Abstract
The arrival of an invasive species can have wide-ranging ecological impacts on native taxa, inducing rapid evolutionary responses in ways that either reduce the invader's impact or exploit the novel opportunity that it provides. The invasion process itself can cause substantial evolutionary shifts in traits that influence the invader's dispersal rate (via both adaptive and non-adaptive mechanisms) and its ability to establish new populations. I briefly review the nature of evolutionary changes likely to be set in train by a biological invasion, with special emphasis on recent results from my own research group on the invasion of cane toads (Rhinella marina) through tropical Australia. The toads’ invasion has caused evolutionary changes both in the toads and in native taxa. Many of those changes are adaptive, but others may result from non-adaptive evolutionary processes: for example, the evolved acceleration in toad dispersal rates may be due to spatial sorting of dispersal-enhancing genes, rather than fitness advantages to faster-dispersing individuals. Managers need to incorporate evolutionary dynamics into their conservation planning, because biological invasions can affect both the rates and the trajectories of evolutionary change.
Keywords: adaptation, alien species, ecological impact, spatial sorting
Introduction
As an introduced species spreads outside its native range, it initiates a complex array of evolutionary processes that can produce clear effects over a timeframe of years or decades. The opportunity to measure not only selection, but also its results, has motivated many biologists to explore this intersection of ecology, evolution, and population biology (Cox 2004; Huey et al. 2005). The consequent explosion of information on evolutionary aspects of biological invasions has attracted several excellent reviews (e.g., Thompson 1998; Mooney and Cleland 2001; Cox 2004; Lambrinos 2004; Strauss et al. 2006b; Sax et al. 2007; Vellend et al. 2007; Buswell et al. 2011; Westley 2011). It is clear that evolutionary change can occur rapidly (Reznick and Ghalambor 2001; Hairston et al. 2005; Carroll et al. 2007; Hendry et al. 2008) and can modify traits both in invaders and in the taxa with which they interact. Thus, the proposition that invasion can drive evolutionary change is well supported, and researchers are now asking more detailed questions such as how frequently such changes occur (Buswell et al. 2011) and what genetic mechanisms and adaptive processes underlie them (Lee and Bell 1999; Carroll et al. 1998, 2005; Carroll 2007a,b, 2008). Understanding such topics may provide a basis for novel approaches to controlling the invader, or mitigating its impact, for example, we may be able to identify and exploit adaptive trade-offs and evolutionary traps to curtail invader numbers (Ward-Fear et al. 2010; Lankau and Strauss 2011). In this review, I will examine ideas and evidence on the evolutionary consequences of biological invasions, with a strong focus on one study system – the invasion of cane toads through tropical Australia.
Impacts of biological invasion on the rate and trajectory of evolution
In many cases, the most rapid changes in trait values may occur early in the process of adaptation, as soon as the novel selective challenge is encountered. Fitness differentials are high initially, but reduce through time until the most common genotypes are those that confer highest fitness. The arrival of an invasive species thus can elicit a rapid shift in genotype frequencies until the challenge exerted by the interloper has been blunted by adaptation (e.g., Vermeij 1996; Stockwell et al. 2003; Buswell et al. 2011). Because many invader populations are increasing (ro > 1) whereas those of many native taxa are not, and rapid population growth enhances the opportunities for rapid evolution (Reznick and Ghalambor 2001), invaders may evolve more rapidly than the native taxa they affect.
Adaptation is not inevitable. The potential for evolutionary change can be reduced by low genetic diversity within the invader, as a result of founder effects (Lee et al. 2007; but see Kolbe et al. 2007). Likewise, intense selection exerted by an invader may depress population sizes of the native taxa so greatly that extinction is more likely than adaptation. Other selective forces may oppose the changes favored by the invaders’ presence. Phenotypically, plastic responses to invader cues may generate suboptimal phenotypes, curtailing effective selection (Richards et al. 2006) but potentially serving as a bridge to ultimate adaptive evolution (Ghalambor et al. 2007). Attributing a lack of evolutionary response to such mechanistic constraints is a formidable logistical challenge, requiring sophisticated experimental work to tease apart the genetic underpinnings of adaptive responses, or the lack thereof (Carroll et al. 2005).
Thus, invasive species have the potential to cause rapid evolutionary change, but may not always do so. Proliferating empirical studies on evolutionary shifts associated with biological invasions (Thompson 1998; Westley 2011) mean that it may soon be possible to quantitatively compare rates of evolutionary change between invasive species in their ancestral range versus the newly occupied area, or invasive species in sites that have been colonized for differing lengths of time, or native taxa in areas that have or have not been invaded, or invasive versus native taxa. Such comparisons will clarify the effects of biological invasion on rates of evolution.
Natural ecosystems contain complex webs of interactions among species, and the arrival of an invasive species can reverberate via many pathways. We may see evolutionary changes in the invader, in native species directly impacted by it, and in species influenced indirectly via their interactions with affected native taxa. Many systems are under simultaneous challenge from multiple invaders, adding to the complexity of response. The traits affected also are diverse, ranging through morphology, ecology, life history, physiology, and behavior. The interspecific interactions may involve relationships such as predation, herbivory, pathogen transfer, interference or exploitative competition, evolutionary traps (such as consuming a lethally toxic invader that resembles a harmless native prey species), and hybridization. In total, then, a biological invasion – even by a single species into a relatively species-poor natural system – can impose novel ecological and evolutionary pressures on a vast array of biological traits, via a vast array of direct and indirect pathways (see Schlaepfer et al. 2002; Cox 2004; Strauss et al. 2006a). I review such processes below.
Evolution driven by the process of range expansion
Some of the selective challenges experienced by invaders result from the invasion process per se whereas others involve system-specific interactions with abiotic challenges, with the native biota or with other invaders (Fig. 1).
Figure 1.
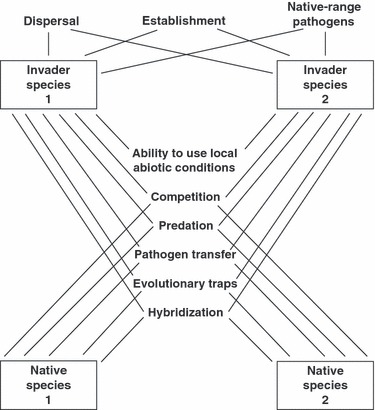
A schematic view of evolutionary processes at work during biological invasions. Lines linking two taxa show potential pathways by which selective forces may be exerted by one species upon the other. Invaders may be subject to selection or sorting for more rapid dispersal and also for traits that facilitate population establishment and minimize dispersal-reducing effects of pathogens. Invaders also interact with each other, and with native species, via a network of processes that include competition, predation, pathogen transfer, toxic ingestion, and hybridization. Each species can interact with others either directly or via indirect effects (mediated by perturbations to other links). The end result is that invasion can unleash a complex array of ecological and evolutionary pressures, even in relatively simple (stable, species-poor) systems.
Establishment success
The ability of a few founders to set up a population depends on the mating system, but generalities may be elusive. Colonizing populations of smooth cordgrass show high rates of self-fertilization, allowing a small number of individuals to found a new population (Brown and Marshall 1981; see also Lavergne and Molofsky 2007 for similar results on vegetative reproduction), but the reverse situation also occurs (outcrossing increases genetic diversity in newly founded populations: Brown and Marshall 1981). The mating system also may be under divergent selection in invasion-front populations compared to those in long-colonized areas, reflecting spatial differences in variables such as population density. Mating systems and patterns of genetic diversity within populations may interact in complex ways with the determinants of dispersal rate. For example, highly dispersive organisms often have multiple introductions to the same site, increasing genetic variation (Kolbe et al. 2007).
High levels of phenotypic plasticity may enhance colonization success, by allowing invaders to adopt the phenotypes best-suited to local conditions (Brown et al. 2011a), but environmentally induced flexibility sometimes may reduce rather than enhance fitness (Price et al. 2003; Yeh and Price 2004; Richards et al. 2006; Ghalambor et al. 2007; Thibert-Plante and Hendry 2011). Some traits may benefit from flexibility whereas others do not. Because colonization success is enhanced by larger relative brain size (in mammals, birds, reptiles, and amphibians: Amiel et al. 2011), we might expect the evolution of larger brain size during colonization of some but not all environments (e.g., smaller brains may be optimal in Australia, reflecting resource constraints: Amiel et al. 2011). Plausibly, the selective advantages of behavioral flexibility (and thus, larger brain size) may shift in complex ways during a biological invasion, with initial benefits reducing through time since colonization, as the challenges to the invader cease to be novel. Trade-off models suggest that invaders will be under selection (and also spatial sorting: Shine et al. 2011) to reduce investment into any processes that constrain dispersal rate. Thus, for example, we might expect lower investment into immune defense in invaders (Lee and Klasing 2004).
Cane toads (Rhinella marina) are large toxic anurans native to Central and South America, but introduced to northeastern Australia in 1935 in a futile attempt at biocontrol (Shine 2010). They have since spread across the Australian tropics. Behavioral plasticity has allowed toads to colonize climatic zones well outside those experienced in the native range (Brown et al. 2011a). Analyses of progeny from adult toads collected at various points across the toads’ invasion history reveal significant evolutionary changes in growth rates, consistent with the hypothesis that selective targets at the invasion front may differ from those in long-colonized areas (Phillips 2009; Phillips et al. 2010c). The prediction of reduced immunocompetence in toads at the invasion front accords with a high incidence of bacterially influenced arthritis in these animals (Brown et al. 2007), as well as weaker responses to subcutaneous injection of phytohemagglutinin (G. P. Brown and R. Shine, unpublished data), and lower metabolic investment in response to a standardized immune challenge (Llewellyn 2009).
Dispersal rate
In a range-expanding population, natural selection can favor the evolution of enhanced rates of dispersal, whereby individuals that disperse most rapidly benefit because their access to resources is not constrained by high densities of conspecifics (Travis and Dytham 2002). Selection for rapid dispersal also can occur at the level of families (variance in dispersal reduces among-progeny competition: Hamilton and May 1977), or groups (if rates of population extinction are high, and all new populations are founded by dispersers, then population-level selection can maintain high frequencies of dispersing individuals: Van Valen 1973). Intriguingly, rapid dispersal also can evolve non-adaptively, by spatial sorting of genes within the invading species (Shine et al. 2011). Any alleles that code for faster dispersal will tend to accumulate at the expanding range edge, whereas alleles that code for slower dispersal will be confined to long-colonized areas (Travis and Dytham 2002). Because slow-dispersing individuals cannot reach the invasion front, accelerated rates of dispersal will evolve even if this trait does not enhance lifetime reproductive success (Shine et al. 2011). It is evolution through space not time and does not depend upon differential fitness.
A wide range of traits that influence rates of dispersal might evolve at an expanding range edge. For plants, traits such as small seed size, short generation time, high fecundity, and reliance on abiotic dispersal mechanisms may enhance dispersal rate (Daehler 1998; Grotkopp et al. 2002; Ridley and Ellstrand 2009). For animals, range expansion may be accelerated by better locomotor ability, high fecundity, rapid growth, and habitat breadth (Lodge 1993; Thomas et al. 2001; Cassey 2002). The traits that enhance dispersal rate are system specific – the features that enable a seed to drift through the air are very different from those that enable it to cling to a mobile bird or mammal, and from the ones enabling that host organism to move further than its conspecifics. One interesting set of traits involves host–pathogen interactions; if pathogens vary in the degree to which they impede host dispersal, we expect to see the evolution of lower-impact pathogens in invasion-front populations of the host (Phillips et al. 2010a).
A growing literature provides examples of dispersal-facilitating traits accumulating at expanding range edges. For example, seed mass of lodgepole pine was lowest at the range edge (Cwynar and Macdonald 1987). Speckled wood butterflies in colonizing populations were larger and had longer thoraxes (where the flight muscles are located) and broader wings than conspecifics in more central parts of the species’ range (Hill et al. 1999). Two species of bush crickets showed more of the long-winged morph than the short-winged morph in range-expanding populations (Simmons and Thomas 2004). Similar trends occur in populations of ground beetles colonizing northwards in southern Canada (Niemala and Spence 1991). Work on allozyme variants in the flight abilities of butterflies has shown how the genetic underpinnings of differential dispersal rates can influence extinction and colonization rates in metapopulations (Hanski and Saccheri 2006; Saccheri and Hanski 2006; Zheng et al. 2009). In some cases at least, selection imposed during the process of dispersal may create a distinctive subset of traits that facilitate colonization: for example, the individuals surviving a long and rigorous migration episode to a new habitat patch are likely to exhibit above-average migratory efficiency and/or energy utilization (Kinnison and Hairston's 2007‘favored-founder’ hypothesis).
As predicted from the ideas mentioned earlier, cane toads in Australia have evolved faster dispersal during their invasion. Annual rates of spread have increased about fivefold within 75 years (from 10–15 to 55–60 km per annum: Urban et al. 2008), driven by evolved changes in behavior, morphology, and physiology (activity levels, relative leg length, stamina: Phillips et al. 2006; Llewelyn et al. 2010). Mean daily dispersal distances are about 10-fold higher for invasion-front toads than for conspecifics from long-colonized areas (Alford et al. 2009; Fig. 2A). Raising offspring in common-garden conditions has confirmed significant heritability for dispersal rates (Phillips et al. 2010b). We do not yet know whether faster dispersal has evolved because it enhances individual fitness (i.e., via natural selection) or because of spatial sorting. In keeping with the latter hypothesis, the fastest-dispersing toads are the most likely to be killed by predators (Phillips et al. 2010c), invasion-front toads rarely reproduce (Crossland et al. 2008), and long-legged (fast-dispersing) toads at the invasion front often develop spinal arthritis (Brown et al. 2007; Fig. 2B).
Figure 2.
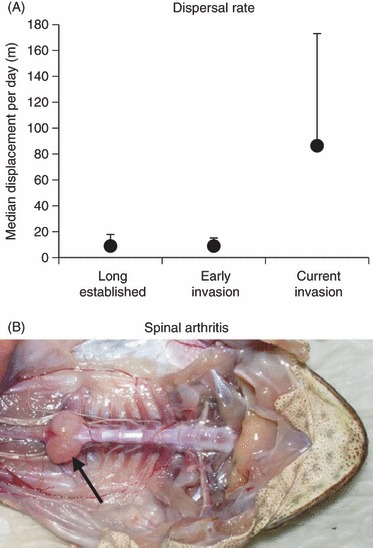
In Australia, cane toads at the invasion front now travel much further per night than was the case early in the toad's invasion process (A); this high dispersal rate puts substantial pressure on the toads’ locomotor apparatus, resulting in spinal arthritis (large bony swellings on posterior spine, indicated by arrow (B). Modified from Alford et al. (2009) and Brown et al. (2007).
Evolution driven by interactions between invaders and native species
A range-expanding species is likely to encounter novel conditions as it spreads outside its previous geographic distribution. If the optimal phenotype to deal with those novel conditions differs from that favored under ancestral conditions, selection likely will result in adjustments that enhance the invader's ability to exploit these novel opportunities.
At first sight, it would seem that local abiotic conditions pose a challenge to the invader (for which they are novel) but not the local taxa (which have evolved in those circumstances). However, the effects of competition can be mediated via shifts in abiotic factors. For example, an invasive woody shrub can alter thermal and light levels on the ground beneath it, as well as reducing nutrient availability and salinity in the soil (Cox 2004; Benkman et al. 2008; Gonzalez et al. 2008). In marine benthic and terrestrial plant communities, invaders may take up open space, thus restricting settlement opportunities. Any such shift in resource availability might impose selection on habitat selection and use by native species.
The parallel effects on invaders and natives of biotic interactions are more clear-cut and may influence establishment success (Strauss et al. 2006b; Tingley et al. 2011) as well as subsequent adaptive shifts (Langkilde 2009). Either or both the invader and the native may be affected by competition, predation, herbivory, toxic ingestion, pathogen transfer, or hybridization between taxa (Fig. 1). The nature of a native taxon's ecological relationship with the invader will necessarily modify the nature of impact. As Carroll (2008, p. 361) notes, ‘both opportunity and catastrophe generate adaptive responses’.
Catastrophes induced by invasive species have attracted extensive research. In the case of invasive predators that consume native prey, selection may favor rapid adaptive responses in the endemic fauna to detect and avoid the unwelcome new arrival. For example, the arrival of mammalian predators (rats, stoats, cats, possums, etc.) may have exerted intense selection on New Zealand lizards. The absence of mammalian predators on these islands over evolutionary time presumably fashioned lizard biology in ways that reduced their vulnerability to visually hunting birds, but were ineffective against mammalian predators that use chemosensory cues for hunting (Hoare et al. 2007). The arrival of predatory mammals thus may have imposed selection on a suite of lizard attributes, with a sudden selective advantage to reducing the production and dissemination of scent cues detectable by such predators, to using retreat sites inaccessible to such predators, and to responding behaviorally to predator cues in ways that enhance lizard survival (e.g., Hoare et al. 2007). Similarly, the arrival of foxes in Australia may have imposed strong selection for arboreal rather than terrestrial nesting in birds and for avoidance of fox cues by edible-sized mammals.
Native taxa with other types of ecological relationships to the invader will be affected in other (and sometimes multiple) ways. For example, an invasive species may consume juveniles of a native species, compete with subadults of the same species, and be consumed by adults of that taxon. The complexity of such interactions will generate equally complex evolutionary routes to impact mitigation. Rather than trying to review this extensive field in detail (see Cox 2004 for examples), I simply note that some invaders will compete with native taxa for resources (potentially favoring adaptive shifts in niche parameters for one or both parties), some will hybridize with native taxa (potentially exerting selection on mating systems and especially, mate choice), and some will exchange pathogens with native taxa (imposing selection on the ability of the novel host to recognize and suppress the newly encountered pathogen: Cox 2004; Pizzatto and Shine 2011a,b). In some cases, the invader may evolve in ways that reduce rather than increase the severity of its impact on native taxa (e.g., reduced allelopathy: Lankau et al. 2009).
The importance of invader-driven catastrophe for conservation issues has distracted attention from the possibility that invasion benefits a subset of native taxa (King et al. 2006; Hagman and Shine 2007). For example, the invader may provide an additional food source for predators and additional hosts for parasites. The net effect of an invasive species on any given native taxon will be the sum total of negative and positive effects. For example, beneficial effects of novel food may outweigh deleterious habitat modifications. If the morphology, physiology, or behavior that allows effective exploitation of this novel resource differs from that exhibited by the native taxon at the time of invasion, then we may see rapid shifts in traits that allow more successful exploitation of the new opportunity. Carroll's work on soapberry bugs provides elegant experimental evidence of the evolutionary processes that have enabled native insects to exploit invading plants (Carroll et al. 1998, 2005; Carroll 2007a,b, 2008). The actual changes likely will be complex and spatially heterogeneous and reflect adaptation in the invader (in ways that reduce its vulnerability to the native taxon) as well as adaptive responses of the endemic biota to the invader.
The main ecological impact of cane toads on the Australian native fauna is via lethal toxic ingestion by predators (and not, for example, by competition, predation, or pathogen transfer), and only a few predator species are affected at the population level (mostly large species: Shine 2010). Rapid aversion learning reduces mortality levels for most predator species and thus reduces the intensity of selection on toad-smart traits (Shine 2010; Somaweera et al. 2011). Nonetheless, at least one species of frog-eating snake (the death adder, Acanthophis praelongus) experiences strong selection on behavior (avoidance of toads as prey) and morphology (reduced head size relative to body size, a trait influencing the snake's ability to consume a toad large enough to kill it: Phillips et al. 2010d; Fig. 3A). In another toad-vulnerable species (the red-bellied blacksnake, Pseudechis porphyriacus), snakes from toad-colonized areas are less likely to eat a toad (Fig. 3B), and more tolerant to the toads’ toxin, than are conspecifics from toad-free areas. Blacksnakes also show a reduction in relative head size as a function of the duration of sympatry with cane toads (Phillips and Shine 2006).
Figure 3.
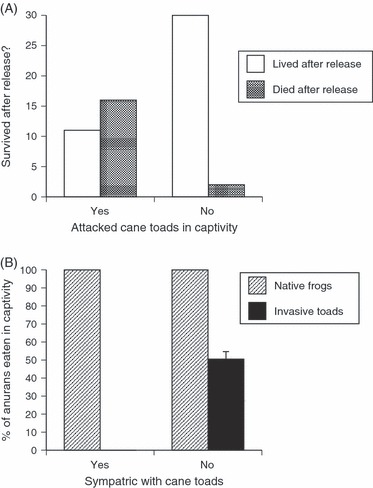
Radio-tracking of death adders (Acanthophis praelongus) after cane toad invasion showed that a snake's fate in the wild could be predicted from its behavioral responses to cane toads (Rhinella marina) in laboratory tests: snakes that attempted to eat toads in the laboratory also did so in the field after release and were killed by the toads’ toxins (A). This selective force has resulted in adaptive shifts in prey choice in snake species exposed to cane toads. (B) Geographic comparisons in blacksnakes, Pseudechis porphyriacus, show that snakes from toad-infested areas refuse to consume toads when offered them in captivity, whereas toad-naïve snakes readily attack toads (and thus are likely to be fatally poisoned). Modified from Phillips et al. (2010d) and Phillips and Shine (2006).
Evolution driven by the invader's impact on interactions among native species
An invader's arrival may affect not only ecological (and thus evolutionary) interactions between an invader and a native species but also interactions between native species. Adaptive shifts can be driven by changes in the abundance, behavior, ecology, morphology, or physiology of key species. For example, reduced abundance of some native taxon may force its main predator to shift in dietary habits, or a change in habitat use by that prey taxon may force the predator to forage elsewhere. Reduced abundance of a predator may allow a native prey taxon to expand its ecological niche. It is easy to envisage long and complex chains of causation ramifying through trophic levels, but documenting such changes poses a formidable logistical challenge. Examples include increased hatching success of turtle eggs because of invasive-toad-induced mortality of natural predators (varanid lizards: Doody et al. 2006), and an introduced leafhopper causing a population expansion in a parasitoid wasp, thereby increasing rates of predation on a native leafhopper (Settle and Wilson 1990). The invader also may act as a bridge to connect two native taxa, for example, through gene flow (if native taxa can interbreed with the invader but not with each other) or pathogen transfer (if the invader can take parasites from native taxa into situations where they can infect other native taxa). Any such changes could enforce selection on the native species. The myriad ecological connections within natural food webs mean that the potential complexities of indirect effects of invasion are enormous.
The destabilizing effects of biological invasions on host–parasite relationships remain a substantial challenge for future research. Some parasites of native species may virtually disappear after an invader arrives, for example, a tapeworm of Australian pythons has declined since arrival of cane toads, apparently because the (virtually inedible) toad provides a terminal host within which adult tapeworms can develop, but are never passed on to snake predators (Freeland et al. 1986). Other parasites may benefit from the invader's arrival, for example, myxosporidians that occupy anuran bladders have increased in frequency among Australian frogs since the cane toad's arrival (Hartigan et al. 2010). Parasites that accompany an invader may host-switch to native taxa, sometimes with devastating results, and the reverse may occur also (transfer of parasites from native taxa to the invader). Such disruptions of existing host–parasite systems may impose selection both on the novel hosts (to better recognize and destroy the parasite) and on the parasite (to evade the novel host's immune responses). Invasive species allow us to explore the initial stages of parasite–host coevolution, before adaptive shifts obscure interactions (Pizzatto et al. 2010; Pizzatto and Shine 2011a,b).
Applications of an evolutionary perspective
How does an evolutionary perspective help us to manage invasion biology systems (see also Ashley et al. 2003; Stockwell et al. 2003; Carroll 2011)? My own group's research on invasive cane toads has suggested the following practical applications of evolutionary thinking:
Predicting the rate of invader spread– Both selection and sorting can favor rapid acceleration of the invasion front's spread, as well as potentially favoring broader habitat use. Managers in advance of the invasion front thus are likely to overestimate the time lag before invaders arrive. The magnitude of this increase in cane toad invasion rate (10-fold shift in mean daily displacement within 70 years: Alford et al. 2009; see Fig. 2A) suggests that such effects often may be substantial.
Predicting the attributes of invaders– Rapid adaptive or non-adaptive shifts associated with the invasion process may change many attributes of the invader, such that information and approaches developed from long-colonized areas may provide an unreliable basis from which to predict the attributes, impacts, and interactions of the invasion vanguard.
Novel control approaches based on evolved traits of invaders – If selection or sorting for accelerated dispersal results in lower investment in dispersal-constraining traits (such as immunocompetence: Lee and Klasing 2004), or lower investment into defensive compounds (Siemann and Rogers 2003), we might be able to target control at such evolved vulnerabilities (Brown et al. 2007).
Novel control approaches based on phylogenetic conservatism– If the invader belongs to a phylogenetic lineage not present in the invaded region, it may differ from native taxa in basic facets of biology. Such divergences provide opportunities for species-selective control. For example, the tadpoles of invasive cane toads use pheromones to communicate alarm and food location, and we might be able to utilize such species-specific communication systems to control toads without influencing native anurans (Hagman and Shine 2009; Crossland and Shine 2011).
Novel control approaches based on evolutionary mismatches – A species that evolves in one part of the world is unlikely to be perfectly suited to conditions within some other area that it invades. Identifying and exacerbating those mismatches may provide opportunities for target-specific control (Stockwell et al. 2003; Hendry et al. 2011). For example, cane toads in Australia do not recognize large predatory local ants as dangerous and are more vulnerable to ant attack than are native frogs; thus, we might be able to exploit the ants’ selective predation to help control toad numbers (Ward-Fear et al. 2010). Traits with strong phylogenetic conservatism likely will respond less rapidly to selection than less conservative traits, enhancing the feasibility of exploiting such traits for biocontrol.
Prioritizing vulnerable native taxa for active management– The traits determining a native species’ vulnerability to an invader, and the mechanisms by which it eventually adapts to the invader's presence, likely will show strong phylogenetic conservatism. Thus, we can predict which native taxa are most vulnerable and allocate management to those species for which the magnitude of impact will be greatest. We can also predict the duration of impact, based on the mechanisms by which native taxa adjust to invader presence. In the case of cane toad impacts, a capacity for taste aversion learning enables a rapid recovery from initial toad impact; a capacity for adaptive (genetically based) shifts allows recovery over a much longer timescale; and an inability to modify responses by either mechanism results in persistent high vulnerability to the invader (Shine 2010).
Summary
Understanding the powerful evolutionary forces unleashed by biological invasions can assist managers to predict and mitigate undesirable impacts of the invasion process. Although the study of invasion biology reveals many catastrophes, the emerging evidence of dynamic responses to invasion provides a glimmer of encouragement. Given the opportunity, many native taxa may prove surprisingly capable of dealing with – or even exploiting – the arrival of invaders. If we understand those evolutionary adjustments, we may be able to assist vulnerable taxa to withstand the challenges that we have imposed upon them by translocating so many organisms around the globe.
Acknowledgments
I thank the members of Team Bufo for all their ideas and hard work, the Australian Research Council for funding, the editors for inviting me to prepare this review, and two anonymous reviewers for helpful comments.
Literature cited
- Alford RA, Brown GP, Schwarzkopf L, Phillips BL, Shine R. Comparisons through time and space suggest rapid evolution of dispersal behaviour in an invasive species. Wildlife Research. 2009;36:23–28. [Google Scholar]
- Amiel JJ, Tingley R, Shine R. Effects of relative brain size on establishment success of invasive amphibians and reptiles. PLoS ONE. 2011;6:e18277. doi: 10.1371/journal.pone.0018277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley MV, Willson MF, Pergams ORW, O'Dowd DJ, Gende SM, Brown SJ. Evolutionarily enlightened management. Biological Conservation. 2003;111:115–123. [Google Scholar]
- Benkman CW, Siepelski AM, Parchman TL. The local introduction of strongly interacting species and the loss of geographic variation in species and species interactions. Molecular Ecology. 2008;17:395–404. doi: 10.1111/j.1365-294X.2007.03368.x. [DOI] [PubMed] [Google Scholar]
- Brown AHD, Marshall DR. Evolutionary changes accompanying colonization in plants. In: Scudder GGT, Reveal JL, editors. Evolution Today. Proceedings of the Second International Conference of Systematic and Evolutionary Biology. Pittsburgh, PA: Carnegie-Mellon University; 1981. pp. 351–363. [Google Scholar]
- Brown GP, Shilton CM, Phillips BL, Shine R. Invasion, stress, and spinal arthritis in cane toads. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17698–17700. doi: 10.1073/pnas.0705057104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GP, Kelehear C, Shine R. Effects of seasonal aridity on the ecology and behaviour of invasive cane toads (Rhinella marina) in the Australian wet-dry tropics. Functional Ecology. 2011a doi: 10.1111/j.1365-2435.2011.01888.x. [DOI] [Google Scholar]
- Brown GP, Shilton CM, Shine R. Measuring amphibian immunocompetence: validation of the phytohemagglutinin skin-swelling assay in the cane toad, Bufo marinus. Methods in Ecology and Evolution. 2011b;2:341–348. [Google Scholar]
- Buswell JM, Moles AT, Hartley S. Is rapid evolution common in introduced plant species? Journal of Ecology. 2011;99:214–224. [Google Scholar]
- Carroll SP. Brave New World: the epistatic foundations of natives adapting to invaders. Genetica. 2007a;129:193–204. doi: 10.1007/s10709-006-9014-8. [DOI] [PubMed] [Google Scholar]
- Carroll SP. Natives adapting to invasive species – ecology, genes, and the sustainability of conservation. Ecological Research. 2007b;22:892–901. [Google Scholar]
- Carroll SP. Facing change: forms and foundations of contemporary adaptation to biotic invasions. Molecular Ecology. 2008;17:361–372. doi: 10.1111/j.1365-294X.2007.03484.x. [DOI] [PubMed] [Google Scholar]
- Carroll SP. Conciliation biology: the eco-evolutionary management of permanently invaded biotic systems. Evolutionary Applications. 2011;4:184–199. doi: 10.1111/j.1752-4571.2010.00180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SP, Klassen SP, Dingle H. Rapidly evolving adaptations to host ecology and nutrition in the soapberry bug. Evolutionary Ecology. 1998;12:955–968. [Google Scholar]
- Carroll SP, Loye JE, Dingle H, Mathieson M, Famula TR, Zalucki M. And the beak shall inherit – evolution in response to invasion. Ecology Letters. 2005;8:944–951. doi: 10.1111/j.1461-0248.2005.00800.x. [DOI] [PubMed] [Google Scholar]
- Carroll SP, Hendry AH, Reznick D, Fox CE. Evolution on ecological time scales. Functional Ecology. 2007;21:387–393. [Google Scholar]
- Cassey P. Life history and ecology influences establishment success of introduced land birds. Biological Journal of the Linnean Society. 2002;76:465–480. [Google Scholar]
- Cox GW. Alien Species and Evolution. Washington: Island Press; 2004. [Google Scholar]
- Crossland MR, Shine R. Cues for cannibalism: cane toad tadpoles use chemical signals to locate and consume conspecific eggs. Oikos. 2011;120:327–332. [Google Scholar]
- Crossland MR, Brown GP, Anstis M, Shilton C, Shine R. Mass mortality of native anuran tadpoles in tropical Australia due to the invasive cane toad (Bufo marinus. Biological Conservation. 2008;141:2387–2394. [Google Scholar]
- Cwynar LC, Macdonald GM. Geographical variation of lodgepole pine in relation to population history. American Naturalist. 1987;129:463–469. [Google Scholar]
- Daehler CC. The taxonomic distribution of invasive angiosperm plants: ecological insights and comparison to agricultural weeds. Biological Conservation. 1998;84:167–180. [Google Scholar]
- Doody JS, Green B, Sims R, Rhind D, West P, Steer D. Indirect impacts of invasive cane toads (Bufo marinus) on nest predation in pignosed turtles (Carettochelys insculpta. Wildlife Research. 2006;33:49–54. [Google Scholar]
- Freeland WJ, Delvinquier BLJ, Bonnin B. Food and parasitism of the cane toad, Bufo marinus, in relation to time since colonisation. Australian Wildlife Research. 1986;13:489–499. [Google Scholar]
- Ghalambor CK, McKay JK, Carroll S, Reznick DN. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation to new environments. Functional Ecology. 2007;21:394–407. [Google Scholar]
- Gonzalez A, Lambert A, Ricciardi A. When does ecosystem engineering cause invasion and species replacement? Oikos. 2008;117:1247–1257. [Google Scholar]
- Grotkopp EM, Rejmanek M, Rost TL. Towards a causal explanation of plant invasiveness: seedling growth and life-history strategies of 29 pine (Pinus) species. American Naturalist. 2002;159:396–419. doi: 10.1086/338995. [DOI] [PubMed] [Google Scholar]
- Hagman M, Shine R. Effects of invasive cane toads on Australian mosquitoes: does the dark cloud have a silver lining? Biological Invasions. 2007;9:445–452. [Google Scholar]
- Hagman M, Shine R. Larval alarm pheromones as a potential control for invasive cane toads (Bufo marinus) in tropical Australia. Chemoecology. 2009;19:211–217. [Google Scholar]
- Hairston NG, Jr, Ellner SP, Geber MA, Yoshida T, Fox JA. Rapid evolution and the convergence of ecological and evolutionary time. Ecology Letters. 2005;8:1114–1127. [Google Scholar]
- Hamilton WD, May RM. Dispersal in stable habitats. Nature. 1977;269:578–581. [Google Scholar]
- Hanski I, Saccheri I. Molecular-level variation affects population growth in a butterfly metapopulation. PLoS Biology. 2006;4:e129. doi: 10.1371/journal.pbio.0040129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartigan A, Phalen DN, Slapeta J. Museum material reveals a frog parasite emergence after the invasion of the cane toad in Australia. Parasites and Vectors. 2010;3:50–55. doi: 10.1186/1756-3305-3-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry AP, Farrugia TJ, Kinnison MT. Human influences on rates of phenotypic change in wild animal populations. Molecular Ecology. 2008;17:20–29. doi: 10.1111/j.1365-294X.2007.03428.x. [DOI] [PubMed] [Google Scholar]
- Hendry AP, Kinnison MT, Heino M, Day T, Smith TB, Fitt G, Bergstrom CT, et al. Evolutionary principles and their practical application. Evolutionary Applications. 2011;4:159–183. doi: 10.1111/j.1752-4571.2010.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JK, Thomas CD, Blakely DS. Evolution of flight morphology in a butterfly that has recently expanded its geographic range. Oecologia. 1999;121:165–170. doi: 10.1007/s004420050918. [DOI] [PubMed] [Google Scholar]
- Hoare JM, Pledger S, Nelson NJ, Daugherty CH. Avoiding aliens: behavioural plasticity in habitat use enables large, nocturnal geckos to survive Pacific rat invasions. Biological Conservation. 2007;136:510–519. [Google Scholar]
- Huey RB, Gilchrist GW, Hendry AP. Using invasive species to study evolution. In: Sax DF, Stachowicz JJ, Gaines SD, editors. Species Invasions: Insights into Ecology, Evolution and Biogeography. Sunderland, MA: Sinauer Associates; 2005. pp. 139–164. [Google Scholar]
- King RB, Ray JM, Stanford KM. Gorging on gobies: beneficial effects of alien prey on a threatened vertebrate. Canadian Journal of Zoology. 2006;84:108–115. [Google Scholar]
- Kinnison MT, Hairston NG., Jr Eco-evolutionary conservation biology: contemporary evolution and the dynamics of persistence. Functional Ecology. 2007;21:444–454. [Google Scholar]
- Kolbe JJ, Glor RE, Schetino LR, Lara AC, Larson A, Losos JB. Multiple sources, admixture, and genetic variation in introduced Anolis lizard populations. Conservation Biology. 2007;221:1612–1625. doi: 10.1111/j.1523-1739.2007.00826.x. [DOI] [PubMed] [Google Scholar]
- Lambrinos JG. How interactions between ecology and evolution influence contemporary invasion dynamics. Ecology. 2004;85:2061–2070. [Google Scholar]
- Langkilde T. Invasive fire ants alter behaviour and morphology of native lizards. Ecology. 2009;90:208–217. doi: 10.1890/08-0355.1. [DOI] [PubMed] [Google Scholar]
- Lankau RA, Strauss SY. Newly rare or newly common: evolutionary feedbacks through changes in population density and relative species abundance, and their management implications. Evolutionary Applications. 2011;4:338–353. doi: 10.1111/j.1752-4571.2010.00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankau RA, Nusso V, Spyreas G, Davis AS. Evolutionary limits ameliorate the negative impact of an invasive plant. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15362–15367. doi: 10.1073/pnas.0905446106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavergne S, Molofsky J. Increased genetic variation and evolutionary potential drive the success of an invasive grass. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3883–3888. doi: 10.1073/pnas.0607324104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CE, Bell MA. Causes and consequences of recent freshwater invasions by saltwater animals. Trends in Ecology and Evolution. 1999;14:284–288. doi: 10.1016/S0169-5347(99)01596-7. [DOI] [PubMed] [Google Scholar]
- Lee KA, Klasing KC. A role for immunology in invasion biology. Trends in Ecology and Evolution. 2004;19:523–529. doi: 10.1016/j.tree.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Lee CE, Remfert JL, Chang Y-M. Response to selection and evolvability of invasive populations. Genetica. 2007;129:179–192. doi: 10.1007/s10709-006-9013-9. [DOI] [PubMed] [Google Scholar]
- Llewellyn DCC. 2009. Behavioural and physiological responses to immune system activation in cane toads (Bufo marinus) during a biological invasion. BSc (Honours) thesis, Biological Sciences, University of Sydney.
- Llewelyn J, Phillips BL, Alford RA, Schwarzkopf L, Shine R. Locomotor performance in an invasive species: cane toads from the invasion front have greater endurance, but not speed, compared to conspecifics from a long-colonised area. Oecologia. 2010;162:343–348. doi: 10.1007/s00442-009-1471-1. [DOI] [PubMed] [Google Scholar]
- Lodge DM. Biological invasions: lessons for ecology. Trends in Ecology and Evolution. 1993;8:133–137. doi: 10.1016/0169-5347(93)90025-K. [DOI] [PubMed] [Google Scholar]
- Mooney HA, Cleland EE. The evolutionary impact of invasive species. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5446–5451. doi: 10.1073/pnas.091093398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemala J, Spence JR. Distribution and abundance of an exotic ground-beetle (Carabidae): a test of community impact. Oikos. 1991;62:351–359. [Google Scholar]
- Phillips BL. The evolution of growth rates on an expanding range edge. Biology Letters. 2009;5:802–804. doi: 10.1098/rsbl.2009.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips BL, Shine R. An invasive species induces rapid adaptive change in a native predator: cane toads and black snakes in Australia. Proceedings of the Royal Society B. 2006;273:1545–1550. doi: 10.1098/rspb.2006.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips BL, Brown GP, Webb JK, Shine R. Invasion and the evolution of speed in toads. Nature. 2006;439:803. doi: 10.1038/439803a. [DOI] [PubMed] [Google Scholar]
- Phillips BL, Kelehear C, Pizzatto L, Brown GP, Barton D, Shine R. Parasites and pathogens lag behind their host during periods of host range-advance. Ecology. 2010a;91:872–881. doi: 10.1890/09-0530.1. [DOI] [PubMed] [Google Scholar]
- Phillips BL, Brown GP, Shine R. Evolutionarily accelerated invasions: the rate of dispersal evolves upwards during the range advance of cane toads. Journal of Evolutionary Biology. 2010b;23:2595–2601. doi: 10.1111/j.1420-9101.2010.02118.x. [DOI] [PubMed] [Google Scholar]
- Phillips BL, Brown GP, Shine R. The evolution of life-histories during range-advance. Ecology. 2010c;91:1617–1627. doi: 10.1890/09-0910.1. [DOI] [PubMed] [Google Scholar]
- Phillips BL, Greenlees MJ, Brown GP, Shine R. Predator behaviour and morphology mediates the impact of an invasive species: cane toads and death adders in Australia. Animal Conservation. 2010d;13:53–59. [Google Scholar]
- Pizzatto L, Shine R. The effects of experimentally infecting Australian tree frogs with lungworms from invasive cane toads. International Journal of Parasitology. 2011a;41:943–949. doi: 10.1016/j.ijpara.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Pizzatto L, Shine R. Ecological impacts of invading species: do parasites of the cane toad imperil Australian frogs? Austral Ecology. 2011b doi: 10.1111/j.1442-9993.2010.02231.x. [DOI] [Google Scholar]
- Pizzatto L, Shilton CM, Shine R. Infection dynamics of the lungworm Rhabdias pseudosphaerocephala in its natural host, the cane toad Bufo marinus, and in novel hosts (Australian frogs) Journal of Wildlife Diseases. 2010;46:1152–1164. doi: 10.7589/0090-3558-46.4.1152. [DOI] [PubMed] [Google Scholar]
- Price TD, Qvarnstrom A, Irwin DE. The role of phenotypic plasticity in driving genetic evolution. Proceedings of the Royal Society B. 2003;270:1433–1440. doi: 10.1098/rspb.2003.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick DN, Ghalambor CK. The population ecology of contemporary adaptations: what empirical studies reveal about the conditions that promote adaptive radiation. Genetica. 2001;112–113:183–189. [PubMed] [Google Scholar]
- Richards CL, Bossdorf O, Muth NZ, Gurevitch J, Pigliucci M. Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecology Letters. 2006;9:981–993. doi: 10.1111/j.1461-0248.2006.00950.x. [DOI] [PubMed] [Google Scholar]
- Ridley CE, Ellstrand NC. Evolution of enhanced reproduction in the hybrid-derived invasive, California wild radish (Raphanus sativus. Biological Invasions. 2009;11:2251–2264. [Google Scholar]
- Saccheri I, Hanski I. Natural selection and population dynamics. Trends in Ecology and Evolution. 2006;6:341–347. doi: 10.1016/j.tree.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Sax DF, Stanchowicz JJ, Brown JH, Bruno JF, Dawson MN, Gaines SD, Grosberg RK, et al. Ecological and evolutionary insights from species invasions. Trends in Ecology and Evolution. 2007;22:466–471. doi: 10.1016/j.tree.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Schlaepfer MA, Runge MC, Sherman PW. Ecological and evolutionary traps. Trends in Ecology and Evolution. 2002;17:474–480. [Google Scholar]
- Settle WH, Wilson LT. Invasion by the variegated leafhopper and biotic interactions: parasitism, competition, and apparent competition. Ecology. 1990;71:1461–1470. [Google Scholar]
- Shine R. The ecological impact of invasive cane toads (Bufo marinus) in Australia. Quarterly Review of Biology. 2010;85:253–291. doi: 10.1086/655116. [DOI] [PubMed] [Google Scholar]
- Shine R, Brown GP, Phillips BL. An evolutionary process that assembles phenotypes through space rather than time. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5708–5711. doi: 10.1073/pnas.1018989108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemann E, Rogers WE. Reduced resistance of invasive varieties of the alien tree Sapium sebiferum to a generalist herbivore. Oecologia. 2003;135:451–457. doi: 10.1007/s00442-003-1217-4. [DOI] [PubMed] [Google Scholar]
- Simmons AD, Thomas CD. Changes in dispersal during species’ range expansions. American Naturalist. 2004;164:378–395. doi: 10.1086/423430. [DOI] [PubMed] [Google Scholar]
- Somaweera R, Webb JK, Brown GP, Shine R. Hatchling Australian freshwater crocodiles rapidly learn to avoid toxic invasive cane toads. Behaviour. 2011;148:501–517. [Google Scholar]
- Stockwell CA, Hendry AP, Kinnison MT. Contemporary evolution meets conservation biology. Trends in Ecology and Evolution. 2003;18:94–101. [Google Scholar]
- Strauss SY, Lau JA, Carroll SP. Evolutionary responses of natives to introduced species: what do introductions tell us about natural communities? Ecology Letters. 2006a;9:357–374. doi: 10.1111/j.1461-0248.2005.00874.x. [DOI] [PubMed] [Google Scholar]
- Strauss SY, Webb CO, Salamin N. Exotic taxa less related to native species are more invasive. Proceedings of the National Academy of Sciences of the United States of America. 2006b;103:5841–5845. doi: 10.1073/pnas.0508073103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibert-Plante X, Hendry AP. Factors influencing progress toward sympatric speciation. Journal of Evolutionary Biology. 2011 doi: 10.1111/j.1420-9101.2011.02348.x. [DOI] [PubMed] [Google Scholar]
- Thomas CD, Bodsworth EJ, Wilson RJ, Simmons AD, Davies ZG, Musche M, Coonradt L. Ecological and evolutionary processes at expanding range margins. Nature. 2001;411:577–581. doi: 10.1038/35079066. [DOI] [PubMed] [Google Scholar]
- Thompson JN. Rapid evolution as an ecological process. Trends in Ecology and Evolution. 1998;13:329–332. doi: 10.1016/s0169-5347(98)01378-0. [DOI] [PubMed] [Google Scholar]
- Tingley R, Phillips BL, Shine R. Establishment success of introduced amphibians increases in the presence of congeneric species. American Naturalist. 2011;177:382–388. doi: 10.1086/658342. [DOI] [PubMed] [Google Scholar]
- Travis JMJ, Dytham C. Dispersal evolution during invasions. Evolutionary Ecology Research. 2002;4:1119–1129. [Google Scholar]
- Urban M, Phillips BL, Skelly DK, Shine R. A toad more traveled: the heterogeneous invasion dynamics of cane toads in Australia. American Naturalist. 2008;171:E134–E148. doi: 10.1086/527494. [DOI] [PubMed] [Google Scholar]
- Van Valen L. A new evolutionary law. Evolutionary Theory. 1973;1:1–30. [Google Scholar]
- Vellend M, Harmon LJ, Lockwood JL, Mayfield MM, Hughes AR, Wares JP, Sax DF. Effects of exotic species on evolutionary diversification. Trends in Ecology and Evolution. 2007;22:481–488. doi: 10.1016/j.tree.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Vermeij GJ. An agenda for invasion biology. Biological Conservation. 1996;78:3–9. [Google Scholar]
- Ward-Fear G, Brown GP, Shine R. Using a native predator (the meat ant, Iridomyrmex reburrus) to reduce the abundance of an invasive species (the cane toad, Bufo marinus) in tropical Australia. Journal of Applied Ecology. 2010;47:273–280. [Google Scholar]
- Westley PA. What invasive species reveal about the rate and form of contemporary phenotypic change in nature. American Naturalist. 2011;177:496–509. doi: 10.1086/658902. [DOI] [PubMed] [Google Scholar]
- Yeh PJ, Price TD. Adaptive phenotypic plasticity and the successful colonization of a novel environment. American Naturalist. 2004;164:531–542. doi: 10.1086/423825. [DOI] [PubMed] [Google Scholar]
- Zheng C, Ovaskainen O, Hanski I. Modelling single nucleotide effects in phosphoglucose isomerase on dispersal in the Glanville fritillary butterfly: coupling of ecological and evolutionary dynamics. Philosophical Transactions of the Royal Society B. 2009;364:1519–1532. doi: 10.1098/rstb.2009.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]


