Tendency to form colonies |
|---|---|---|---|---|---|
| ML76 | Leeuwenhof | 9/2004 | 17.9 | 0.478 | Never |
| ML50 | Leeuwenhof | 9/2004 | 2.8 | 0.388 | Always |
| ML49 | Leeuwenhof | 9/2004 | 0 | 0.409 | Never |
| ML14 | Leeuwenhof | 9/2004 | 0 | 0.453 | Sometimes |
| MT45 | Tiens Broek | 8/2005 | 4.00 | 0.468 | Sometimes |
| MT50 | Tiens Broek | 8/2005 | 10.61 | 0.376 | Sometimes |
| MT38 | Tiens Broek | 8/2005 | 0 | 0.435 | Always |
| MT6 | Tiens Broek | 8/2005 | 0 | 0.500 | Sometimes |
| MW24 | Park Westveld | 7/2007 | 5.56 | 0.427 | Sometimes |
| MW31 | Park Westveld | 7/2008 | 13.88 | 0.517 | Sometimes |
Data from I. van Gremberghe, personal observation; van Gremberghe et al. 2009a; Van Wichelen et al. 2010.
We used six Daphnia magna Straus, 1820 clones from Belgium and four Daphnia similis Claus, 1876 clones from Ethiopia in our experiment (Table 2). Two of the Belgian D. magna clones, DWEST_02 and DWEST_04, were isolated from the same pond (Westveld Park) as Microcystis strains MW24 and MW31. This pond was recently drained in an effort to control cyanobacteria blooms, and while the Microcystis strains were isolated from the pond before this event (in July 2007 and 2008), the Daphnia clones were collected after pond restoration (in September 2009). The other four Belgian clones were hatched in fall 2009 from the upper 2–3 cm of four different pond sediments collected in winter 2007: clone DBLAIN_NF6 was hatched from the sediments of a small pond in the nature reserve De Blankaart (Woumen); DTER1_12 was derived from Tersaert, a pond in Neerijse; DLRV_F2 was hatched from Langerodevijver, a 21-ha shallow lake in the natural reserve of Doode Bemde (Korbeek-Dijle); DMO_F15 came from a pond in Moorsel (Tervuren); with the exception of Langerodevijver, all these ponds are also referred to in the study by Jansen et al. (2010a,b) and have been characterized for their abiotic conditions (Rousseaux 2011). In addition to the six D. magna clones, we also worked with four D. similis clones that were collected in 2009 from artificial reservoirs in the semiarid highlands of Tigray, Northern Ethiopia (see Dejenie et al. 2008). The four clones were isolated from three different but neighbouring populations: DMG1_01 from the 14.7 ha reservoir Mai Gassa I; DMG2_01 and DMG2_02 from the 9.1 ha reservoir Mai Gassa II; and DGM2_05 from the reservoir Gereb Mihiz (17.7 ha). These reservoirs are described in the study by Dejenie et al. (2008). Owing to exceptionally dry weather in 2008–2009, Mai Gassa I and II dried up completely in May–August 2009. Mai Gassa I and II normally contain intense Microcystis blooms. In 2009, however, probably associated with the fish kill induced by the drystands, Microcystis densities were relatively low and Daphnia densities were relatively high. We included Ethiopian D. similis in our analysis because the Ethiopian reservoirs suffer from far more intense Microcystis blooms than most ponds in Belgium. Also, in an enclosure experiment carried out in two reservoirs, we obtained clear indications that local Daphnia populations may contribute to a suppression of the growth of Microcystis (Dejenie et al. 2009). All Daphnia clones were kept for multiple generations in the laboratory before using them in experiments. Prior to the start of the experiment, Daphnia clones were kept under optimal conditions for two generations to reduce the interference of maternal effects with our results and to obtain enough individuals for the experiment. Animals were cultured individually in 210-mL jars in a climatic room at 20 ± 1°C and a light/dark cycle of 16 h:8 h; food levels were restored daily to 5 × 104Scenedesmus obliquus cells mL−1 and their medium, consisting of 24 h aged tap water, was refreshed twice weekly.
Table 2.
Characterization of the Daphnia clones used in the experiment
| Name | Daphnia spp. | Origin | Sampling year |
|---|---|---|---|
| DWEST_02 | D. magna | Park Westveld (Belgium) | 2009 |
| DWEST_04 | D. magna | Park Westveld (Belgium) | 2009 |
| DBLAIN_NF6 | D. magna | De Blankaart (Belgium) | 2007 |
| DTER1_12 | D. magna | Tersaert 1 (Belgium) | 2007 |
| DLRV_F2 | D. magna | Langerode (Belgium) | 2007 |
| DMO_F15 | D. magna | Moorsel (Belgium) | 2007 |
| DMG1_01 | D. similis | Mai Gassa I (Ethiopia) | 2009 |
| DMG2_01 | D. similis | Mai Gassa II (Ethiopia) | 2009 |
| DMG2_02 | D. similis | Mai Gassa II (Ethiopia) | 2009 |
| DGM2_05 | D. similis | Gereb Mihiz (Ethiopia) | 2009 |
For convenience, in the remainder of the paper, we use the term ‘strain’ when we refer to a Microcystis genotype and the term ‘clone’ when we refer to a Daphnia genotype.
Experimental design and procedures
A full factorial design was used combining the 10 Daphnia clones with the 10 Microcystis strains in a food gradient of Microcystis: Scenedesmus in 0:100, 20:80, 40:60, 60:40, 80:20, 100:0 carbon ratios. Scenedesmus obliquus was used as a standard good-quality food to prepare food mixtures with Microcystis. During the experiment, total food concentration was restored daily to 1.0 mg C L−1Microcystis + Scenedesmus. According to literature (Lampert 1977; Gustafsson and Hansson 2004), this is a sufficiently high food concentration for rapid growth and good reproduction of daphnids. For Microcystis, we estimated cell volumes using a sphere as an approximation of the coccoid form of Microcystis cells (Holm and Armstrong 1981). We converted the biovolume to amount of carbon using the formula 0.216·cell volume0.939 = pgC·cell−1 (Menden-Deuer and Lessard 2000). We harvested fresh Microcystis suspensions for all 10 strains every 2 days by centrifugation of exponentially growing cultures. We established the concentration and average cell diameter of the resulting suspensions using a Coulter counter (Multisizer™ 3 COULTER COUNTER®; Beckman Coulter Inc., Brea, CA, USA), and diluted appropriate amounts in 200 mL dechlorinated water to obtain a total carbon content of 1.0 mg C L−1 food concentrations in all experimental jars. In treatments with a 100%Microcystis diet, cell concentrations ranged in between 150 000 and 200 000 cells mL−1, depending on the cell size of the strain used. Such cell concentrations of Microcystis (and higher) often occur in nature (e.g. Kurmayer et al. 2003; Kann 2006); similarly, relative proportions of >80%Microcystis in the phytoplankton community have regularly been reported (e.g. Zurawell et al. 1999; Downing et al. 2001; Song et al. 2010). To count Microcystis in the Coulter counter, we first boiled subsamples of each strain for 6 min in a hot water bath, to disperse colonies and obtain single intact cells for counting and measuring (Joung et al. 2006). Daphnia were fed with suspensions of Microcystis that were at most 2 days old. Prior quantification of the total organic carbon levels in our Scenedesmus strain showed that 1.0 mg C L−1 corresponds to 118 000 cells mL−1.
All treatments were replicated three times. In total, the experiment consisted of a combination of 10 Daphnia clones × 10 Microcystis strains × 6 Microcystis concentrations × 3 replicate units of 10 Daphnia individuals each, for a total of 1800 experimental units. For each unit, 10 2-day-old Daphnia juveniles were used. The animals were exposed to the different food treatments for 48 h; this is a standard period for acute aquatic ecotoxicity tests with Daphnia (OECD Adopted 2004). After this time, surviving individuals were counted and mortality was recorded. All experiments were conducted in a light chamber with light intensity of ca. 35 μmol photons m−2 s−1, temperature of 20 ± 1°C, and a light/dark cycle of 16 h:8 h. Because of the size of the experiment, exposures were spread over a period of 27 days. The starting day for each clone (combined with all strains and concentrations) was randomized, and replicate units of a given clone were always started on at least two different days.
Statistical analysis
Mortality count data were analysed with generalized linear mixed models using ‘R’ (lmer function in package lme4, software version 2.12.1, R Development Core Team 2005). We used a model with binomial error distribution and logit link function to test for main effects and relevant interaction terms of Microcystis strain, Daphnia clone, and Microcystis concentration on the proportion of dead animals. As we were primarily interested in our Microcystis strains and Daphnia clones as representatives of the entire population of possible strains and clones, they were both considered random categorical factors in this analysis. Microcystis concentration was incorporated as a continuous variable. The day on which each jar entered the experiment was inserted into the model as a random categorical blocking factor, ‘day’, to correct for handling differences. We acknowledge that this first model does not include the full complexity of our design, as it ignores the origin of clones and strains in the analysis (but see below for an analysis that does take origin into account). This simplification was done because the full model resulted in a too high computational complexity.
As overall mortality was low (see Results), we constructed a second model on a data set omitting the treatments with low Microcystis concentrations (where mortality was almost zero). Only data corresponding to the 80% and 100%Microcystis diet for each clone × strain combination were included. We still included the %Microcystis in the model, but merely as (fixed) categorical blocking factor. In this second analysis, we included the Daphnia species as a fixed factor, with Daphnia clone nested in Daphnia species. The Daphnia clones used in our experiment indeed belong to two different species, D. similis and D. magna, originating from two different countries, Ethiopia and Belgium, respectively. Similarly, we included the lake where Microcystis was isolated from as a fixed factor, with Microcystis strain nested in lake. We acknowledge that the second model is to be preferred over the first model that ignores origin. We merely report the results of the first model to demonstrate there are no substantial differences between the results of an analysis of the whole range of Microcystis concentrations versus a subset including only the highest concentrations.
To take a closer look at the pairwise differences between clones and between strains and at genotype × genotype interactions, we performed post hoc Tukey's HSD tests following a linear model (anova) in ‘R’ (R Development Core Team 2005). In this analysis, we included Daphnia clone and Microcystis strain as fixed independent variables, and the proportion of dead individuals in each jar in the 80% and 100%Microcystis treatments as dependent variable. Treatment of Daphnia clone and Microcystis strain in this analysis as fixed categorical variables is justified by the fact that here we are interested in the differences between specific pairs of clones or strains. Treatment was implemented as a fixed categorical blocking factor. We only included levels of 80% and 100%Microcystis, because the other levels induced very low levels of mortality. We are aware that our data do not fulfil the assumptions of anova (arcsin transformations did not improve the fit) but are confident that the model is sufficiently robust for this analysis of pairwise comparisons. This is supported by the fact that the basic model results of the anova (See Table S4) do not differ substantially from the results generated by our generalized linear mixed models. We here resorted to a basic and simplified anova as the complexity of the generalized linear model prevents a straightforward analysis of post hoc comparisons.
Finally, to analyse whether mortality induced by Microcystis strains is related to their microcystin concentration, the Pearson's correlation coefficient was calculated between the average mortality imposed by the different Microcystis strains (considering 80% and 100%Microcystis treatments only) and the microcystin concentration of those strains.
Results
Daphnia mortality induced by Microcystis
In general, the mortality we observed was rather low (mean <5%; n = 1782) but increased with the concentration of Microcystis (Fig. 1). At the lowest concentrations (20–40%) of Microcystis, mortality was around 3.6% and at the highest concentrations (80–100%), mortality was on average 7% (Fig. 1) with values ranging from 0% to 18.5% depending on the Daphnia clone and Microcystis strain combination (Fig. 2). All factors and interaction terms that were included in our analysis have a significant impact on Daphnia mortality in our experiment (Table 3). There is a significant Microcystis concentration effect confirming that mortality in Daphnia increases with increasing Microcystis concentrations in their food (Fig. 1). The significant interactions of clone and strain with concentration (including the three-way interaction, Table 3) are mainly caused by the fact that differences in mortality are especially pronounced at the higher concentrations, and indicate that the extent to which high Microcystis concentrations induce mortality is dependent on the Daphnia clone and Microcystis strain, or their combination. The significant main effect of Daphnia clone (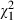 = 111.14, P < 0.001, Table 3, Fig. 2A) confirms that Daphnia genotypes differ in overall susceptibility to Microcystis, while the significant main effect of Microcystis strain (
= 111.14, P < 0.001, Table 3, Fig. 2A) confirms that Daphnia genotypes differ in overall susceptibility to Microcystis, while the significant main effect of Microcystis strain (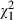 = 118.32, P < 0.001, Table 3, Fig. 2B) indicates that Microcystis genotypes differ in overall toxicity to Daphnia. The Daphnia clone effect seems to attribute more to the total amount of explained variation than the Microcystis strain effect in our analysis (Table 3). A large part of the variation is, however, explained by a highly significant Daphnia clone × Microcystis strain interaction effect (
= 118.32, P < 0.001, Table 3, Fig. 2B) indicates that Microcystis genotypes differ in overall toxicity to Daphnia. The Daphnia clone effect seems to attribute more to the total amount of explained variation than the Microcystis strain effect in our analysis (Table 3). A large part of the variation is, however, explained by a highly significant Daphnia clone × Microcystis strain interaction effect (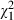 = 132.93, P < 0.001, Table 3, Fig. 3).
= 132.93, P < 0.001, Table 3, Fig. 3).
Figure 1.
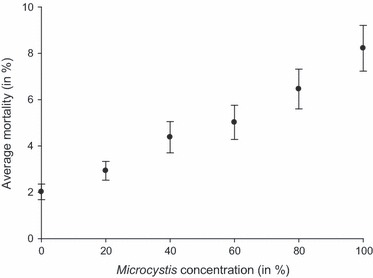
Average Daphnia mortality (%) when exposed to increasing Microcystis concentrations, showing a significant effect of dietary Microcystis concentration on the mortality of Daphnia. Error bars indicate the standard error of mean (n = 297).
Figure 2.
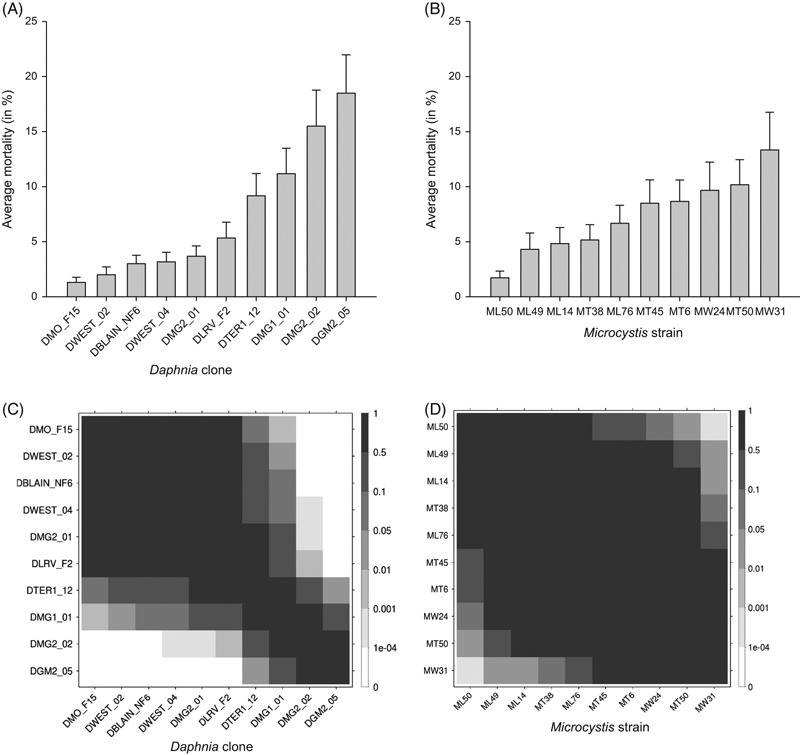
(A,B) The average percentage mortality of each of 10 Daphnia clones when exposed to Microcystis (A) and average mortality in Daphnia caused by each of 10 Microcystis strains (B) in the treatments with 80% and 100%Microcystis. The error bars indicate the standard error of mean (n = 60, except nDMO_F15 = 54, and nML14 = nML49 = nML50 = 58 due to missing values). (C,D) P-values of pairwise comparisons indicating significant differences in susceptibility between Daphnia clones (C) and in induced mortality between Microcystis strains (D).
Table 3.
Results of the generalized linear mixed model with binomial error distribution and logit link function on Daphnia mortality over the complete range of Microcystis concentrations
| Effect | χ2 | χ df | % of Variance | P-value | |
|---|---|---|---|---|---|
| Clone | Random | 111.14 | 1 | 3.78 | <0.001*** |
| Strain | Random | 118.32 | 1 | 0.44 | <0.001*** |
| Concentration | Continuous | 184.32 | 1 | <0.001*** | |
| Clone × Strain | Random | 132.93 | 1 | 12.42 | <0.001*** |
| Clone × Concentration | Random | 13.68 | 1 | <0.01 | 0.002** |
| Strain × Concentration | Random | 10.54 | 1 | <0.01 | 0.001** |
| Clone × Strain × Concentration | Random | 17.87 | 1 | <0.01 | <0.001*** |
| Day effect | Random | 447.98 | 1 | 38.80 | <0.001*** |
| Error | 44.56 |
P-values lower than 0.001 are marked with ‘***’, between 0.001 and 0.01 with ‘**’.
Figure 3.
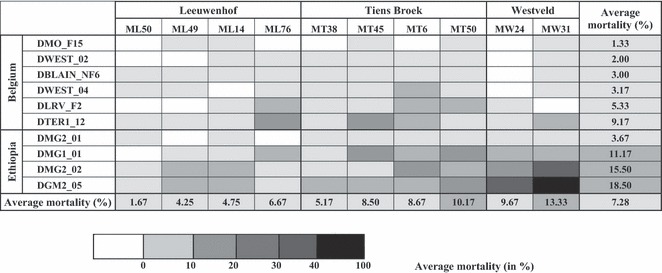
Interaction plot showing average mortalities of Daphnia in the 80% and 100%Microcystis concentrations in all 100 combinations of 10 different Daphnia clones (rows) and 10 different Microcystis strains (columns). Mortalities are coded as grey scales from 0–10%, 10–20%, 20–30%, 30–40% and >40% mortality. The genotypes of both interacting species are ordered along their overall susceptibility and toxicity, in addition to a grouping according to origin (Daphnia: country; Microcystis: lake). The resulting pattern shows, in addition to differences in susceptibility among Daphnia genotypes and in toxicity among Microcystis strains, strong genotype × genotype interactions (see also Table 4).
Ranking of the Daphnia clones and pairwise comparisons of mortality in the 80–100%Microcystis concentrations (Fig. 2A,C) reveal that clones DMO_F15 and DWEST_02 are the most resistant clones, while DMG2_02 and DGM2_05 are the most sensitive to exposure to Microcystis. Ranking of the Microcystis strains (Fig. 2B,D) shows that MW31 and MT50 are the most toxic to Daphnia, while ML50 and ML49 induce the least mortality. The interaction between clone and strain is clearly visible by the patchiness of the grey shades in Fig. 3 where we plotted the mortality intensity for each Daphnia clone–Microcystis strain combination (See also Fig. S1 for the pairwise differences in mortality among Daphnia clone–Microcystis strain combinations). Three clone × strain combinations differ significantly from most of the others, namely DMG2_02-MW31, DGM2_05-MW31, and DGM2_05-MW24. These are combinations that result in particularly high mortalities (>30%, Fig. 3).
The generalized linear mixed model based on the truncated data set including only data from the highest two Microcystis concentrations (Table 4), which takes into account the nested design, confirms the significance of our main effects Daphnia clone (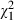 = 96.91, P < 0.001), Microcystis strain (
= 96.91, P < 0.001), Microcystis strain (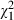 = 16.64, P < 0.001) and their interaction (
= 16.64, P < 0.001) and their interaction (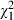 = 44.47, P < 0.001). The explained variance by the clone and strain effect is here even greater, and the high importance of the clone × strain interaction is confirmed. Furthermore, the interaction between Microcystis lake and Daphnia species proves to be significant (
= 44.47, P < 0.001). The explained variance by the clone and strain effect is here even greater, and the high importance of the clone × strain interaction is confirmed. Furthermore, the interaction between Microcystis lake and Daphnia species proves to be significant (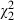 = 13.25, P = 0.001, Fig. 4C). Indeed, while there is a significant effect of the lake Microcystis was isolated from (
= 13.25, P = 0.001, Fig. 4C). Indeed, while there is a significant effect of the lake Microcystis was isolated from (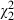 = 8.99, P = 0.011, Fig. 4A), it is clear from Fig. 4C that this lake effect is strongly dependent on whether we consider Belgian or Ethiopian Daphnia. Daphnia mortality is highest when Ethiopian D. similis clones are exposed to Microcystis strains from Westveld park, followed by combinations of Ethiopian D. similis and Tiensbroek Microcystis. The four remaining combinations are less lethal. Excluding the Microcystis strain belonging to the different morphospecies M. viridis, MW24, from the analysis presented in Table 4 does not change any of these results (see Table S1).
= 8.99, P = 0.011, Fig. 4A), it is clear from Fig. 4C that this lake effect is strongly dependent on whether we consider Belgian or Ethiopian Daphnia. Daphnia mortality is highest when Ethiopian D. similis clones are exposed to Microcystis strains from Westveld park, followed by combinations of Ethiopian D. similis and Tiensbroek Microcystis. The four remaining combinations are less lethal. Excluding the Microcystis strain belonging to the different morphospecies M. viridis, MW24, from the analysis presented in Table 4 does not change any of these results (see Table S1).
Table 4.
Results of the generalized linear mixed model with binomial error distribution and logit link function on Daphnia mortality in the 80% and 100%Microcystis concentrations
| Effect | χ2 | χ df | % of variance | P-value | |
|---|---|---|---|---|---|
| Clone effect (nested in Species) | Random | 96.91 | 1 | 18.83 | <0.001*** |
| Strain effect (nested in Lake) | Random | 16.64 | 1 | 1.89 | <0.001*** |
| Clone × Strain | Random | 44.47 | 1 | 13.46 | <0.001*** |
| Lake effect | Fixed | 8.99 | 2 | 0.011* | |
| Species effect | Fixed | 1.823 | 1 | 0.177 | |
| Species × Lake | Fixed | 13.25 | 2 | 0.001** | |
| Concentration | Fixed | 7.97 | 1 | 0.005** | |
| Day effect | Random | 258.56 | 1 | 35.98 | <0.001*** |
| Error | 29.84 |
P-values lower than 0.001 are marked with ‘***’, between 0.001 and 0.01 with ‘**’, between 0.01 and 0.05 with ‘*’.
Figure 4.
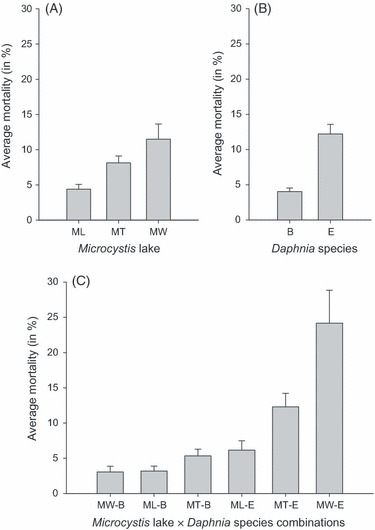
The average percentage mortality for (A) Microcystis strains from each lake. (B) Daphnia clones from each species/locality. (C) Each Daphnia species × Microcystis lake combination, in treatments with Microcystis concentrations, 80% and 100%. The error bars indicate the standard error of mean (nML = 234, nMT = 240, nMW = 120, nB = 354, nE = 240, nMW-B = 72, nML-B = 138, nMT-B = 144, nML-E = 96, nMT-E = 96, nMW-E = 48). ML refers to the lake Leeuwenhof, MT: Tiensbroek, and MW: Westveldpark. B denotes the Belgian species Daphnia magna. E refers to the Ethiopian Daphnia similis.
Mortality and microcystin-LR concentration
The Pearson's correlation coefficient between the average mortality induced by the different Microcystis strains (considering the 80% and 100%Microcystis concentrations only) and the actual microcystin concentration of those strains, as determined by ELISA, is not significant (r = 0.53, P = 0.12, Fig. 5). Figure 5 shows an overall tendency for a relationship between average Daphnia mortality and microcystin content of the Microcystis strains, but the pattern is rendered insignificant by the fact that some strains deviate from the general trend. Microcystis strain ML76 appears less toxic than expected by its microcystin content, while the four nonmicrocystin-producing strains induce some mortality in Daphnia nevertheless. This is particularly striking in strain MT6. If strain ML76 is excluded from the analysis, the Pearson's correlation coefficient becomes significant (r = 0.77, P = 0.015).
Figure 5.
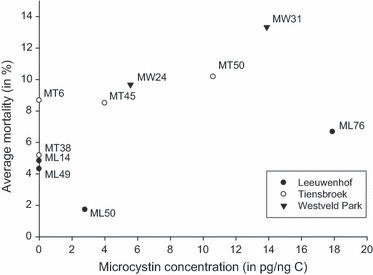
The mortality of the different Microcystis strains, averaged over all Daphnia clones exposed to 80% and 100%Microcystis, was plotted against the microcystin concentration of those strains. Symbols indicate the pond where the Microcystis strains were isolated from. Pearson's correlation coefficient r = 0.53 (P = 0.12).
Discussion
Genotype by genotype interactions
Overall, our experiment confirms the results of earlier studies showing that exposure to Microcystis results in mortality in Daphnia (e.g. Lampert 1981; Nizan et al. 1986; Reinikainen et al. 1994; DeMott 1999). The relatively low mortality rates even at 100%Microcystis diet were unexpected, but may be related to the relatively short exposure time we used (2 days).
Our results confirm earlier work reporting genetic differences in toxicity among Microcystis strains (e.g. Nizan et al. 1986; Jungmann and Benndorf 1994; Czarnecki et al. 2006) and in resistance among Daphnia genotypes (Gustafsson et al. 2005; Sarnelle and Wilson 2005; Blom et al. 2006; Wilson et al. 2006). These genetic differences are well known and indicate that there is ample evolutionary potential for toxicity in Microcystis to evolve and for resistance in Daphnia populations to evolve in response to the occurrence and strain composition of Microcystis populations. Intriguingly, our results indicate that genetic variation in Daphnia resistance explains more variation in mortality than genetic variation in Microcystis toxicity. This is unexpected, as cyanobacteria are known to exhibit a wide variety of grazing avoidance mechanisms and substantial differences in mortality induction could be expected. Yet, the degree to which Microcystis induces mortality in Daphnia upon relatively short-term exposure seems to vary dramatically depending on the Daphnia genotype used. This brings a different perspective to cyanobacteria–zooplankton interactions, which builds further on the studies showing acclimation, maternal effects and genetic adaptation in Daphnia to Microcystis (Hairston et al. 2001; Gustafsson et al. 2005; Sarnelle et al. 2010; Schwarzenberger et al. 2010).
The key observation of our study is that there is an important genotype × genotype interaction effect on mortality in Daphnia: the degree to which Daphnia suffers from exposure to Microcystis does not only depend on the Daphnia and Microcystis genotype, but also on which genotypes interact with each other. While Microcystis strains MW31 and MT50 are overall the most toxic to Daphnia, some Daphnia genotypes suffer little from them, even though they experience significant mortality from exposure to some other Microcystis strains. These genotype × genotype interactions may explain the confusing picture that is provided by the literature on cyanobacteria–zooplankton interactions, in which widely different results are obtained depending on the study. For example, Christoffersen et al. (1993) and Sarnelle (2007) suggest Daphnia can control the development of Microcystis blooms depending on initial conditions and history, while other studies stress the highly deleterious influence that Microcystis exerts on Daphnia, reducing population growth and survival (Gliwicz and Siedlar 1980; Nizan et al. 1986; Ghadouani et al. 2003). Our results imply that there is no general resistance mechanism in Daphnia nor in Microcystis. Given the high diversity of secondary metabolites and the capacity for colony formation in different Microcystis strains, it is likely that there are trade-offs among defences. Similarly, there may be trade-offs against counter-defences in Daphnia. This has important implications for our view on how toxic Microcystis blooms develop, which may not so much be the result of zooplankton grazing in general but rather may reflect the outcome of interactions between defences and counter-defences in auto- and heterotrophs, comediated by the costs imposed by both the development of these defences and counter-defences. Importantly, genotype × genotype interactions provide the raw material for local co-adaptation and thus may lead to a geographic mosaic of coevolution between the cyanobacteria that protect themselves against grazing and their grazers that protect themselves against toxicity. The concept of the geographic mosaic of coevolution (Thompson 2005; or the concept of evolving metacommunities if one takes the broader community into account, see Urban et al. 2008) may offer a strong framework to investigate Microcystis–Daphnia interactions. Genotype × genotype interactions and associated coevolutionary dynamics are well studied in host–parasite systems (Carius et al. 2001; Ebert 2008). The strong interaction effect between Microcystis and Daphnia genotype in our experiment suggests a high potential for a coevolutionary arms race, similar to that among hosts and parasites. There is growing evidence that genetic diversity and evolutionary changes may strongly impact the dynamics of predator–prey interactions, as have been demonstrated by the seminal studies of Yoshida et al. (2003, 2007). The genotype × genotype interactions we report suggest that Daphnia may not develop generalized responses against Microcystis but rather may specifically adapt to local assemblages of cyanobacteria strains and vice versa. This localized coevolutionary arms race may also have practical consequences, as the capacity of zooplankton to genetically adapt to locally occurring Microcystis strains may increase the likelihood that the development of a bloom can be prevented by grazing.
Intriguingly, the Ethiopian D. similis clones tended to be more sensitive to Microcystis than the Belgian D. magna clones, even though the D. similis genotypes were isolated from reservoirs in Ethiopia that are usually heavily infected with Microcystis blooms. Although it is speculative, it is worth mentioning that all Microcystis strains used in our experiment were isolated from Belgium and not from Ethiopia. If there is a strong effect of localized coevolution in Daphnia–Microcystis interactions, it is conceivable that Daphnia species that were not exposed to specific Microcystis strains, not even at a regional level, may be more sensitive to the toxic compounds these strains produce than Daphnia that were exposed earlier to these strains. When we analyse our data building a model containing only Ethiopian Daphnia clones (data from all Microcystis strains and concentrations, see Table S2), the percentage of variance that can be attributed to Microcystis strain identity rises from 0.44% in the analysis containing all Daphnia clones (Table 3) to almost 10% (9.38%, 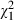 = 162.58, P < 0.001), while the variance of the main effect of Daphnia clone (1.44%,
= 162.58, P < 0.001), while the variance of the main effect of Daphnia clone (1.44%, 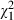 = 83.81, P < 0.001) and the clone × strain interaction effect (3.70%,
= 83.81, P < 0.001) and the clone × strain interaction effect (3.70%, 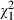 = 32.26, P < 0.001) are relatively low compared to 3.78% and 12.42%, respectively, in the analysis including the Belgian clones. This stronger impact of Microcystis strain identity is expected for first exposures of local grazer populations with novel types of defences. On the contrary, when we only analyse the Belgian data (see Table S3), we observe a relatively high (19.41%) contribution of genotype × genotype interactions in explaining variation in mortality (
= 32.26, P < 0.001) are relatively low compared to 3.78% and 12.42%, respectively, in the analysis including the Belgian clones. This stronger impact of Microcystis strain identity is expected for first exposures of local grazer populations with novel types of defences. On the contrary, when we only analyse the Belgian data (see Table S3), we observe a relatively high (19.41%) contribution of genotype × genotype interactions in explaining variation in mortality (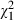 = 43.36, P < 0.001), while the amount of variation explained by Microcystis strain identity is reduced to almost 0% (
= 43.36, P < 0.001), while the amount of variation explained by Microcystis strain identity is reduced to almost 0% (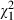 = 22.41, P < 0.001) (Daphnia clone: 7.54%,
= 22.41, P < 0.001) (Daphnia clone: 7.54%, 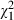 = 28.80, P < 0.001). In a geographic mosaic of coevolution, we indeed expect overall differences between genotypes to be reduced and interaction effects to be important. It is noted that the difference that we observe between the D. similis and D. magna genotypes in terms of resistance may be either a species effect or related to geography, as geography and species identity are confounded in our experiment. Moreover, as we could not expose the Daphnia in our study to Ethiopian Microcystis strains, one should not interpret our results as indicating that Ethiopian Daphnia are less resistant against Microcystis toxins than Belgian Daphnia. First, this would be unexpected given that, overall, the incidence of Microcystis blooms in Ethiopia is much higher than in Belgium. Secondly, we actually observed in an earlier study that local Daphnia populations inhabiting two reservoirs in the highlands of Tigray were able to suppress a developing Microcystis population in enclosures (Dejenie et al. 2009).
= 28.80, P < 0.001). In a geographic mosaic of coevolution, we indeed expect overall differences between genotypes to be reduced and interaction effects to be important. It is noted that the difference that we observe between the D. similis and D. magna genotypes in terms of resistance may be either a species effect or related to geography, as geography and species identity are confounded in our experiment. Moreover, as we could not expose the Daphnia in our study to Ethiopian Microcystis strains, one should not interpret our results as indicating that Ethiopian Daphnia are less resistant against Microcystis toxins than Belgian Daphnia. First, this would be unexpected given that, overall, the incidence of Microcystis blooms in Ethiopia is much higher than in Belgium. Secondly, we actually observed in an earlier study that local Daphnia populations inhabiting two reservoirs in the highlands of Tigray were able to suppress a developing Microcystis population in enclosures (Dejenie et al. 2009).
Daphnia survival in our experiment is primarily because of physiological adaptations rather than to, for example, behavioural responses. With the short exposure times used, one way for the grazers to survive in principle might be to stop feeding so that exposure to toxins is minimized. However, in an additional experiment in which we monitored three of the here studied Daphnia genotypes and Microcystis strains during 5 days, we visually checked gut fullness of animals after 2 and 5 days of feeding on 100%Microcystis, and almost all animals (n = 72) had full guts and thus were actively feeding on Microcystis (V. Lemaire and L. De Meester, unpublished data). The observation that the mortality we observed occurred in a time span of only 2 days strongly points towards the effect of a toxin after ingestion. Indeed, mechanical difficulties in handling colonies or low nutritional value, although also defence strategies of cyanobacteria, are unlikely to lead to mortality within 48 h, as Daphnia juveniles can use a reserve of maternal lipids up until the age of 4 days (Reinikainen et al. 1994).
Even though we find strong genotype × genotype interactions, our data reveal a weak indication that microcystin may still play a role in overall toxicity. We observed a tendency for Microcystis strains to cause higher mortality in Daphnia when they contain higher microcystin levels, but the correlation was not significant (r = 0.53, P = 0.12, see also Fig. 5). Figure 5 shows that some strains showed lower toxicity than expected based on microcystin measurements. We need to interpret this with caution, however, as microcystin was not quantified directly on the stocks used as food in our experiments, but prior to the experiments as part of earlier research (van Gremberghe et al. 2009a,b,c; Van Wichelen et al. 2010). In addition, although ELISA is a sensitive method, it is not able to differentiate between microcystin types (e.g. microcystin-LR, -LY, -LW, -LF, and -RR), which are known to have different impacts on biota (Ibelings and Havens 2008). More importantly, some of the supposedly nontoxic strains clearly induced mortality in Daphnia. These strains are known not to contain the mcy genes A and E (van Gremberghe et al. 2009a), and confirm that toxicity of Microcystis is not only dependent on microcystin but can be mediated by a variety of polypeptides (e.g. Kaebernick et al. 2001; Schwarzenberger et al. 2010). These results are consistent with the rejection of microcystins as a general determinant of toxicity by Wilson et al. (2006) in their meta-analysis. Our results thus sketch a subtle picture in which there are strong genotype × genotype interactions, but still certain defence systems may have more impact than others. Our results emphasize the complexity of toxicity mechanisms in Microcystis, without ignoring the role microcystin has to play. Future research might contribute to our understanding of the mechanistic basis of the genotype × genotype interactions we report in this study, by quantifying and differentiating among the different microcystin types and other polypeptides in the used Microcystis strains.
Our experiment is to our knowledge unique in combining a set of Microcystis strains and Daphnia genotypes in all pairwise combinations and provides strong evidence for genotype × genotype interactions shaping defences and counter-defences in auto- and heterotrophs. Yet there are a number of methodological limitations. First, although we document strong genotype × genotype interactions, the design of our study does not allow to directly test for local genetic adaptation by comparing sympatric and allopatric combinations and reciprocal exposures. We could not work with Ethiopian Microcystis strains, and we had only one habitat from which we had Microcystis strains and Daphnia genotypes (Westveld park pond), and these were isolated in different years. Intriguingly, while the Westveld Microcystis strains were among the most toxic to Daphnia among all Microcystis strains, the two Daphnia clones from that same pond showed very low levels of susceptibility to these two strains. Given that we only have one such data point, however, this remains an anecdotal observation. Thus, although in documenting genotype × genotype interactions we provide evidence for an important condition for a geographic mosaic of coevolution to develop, our results do not provide direct evidence for local genetic coadaptation between Microcystis and Daphnia in nature. Secondly, both of our statistical models attribute a substantial amount of the variance to the random factor ‘day’ (see Tables 3 and 4). We cannot explain this effect without some speculation, but the most obvious explanation is that there was some day-to-day variation in chemical composition of the Microcystis cultures (toxin concentrations or other). This might be due to the fact that batch cultures are intrinsically never fully standardized. It is known that chemical composition of Microcystis is impacted, for example, by population growth rates (Long et al. 2001). Yet this effect of day does not interfere with the conclusions of our study, because for each Daphnia–Microcystis combination treatment, there were three replicates that were by design spread over time. Furthermore, we used all of the Microcystis strains every day during the entire experimental period so that the day effect cannot hide an effect of strain identity.
Applications in the control of cyanobacteria blooms
The emerging picture from our work is that genotype × genotype interactions may be an important determinant of Microcystis–Daphnia interactions, which is expected to result in complex dynamics of coadaptation. Our results add to the evidence that genotype identity and genetic diversity may impact the dynamics of predator–prey interactions in zooplankton feeding on phytoplankton (Yoshida et al. 2003, 2007). Although we do not measure bio-control directly, our results are expected to have important implications for the prevention and control of cyanobacteria blooms.
As mentioned in the introduction, top–down control of cyanobacteria blooms by zooplankton has received relatively little attention in recent years, mainly because of the observation that cyanobacteria may intoxicate zooplankton so that the latter are not capable of suppressing an existing bloom (Gulati et al. 2008). Gulati et al. (2008) in their review on lake restorations in north-western Europe identified the incapability of daphnids to graze on filamentous or colonial cyanobacteria as one of key bottlenecks that can explain the failure of biomanipulation measures. Yet, our results on genotype by genotype interactions may explain why studies on Daphnia–cyanobacteria interactions have yielded contradicting results in the past and offer new perspectives to exploit the adaptive potential of Daphnia populations to control cyanobacterial blooms. Our results suggest that biomanipulation, involving a massive reduction in fish biomass to boost development of large-bodied Daphnia, might work even in lakes that are prone to cyanobacteria blooms if the genotype composition of both Daphnia and Microcystis is taken into account. Indeed, given the high population growth rate of Daphnia and associated high phytoplankton clearance rates, top–down control by Daphnia may be possible on the condition that one can boost the development of Daphnia populations that are adapted to the local strain composition of Microcystis. Experimental evolution in Daphnia has been shown to result in genetic shifts leading to adaptation to the stressor within a time period as short as a few months (Van Doorslaer et al. 2009; Jansen et al. 2011a,b). Yet, it is probably important that the phytoplankton community is not entirely dominated by cyanobacteria when the Daphnia are expected to develop, as cyanobacteria are nutritionally poor food (Brett and Müller-Navarra 1997; von Elert et al. 2003) and may not support the rapid development of dense Daphnia populations when dominant. In practice, this implies that cyanobacteria control in lakes by top–down impact may only work after sufficiently strong winters, when the cyanobacteria fail to remain abundant year-round, creating a window of opportunity in the spring for Daphnia to develop to sufficiently high densities. In sufficiently small systems (e.g. garden ponds or open-air water reservoirs for horticulture), one could contemplate to inoculate Daphnia early in the season to boost population development and top–down control. In doing this, however, it will be important to carefully select the Daphnia population for its capacity to control the local set of Microcystis strains. One obvious way to do this would be to sample dormant egg banks of Daphnia from different water bodies in the region and use experimental evolution to select for genotypes that can cope with the local strains of Microcystis and culture them in the laboratory to sufficient densities for re-inoculation. Obviously, a targeted approach involving the inoculation of Daphnia would only work for small systems: if we accept that it is feasible to obtain 1 × 106 (juvenile) Daphnia in controlled outdoor mesocosm systems, this would yield a density of 0.1 Daphnia L−1 in a pond of 1 ha and 1 m deep. This might be sufficient to prevent a cyanobacteria bloom to develop, but the effort would be substantial.
Conclusions
The genotype × genotype interactions reported by the present study may be an important determinant of Microcystis–Daphnia interactions, which is expected to result in complex dynamics of coadaptation. These dynamics have implications both for the characteristics of cyanobacteria blooms that may develop in a given system, as these will also be influenced by the genetic characteristics of the grazer population, and with respect to the potential for top–down control of cyanobacteria blooms. Future studies should focus on confirming genotype × genotype interactions and further characterizing the potential occurrence of a geographic mosaic of coevolution between Daphnia and Microcystis, and should address both the dynamics through time at the local and regional scale as well as the potential of top–down control that is implied by our results.
Acknowledgments
We acknowledge financial support by K.U.Leuven Research Fund projects GOA/2008/06 and PF/2010/07. JV is a postdoctoral researcher with the National Fund for Scientific Research, Flanders (FWO). Data Archiving Statement: Data for this study are available as Online Supplementary Materials.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Results of pairwise comparisons (Tukey HSD, based on the anova in Table S4) between all clone × strain combinations: The P-values (A) and the absolute differences in average mortality (B) observed in the 80% and 100% Microcystis concentrations.
Table S1. Results of the generalized linear mixed model with binomial error distribution and logit link function on Daphnia mortality in the 80% and 100% Microcystis concentrations, without combinations including the Microcystis viridis strain MW24.
Table S2. Results of the generalized linear mixed model with binomial error distribution and logit link function on Daphnia mortality of Ethiopian clones over the complete range of Microcystis concentrations.
Table S3. Results of the generalized linear mixed model with binomial error distribution and logit link function on Daphnia mortality of Belgian clones over the complete range of Microcystis concentrations.
Table S4. Results of an anova on a linear model of the proportion Daphnia mortality in the 80% and 100% Microcystis concentration.
Table S5. Full data with the number of dead and surviving individuals (out of 10) for the three replicates of each combination of 10 Daphnia clones × 10 Microcystis strains × 6 concentrations of Microcystis (in %).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Literature Cited
- Anderson DM, Glibert PM, Burkholder JM. Harmful algal blooms and eutrophication: nutrient sources, composition, and consequences. Estuaries. 2002;25:704–726. [Google Scholar]
- Bell SG, Codd GA. Cyanobacterial toxins and human health. Reviews in Medical Microbiology. 1994;5:256–264. [Google Scholar]
- Blom JF, Baumann HI, Codd GA, Jüttner F. Sensitivity and adaptation of aquatic organisms to oscillapeptin J and (D-Asp3, (E)-Dhb7) microcystin-RR. Archiv für Hydrobiologie. 2006;167:547–559. [Google Scholar]
- Brett M, Müller-Navarra D. The role of highly unsaturated fatty acids in aquatic foodweb processes. Freshwater Biology. 1997;38:483–499. [Google Scholar]
- Carius HJ, Little TJ, Ebert D. Genetic variation in host-parasite association: potential for coevolution and frequency-dependent selection. Evolution. 2001;55:1136–1145. doi: 10.1111/j.0014-3820.2001.tb00633.x. [DOI] [PubMed] [Google Scholar]
- Chen W, Song L, Ou D, Gan N. Chronic toxicity and responses of several important enzymes in Daphnia magna on exposure to sublethal microcystin-LR. Environmental Toxicology. 2005;20:323–330. doi: 10.1002/tox.20108. [DOI] [PubMed] [Google Scholar]
- Choi H, Kim B, Kim J, Han M. Streptomyces neyagawaensis as a control for the hazardous biomass of Microcystis aeruginosa (Cyanobacteria) in eutrophic freshwaters. Biological Control. 2005;33:335–343. [Google Scholar]
- Chorus I, Mur L. Preventative measures. In: Chorus I, Bertram J, editors. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management. London and New York: E & FN Spon; 1999. pp. 232–266. [Google Scholar]
- Christoffersen K, Riemann B, Klyser A, Søndergaard M. Potential role of fish predation and natural populations of zooplankton in structuring a plankton community in eutrophic lake water. Limnology and Oceanography. 1993;38:561–573. [Google Scholar]
- Codd GA. Cyanobacterial toxins, the perception of water quality, and the prioritisation of eutrophication control. Ecological Engineering. 2000;16:51–60. [Google Scholar]
- Codd GA, Lindsay J, Young FM, Morrison LF, Metcalf JS. Harmful Cyanobacteria: from mass mortalities to management measures. In: Huisman J, Matthijs HCP, Visser PM, editors. Harmful Cyanobacteria. Dordrecht: Springer; 2005. pp. 1–24. [Google Scholar]
- Czarnecki O, Henning M, Lippert I, Welker M. Identification of peptide metabolites of Microcystis (Cyanobacteria) that inhibit trypsin-like activity in planktonic herbivorous Daphnia (Cladocera) Environmental Microbiology. 2006;8:77–87. doi: 10.1111/j.1462-2920.2005.00870.x. [DOI] [PubMed] [Google Scholar]
- Dao TS, Do-Hong L-C, Wiegand C. Chronic effects of cyanobacterial toxins on Daphnia magna and their offspring. Toxicon. 2010;55:1244–1254. doi: 10.1016/j.toxicon.2010.01.014. [DOI] [PubMed] [Google Scholar]
- De Bernardi R, Giussani G, Pedretti EL. The significance of blue-green algae as food for filter feeding zooplankton: experimental studies of Daphnia spp. fed Microcystis aeruginosa. Verhandlungen Internationale Vereinigung für Theoretische und Angewandte Limnologie. 1981;21:477–483. [Google Scholar]
- Dejenie T, Asmelash T, De Meester L, Mulugeta A, Gebrekidan A, Risch S, Pals A, et al. Limnological and ecological characteristics of tropical highland reservoirs in Tigray, Northern Ethiopia. Hydrobiologia. 2008;610:193–209. [Google Scholar]
- Dejenie T, Asmelash T, Rousseaux S, Gebregiorgis T, Gebrekidan A, Teferi M, Nyssen J, et al. Impact of the fish Garra on the ecology of reservoirs and the occurrence of Microcystis blooms in semi-arid tropical highlands: an experimental assessment using enclosures. Freshwater Biology. 2009;54:1605–1615. [Google Scholar]
- DeMott WR. Foraging strategies and growth inhibition in five daphnids feeding on mixtures of a toxic cyanobacterium and a green alga. Freshwater Biology. 1999;42:263–274. [Google Scholar]
- Douglas GB, Adeney JA, Robb MS. A novel technique for reducing bioavailable phosphorus in water and sediments. 1999. pp. 517–523. International Association Water Quality Conference on Diffuse Pollution.
- Downing JA, Watson SB, McCauley E. Predicting cyanobacterial dominance in lakes. Canadian Journal of Fisheries and Aquatic Sciences. 2001;58:1905–1908. [Google Scholar]
- Ebert D. Host-parasite coevolution: insights from the Daphnia-parasite model system. Current Opinion in Microbiology. 2008;11:290–301. doi: 10.1016/j.mib.2008.05.012. [DOI] [PubMed] [Google Scholar]
- von Elert E, Martin-Creuzburg D, Le Coz JR. Absence of sterols constrains carbon transfer between cyanobacteria and a freshwater herbivore (Daphnia galeata. Proceedings of the Royal Society of London, Biological Sciences. 2003;270:1209–1214. doi: 10.1098/rspb.2003.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadouani A, Pinel-Alloul B, Prepas EE. Effects of experimentally induced cyanobacterial blooms on crustacean zooplankton communities. Freshwater Biology. 2003;48:363–381. [Google Scholar]
- Gliwicz ZM. Why do cladocerans fail to control algal blooms. Hydrobiologia. 1990;200–201:83–97. [Google Scholar]
- Gliwicz ZM, Siedlar E. Food size limitation and algae interfering with food collection in Daphnia. Archiv für Hydrobiologie. 1980;88:155–177. [Google Scholar]
- van Gremberghe I, Vanormelingen P, Van der Gucht K, Mancheva A, D'hondt S, De Meester L, Vyverman W. Influence of Daphnia infochemicals on functional traits of Microcystis strains (Cyanobacteria) Hydrobiologia. 2009a;635:147–155. [Google Scholar]
- van Gremberghe I, Vanormelingen P, Van der Gucht K, Souffreau C, Vyverman W, De Meester L. Priority effects in experimental populations of the cyanobacterium Microcystis. Environmental Microbiology. 2009b;11:2564–2573. doi: 10.1111/j.1462-2920.2009.01981.x. [DOI] [PubMed] [Google Scholar]
- van Gremberghe I, Vanormelingen P, Vanelslander B, Van der Gucht K, D'Hondt S, De Meester L, Vyverman W. Genotype-dependent interactions among sympatric Microcystis strains mediated by Daphnia grazing. Oikos. 2009c;118:1647–1658. [Google Scholar]
- Guillard RR, Lorenzen CJ. Yellow-green algae with chlorophyllide c. Journal of Phycology. 1972;8:10–14. [Google Scholar]
- Gulati RD, Pires LMD, van Donk E. Lake restoration studies: failures, bottlenecks and prospects of new ecotechnological measures. Limnologica. 2008;38:233–247. [Google Scholar]
- Gustafsson S, Hansson L-A. Development of tolerance against toxic cyanobacteria in Daphnia. Aquatic Ecology. 2004;38:37–44. [Google Scholar]
- Gustafsson S, Rengefors K, Hansson L-A. Increased consumer fitness following transfer of toxin tolerance to offspring via maternal effects. Ecology. 2005;86:2561–2567. [Google Scholar]
- Hairston NG, Lampert W, Caceres CE, Holtmeier CL, Weider LJ, Gaedke U, Fischer JM, et al. Rapid evolution revealed by dormant eggs. Nature. 1999;401:446. [Google Scholar]
- Hairston NG, Holtmeier CL, Lampert W, Weider LJ, Post DM, Fischer JM, Cáceres CE, et al. Natural selection for grazer resistance to toxic cyanobacteria: evolution of phenotypic plasticity? Evolution. 2001;55:2203–2214. doi: 10.1111/j.0014-3820.2001.tb00736.x. [DOI] [PubMed] [Google Scholar]
- Hietala J, Reinikainen M, Walls M. Variation in life history responses of Daphnia to toxic Microcystis aeruginosa. Journal of Plankton Research. 1995;17:2307–2318. [Google Scholar]
- Holm NP, Armstrong DE. Role of nutrient limitation and competition in controlling the populations of Asterionella formosa and Microcystis aeruginosa and in semicontinuous culture. Limnology and Oceanography. 1981;26:622–634. [Google Scholar]
- Honjo M, Matsui K, Ueki M, Nakamura R, Fuhrman JA, Kawabata Z. Diversity of virus-like agents killing Microcystis aeruginosa in a hyper-eutrophic pond. Journal of Plankton Research. 2006;28:407–412. [Google Scholar]
- Huisman J, Sharples J, Stroom JM, Visser PM, Kardinaal WEA, Verspagen JMH, Sommeijer B. Changes in turbulent mixing shift competition for light between phytoplankton species. Ecology. 2004;85:2960–2970. [Google Scholar]
- Huisman J, Matthijs HCP, Visser PM. Harmful Cyanobacteria. Dordrecht: Springer; 2005. [Google Scholar]
- Ibelings BW, Havens KE. Cyanobacterial toxins: a qualitative meta-analysis of concentrations, dosage and effects in freshwater, estuarine and marine biota. In: Hudnell HK, editor. Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs. Berlin: Springer-Verlag; 2008. pp. 675–732. [DOI] [PubMed] [Google Scholar]
- Janse I, Kardinaal WEA, Meima M, Fastner J, Visser PM, Zwart G. Toxic and nontoxic Microcystis colonies in natural populations can be differentiated on the basis of rRNA gene internal transcribed spacer diversity. Applied and Environmental Microbiology. 2004;70:3979–3987. doi: 10.1128/AEM.70.7.3979-3987.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen M, Stoks R, Coors A, De Meester L. No evidence for a cost of selection by carbaryl exposure in terms of vulnerability to fish predation in Daphnia magna. Hydrobiologia. 2010a;643:123–128. [Google Scholar]
- Jansen M, Stoks R, Decaestecker E, Coors A, Van de Meutter F, De Meester L. Local exposure shapes spatial patterns in infectivity and community structure of Daphnia parasites. The Journal of Animal Ecology. 2010b;79:1023–1033. doi: 10.1111/j.1365-2656.2010.01718.x. [DOI] [PubMed] [Google Scholar]
- Jansen M, Coors A, Stoks R, De Meester L. Evolutionary ecotoxicology of pesticide resistance: a case study in Daphnia. Ecotoxicology. 2011a;20:543–551. doi: 10.1007/s10646-011-0627-z. [DOI] [PubMed] [Google Scholar]
- Jansen M, Stoks R, Coors A, Doorslaer W, De Meester L. Collateral damage: rapid exposure-induced evolution of pesticide resistance leads to increased susceptibility to parasites. Evolution. 2011b;65:2681–2691. doi: 10.1111/j.1558-5646.2011.01331.x. [DOI] [PubMed] [Google Scholar]
- Jeppesen E, Meerhoff M, Jacobsen B, Hansen R, Søndergaard M, Jensen J, Lauridsen T, et al. Restoration of shallow lakes by nutrient control and biomanipulation – the successful strategy varies with lake size and climate. Hydrobiologia. 2007;581:269–285. [Google Scholar]
- Jochimsen EM, Carmichael WW, An J, Cardo DM, Cookson ST, Holmes CEM, Antunes MB, et al. Liver failure and death after exposure to microcystins at a hemodialysis center in Brazil. New England Journal of Medicine. 1998;338:873–878. doi: 10.1056/NEJM199803263381304. [DOI] [PubMed] [Google Scholar]
- Jöhnk KD, Huisman J, Sharples J, Sommeijer B, Visser PM, Stroom JM. Summer heatwaves promote blooms of harmful cyanobacteria. Global Change Biology. 2008;14:495–512. [Google Scholar]
- Joung S-H, Kim C-J, Ahn C-Y, Jang K-Y, Boo S-M, Oh H-M. Simple method for a cell count of the colonial cyanobacterium, Microcystis sp. The Journal of Microbiology. 2006;44:562–565. [PubMed] [Google Scholar]
- Jungmann D. Toxic compounds isolated from Microcystis PCC7806 that are more active against Daphnia than two microcystins. Limnology and Oceanography. 1992;37:1777–1783. [Google Scholar]
- Jungmann D, Benndorf J. Toxicity to Daphnia of a compound extracted from laboratory and natural Microcystis spp., and the role of microcystins. Freshwater Biology. 1994;32:13–20. [Google Scholar]
- Kaebernick M, Rohrlack T, Christoffersen K, Neilan BA. A spontaneous mutant of microcystin biosynthesis: genetic characterization and effect on Daphnia. Environmental Microbiology. 2001;3:669–679. doi: 10.1046/j.1462-2920.2001.00241.x. [DOI] [PubMed] [Google Scholar]
- Kann J. 2006. Microcystis aeruginosa occurrence in the Klamath river system of Southern Oregon and Northern California. Report for the Yurok Tribe Environmental Program and Fisheries Department, CA by Aquatic Ecosystem Sciences, Ashland, OR. 26p.
- Kardinaal WEA, Visser PM. Dynamics of cyanobacterial toxins: sources of variability in microcystin-concentrations. In: Huisman J, Matthijs HCP, Visser PM, editors. Harmful Cyanobacteria. Dordrecht: Springer; 2005. pp. 41–63. [Google Scholar]
- Kasprzak P, Benndorf J, Gonsiorczyk T, Koschel R, Krienitz L, Mehner T, Hulsmann S, et al. Reduction of nutrient loading and biomanipulation as tools in water quality management: longterm observations on Bautzen Reservoir and Feldberger Haussee (Germany) Lake and Reservoir Management. 2007;23:410–427. [Google Scholar]
- Kosten S, Huszar VLM, Bécares E, Costa LS, van Donck E, Hansson L-A, Jeppesen E, et al. Warmer climates boost cyanobacterial dominance in shallow lakes. Global Change Biology. 2011 doi: 10.1111/j.1365-2486.2011.02488.x. [DOI] [Google Scholar]
- Kuiper-Goodman T, Falconer I, Fitzgerald J. Human health aspects. In: Chorus I, Bartram J, editors. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management. London: Spon Press; 1999. pp. 113–154. [Google Scholar]
- Kurmayer R, Jüttner F. Strategies for the co-existence of zooplankton with the toxic cyanobacterium Planktothrix rubescens in Lake Zurich. Journal of Plankton Research. 1999;21:659–683. [Google Scholar]
- Kurmayer R, Dittmann E, Fastner J, Chorus I. Diversity of microcystin genes within a population of the toxic cyanobacterium Microcystis spp. in Lake Wannsee (Berlin, Germany) Microbial Ecology. 2001;43:107–118. doi: 10.1007/s00248-001-0039-3. [DOI] [PubMed] [Google Scholar]
- Kurmayer R, Christiansen G, Chorus I. The abundance of microcystin-producing genotypes correlates positively with colony size in Microcystis sp. and determines its microcystin net production in Lake Wannsee. Applied and Environmental Microbiology. 2003;69:787–795. doi: 10.1128/AEM.69.2.787-795.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert W. Studies on the carbon balance of Daphnia pulex De Geer as related to environmental conditions. II. The dependence of carbon assimilation on animal size, temperature, food concentration and diet species. Archiv für Hydrobiologie. 1977;48:310–335. [Google Scholar]
- Lampert W. Inhibitory and toxic effects of blue-green algae on Daphnia. Internationale Revue der gesamten Hydrobiologie und Hydrographie. 1981;66:285–298. [Google Scholar]
- Lampert W. Further studies on the inhibitory effect of the toxic blue-green Microcystis aeruginosa on the filtering rate of zooplankton. Archiv für Hydrobiologie. 1982;95:207–220. [Google Scholar]
- Long BM, Jones GJ, Orr PT. Cellular microcystin content in N-limited Microcystis aeruginosa can be predicted from growth rate. Applied and Environmental Microbiology. 2001;67:278–283. doi: 10.1128/AEM.67.1.278-283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madgwick FJ. Restoring nutrient-enriched shallow lakes: integration of theory and practice in the Norfolk Broads, UK. Hydrobiologia. 1999;408:1–12. [Google Scholar]
- Maier HR, Kingston GB, Clark T, Frazer A, Sanderson A. Risk-based approach for assessing the effectiveness of flow management in controlling cyanobacterial blooms in rivers. River Research and Applications. 2004;20:459–471. [Google Scholar]
- Martin-Creuzburg D, von Elert E, Hoffmann KH. Nutritional constraints at the cyanobacteria–Daphnia magna interface: the role of sterols. Limnology and Oceanography. 2008;53:456–468. [Google Scholar]
- Martínez Hernandez J, Lopez-Rodas V, Costas E. Microcystins from tap water could be a risk factor for liver and corectal cancer: a risk intensified by global change. Medical Hypotheses. 2009;72:539–540. doi: 10.1016/j.mehy.2008.11.041. [DOI] [PubMed] [Google Scholar]
- Matveev V, Matveeva L, Jones GJ. Study of the ability of Daphnia carinata King to control phytoplankton and resist cyanobacterial toxicity: implications for biomanipulation in Australia. Australian Journal of Marine and Freshwater Research. 1994;45:889–904. [Google Scholar]
- Menden-Deuer S, Lessard EJ. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnology and Oceanography. 2000;45:569–579. [Google Scholar]
- Moss B. Climate change, nutrient pollution and the bargain of Dr Faustus. Freshwater Biology. 2010;55:175–187. [Google Scholar]
- Moss B, Madgwick FJ, Phillips GL. A Guide to the Restoration of Nutrient-Enriched Shallow Lakes. Norwich: Broads Authority and Environment Agency; 1996. [Google Scholar]
- Nishibe Y, Manage PM, Kawabata Z, Nakano S. Trophic coupling of a testate amoeba and Microcystis species in a hypertrophic pond. Limnology. 2004;5:71–76. [Google Scholar]
- Nizan S, Dimentman C, Shilo M. Acute toxic effects of the cyanobacterium Microcystis aeruginosa on Daphnia magna. Limnology and Oceanography. 1986;31:497–502. [Google Scholar]
- van Oosterhout F, Lürling M. Effects of the novel ‘Flock & Lock’ lake restoration technique on Daphnia in Lake Rauwbraken (The Netherlands) Journal of Plankton Research. 2011;33:255–263. [Google Scholar]
- Ouellette AJA, Wilhelm SW. Toxic cyanobacteria: the evolving molecular toolbox. Frontiers in Ecology and the Environment. 2003;1:359–366. [Google Scholar]
- Paerl HW, Huisman J. Blooms like it hot. Science. 2008;320:57–58. doi: 10.1126/science.1155398. [DOI] [PubMed] [Google Scholar]
- Paerl HW, Fulton RSI, Moisander PH, Dyble J. Harmful freshwater algal blooms, with an emphasis on cyanobacteria. The Scientific World Journal. 2001;1:76–113. doi: 10.1100/tsw.2001.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretyatko A, Teissier S, De Backer S, Triest L. Biomanipulation of hypereutrophic ponds: when it works and why it fails. Environmental Monitoring and Assessment. 2010 doi: 10.1007/s10661-10011-12057-z. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing, Reference Index Version 2.12.1. Vienna: R foundation for statistical computing; 2005. http://www.R-project.org (accessed on 26 January 2011) [Google Scholar]
- Reinikainen M, Ketola M, Walls M. Effects of the concentrations of toxic Microcystis aeruginosa and an alternative food on the survival of Daphnia pulex. Limnology and Oceanography. 1994;39:424–432. [Google Scholar]
- Reinikainen M, Meriluoto JAO, Spoof L, Harada K-I. The toxicities of a polyunsaturated fatty acid and a microcystin to Daphnia magna. Environmental Toxicology. 2001;16:444–448. doi: 10.1002/tox.10003. [DOI] [PubMed] [Google Scholar]
- Robb MS, Greenop B, Goss Z, Douglas GB, Adeney JA. Application of Phoslock™, an innovative phosphorus binding clay, to two Western Australian waterways: preliminary findings. Hydrobiologia. 2003;494:237–243. [Google Scholar]
- Rousseaux S. 2011. The importance of genetic diversity and evolution in metacommunities (PhD diss)., K. U. Leuven.
- Sarnelle O. Initial conditions mediate the interaction between Daphnia and bloom-forming cyanobacteria. Limnology and Oceanography. 2007;52:2120–2127. [Google Scholar]
- Sarnelle O, Wilson AE. Local adaptation of Daphnia pulicaria to toxic cyanobacteria. Limnology and Oceanography. 2005;50:1565–1570. [Google Scholar]
- Sarnelle O, Gustafsson S, Hansson L-A. Effects of cyanobacteria on fitness components of the herbivore Daphnia. Journal of Plankton Research. 2010;32:471–477. [Google Scholar]
- Schwarzenberger A, Zitt A, Kroth P, Mueller S, Von Elert E. Gene expression and activity of digestive proteases in Daphnia: effects of cyanobacterial protease inhibitors. BMC Physiology. 2010;10:6–20. doi: 10.1186/1472-6793-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigee DC, Glenn R, Andrews MJ, Bellinger EG, Butler RD, Epton HAS, Hendry RD. Biological control of cyanobacteria: principles and possibilities. Hydrobiologia. 1999;395–396:161–172. [Google Scholar]
- Sivonen K, Jones G. Cyanobacterial toxins. In: Chorus I, Bartram J, editors. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management. London: Spon Press; 1999. pp. 41–111. [Google Scholar]
- Song X, Liu Z, Yang G, Chen Y. Effects of resuspension and eutrophication level on summer phytoplankton dynamics in two hypertrophic areas of Lake Taihu, China. Aquatic Ecology. 2010;44:41–54. [Google Scholar]
- Stewart I, Seawright AA, Shaw GR. Cyanobacterial poisoning in livestock, wild mammals, and birds – an overview. In: Hudnell HK, editor. Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs. New York: Springer Science and Business Media, LLC; 2008. pp. 613–637. [DOI] [PubMed] [Google Scholar]
- Thompson JN. The Geographic Mosaic of Coevolution. Chicago: University of Chicago Press; 2005. [Google Scholar]
- Tucker S, Pollard P. Identification of cyanophage Ma-LBP and infection of the cyanobacterium Microcystis aeruginosa from an Australian subtropical lake by the virus. Applied and Environmental Microbiology. 2005;71:629–635. doi: 10.1128/AEM.71.2.629-635.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban MC, Leibold MA, Amarasekare P, De Meester L, Gomulkiewicz R, Hochberg ME, Klausmeier CA, et al. The evolutionary ecology of metacommunities. Trends in Ecology & Evolution. 2008;23:311–317. doi: 10.1016/j.tree.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Van Doorslaer W, Stoks R, Duvivier C, Bednarska A, De Meester L. Population dynamics determine genetic adaptation to temperature in Daphnia. Evolution. 2009;63:1867–1878. doi: 10.1111/j.1558-5646.2009.00679.x. [DOI] [PubMed] [Google Scholar]
- Van Wichelen J, Van Gremberghe I, Vanormelingen P, Debeer A-E, Leporcq B, Menzel D, Codd GA, et al. Strong effects of amoebae grazing on the biomass and genetic structure of a Microcystis bloom (Cyanobacteria) Environmental Microbiology. 2010;12:2797–2813. doi: 10.1111/j.1462-2920.2010.02249.x. [DOI] [PubMed] [Google Scholar]
- Wilson AE, Sarnelle O, Neilan BA, Salmon TP, Gehringer MM, Hay ME. Genetic variation of the bloom-forming cyanobacterium Microcystis aeruginosa within and among lakes: implications for harmful algal blooms. Applied and Environmental Microbiology. 2005;71:6126–6133. doi: 10.1128/AEM.71.10.6126-6133.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AE, Sarnelle O, Tillmanns AR. Effects of cyanobacterial toxicity and morphology on the population growth of freshwater zooplankton: meta-analyses of laboratory experiments. Limnology and Oceanography. 2006;51:1915–1924. [Google Scholar]
- Wu YH, Liu JT, Yang LZ, Chen H, Zhang SQ, Zhao HJ, Zhang NM. Allelopathic control of cyanobacterial blooms by periphyton biofilms. Environmental Microbiology. 2011;13:604–615. doi: 10.1111/j.1462-2920.2010.02363.x. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Jones LE, Ellner SP, Fussmann GF, Hairston NG., Jr Rapid evolution drives ecological dynamics in a predator-prey system. Nature. 2003;424:303–306. doi: 10.1038/nature01767. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Ellner SP, Jones LE, Bohannan BJM, Lenski RE, Hairston NG., Jr Cryptic population dynamics: rapid evolution masks trophic interactions. PLoS Biology. 2007;5:1868–1879. doi: 10.1371/journal.pbio.0050235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Hu H-Y, Hong Y, Yang J. Isolation of a Poterioochromonas capable of feeding on Microcystis aeruginosa and degrading microcystin-LR. FEMS Microbiology Letters. 2008;288:241–246. doi: 10.1111/j.1574-6968.2008.01355.x. [DOI] [PubMed] [Google Scholar]
- Zimba PV, Camus A, Allen EH, Burkholder JM. Co-occurrence of white shrimp, Litopenaeus vannamei, mortalities and microcystin toxin in a southeastern USA shrimp facility. Aquaculture. 2006;261:1048–1055. [Google Scholar]
- Zurawell RW, Kotak BG, Prepas EE. Influence of lake trophic status on the occurrence of microcystin-LR in the tissue of pulmonate snails. Freshwater Biology. 1999;42:707–718. [Google Scholar]
- Zurawell RW, Chen H, Burke JM, Prepas EE. Hepatotoxic cyanobacteria: a review of the biological importance of microcystins in freshwater environments. Journal of Toxicology and Environmental Health. Part B, Critical reviews. 2005;8:1–37. doi: 10.1080/10937400590889412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


