Abstract
Arguably the most fundamental of trade-offs in life-history evolution is the increase in natural mortality resulting from sexual maturity and reproduction. Despite its central importance, this increase in mortality, a survival cost, garners surprisingly little attention in fish and fisheries modeling studies. We undertook an exploratory analysis to evaluate the consequences of this omission for life-history projections. To this end, we developed a simulation approach that integrates quantitative genetics into the ecological dynamics of a fish population and parameterized the model for Atlantic cod (Gadus morhua, L.). When compared to simulations in which the mortality of immature and mature individuals is equal, the inclusion of a survival cost results in larger asymptotic body size, older age at maturity, and larger size at maturity. We also find that measures of population productivity (spawning stock biomass, recruits-per-spawner) are overestimated if the survival cost is excluded. This sensitivity of key metrics of population growth rate and reproductive capacity to the magnitude of the survival cost of reproduction underscores the need to explicitly account for this trade-off in projections of fish population responses to natural and anthropogenic environmental change, including fisheries.
Keywords: Atlantic cod, life-history evolution, natural mortality, recruitment, spawning stock, survival cost of reproduction
Introduction
Natural mortality is one of the key parameters responsible for shaping life histories through natural and human-induced selection (Roff 2002). From an ecological perspective, natural mortality plays a fundamental role in the year-to-year dynamics of a population and its long-term renewal capability. Natural mortality also strongly affects the harvest pressure that a population can sustain, and natural mortality rate estimates strongly affect harvest rate recommendations (Hilborn and Walters 1992), such that understanding the components of natural mortality and quantifying them has become increasingly important for the management of depleted populations (Swain 2011). This is particularly pronounced in the context of fisheries: Overfishing is a globally acknowledged problem (FAO 2010), and accurate estimation of natural mortality is essential for sustainable fisheries management (Hilborn and Walters 1992).
To this end, understanding the components and correlates of natural mortality in fish, as well as how different mortality regimes can affect population status, is necessary from both a conservation and a management perspective. Although numerous studies have linked the rate of natural mortality to fish life-history traits such as body size, growth rate, and asymptotic body size (e.g., Charnov 1993; Quinn and Deriso 1999; Hutchings 2002; Gislason et al. 2010), one component of natural mortality that has garnered little attention in the fisheries context is the survival cost of reproduction, that is, the increase in the natural mortality owing to an individual being sexually mature and reproducing (Fisher 1930; Cole 1954; Charlesworth 1980). The exclusion of this component of mortality is surprising given that across taxa it has considerable influence on life-history evolution (Bell 1980; Roff 1984; Reznick 1985), the size and quality of the mature population (e.g., Silvertown and Dodd 1999; Proaktor et al. 2008), and its overall effect on longevity (Hutchings 2005; Jørgensen and Fiksen 2010; Swain 2011).
Generally, the survival cost of reproduction arises jointly from the energetic investment to reproduction and increased mortality associated with reproductive behavior (Bell 1980). For example, a reproductive individual might not forage for food as efficiently as a nonreproductive individual, thus lowering energy intake, in addition to which reproduction consumes energy reserves owing to gonad development and egg production (e.g., Adams and Huntingford 1997; Hutchings 2002; Vinyard and Winzeler 2002; Hendry and Beall 2004, Scarnecchia et al. 2007). On the other hand, increased energetic demands of reproduction can also increase the need for foraging, potentially increasing the risk of predation (Metcalfe et al. 1999). In males, aggressive behavior related to competition for space, mates, and parental care is also often associated with reproduction, leading to elevated mortality and energy loss (e.g., Dufresne et al. 1990). Survival costs associated with reproduction can also be highly species specific. For example, in migratory fishes, such as Atlantic salmon (Salmo salar), individuals can undertake migrations of thousands of kilometers from feeding to spawning grounds (Fleming 1996), which substantially decreases their survival owing to high energetic requirements and elevated risk of predation (Schaffer and Elson 1975; Jonsson et al. 1991; Berg et al. 2001).
Although a life-history trade-off between current and future reproduction must exist (Bell 1980; Roff 1984, 2002; Stearns 1989), the magnitude of the survival cost can vary considerably among environments, individuals, populations, and years. Individual quality matters insofar as those that have accumulated the largest energy reserves and that are large in body size tend to experience lower survival costs of reproduction (e.g., Hutchings 1993, 1994; Bertschy and Fox 1999). At high food availability and, more generally, at good growth conditions, survival costs are likely to be lower than in poor growth conditions (Bell and Koufopanou 1986). Therefore, annual fluctuations in prey abundance or temperature can induce temporal variations in the magnitude of this trade-off. If coupled with parallel changes in overall natural mortality (as would be expected in association with fluctuations in prey abundance; e.g., Stige et al. 2010), the risk for a rapid population decline might then rapidly increase.
From an evolutionary perspective, the survival cost of reproduction affects the fitness of alternative life histories and thereby their adaptive optima (Bell 1980; Stearns 1989; Roff 2002). If the survival cost of reproduction is high, an individual is not likely to reproduce many times before its death. In such cases, selection may favor individuals that mature at an older age, but at a larger body size, to maximize lifetime reproductive success. In contrast, if the survival cost is relatively minor, it may be beneficial to start reproducing early in life, even though body size at subsequent ages would then be smaller (Bell 1980; Stearns 1989). However, the final fitness optimum depends not only on the survival cost but also on the overall level of natural mortality. If mortality of immature individuals is high, the optimal strategy may be to reproduce as early as possible to ensure at least some offspring production before death (Bell 1980). Variations in natural mortality and the survival cost of reproduction can thereby jointly give rise to differing local adaptations and lead to population-specific differences in the age and size at maturation as well as in correlated life-history traits (e.g., Hutchings 1993, 1994; Bertschy and Fox 1999).
Most populations of Atlantic cod (Gadus morhua, L.) provide well-known examples of the consequences of overfishing (Walters and Maguire 1996; Hutchings and Reynolds 2004). Despite fishery closures and dramatic reductions in fishing mortality, most Northwest Atlantic populations have exhibited few signs of recovery (Hutchings and Rangeley 2011). Among the hypothesized causes of the slow recovery is reduced reproductive rate caused by life-history change (Hutchings and Reynolds 2004; Hutchings 2005; Walsh et al. 2006). Namely, owing to a life-history shift toward earlier maturation at smaller size (Olsen et al. 2004; Swain et al. 2007), the juvenile production of current spawners is predicted to be lower than what it was formerly (Hutchings 2005; Walsh et al. 2006). Earlier maturation, independently of the cause of this life-history change, is also predicted to increase overall natural mortality owing to the survival cost of maturation (Hutchings 2005; Jørgensen and Fiksen 2010; Swain 2011). Given its prominence in studies of overfishing, fish stock collapse and recovery (Hutchings and Reynolds 2004), as well as in studies of fisheries-induced evolution (e.g., Olsen et al. 2004; Kuparinen and Merilä 2007; Law 2007; Swain et al. 2007), Atlantic cod provides an appropriate model species for the investigation of phenotypic and population level consequences of the survival cost of reproduction. To this end, we develop a simulation approach that integrates quantitative genetics into ecological dynamics of a fish population and investigate the role of the survival cost of reproduction in cod life histories and population reproductive capacity, with particular focus on population and life-history metrics of direct interest in the fisheries context.
Materials and methods
Simulation approach
We developed a mechanistic, individual-based, quantitative genetic life-history model to explore how fish life histories adapt to different scenarios for the survival cost of reproduction and how these adaptations feed back to the reproductive capacity of the population. In this model, fish in a population were traced at annual time steps, and at every time step, the processes of mortality, growth, and reproduction were simulated at an individual basis.
Individual fish life histories were characterized through von Bertalanffy (1938) growth trajectories
| (1) |
where l(t) is length at age t, L∞ is the asymptotic length, k is the intrinsic growth rate (rate at which L∞ is reached), and L0 is the length at birth (at time point t = 0). In our model, we considered the growth trajectories to be heritable, so that at birth, each individual is assigned its own k and L∞ parameters. L0 was not relevant in the present study and was thus set constant to every individual. Maturation was assumed to occur when an individual had reached 66% of its L∞, a threshold (Fig. 1) that has been found to provide a good proxy of the timing and the size at maturation (Jensen 1997). As von Bertalanffy curve parameters k and L∞ are known to be strongly and negatively correlated (Charnov 1993), inheritance of the parameters was done by first assigning an individual a value of L∞ based on its genotype and then generating the value of k using a statistical regression for k with L∞ as an explanatory variable, and by adding random variation to the value of k predicted by the model. Random variation was generated from a normal distribution with mean 0 and standard deviation matching that of the residuals in the regression model (see below for details of the model parameterization).
Figure 1.
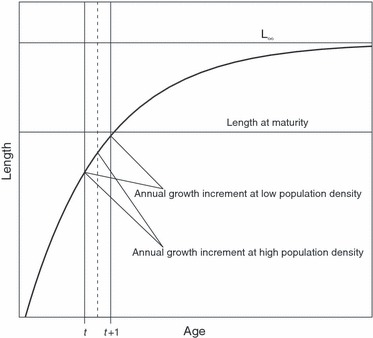
A schematic illustration of the use of von Bertalanffy (VB) growth curves in the simulation model. Individuals were assumed to mature at a body size that was 66% of L∞. At optimal growth conditions (i.e., low population density), an individual's progress along its VB curve according to its age increase from t to t + 1, whereas at high population density, resources allocated to growth were limited and progress along the VB curve was reduced from that at the optimal conditions.
Given that quantitative traits are influenced by a large number of loci each with small effect (Roff 2002), we described the genotypes of the individuals through 10 diploid loci with two alleles in each. (This number of loci was sufficient in describing the trait distribution smoothly, and adding further loci did not affect the simulations.) The alleles were coded with 0 and 1, and the impacts of the loci were assumed to be equal and additive. Inheritance of the alleles followed classical Mendelian heritance, such that at each locus, an offspring received one randomly sampled allele from its mother and one from its father. A genetic trait value was derived by summing the allelic values (ranging from 0 to 20). To allow for some phenotypic variation around the genetic trait value, a normally distributed random number was added to the genetic trait value which then yielded the final phenotypic trait value that coded the value of L∞. To avoid unrealistic growth parameter values, the phenotypic trait values were bounded to ±5 from the extremes of the genotypic trait value.
Instantaneous mortality rate of an individual depended on its maturity status, so that an immature individual experienced the overall mortality M, and the survival cost of reproduction (SC) was added to M if the individual was mature. The final instantaneous mortality rate was then transformed to proportion scale (by 1 − e−c, where c is the instantaneous mortality rate), and a Bernoulli trial was used to decide whether an individual dies. The growth of an individual was derived from its von Bertalanffy growth curve, but to account for the effect of population density on growth, the time available for growth within 1 year was set to range between 0 and 1 (Fig. 1). If the population was far from its carrying capacity, the time spent on growth within 1 year was very close to 1. However, if the population was very close to or above its carrying capacity, we assumed that the time available for growth was reduced according to a logistic equation growth time = e15 − 17.6 × c (1 + e15 − 17.6 × c)−1, where c is the ratio of the population's biomass and its carrying capacity, e.g., Δt = 0.5 if the population is at 85% of its carrying capacity. While the choice of this equation is somewhat arbitrary, its purpose is to ensure that the population biomass is bounded by the carrying capacity by constraining growth at high population densities. As density and intraspecific competition increase, they will lower the available energy per capita for somatic growth. While the choice of the parameters in the logistic growth time equation was arbitrary, the ability of our growth model to describe cod life histories was evaluated by comparison of the observed and predicted growth histories (see Model parameterization and Results).
At every reproductive episode, each mature female was randomly assigned to a mature male, and the number of juveniles produced depended on the female's body size and the density of the population such that half of the juvenile production was density-independent and the other half depended on the population density through the same logistic equation as the density-dependent growth. Predicted number of juveniles was then rounded to the closest integer. Inheritance of genetic traits was modeled as described above, and the sex of each individual was drawn randomly from a Bernoulli trial with the probability of 0.5.
Our model is intended to describe the evolution of alternative life-history types. For example, low L∞ characterizes a life-history type where individual growth is initially fast (high value of k) but, after early maturation at a small size, the rate of growth levels off. In contrast, individuals with high L∞ have lower initial growth rates and mature late at a large body size (Fig. 1). These patterns are consistent with those incorporated in general life-history models in fishes (e.g., Roff 1984): After maturation, energy available for somatic growth reduces, leading to slower growth after maturation, such that the L∞ of small maturing individuals remains lower than the L∞ of large maturing individuals. Therefore, our model is not based on genetic correlations among the life-history traits but rather the realized phenotypic correlations between L∞ and k, and between L∞ and the size at maturity. The fact that L∞ is the trait directly coded by the phenotypic trait value (and that other parameters are derived based on it) is a convenient technical solution but does not reflect true causal effect of this parameter on the others. However, in practice, it was convenient to construct the model in this way, as data on L∞ and k are most abundantly available and the 66% threshold provides a way to further link size and age at maturity to the von Bertalanffy growth parameters.
Model parameterization
To parameterize the model, we utilized individual-based data on Atlantic cod that inhabit meromictic Ogac Lake on Baffin Island in the Canadian Arctic (Hardie and Hutchings 2011). A key advantage to using data from a near-pristine, negligibly exploited population is that the variability in individual growth trajectories reflects natural phenotypic variability in growth, rather than being affected by fishing (see Hardie and Hutchings 2011 for additional details). Length-at-age trajectories of the individuals were measured from otoliths (N = 258), and von Bertalanffy growth curves (eqn 1) were fitted to the trajectories through nonlinear least-squared regression. We restricted the data to those growth trajectories for which L∞ was <130 cm, as some individuals were caught at such a young age that their growth had not yet started to level off, which then resulted in unrealistically high values of L∞. Log transformation of k (for sake of normality) was modeled through linear regression with L∞ and its square as explanatory variables, and the model was then reduced in a stepwise manner. The final model was log(k) = −0.609 − 0.013 × L∞, and the standard deviation of the residuals was 0.305. Pearson's correlation coefficient between log(k) and L∞ was 0.688 (t = 15.2, df = 256, P < 0.01). In the simulations, L∞ values were linearly calculated from the phenotypic trait values on the range from 30 to 130 cm and k predicted using the statistical model, and the value of L∞; L0 was set to be 4 cm for every growth curve.
The juvenile production was described by directly modeling the number of juveniles surviving up to 3 years of age. To do this, we combined a model for egg production with the life tables for larvae survival given by Hutchings (2005), so that the number of juveniles surviving up to 3 years of age was predicted to be 0.37 × female weight + 0.27. The weight–length relationship was obtained by fitting a power-law function to measured weight (in kg) at length (in cm) data through nonlinear least-squared regression, with the relations being described through weight = 3.52 × 10−6 × (length)3.19. Standard deviation of the phenotypic variation around the genetic trait value was calibrated, so that the resulting heritabilities were realistic (0.2–0.3; Mousseau and Roff 1987; Law 2007), and was thereby set to 3.5. Maximum lifetime was set to 25 years in this analysis.
Simulation design
The above-mentioned model was utilized to investigate how cod life histories adapt to alternative mortality regimes, given our interest in investigating how survival costs of reproduction affect the adaptation of life histories and vital fitness-related life-history traits. To this end, we ran the model in a full-factorial design with two scenarios for M (0.1 and 0.12) and three scenarios for SC (0.05, 0.1, and 0.15), in accordance with the parameter estimates used by Hutchings (2005). By having M vary in this manner, we are following the recommendation by Roff (1984) that preferred life-history models are those for which there is a clear separation between life-history parameters before and after maturity. The values of M were chosen close to each other intentionally, as otherwise adaptive differences in life histories would be obscured by large differences in population abundance (as affected through density-dependent growth), thus making a comparison between the scenarios difficult. Overall rates of natural mortality (M + SC; see Table 1) were also kept reasonably low, as a population would not sustain high mortality for many subsequent generations, and, in such cases, there would be little sense to investigate the adaptation of life histories as direct ecological consequences of mortality would be overwhelming. Each parameter combination was run for over 1500 simulation steps (years) to ensure that populations became fully adapted to their conditions, as reflected in our simulations by temporal stability in life-history trait values. In these runs, the overall rate of mortality was recorded, and each run was then repeated with SC being 0 and M equal to the average overall mortality rate over the last 100 years (fully adapted population at its equilibrium). In this way, each simulation run with the survival cost of reproduction produced a counterpart run that had the same overall mortality but no difference in mortality between mature and immature individuals.
Table 1.
Simulation scenarios as well as the average life-history traits in the adapted populations. Values for the simulations without a survival cost* are given inside brackets
| Scenario† | L∞ (cm)‡ | k‡ | Age at maturity (year) | Length at maturity (cm) |
|---|---|---|---|---|
| M = 0.1, SC = 0.05 (M = 0.122) | 88.4 (83.9) | 0.178 (0.191) | 9.7 (9.0) | 57.9 (55.0) |
| M = 0.1, SC = 0.1 (M = 0.132) | 92.5 (83.5) | 0.168 (0.192) | 9.2 (8.4) | 61.0 (54.9) |
| M = 0.1, SC = 0.15 (M = 0.143) | 94.8 (84.1) | 0.161 (0.191) | 8.6 (7.9) | 62.9 (55.4) |
| M = 0.12, SC = 0.05 (M = 0.141) | 86.7 (84.0) | 0.182 (0.191) | 8.3 (7.9) | 57.2 (55.4) |
| M = 0.12, SC = 0.1 (M = 0.154) | 89.6 (83.7) | 0.175 (0.191) | 7.7 (7.4) | 59.5 (55.3) |
| M = 0.12, SC = 0.15 (M = 0.169) | 92.3 (81.8) | 0.168 (19.6) | 7.2 (6.7) | 61.5 (54.3) |
Simulations without the survival cost have the same overall rate of mortality but without the survival cost of reproduction; i.e., mortality among mature and immature individuals is the same.
Natural mortality is denoted with M and the survival cost of reproduction with SC.
von Bertalanffy growth parameters [see eqn (1) in the methods section].
At the beginning of each simulation run, the population size was set at 2000 individuals (∼ 1700–2100 kg) and the carrying capacity to 5000 kg. Initial genetic trait values (sum of allelic values) were generated from discrete uniform distributions to allow large genotypic diversity, and matching sets of allele values were then generated using Bernoulli trials. Ranges of the uniform distributions for each simulation scenario were set based on initial simulation runs, so that the adaptation time would not become overly long.
At each simulation step, the following variables were recorded: number of individuals in the population, number of mature individuals, population's biomass, spawning stock biomass, average L∞, average k, age at maturity, length at maturity, phenotypic and genetic trait values, and trait heritabilities (variance of the genetic trait value divided with the variance of the phenotypic trait value). All the simulations and analyses were conducted with R 2.10.0 (R Development Core Team 2009).
Results
At the beginning of the simulation runs, the population size increased until it reached an equilibrium, which took a few decades. Evolutionary adaptation of growth strategies to the mortality conditions was much slower, but within 1400 years (roughly 125 cod generations; Hutchings 2005), the life histories had stabilized (Fig. 2; each simulation run was checked separately), although year-to-year stochastic fluctuations still existed owing to demographic stochasticity in the model. Comparison of the length-at-age trajectories in the adapted populations (recorded at eight time points) and the empirical lengths-at-age measured from otoliths showed that the simulations appeared to mimic cod life histories very realistically (Fig. 3). Closest matches with the otolith data were provided by the parameter combinations of M = 0.12 and SC = 0.15, M = 0.12 and SC = 0.1, and M = 0.1 and SC = 0.15, suggesting that these mortality conditions might be closest to those typically experienced by the study population. The values at which the traits stabilized, such as L∞, k, age at maturity, and length at maturity, are characteristic of many cod populations, particularly at northern latitudes (e.g., Northeast Arctic cod, northern cod; Myers et al. 1997; COSEWIC 2010).
Figure 2.
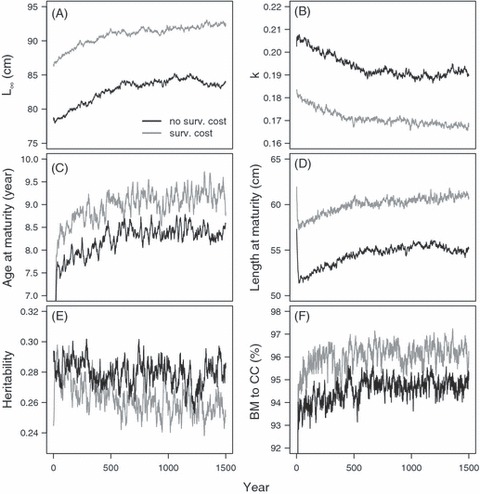
Illustration of simulation runs and the effect of the survival cost of reproduction on the adaptation of two cod populations. In the first scenario (gray line; survival cost), the overall natural mortality is split into two components: natural mortality experiences by all individuals (M) and the survival cost of reproduction (SC) both of which were set to 0.1. In the second scenario with no survival cost of reproduction (black line), M is set to the average of the overall mortality over the last 100 years in the first simulation scenario, so that M = 0.132. Therefore, the overall (instantaneous) rate of natural mortality is equal in both the simulation scenarios, but in the first scenario, immature fish experience mortality lower than the average, and mature fish experience higher mortality than the average. In contrast, in the second scenario, mortality experience by all the fish is the same. The panels show the average von Bertalanffy parameters (A) L∞, (B) k, (C) average age, (D) length at maturity, (E) heritability of the growth strategy (see Methods for further details), and (F) the biomass to carrying-capacity ratio.
Figure 3.
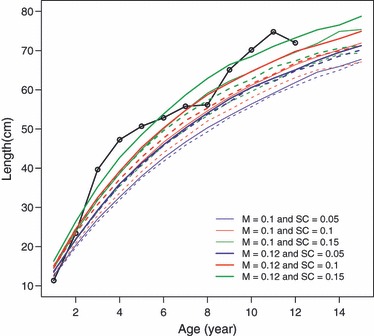
Average length-at-age measured from cod otoliths (black solid line with bullets) and average length-at-age predicted by the simulation model. In simulations, the averages are calculated over eight recording points at 10-year intervals in the adapted population (last 100 years of simulated 1500 years). Natural mortality (M) and survival cost of reproduction (SC) scenarios are given in the legend. Dashed lines show predictions for the simulation scenarios, where the overall rates of mortality are the same as in the scenarios indicated in the legend but with no survival cost of reproduction, so that mortality does not differ between immature and mature individuals.
To explore the role of the survival cost of reproduction, we compared simulation runs that incorporated a survival cost with those having the same overall rate of natural mortality but no survival cost (Table 1). To this end, we calculated the difference between the runs with and without the survival cost of reproduction in the average L∞, k, age at maturity, length at maturity, spawning stock biomass (SSB), and the recruit-per-spawner ratio (recruits/SSB) in the adapted population (over the last 100 simulation steps). For spawning stock biomass and the recruit-per-spawner ratio, we only estimated relative differences, given that the absolute values depend on the arbitrarily chosen carrying capacity of the population.
In general, a survival cost of reproduction increased L∞ and both the age and length at maturity. Because of the negative correlation between L∞ and k, the increase in L∞ was accompanied with a decrease in k (Fig. 4A–D). The increase in L∞ attributable to the survival cost was on the scale of 5–10 cm, while age at maturity increased about 0.4–0.8 years and the length at maturity from 2 to 8 cm. In the presence of the survival cost of reproduction, mature individuals experienced higher mortality than in the absence of this cost. This was reflected in the spawning stock biomass and the recruit-per-spawner ratio, such that both were considerably lower in the presence of the survival cost (Fig. 4E,F). Reductions in the spawning stock biomass were on the order of 4–14%, whereas the recruit-per-spawner ratio was reduced by 25–35%.
Figure 4.
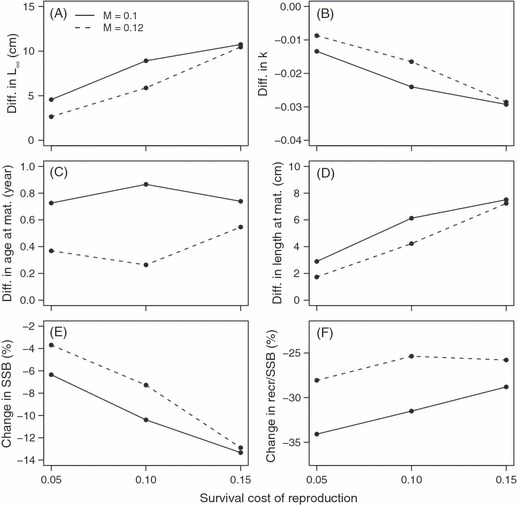
Differences in life-history traits and population characteristics owing to the survival cost of reproduction (SC). These were obtained by comparing two simulation runs with the same rate of overall mortality but either mortality being equal among immature and mature individuals (no SC) or increased among mature (due to SC). The differences were calculated by reducing an average value in the simulation run without SC from the average in the simulation run with SC. Both averages are calculated from the adapted populations, so over the last 100 years of the simulated 1500 year. Survival costs of reproduction are indicated in the x-axis, while the natural mortality experienced by all individuals (M) is given in legend. Differences are given for (A) L∞, (B) k, (C) average age, and (D) length at maturity. Panels (E) and (F) give relative differences in the spawning stock biomass (SSB) and the recruits-per-spawner ratio (recruits/SSB), respectively, between the simulations with and without SC. For example, panel A indicates that at every combination of M (line type) and SC (x-axis), L∞ is higher in the presence of SC compared to the simulation without SC (the difference is always >0).
Discussion
Our study illustrates how elevated mortality among mature individuals can significantly affect projections of population productivity and life histories. When the survival cost of reproduction is accounted for, life histories evolved toward older age and larger size at maturation and larger maximum body sizes (Figs 3 and 4, Table 1). In contrast, both spawning stock biomass and recruits-per-spawner were lower when a survival cost of reproduction was incorporated, compared to the unrealistic life-history scenario for which mortality among mature and immature individuals is assumed equal (Fig. 4). These findings highlight the importance of considering dissimilarities in natural mortality between juveniles and reproducing adults to realistically predict juvenile production and recovery potential (Hutchings and Reynolds 2004; Hutchings 2005), to forecast how changes in age and size composition affect overall natural mortality (Jørgensen and Fiksen 2010; Swain 2011), and to generally understand the causal factors underlying phenotypic variation in fish life histories (Bell 1980; Stearns 1989).
Estimation of the survival cost of reproduction can be inherently difficult, as it can be masked by variation in local resources (Bell and Koufopanou 1986), such that a full quantification of the cost requires replicated experiments across a range of species’ natural environments (Stearns 1989). In fish, empirical studies of the costs associated with reproduction have largely focused on growth and energy reserves (e.g., Adams and Huntingford 1997; Scarnecchia et al. 2007); attempts to estimate the survival cost of reproduction in fishes are rare. In brook trout (Salvelinus fontinalis, Mitchill), the survival cost has been estimated in three populations in Newfoundland, using a mark–recapture experiment. In this study, overwinter mortality was found to be increased by 17–89% among reproducing individuals compared to nonreproductive ones (Hutchings 1994). This survival cost of reproduction was also further affected by phenotypic traits, such that it was negatively correlated with body size but positively associated with age (Hutchings 1994). By combining demographic data and modeling techniques, Bertschy and Fox (1999) similarly investigated the magnitude of the survival cost of reproduction and its life history correlates in pumpkinseed sunfish (Lepomis gibbosus, L.). They found that in a normally growing population, the survival costs of reproduction were on the scale of 5–10%, but in stunted populations, the cost was at least 2.5 times higher, suggesting that growth rate and age and size at maturity can strongly affect the magnitude of the cost. In Atlantic cod, and based on their exhaustive examination of Northeast Arctic cod otoliths, Beverton et al. (1994) concluded that the instantaneous rate of natural mortality (M) declines as age at maturity increases, concluding that M was equal to 0.25, 0.17, and 0.15 for individuals maturing at ages 6, 7, and 8 years, respectively. These case studies of fishes reflect a pattern evident across multiple taxa and demonstrate that not only the survival cost of reproduction is expected from the basis of life-history theory (Bell 1980; Stearns 1989) but that it is also commonly detected in natural populations (e.g., Sinervo and DeNardo 1996; Silvertown and Dodd 1999; Proaktor et al. 2008; for reviews see Bell 1980; Reznick 1985).
In the management of fisheries and fish stocks, reliable estimation of metrics of population productivity (e.g., spawning stock size, recruitment) is vital for determining the fishing mortality that a population can sustain and how it should be modified to attain a targeted population size (e.g., the biomass at which maximum sustainable yield is realized, or BMSY; Hilborn and Walters 1992). From this perspective, the role of the survival cost of reproduction can be crucial as it effectively shapes the age and size structure of the spawning stock: The greater the survival cost, the lower the expected number of times a mature fish spawns before its death. Therefore, a survival cost of reproduction reduces the abundance of old large spawners from what would have been predicted in the absence of the survival cost. The spawning contribution of larger, older spawners can be disproportionally high relative to that of younger, smaller individuals (Berkeley et al. 2004; Birkeland and Dayton 2005) and therefore directly reflects the spawning stock biomass and recruit-per-spawner ratio, as we observed in our simulations (Fig. 4). As a result, the fishing mortality that a population can safely sustain can be lower than what would be predicted if the elevated mortality among spawners was not accounted for. Another aspect related to fisheries stock assessment is that any process affecting age-class-specific maturity ogives (i.e., the proportion of mature individuals) can also alter the age-class-specific overall rate of natural mortality. Such drivers can, for example, be increased temperature (Dhillon and Fox 2004; Pörtner and Peck 2010; Kuparinen et al. 2011), changes in food abundance (Uusi-Heikkilä et al. 2011) or fisheries-induced evolution toward earlier maturation (e.g., Olsen et al. 2004). Omitting such environmental drivers can thus lead to an underestimation of natural mortality and, thus, overestimation of the reproductive ability of the fish stock (Jørgensen and Fiksen 2010; Swain 2011). Particularly in the context of fisheries-induced evolution, the impact of advanced maturation on population productivity might be underestimated if the increase in natural mortality owing to the survival cost of reproduction is excluded (see Andersen and Brander 2009; Kinnison et al. 2009).
As shown here through the simulated evolution of fish life histories, the survival cost of reproduction affects the optimality of alternative life-history strategies and can, therefore, be of primary importance in life-history evolution. Given its influence on per capita population growth rate (Hutchings 2005; Swain 2011), it can be expected to influence recovery rates as well. Habitat-specific variation in survival costs can partly explain local adaptation in fitness-related life-history traits such as age and size at maturation or body size that are commonly seen in salmonids (e.g., Taylor 1991; Garcia de Leaniz et al. 2007) but also in many other fish species, such as guppies (Poecilia reticulata) (Reznick et al. 1997), grayling (Thymallus thymallus) (Haugen 2000), and lemon shark (Negaprion brevirostris) (DiBattista et al. 2011). Conversely, adaptive differences in life-history traits among populations can also serve as an indicator of possible habitat-related differences in the survival cost of reproduction, although distinguishing this from other sources of mortality such as predation can be difficult (Stearns 1989). In the evolutionary responses of life histories to fishing (e.g., Heino and Godø 2002; Kuparinen and Merilä 2007; Law 2007; Johnson et al. 2011), the survival cost of reproduction can be an important component of natural selection opposing the evolutionary pressures induced by fishing: while high fishing mortality would favor early maturation at a small body size, the survival cost of reproduction still increases the fitness of life histories with late maturation at large body sizes (Figs 2 and 4). It is the relative strengths of fisheries-induced and natural selection that eventually determine how fish life histories might evolve (Edeline et al. 2007; Kuparinen et al. 2009). While the survival cost of reproduction is often not included in predictions on evolutionary responses to fishing (e.g., Dunlop et al. 2009, Wang and Höök 2010, but see Hutchings 2009 and Poos et al. 2011), its potentially substantial role as a component of natural selection suggests that it should be accounted for, at least at a first approximation, if quantitative estimates of the survival cost are not available.
As a theoretical simulation approach, our study is subject to many restrictive assumptions and thereby the results must be viewed in light of the assumed model. As models always are (Box 1979), ours constitutes a vast simplification of reality. Nonetheless, our modeling approach is directly built on empirically observed growth histories and their natural phenotypic variability. While the model clearly omits a great deal of mechanistic details underlying fish growth and maturation, the growth histories predicted by the model match well with those observed empirically, such that the model appears to provide a transparent and data-supported way of exploring ecological and evolutionary dynamics. Although the applied growth history data were collected from a nonstandard environment for cod, this does not affect our results regarding the role of the survival cost of reproduction in the evolution of life histories. Importantly, it allowed us to investigate natural variability in growth for a very lightly exploited cod population. Nonetheless, one should bear in mind that differences among populations are likely to exist and that generalizations to other cod populations should be done cautiously. One clear simplification of our model was the assumption that growth and maturation were considered to be the only coevolving traits, whereas correlates of reproductive effort are also expected to evolve as a response to changes in the survival cost of reproduction (Reznick 1985; Bertschy and Fox 1999). Mortalities considered in our simulations (both M and SC) were empirically realistic (e.g., Hutchings 1993, 1994; Bertschy and Fox 1999), with the overall mortalities corresponding to those estimated for Southern Gulf of St. Lawrence cod in the 1970s (0.1–0.2; Swain 2011). In other systems, however, M and the survival cost of reproduction can sometimes be much higher; in fisheries stock assessments, the overall rate of natural mortality is often assumed to be 0.2 and estimates of the survival cost of reproduction can easily be much higher than what was assumed here. Moreover, as it remained beyond the scope of this study, we did not consider phenotypic, spatial, or temporal fluctuations in M or SC or trends in environmental drivers affecting growth and maturation (e.g., temperature or food). In all, while our study provides insights into ecological and evolutionary implications of the survival cost of reproduction in fishes, we acknowledge the need for further analyses in this respect.
Taken together, through our simulation approach that incorporated ecological and evolutionary dynamics of a fish population, we demonstrated the fundamental role of the survival cost of reproduction both on fitness-related life-history traits, such as the age and size at maturity, as well as on the reproductive capacity of the population as seen in the spawning stock biomass and the recruit-per-spawner ratio. These results urge careful consideration of the survival cost of reproduction in predictions of a fish stock's reproductive and recovery capacity and in model forecasts of fish life-history evolution.
Acknowledgments
The research leading to these results has received funding from the Academy of Finland (AK), the Natural Sciences and Engineering Research Council of Canada and Polar Continental Shelf Programme (DH, JH) and from the European Union's Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 244706/ECOKNOWS project (AK). However, the paper does not necessarily reflect European Commission's views and in no way anticipates the Commission's future policy in the area. We thank Sakari Kuikka, the associate editor, and two anonymous referees for their helpful comments on an earlier version of this article.
Literature cited
- Adams CE, Huntingford FA. Growth, maturation and reproductive investment in Arctic charr. Journal of Fish Biology. 1997;51:750–759. [Google Scholar]
- Andersen KH, Brander K. Expected rate of fisheries-induced evolution is slow. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11657–11660. doi: 10.1073/pnas.0901690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. The costs of reproduction and their consequences. The American Naturalist. 1980;116:45–76. [Google Scholar]
- Bell G, Koufopanou V. The cost of reproduction. In: Dawkins R, Ridley M, editors. Oxford Surveys in Evolutionary Biology. Vol. 3. Oxford: Oxford University Press; 1986. pp. 83–131. [Google Scholar]
- Berg OK, Hendry AP, Svendsen B, Bech C, Arnekleiv JV, Lohrmann A. Maternal provisioning of offspring and the use of those resources during ontogeny: variation within and between Atlantic Salmon families. Functional Ecology. 2001;15:13–23. [Google Scholar]
- Berkeley SA, Chapman C, Sogard SM. Maternal age as a determinant of larval growth and survival in a marine fish, Sebastes melanops. Ecology. 2004;85:1258–1264. [Google Scholar]
- von Bertalanffy L. A quantitative theory of organic growth (inquiries on growth laws II) Human Biology. 1938;10:181–213. [Google Scholar]
- Bertschy KA, Fox MG. The influence of age-specific survivorship on pumpkinseed sunfish life histories. Ecology. 1999;80:2299–2313. [Google Scholar]
- Beverton RJH, Hylen A, Ostvedt OJ. Growth, maturation, and longevity of maturation cohorts of Northeast Arctic cod. ICES Journal of Marine Science Symposium. 1994;198:482–501. [Google Scholar]
- Birkeland C, Dayton PK. The importance in fishery management of leaving the big ones. Trends in Ecology and Evolution. 2005;20:356–358. doi: 10.1016/j.tree.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Box GEP. Robustness in the strategy of scientific model building. In: Launer RL, Wilkinson GN, editors. Robustness in Statistics. Vol. 1. New York, NY: Academic Press; 1979. pp. 201–236. [Google Scholar]
- Charlesworth B. Evolution in Age-Structured Populations. Cambridge: Cambridge University Press; 1980. [Google Scholar]
- Charnov E. Life History Invariants: Some Explorations of Symmetry in Evolutionary Ecology. Oxford: Oxford University Press; 1993. [Google Scholar]
- Cole LC. The population consequences of life history phenomena. Quarterly Review of Biology. 1954;29:103–137. doi: 10.1086/400074. [DOI] [PubMed] [Google Scholar]
- COSEWIC. 2010. COSEWIC assessment and status report on the Atlantic cod, Gadus morhua, in Canada. Committee on the Status of Endangered Wildlife in Canada, Ottawa. http://www.sararegistry.gc.ca/virtual_sara/files/cosewic/sr_Atlantic%20Cod_0810_e.pdf (accessed on 18 June 11)
- Dhillon RS, Fox MG. Growth-independent effects of temperature on age and size at maturity in Japanese Medaka (Oryzias latipes. Copeia. 2004;2004:37–45. [Google Scholar]
- DiBattista JD, Feldheim KA, Garant D, Gruber SH, Hendry AP. Anthropogenic disturbance and evolutionary parameters: a lemon shark population experiencing habitat loss. Evolutionary Applications. 2011;4:1–17. doi: 10.1111/j.1752-4571.2010.00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufresne F, FitzGerald GJ, Lachance S. Age and size-related differences in reproductive success and reproductive costs in threespine sticklebacks (Gasterosteus aculeatus. Behavioural Ecology. 1990;1:140–147. [Google Scholar]
- Dunlop ES, Heino M, Dieckmann U. Eco-genetic modeling of contemporary life-history evolution. Ecological Applications. 2009;19:1815–1834. doi: 10.1890/08-1404.1. [DOI] [PubMed] [Google Scholar]
- Edeline E, Carlson SM, Stige LC, Winfield IA, Fletcher JM, James JB, Haugen TO, et al. Trait changes in a harvested population are driven by a dynamic tug-of-war between natural and harvest selection. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15799–15804. doi: 10.1073/pnas.0705908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations) The State of World Fisheries and Aquaculture. Rome: FAO; 2010. [Google Scholar]
- Fisher RA. The Genetical Theory of Natural Selection. Oxford: Oxford University Press; 1930. [Google Scholar]
- Fleming IA. Reproductive strategies of Atlantic salmon: ecology and evolution. Reviews in Fish Biology and Fisheries. 1996;6:379–416. [Google Scholar]
- Gislason H, Daan N, Rice JC, Pope JG. Size, growth, temperature and the natural mortality of marine fish. Fish and Fisheries. 2010;11:149–158. [Google Scholar]
- Hardie DC, Hutchings JA. The ecology of Atlantic cod (Gadus morhua) in Canadian Arctic lakes. Arctic. 2011;64:137–150. [Google Scholar]
- Haugen TO. Growth and survival effects on maturation pattern in populations of grayling with recent common ancestors. Oikos. 2000;90:107–118. [Google Scholar]
- Heino M, Godø OR. Fisheries-induced selection pressures in the context of sustainable fisheries. Bulletin of Marine Science. 2002;70:639–656. [Google Scholar]
- Hendry AP, Beall E. Energy use in spawning Atlantic salmon. Ecology of Freshwater Fish. 2004;13:185–196. [Google Scholar]
- Hilborn R, Walters CJ. Quantitative Fisheries Stock Assessment. New York, NY: Chapman and Hall; 1992. [Google Scholar]
- Hutchings JA. Adaptive life histories effected by age-specific survival and growth rate. Ecology. 1993;74:673–684. [Google Scholar]
- Hutchings JA. Age- and size-specific costs of reproduction within populations of brook trout, Salvelinus fontinalis. Oikos. 1994;70:12–20. [Google Scholar]
- Hutchings JA. Life histories of fish. In: Hart PJB, Reynolds JD, editors. Handbook of Fish Biology and Fisheries Vol. 1. Fish Biology. Oxford: Blackwell Science; 2002. pp. 149–174. [Google Scholar]
- Hutchings JA. Life-history consequences of overexploitation to population recovery in Northwest Atlantic cod (Gadus morhua. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:824–832. [Google Scholar]
- Hutchings JA. Avoidance of fisheries-induced evolution: management implications for catch selectivity and limit reference points. Evolutionary Applications. 2009;2:324–334. doi: 10.1111/j.1752-4571.2009.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings JA, Rangeley RW. Correlates of recovery for Canadian Atlantic cod. Canadian Journal of Zoology. 2011;89:386–400. [Google Scholar]
- Hutchings JA, Reynolds JD. Marine fish population collapses: consequences for recovery and extinction risk. BioScience. 2004;54:297–309. [Google Scholar]
- Jensen AL. Origin of the relation between K and Linf and synthesis of relations among life history parameters. Canadian Journal of Fisheries and Aquatic Sciences. 1997;54:987–989. [Google Scholar]
- Johnson DW, Christie MR, Moye J, Hixon MA. Genetic correlations between adults and larvae in a marine fish: potential effects of fishery selection on population replenishment. Evolutionary Applications. 2011;4:621–633. doi: 10.1111/j.1752-4571.2011.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson N, Jonsson B, Hansen LP. Energetic cost of spawning in male and female Atlantic salmon (Salmo salar L.) Journal of Fish Biology. 1991;39:739–744. [Google Scholar]
- Jørgensen C, Fiksen Ø. Modelling fishing-induced adaptations and consequences for natural mortality. Canadian Journal of Fisheries and Aquatic Sciences. 2010;67:1086–1097. [Google Scholar]
- Kinnison MT, Palkovacs EP, Darimont CT, Carlson SM, Paquet PC, Wilmers CC. Some cautionary notes on fisheries evolutionary impact assessments. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:E115. doi: 10.1073/pnas.09007871106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuparinen A, Merilä J. Detecting and managing fisheries-induced evolution. Trends in Ecology and Evolution. 2007;22:652–659. doi: 10.1016/j.tree.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Kuparinen A, Kuikka S, Merilä J. Estimating fisheries-induced selection: traditional gear selectivity research meets fisheries-induced evolution. Evolutionary Applications. 2009;2:234–243. doi: 10.1111/j.1752-4571.2009.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuparinen A, Cano Arias JM, Loehr J, Herczeg G, Gonda A, Merilä J. Fish age at maturation is influenced by temperature independently of growth. Oecologia. 2011;167:435–443. doi: 10.1007/s00442-011-1989-x. [DOI] [PubMed] [Google Scholar]
- Law R. Fisheries-induced evolution: present status and future directions. Marine Ecology Progress Series. 2007;335:271–277. [Google Scholar]
- de Leaniz C, Fleming IA, Einum S, Verspoor E, Jordan WC, Consuegra S, Aubin-Horth N, et al. A critical review of adaptive genetic variation in Atlantic salmon: implications for conservation. Biological Reviews. 2007;82:173–211. doi: 10.1111/j.1469-185X.2006.00004.x. [DOI] [PubMed] [Google Scholar]
- Metcalfe NB, Fraser NHC, Burns MD. Food availability and the nocturnal vs. diurnal foraging trade-off in juvenile salmon. Journal of Animal Ecology. 1999;68:371–381. [Google Scholar]
- Mousseau TA, Roff DA. Natural selection and the heritability of fitness components. Heredity. 1987;59:181–197. doi: 10.1038/hdy.1987.113. [DOI] [PubMed] [Google Scholar]
- Myers RA, Mertz G, Fowlow PS. Maximum population growth rates and recovery times for Atlantic cod, Gadus morhua. Fishery Bulletin. 1997;95:762–772. [Google Scholar]
- Olsen ME, Heino M, Lilly RG, Morgam MJ, Brattey J, Ernande B, Dieckmann U. Maturation trends indicative of rapid evolution preceded the collapse of northern cod. Nature. 2004;428:932–935. doi: 10.1038/nature02430. [DOI] [PubMed] [Google Scholar]
- Poos JJ, Brännström Å, Dieckmann U. Harvest-induced maturation evolution under different life-history trade-offs and harvesting regimes. Journal of Theoretical Biology. 2011;279:102–112. doi: 10.1016/j.jtbi.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Pörtner HO, Peck MA. Climate change effects on fishes and fisheries: towards a cause-and-effect understanding. Journal of Fish Biology. 2010;77:1745–1779. doi: 10.1111/j.1095-8649.2010.02783.x. [DOI] [PubMed] [Google Scholar]
- Proaktor G, Coulson T, Milner-Gulland EJ. The demographic consequences of the cost of reproduction in ungulates. Ecology. 2008;89:2604–2611. doi: 10.1890/07-0833.1. [DOI] [PubMed] [Google Scholar]
- Quinn TJ, Deriso RB. Quantitative Fish Dynamics. Oxford: Oxford University Press; 1999. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. ISBN 3-900051-07-0; http://www.R-project.org. [Google Scholar]
- Reznick DN. Cost of reproduction: an evaluation of the empirical evidence. Oikos. 1985;44:257–267. [Google Scholar]
- Reznick DN, Shaw FH, Rodd FH, Shaw RG. Evaluation of the rate of evolution in natural populations of guppies (Poecilia reticulata. Science. 1997;275:1934–1937. doi: 10.1126/science.275.5308.1934. [DOI] [PubMed] [Google Scholar]
- Roff DA. The evolution of life history parameters in teleosts. Canadian Journal of Fisheries and Aquatic Sciences. 1984;41:989–1000. [Google Scholar]
- Roff DA. Life History Evolution. Sunderland, MA: Sinauer; 2002. [Google Scholar]
- Scarnecchia DL, Ryckman LF, Lim Y, Power GJ, Schmitz BJ, Firehammer JA. Life-history and the costs of reproduction in Northern Great Plains Paddlefish (Polyodon spathula) as a potential framework for other Acipenseriform fishes. Reviews in Fisheries Science. 2007;15:211–263. [Google Scholar]
- Schaffer WM, Elson PF. The adaptive significance of variations in life history among local populations of Atlantic salmon in North America. Ecology. 1975;56:577–590. [Google Scholar]
- Silvertown J, Dodd M. The demographic cost of reproduction and its consequences in Balsam Fir (Abies balsamea. The American Naturalist. 1999;29:321–332. doi: 10.1086/303238. [DOI] [PubMed] [Google Scholar]
- Sinervo B, DeNardo DF. Costs of reproduction in the wild: path analysis of natural selection and experimental tests of causation. Evolution. 1996;50:1299–1313. doi: 10.1111/j.1558-5646.1996.tb02370.x. [DOI] [PubMed] [Google Scholar]
- Stearns SC. Trade-offs in life-history evolution. Functional Ecology. 1989;3:259–268. [Google Scholar]
- Stige LC, Ottersen G, Dalpadado P, Chan KS, Hjermann DO, Lajus DL, Yaragina NA, et al. Direct and indirect climate forcing in a multi-species marine system. Proceedings of the Royal Society B series. 2010;277:3411–3420. doi: 10.1098/rspb.2010.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain DP. Life-history evolution and elevated natural mortality in a population of Atlantic cod (Gadus morhua. Evolutionary Applications. 2011;4:18–29. doi: 10.1111/j.1752-4571.2010.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain DP, Sinclair AF, Hanson JM. Evolutionary response to size-selective mortality in an exploited fish population. Proceedings of the Royal Society B series. 2007;274:1015–1022. doi: 10.1098/rspb.2006.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor EB. A review of local adaptation in Salmonidae, with particular reference to Pacific and Atlantic salmon. Aquaculture. 1991;98:185–207. [Google Scholar]
- Uusi-Heikkilä S, Kuparinen A, Wolter C, Meinelt T, O'Toole AC, Arlinghaus R. Experimental assessment of the probabilistic maturation reaction norm: condition matters. Proceedings of the Royal Society B series. 2011;278:709–717. doi: 10.1098/rspb.2010.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinyard GL, Winzeler A. Lahontan cutthroat trout (Oncorhynchus clarki henshawi) spawning and downstream migration of juveniles into Summit Lake, Nevada. Western North American Naturalist. 2002;60:333–341. [Google Scholar]
- Walsh MR, Munch SB, Chiba S, Conover DO. Maladaptive changes in multiple traits caused by fishing: impediments to population recovery. Ecology Letters. 2006;9:142–148. doi: 10.1111/j.1461-0248.2005.00858.x. [DOI] [PubMed] [Google Scholar]
- Walters C, Maguire JJ. Lessons for stock assessment from the northern cod collapse. Reviews in Fish Biology and Fisheries. 1996;6:125–137. [Google Scholar]
- Wang H-Y, Höök TO. Eco-genetic model to explore fishing-induced ecological and evolutionary effects on growth and maturation schedules. Evolutionary Applications. 2010;2:438–455. doi: 10.1111/j.1752-4571.2009.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


