Abstract
Finding a way to block the evolution insecticide resistance would be a major breakthrough for the control of malaria. We suggest that this may be possible by introducing a stress into mosquito populations that restores the sensitivity of genetically resistant mosquitoes and that decreases their longevity when they are not exposed to insecticide. We use a mathematical model to show that, despite the intense selection pressure imposed by insecticides, moderate levels of stress might tip the evolutionary balance between costs and benefits of resistance toward maintaining sensitivity. Our experimental work with the microsporidian parasite Vavraia culicis infecting two lines of resistant mosquitoes and a sensitive line suggests that it may indeed be possible to stress the mosquitoes in the required way. The mortality of resistant mosquitoes 24 h after exposure to the insecticide was up to 8.8 times higher in infected than in uninfected ones; if mosquitoes were not exposed to the insecticide, resistant mosquitoes infected by the microsporidian lived about half as long as uninfected ones and insecticide-sensitive mosquitoes (with or without the parasite). Our results suggest that biopesticides or other insecticides that interfere with the expression of resistance may help to manage insecticide resistance in programs of malaria control.
Keywords: disease biology, evolutionary medicine, evolution-proof control, host parasite interactions
Introduction
Insecticides used for indoor residual spraying (IRS) or on bed nets are among the most effective ways of controlling malaria, cutting the malaria burden by as much as half in several African countries (WHO 2008). However, as they kill mosquitoes shortly after exposure, it seems inevitable that higher insecticide coverage in scaled-up control efforts will speed up the evolution of insecticide resistance (e.g., Vulule et al. 1994; Pennetier et al. 2008; Ranson et al. 2009). Indeed, it is difficult to imagine stronger selection pressure than that imposed by insecticides. If coverage is high, most mosquitoes are exposed at their first attempt at biting and therefore die before having any offspring; their evolutionary fitness is 0. The rare mosquitoes harboring a mutation that makes them resistant to the insecticide, therefore, live much longer than sensitive mosquitoes, and their descendants will soon be the sole survivors of malaria control. Discovering a way to block the evolution of insecticide resistance would be a major breakthrough for public health.
We suggest that it might be possible to do so by manipulating the environment in a way that increases the sensitivity of resistant mosquitoes to insecticides and that increases the evolutionary cost of resistance. Both effects decrease the benefit of carrying the genes responsible for resistance and might therefore tip the evolutionary balance toward maintaining sensitivity.
The environment does indeed affect the sensitivity to insecticides. For instance, the resistance of Culex pipiens to chlorpyrifos is influenced by several environmental parameters: the food used to rear the larvae, the type of water, and the type of cups used to perform the bioassays (Bourguet et al. 1996), and the resistance of cotton aphids to bifenthrin, chlorpyrifos, endosulfan, and triazamate depends on the characteristics of their host plant (Godfrey and Fuson 2001). Most relevant to our suggestion is that infecting DDT- or pyrethroid-resistant adult Anopheles with pathogenic fungi Beauveria bassiana or Metarhizium anisopliae restores their sensitivity to the insecticides (Farenhorst et al. 2009).
Furthermore, the cost of resistance of C. pipiens to organophosphates is increased if the mosquitoes are infected by the microsporidian parasite Vavraia culicis (Agnew et al. 2004) or reared at high larval densities (Bourguet et al. 2004); the fitness cost of permethrin resistance of C. pipiens is enhanced if the mosquito is exposed to temephos, another insecticide (Hardstone et al. 2009); and in the diamondback moth, the cost of resistance to spinosad is low at the optimal temperature and increases at unfavorably low and high temperatures (Li et al. 2007), and the cost of resistance to Bacillus thuringiensis increases in harsh and competitive environments (Raymond et al. 2005).
Thus, stress caused by environmental conditions, by other insecticides, or by parasitic infection can affect the expression and the evolutionary cost of resistance. We include this interaction in a mathematical model to show that, despite very intense selection pressure induced by insecticides, it may be possible to block resistance with moderate levels of stress influencing the expression and cost of resistance. We then use the microsporidian V. culicis as an example to show that, at least in simple laboratory situations, we can manipulate a single environmental factor to influence both parameters – the benefit and the cost of resistance – in a way that might tip the evolutionary balance sufficiently to block the evolution of resistance. Thus, while our experiments should not be taken as a conclusive test of our idea, our suggestion – realized with V. culicis or another stress factor – is a promising approach to manage the growing problem of insecticide resistance.
Materials and methods
Study organisms
Our experiments involved three colonies of Anopheles gambiae: a DDT-resistant colony (ZANU) from Zanzibar with increased metabolism of the insecticide, catalyzed by members of the glutathione S-transferase enzyme family (Ranson et al. 2000); a mildly pyrethroid-resistant colony (RSP) from western Kenya with elevated esterase and oxidase levels (Vulule et al. 1999); and a sensitive colony (Kisumu), which was also colonized from western Kenya and is sensitive to all insecticides (Vulule et al. 1994).
As a biopesticide, we chose the microsporidian V. culicis, an obligate, intracellular parasite of several mosquito species (Becnel et al. 2005; Andreadis 2007), with a life cycle typical of microsporidians (Andreadis 2007). Mosquito larvae are infected when they ingest the parasite's spores along with their food. Some infected larvae and pupae die and release a new generation of the parasite's spores for horizontal transmission to other larvae. If the mosquitoes survive to emerge, the adults remain infected. The parasite has several effects on the adult, including a shorter life span (Koella et al. 2009a; Lorenz and Koella 2011) and reduced susceptibility to malaria (Bargielowski and Koella 2008). Although there is no transovarial vertical transmission, spores harbored by adult females can infest a new breeding site when they are released together with eggs (Andreadis 2007). The prevalence of only a few microsporidian species in natural populations has been estimated; in populations of Aedes mosquitoes, it ranges from 0% to about 50%, while the only study on A. gambiae found 6.6% prevalence in larvae (Andreadis 2007).
Experimental procedures
We reared uninfected and infected mosquitoes according to our standard laboratory practices (e.g., Hansen and Koella 2003; Lambrechts et al. 2006; Fellous and Koella 2009). Briefly, mosquitoes were reared individually in 12-well plates and fed with a standard amount of Tetramin fish food. We obtained microsporidian spores by homogenizing infected adult mosquitoes and then counting the spores at 400× magnification with a haemacytometer. The solution was diluted to 20 000 spores/100 μL. Each well obtained 100 μL of this solution when larvae were 2 days old. In earlier experiments, this infectious dose infected more than 95% of the larvae, but killed them only rarely. Controls received 100 μL of solution containing the same number of uninfected adults.
Restored sensitivity
Resistance of the ZANU mosquitoes to DDT and resistance of the RSP mosquitoes to permethrin were measured in separate experiments with the standard World Health Organisation test-kit according to WHO guidelines (WHO 1998). Mosquitoes were exposed to the insecticide 2 or 3 days after emergence in the WHO testing-tubes containing between 2 and 11 mosquitoes. To measure DDT resistance of ZANU, we exposed the mosquitoes to DDT-treated filter paper (4%) for 0, 45, 90, or 135 min. For permethrin resistance, we exposed the mosquitoes to permethrin-treated filter paper (0.75%) for 0, 15, or 30 min. We chose the times based on earlier experiments, so that we could expect (i) that most uninfected, resistant mosquitoes die at the longest exposure and (ii) that more than 75% of uninfected, sensitive mosquitoes die at the shortest (non-zero) exposure. After exposure, the mosquitoes were transferred to clean holding tubes and provided with cotton balls moistened with saturated sugar solution. The number of dead mosquitoes in each tube was scored 24 h after exposure, and the number of deaths within each tube was analyzed with the statistical package JMP 8.0.2 (SAS Institute, Cary, NC) with a GLM (binomial distribution and logit link) that included replicate (for ZANU), infection status as a nominal factor, time of exposure as an ordinal factor, and the interaction of infection and exposure. For DDT resistance, we ran the experiment twice, one replicate with 504 and the other replicate with 398 mosquitoes. For permethrin resistance, we had one replicate and analyzed 152 mosquitoes. (Mosquitoes in several tubes were inadvertently not sugar-fed and were therefore left out of the analysis.)
Increased cost of resistance
The longevity of unexposed mosquitoes was measured for sensitive, ZANU, and RSP mosquitoes in a single experiment. We reared uninfected and infected mosquitoes individually. Pupae were placed into open 1.5-mL Eppendorf tubes within 50-mL Falcon tubes covered with mosquito netting. Females were provided with cotton balls moistened with saturated sugar solution; males were discarded. Survival was assayed every 24 h. The lengths of the wings of the dead individuals were measured from the alula notch to the wing tip with a dissecting microscope. There were between 23 and 96 mosquitoes per treatment (mosquito line and infection status); differences were because of variation among treatments in number of mothers in the colonies, in fecundity, and in larval survival. Longevity was analyzed with JMP 8.0.2 (SAS Institute) with a survival analysis [Weibull distribution; using proportional hazards (results not shown) gave identical conclusions] that included mosquito line, Vavraia-infection, wing length, and the interactions between the three traits.
Theory
We modeled the evolution of resistance by assuming that, over evolutionary time, the mosquitoes with the highest lifetime reproductive success will replace the others. We further assumed that fecundity and mortality rate do not change with age, so a mosquito's expected lifetime reproductive success is proportional to the average number of gonotrophic cycles it lives, which in turn is the inverse of mortality per gonotrophic cycle.
To calculate the survival from one gonotrophic cycle to the next, we assumed that the insecticide is used in IRS and that the proportion of sensitive mosquitoes killed by the insecticide within a gonotrophic cycle, which we call ‘effective coverage’c, is determined by a combination of the proportion of houses sprayed (the ‘population coverage’), the proportion of mosquitoes that enter houses to bite (i.e., that are not repelled), and the proportion of the mosquitoes biting indoors that are killed (the ‘efficacy’ of the insecticide). (See below for details about how to combine these factors to obtain effective coverage.) Thus, IRS decreases the survival of sensitive mosquitoes from one gonotrophic cycle to the next from (1 − μ)τ to (1 − μ)τ(1 − c), where μ, the daily background mortality (unrelated to the insecticide), is set to 0.1 (Costantini et al. 1996; Charlwood et al. 1997; Takken et al. 1998; Killeen et al. 2000; Midega et al. 2007; Okech et al. 2007) and where the gonotrophic period of τ days is not influenced by the use of insecticides (Quinones et al. 1997). We further assume that the cost of resistance of uninfected mosquitoes is negligible and thus that the survival of resistant mosquitoes over a gonotrophic cycle is equal to that of unexposed, sensitive mosquitoes: (1 − μ)τ.
We considered two effects of infection by the microsporidians. First, it restores insecticide sensitivity to some degree, so that the survival of genetically resistant mosquitoes is (1 − μ)τ(1 − cβ), where β is the extent to which the microsporidian restores sensitivity. Thus, if β = 0, the microsporidian has no effect on resistance, so the insecticide cannot kill the resistant mosquitoes, and their survival is (1 − μ)τ. If β = 1, the insecticide affects genetically resistant mosquitoes to the same extent as sensitive ones. (Note that our definition of sensitivity, β, incorporates all mechanisms, including behavioral ones, that are involved in the mosquitoes’ response to insecticides. See discussion of Effective coverage, below.) Second, in the absence of the insecticide, resistant mosquitoes are more susceptible to the damaging effects of microsporidian infection than sensitive ones. This changes the background survival of resistant mosquitoes from (1 − μ)τ when they are not infected to (1 − μγ)τ when they are, where γ indicates the extent to which the microsporidian increases the background mortality. Overall, the survival of mosquitoes thus changes from (1 − μ)τ(1 − c) for insecticide-sensitive mosquitoes to (1 − μγ)τ(1 − cβ) for microsporidian-infected resistant ones.
The longevity of sensitive mosquitoes is thus 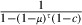 . The average longevity of resistant mosquitoes is the mean of the longevity of microsporidian-infected and uninfected mosquitoes. If the microsporidian infects a proportion π of the mosquitoes, the average longevity of resistant mosquitoes is
. The average longevity of resistant mosquitoes is the mean of the longevity of microsporidian-infected and uninfected mosquitoes. If the microsporidian infects a proportion π of the mosquitoes, the average longevity of resistant mosquitoes is 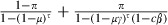 .
.
Effective coverage
As effective coverage (the degree to which the mosquito population is affected by the insecticide) is one of the main parameters in our model, we need a way to estimate it and, in particular, to relate it to population coverage (the proportion of the human population covered by the insecticide). We describe two ways to do so. One is based on a feeding cycle model (Le Menach et al. 2007) that calculates the survival of insecticide-sensitive mosquitoes per gonotrophic cycle as a function of the behavioral response of the mosquitoes to insecticides. The model assumes that the feeding cycle is divided into two parts. First, mosquitoes search for hosts until they are successful or die; mosquitoes complete this part of the cycle in τ1 days and survive it with a probability p1. At each biting attempt, they bite outdoors with probability H; if not, they encounter an insecticide-treated house with a probability ϕ. Mosquitoes are then either repelled and repeat the host-searching cycle at a probability r, or they are not repelled and successfully feed with probability (1 − r)s or are killed by the insecticide with probability (1 − r)(1 − s). Second, once mosquitoes have successfully fed, they rest during τ2 days before laying eggs, and they survive this resting period at a probability p2. With these assumptions, the proportion of mosquitoes that survive the gonotrophic cycle and thus lay eggs is 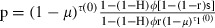 [where τ1(0) is the searching period and τ(0) is the time of the gonotrophic cycle if mosquitoes are not repelled by an insecticide (Le Menach et al. 2007)]. It should be noted that the parameters used in these equations are defined differently than the ones in Le Menach et al. (2007), so that the form of our equation is slightly different. To relate this equation to our evolutionary model, described above, we set p equal to (1 − μ)τ(1 − c), enabling us to calculate the effective coverage c as a function of the mosquitoes’ response to insecticides. According to the model's assumptions, repeated repellency increases the duration of the gonotrophic cycle from τ(0) to
[where τ1(0) is the searching period and τ(0) is the time of the gonotrophic cycle if mosquitoes are not repelled by an insecticide (Le Menach et al. 2007)]. It should be noted that the parameters used in these equations are defined differently than the ones in Le Menach et al. (2007), so that the form of our equation is slightly different. To relate this equation to our evolutionary model, described above, we set p equal to (1 − μ)τ(1 − c), enabling us to calculate the effective coverage c as a function of the mosquitoes’ response to insecticides. According to the model's assumptions, repeated repellency increases the duration of the gonotrophic cycle from τ(0) to 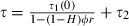 (Le Menach et al. 2007). However, unless the parameters associated with host searching – indoor biting 1 − H, coverage ϕ, and repellency r– are all close to 1, the gonotrophic cycle will be close to that observed in the absence of insecticide use, which is about 3 days. We, therefore, assume τ = τ(0) = 3 for the analysis.
(Le Menach et al. 2007). However, unless the parameters associated with host searching – indoor biting 1 − H, coverage ϕ, and repellency r– are all close to 1, the gonotrophic cycle will be close to that observed in the absence of insecticide use, which is about 3 days. We, therefore, assume τ = τ(0) = 3 for the analysis.
The second way is to use studies that estimate the selection coefficient of insecticide resistance from the rate of evolution of resistance (e.g., Curtis et al. 1978; Wood and Cook 1983; Lynd et al. 2010). In basic population genetic models, the selection coefficient measures the difference of the reproductive successes of resistant and sensitive mosquitoes relative to that of sensitive ones (see Lynd et al. 2010), so the selection coefficient can be written as 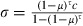 . Effective coverage can thus be estimated from published estimates of selection coefficients and the background mortality of mosquitoes.
. Effective coverage can thus be estimated from published estimates of selection coefficients and the background mortality of mosquitoes.
Results
Experiments
Restored sensitivity
Our ZANU line showed considerable resistance to DDT. While WHO defines sensitive mosquitoes as those that die within 24 h after having been exposed to the concentration used in our study for 60 min, only 45% died after 135 min of exposure (Fig. 1A). Infection by the microsporidian increased the mortality in particular at intermediate exposures (Table 1): from 4% to 28% after 45 min of exposure and from 18% to 54% after 90 min of exposure.
Figure 1.
Experimental effects of Vavraia on mortality of insecticide-resistant mosquitoes. In both panels, red symbols and lines represent permethrin-resistant mosquitoes (RSP line) and blue symbols and lines represent DDT-resistant mosquitoes (ZANU line); in panel B, black lines represent sensitive mosquitoes (Kisumu line). Solid symbols and lines represent microsporidian-infected mosquitoes; open symbols and dotted lines represent uninfected mosquitoes. (A) The proportion of insecticide-resistant mosquitoes dying within 24 h after exposure to permethrin (RSP line) or DDT (ZANU line), as a function of Vavraia-infection. Exposure time is shown on the x-axis. The vertical lines show the 95% confidence intervals of the proportion. (B) Survival curves of permethrin-resistant, DDT-resistant and insecticide–sensitive mosquitoes not exposed to the insecticide, as a function of Vavraia-infection.
Table 1.
GLM (with binomial distribution) of 24-h mortality after exposure of uninfected and Vavraia-infected mosquitoes to insecticides
| ZANU: DDT | RSP: permethrin | |||||
|---|---|---|---|---|---|---|
| df | χ2 | P | df | χ2 | P | |
| Replicate | 1 | 9.6 | 0.002 | |||
| Exposure time | 3 | 178.7 | <0.001 | 1 | 10.7 | 0.005 |
| Infection | 1 | 2.8 | 0.09 | 2 | 1.9 | 0.166 |
| Exposure time × infection | 3 | 10.3 | 0.017 | 2 | 6.2 | 0.046 |
As permethrin kills mosquitoes after a shorter exposure than does DDT, exposures were shorter for RSP than for ZANU. RSP was moderately resistant to permethrin: after 30 min of exposure, 50% died within 24 h (compared with 95% in our sensitive colony, unpublished data) (Fig. 1A). Again, infection by the microsporidian increased mortality in particular at the intermediate exposure (Table 1): after 15 min mortality increased from 7% to 63%, while after 30 min mortality increased from 50% to 67%.
Increased cost of resistance
In the absence of the insecticides, there was little difference between the survival curves of the three lines of mosquitoes (Fig. 1B). In the sensitive Kisumu line, the microsporidian decreased longevity slightly, but in both resistant lines (RSP and ZANU), infection decreased longevity by more than a third: from 9.9 to 6.0 days for ZANU and from 10.4 to 6.7 for RSP, respectively (Table 2). We controlled for wing length in the analysis, as longevity decreased with increasing size (for infected mosquitoes about 5.4 days shorter life per 1 mm longer wings; for uninfected mosquitoes about 4.9 days shorter life per 1 mm longer wings) and as microsporidian infection slightly increased the wing length of RSP (from 2.9 to 3.0 mm) and of ZANU (from 2.8 to 2.9) mosquitoes and slightly decreased wing length of Kisumu mosquitoes (from 3.1 to 3.0 mm). In an analysis that did not control for wing length, the results (not shown) were similar.
Table 2.
Survival analysis (Weibull distribution) of the three lines of mosquitoes (sensitive Kisumu, DDT-resistant ZANU and permethrin-resistant RSP) with and without infection by Vavraia culicis
| df | χ2 | P | |
|---|---|---|---|
| Mosquito line | 2 | 20.7 | <0.001 |
| Infection | 1 | 34.8 | <0.001 |
| Wing length | 1 | 29.7 | <0.001 |
| Line × infection | 2 | 36.4 | <0.001 |
| Line × wing length | 2 | 15.1 | <0.001 |
| Infection × wing length | 1 | 2.0 | 0.153 |
| Line × infection × wing length | 2 | 0.4 | 0.817 |
Theory
Restored sensitivity (i.e., smaller evolutionary benefit of carrying resistance genes) and increased cost of resistance in the absence of exposure can shift the evolutionary balance to a degree that resistance disappears from the population. To illustrate this, we assumed that the mosquitoes with greater reproductive success would replace the others. We further assumed that fecundity is independent of age and that we could therefore estimate reproductive success of sensitive and resistant mosquitoes from their average life spans. As described in the methods, the average longevity of sensitive mosquitoes is 1/[1−(1 − μ)3(1 − c)] and that of resistant mosquitoes is (1 − π)/[1−(1 − μ)3] + π/[1−(1 − μγ)3(1 − cβ)], where π is the proportion of mosquitoes infected by the biopesticide, μ is the background mortality per day of the mosquitoes, c is the effective coverage, γ indicates the extent to which the biopesticide increases the background mortality rate (i.e., the increased cost of resistance) and β is the extent to which the biopesticide restores sensitivity. Estimating the outcome of evolution is then simply a question of evaluating whether the longevity of resistant mosquitoes is greater or less than the longevity of sensitive ones.
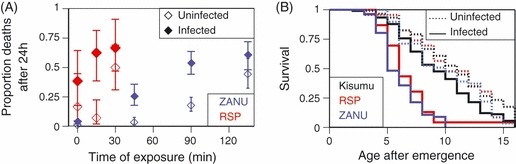
Figure 2A describes a situation where all of the mosquitoes are infected and shows the increased cost of resistance necessary to block the evolution of resistance (i.e., to reduce the longevity of resistant mosquitoes below that of sensitive ones). If sensitivity is completely restored (i.e., if genetically resistant mosquitoes are as sensitive to the insecticide as are genetically sensitive ones; β = 1), any reduction of the resistant mosquitoes’ life span prevents the evolution of resistance (horizontal, dotted line). As the extent to which sensitivity is restored decreases, the effect of the biopesticide required to block the evolution of resistance increases. It should be noted that, even if the biopesticide has no effect on sensitivity, it can block the evolution of resistance, if it sufficiently increases the cost of resistance (solid line). It should also be noted that with increasing coverage by the insecticide, the required cost of resistance imposed by the biopesticide increases more than linearly.
Figure 2.
Evolutionary predictions. The lines show, as a function of effective coverage, the effect of the biopesticide on the mortality of insecticide-resistant mosquitoes where the reproductive successes (estimated as expected longevity, see Materials and methods) of resistant and sensitive mosquitoes are equal. Above the line, the success of resistant mosquitoes is lower than that of sensitive ones, so the evolution of resistance is blocked. In both panels, the lines shows the situation where the biopesticide has no effect on the expression of resistance (β = 0, solid line), sensitivity is partially restored (β = 0.5 or 075, long- and short-dashed lines), and where sensitivity us completely restored, i.e., genetically resistant mosquitoes are as sensitive as genetically sensitive mosquitoes (β = 1, dotted line). (A) The extent to which the biopesticide must increase mortality rate (decrease longevity) of resistant mosquitoes to block resistance, if all mosquitoes can be infected by the biopesticide. (B) The proportion of the mosquitoes that must be infected by the biopesticide to block resistance, if the biopesticide increases the cost of resistance two-fold (γ = 2).
Figure 2B shows the prevalence of the biopesticide required to block resistance for a biopesticide-induced increase of the cost of resistance γ = 2, which is similar to what was observed in our experiment. At high effective coverage, it is not possible to block the evolution of resistance unless the restoration of sensitivity is complete (β = 1, dotted line). Yet, even with complete restoration of sensitivity, the strong selection imposed by insecticides means that over most of the range of effective coverage a large proportion of mosquitoes must be reached by the biopesticide so that resistance can be blocked. Nevertheless, even if the biopesticide has no effect on resistance (β = 0, solid line), an increased cost of resistance means that resistance can be prevented if the effective coverage is sufficiently low.
Effective coverage
From epidemiological model
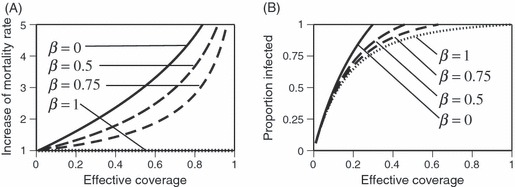
Figure 3 shows the effective coverage as a function of population coverage (the proportion of houses treated with the insecticide) predicted from a detailed model of the mosquito's feeding cycle. The curves illustrate the situations for realistic parameters: 60% repellency (Le Menach et al. 2007) to 90% repellency (Roberts et al. 2010) and no outdoor biting or 10% outdoor biting per feeding attempt (Reddy et al. 2011). The other parameters are as in Le Menach et al. (2007): 10% daily mortality and 25% biting success of the mosquitoes that are not repelled (Note that, we describe biting success relative to the mosquitoes that are not repelled and thus enter the house to feed; Le Menarch et al. describe it relative to all blood-seeking mosquitoes. Therefore, the numerical value for success differs in the two approaches.) With these parameters, effective coverage is generally considerably lower than population coverage, and exceeds 50% only when population coverage is close to 100% (Fig. 3). If outdoor biting or the biting success was higher, effective coverage would be even lower (results not shown).
Figure 3.
The relationship between effective coverage (the proportion of mosquitoes killed by the insecticide during a gonotrophic cycle) and population coverage (the proportion of houses treated with the insecticide) predicted by a model describing the effects of an insecticide on the feeding cycle of mosquitoes. The model takes into account that some mosquitoes bite outdoors (and are thus not exposed to the insecticide), that some mosquitoes are repelled by the insecticide (and thus are unlikely to enter a treated house) and that some of the mosquitoes that bite within houses are not exposed to the insecticide long enough to be killed by it.
From selection coefficient
The low effective coverage is corroborated by population genetic studies, showing that even in areas with high population coverage, the selection coefficient is usually lower than 0.5, that is, resistant mosquitoes have less than 50% higher fitness than sensitive ones (e.g., Curtis et al. 1978; Wood and Cook 1983; Lynd et al. 2010). Relating effective coverage and the selection coefficient as 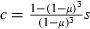 (see Materials and methods), with μ = 0.1/day, implies that effective coverage is generally less than about 30%.
(see Materials and methods), with μ = 0.1/day, implies that effective coverage is generally less than about 30%.
Discussion
Our models suggest that, by partially restoring the sensitivity of genetically resistant mosquitoes to the insecticide and decreasing their longevity when they are not exposed to the insecticide, increased stress (e.g., through a biopesticide such as V. culicis) may change the evolutionary balance sufficiently to block the evolution of resistance. Our idea is similar to a recent approach to manage the evolution of antimalarial resistance. Dual function acridones have an antimalarial function and sensitize parasites that are genetically resistant to chloroquine and other drugs. Therefore, using them in combination therapy with a traditional antimalarial should help to enhance their efficacy and prolong their effective life span (Kelly et al. 2009).
The four relevant parameters underlying the possible success of our suggestion are the degree to which stress restores sensitivity and increases the evolutionary cost of resistance, the effective coverage by the insecticide, and the prevalence of the stress. Below we discuss these in turn.
While several studies (see Introduction) have shown that various aspects of stress influence sensitivity to insecticides and the cost of resistance, our experimental results are particularly promising in showing that a single factor – infection by V. culicis– can influence both parameters to a degree that suggests it could block the evolution of resistance. These experimental results, however, should be accepted with caution. A laboratory-based comparison of three colonies of mosquitoes, which probably differ in other aspects than insecticide resistance, is not necessarily a good indication of the effect of resistance on the measured traits. An essential next step is to run similar experiments in natural situations with mosquitoes that vary in the degree of insecticide resistance.
Insecticides can impose very strong selection, as at a high coverage almost all mosquitoes are exposed to the insecticide at their first biting attempt and therefore, die without having laid any eggs. This is reflected in the prediction that at high coverage, resistance can only be blocked if the biopesticide (or any other mechanism with which stress is manipulated) affects close to 100% of the mosquitoes (Fig. 2B) and has substantial impact on at least one of the two parameters – restored sensitivity or increased cost of resistance. However, what is relevant for evolution is what we call ‘effective coverage’, which is the proportion of mosquitoes that are killed by the insecticide during a single gonotrophic cycle. This is generally much lower than population coverage. As the insecticides currently used for the control of adult mosquitoes repel mosquitoes, many mosquitoes do not fly into treated houses, leave them shortly after entering them or, after having obtained a blood-meal in a treated house, leave it before having been exposed to the insecticide long enough to be killed. Our theoretical prediction of the feeding behavior of mosquitoes (based on Le Menach et al. 2007) suggests that the effective coverage is generally lower than 50% even if most houses are treated. This is corroborated by population genetic studies, which have generally estimated that in areas with high coverage the fitness of resistant mosquitoes is not more than 50% higher than that of sensitive ones (Curtis et al. 1978; Lynd et al. 2010), suggesting that effective coverage is less than about 30%. It is further corroborated by field studies showing that when almost all houses are covered, resistance evolves over a period of several years (Penilla et al. 2007; Mathias et al. 2011), which is considerably more slowly than predicted for high effective coverage (Koella et al. 2009b). Finally, in trials of insecticide-treated nets and IRS with population coverage of close to 100% transmission was reduced less than 10-fold in East and West Africa (reviewed in Curtis and Mnzava 2000), consistent with effective coverage of up to 25% (Koella et al. 2009b). Overall, thus, even in areas where most houses are treated with insecticides, their effective coverage appears to be fairly low, enabling the possibility to block the evolution of insecticide resistance with a biopesticide. Nevertheless, a problem is that, even at moderate effective coverage, a high proportion of the mosquitoes must be reached by the environmental manipulation (Fig. 2B).
Reaching a large number of mosquitoes is a problem for any control agent, but may be more easily achieved with the microsporidian V. culicis or other parasites with a similar life cycle than with other agents, as they offer three possibilities of infection. Spores can be dispersed over breeding sites to infect larvae (Andreadis 2007); they can be fed to adults via sugar traps (Jacob C. Koella, unpublished results, Weiser and Zizka 2004); they are also dispersed by infected females when they are released into breeding sites during oviposition (Andreadis 2007). Thus, while the natural prevalence of V. culicis (like of most microsporidians of mosquitoes) is low (6.6% of the larvae in a West African population of A. gambiae (Andreadis 2007)), a combination of continually distributing the spores to larval sites, infecting the adults through sugar baits (Gu et al. 2011) and auto-dispersal during oviposition, may maintain high prevalence.
A potential problem with our idea is that the shorter life span of infected, insecticide-resistant mosquitoes might select for resistance against the microsporidian, in particular under the intense exposure required, thus making it useless in controlling insecticide resistance. Indeed, experimental exposure of Daphnia (Zbinden et al. 2008) and of Drosophila (Vijendravarma et al. 2008) to microsporidians leads to increased resistance. However, we suggest that the very success of our idea would mean that the selection pressure for resistance against the microsporidian is weak. If the microsporidian can indeed block the evolution of resistance, it will infect almost exclusively sensitive mosquitoes. In these, however, infection had essentially no effect on the longevity (Fig. 1B). This corroborates our other experiments with insecticide-sensitive mosquitoes (Lorenz and Koella 2011). The higher infectious doses used in these experiments had a greater impact on the mosquitoes, but the effects were apparent mostly in old mosquitoes: the infection had no effect on larval survival, had little effect on fecundity up to the third clutch but decreased fecundity in the fourth clutch, and had little effect on mortality rate up to about 2 weeks after emergence but let only few of the mosquitoes survive beyond 3 weeks (Lorenz and Koella 2011). As selection intensity decreases with age (Williams 1957), our results suggest that selection pressure for the resistance against the microaporidian will be weak.
In discussing the possible evolutionary consequences of using a biopesticide, we assumed that the only selection pressure for resistance is caused by the use of the insecticide for malaria control. Indeed, in several areas insecticide resistance evolved as a consequence of IRS (Lines 1988), and in a recent controlled field trial in Mexico, Anopheles populations went from 0% to 20% resistance in 3 years of IRS (Penilla et al. 2007). There are also concerns and some evidence that insecticides on bed nets will drive resistance evolution (Curtis et al. 1998; Kolaczinski et al. 2000; Hemingway et al. 2002; see recent comments in Butler 2011). In western Kenya, for example, the frequency of resistance alleles increased from virtually 0% to 100% between 1996 and 2010, coincident with the substantial scale-up of the use of insecticide-treated nets (Mathias et al. 2011). Nevertheless, much resistance appears to be a consequence of using the insecticides for agricultural purposes (Curtis et al. 1998; Diabate et al. 2002). In such cases, although the ideas underlying the evolutionary consequences of using a biopesticide remain valid, the potential to block resistance may be considerably weakened, as agricultural use imposes additional selection pressure. To predict the evolution of insecticide resistance, we need a quantitative estimate of the relative importance of selection pressures to agricultural and medical use of insecticides. Yet, even if evolution is not blocked, the dual function of restored sensitivity and increased cost (i.e., shorter life of unexposed mosquitoes) target the most important parameter of malaria transmission – the longevity of mosquitoes – and thus would help to maintain the epidemiological efficacy of insecticides even in populations with wide-spread resistance.
In conclusion, we suggest that manipulating the environment in a way that restores sensitivity to insecticides and increases the cost of resistance may help to manage the problem of insecticide resistance, and indeed may block the evolution of resistance. This may be possible in several ways. One possibility is to use a second insecticide that not only kills mosquitoes but also restores their sensitivity against the first one, an analogous approach to the possibility of using PBO, a deltamethrin synergist, to restore sensitivity to deltamethrin (Tungu et al. 2010) and to the suggestion to manage the evolution of antimalarial resistance (Kelly et al. 2009). Another approach may be to use biopesticides such as entomopathogenic fungi, B. bassiana or M. anisopliae, which restore the mosquitoes’ sensitivity to insecticides (Farenhorst et al. 2009), or microsporidians like V. culicis, which in our experiments had several properties that make it a promising candidate.
Much more needs to be done before manipulating the environment to control the evolution of insecticide resistance becomes a reality. In particular, we need a better understanding of the factors underlying sensitivity to insecticides. While science has made considerable progress at understanding some aspects of the genetic basis of resistance (Hemingway et al. 2004), we know much less about the impact of environmental variation (including infection by the biopesticide) on resistance, about the cost of resistance, and about the environment's contribution to the evolution of resistance in natural populations. Without this knowledge, it is difficult to predict how best to manage the environment to block resistance.
Nevertheless, our theoretical and empirical approach suggests that the synergistic effect of a biopesticide and the chemical insecticide can enhance the efficiency and increase the effective life span of the insecticide in malaria control programs. Thus, biopesticides might enable us to use our most effective weapons against malaria – IRS and insecticide-treated bed nets – as long as malaria remains a public health problem.
Acknowledgments
A.S. and T.P.S.K are supported by Leverhulme grant F/07 058/BC. We thank reviewers of previous versions of this manuscript for valuable comments.
Data archiving statement
Data deposited in the Dryad repository: doi:10.5061/dryad.m6g5286d.
Literature cited
- Agnew P, Berticat C, Bedhomme S, Sidobre C, Michalakis Y. Parasitism increases and decreases the costs of insecticide resistance in mosquitoes. Evolution. 2004;58:579–586. [PubMed] [Google Scholar]
- Andreadis TG. Microsporidian parasites of mosquitoes. Journal of the American Mosquito Control Association. 2007;23(Suppl. 2):3–29. doi: 10.2987/8756-971X(2007)23[3:MPOM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bargielowski I, Koella JC. A possible mechanism for the suppression of the development of Plasmodium berghei in the mosquito Anopheles gambiae by the microsporidian Vavraia culicis. PLoS One. 2008;4:e4676. doi: 10.1371/journal.pone.0004676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becnel J, White SE, Shapiro AM. Review of microsporidia-mosquito relationships: from the simple to the complex. Folia Parasitologica. 2005;52:41–50. [PubMed] [Google Scholar]
- Bourguet D, Prout M, Raymond M. Dominance of insecticide resistance presents a plastic response. Genetics. 1996;143:407–416. doi: 10.1093/genetics/143.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguet D, Guillemaud T, Chevillon C, Raymond M. Fitness costs of insecticide resistance in natural breeding sites of the mosquito Culex pipiens. Evolution. 2004;58:128–135. doi: 10.1111/j.0014-3820.2004.tb01579.x. [DOI] [PubMed] [Google Scholar]
- Butler D. Mosquitoes score in chemical war. Growing resistance is threatening global malaria-control efforts. Nature. 2011;475:19. doi: 10.1038/475019a. [DOI] [PubMed] [Google Scholar]
- Charlwood JD, Smith T, Billingsley PF, Takken W, Lyimo EOK, Meuwissen JHET. Survival and infection probabilities of anthropophagic anophelines from an area of high prevalence of Plasmodium falciparum in humans. Bulletin of Entomological Research. 1997;87:445–453. [Google Scholar]
- Costantini C, Li S, della Torre A, Sagnon NF, Coluzzi M, Taylor C. Density, survival and dispersal of Anopheles gambiae complex mosquitoes in a West African Sudan savanna village. Medical and Veterinary Entomology. 1996;10:203–219. doi: 10.1111/j.1365-2915.1996.tb00733.x. [DOI] [PubMed] [Google Scholar]
- Curtis CF, Mnzava AEP. Comparison of house spraying and insecticide-treated nets for malaria control. Bulletin of the World Health Organisation. 2000;78:1389–1400. [PMC free article] [PubMed] [Google Scholar]
- Curtis CF, Cook LM, Wood RJ. Selection for and against insecticide resistance and possible methods of inhibiting the evolution of resistance in mosquitoes. Ecological Entomology. 1978;3:273–287. [Google Scholar]
- Curtis CF, Miller JE, Hodjati MH, Kolaczinski JH, Kasumba I. Can anything be done to maintain the effectiveness of pyrethroid-impregnated bednets against malaria vectors? Philosophical Transactions of the Royal Society of London. Series B, Biological sciences. 1998;353:1769–1775. doi: 10.1098/rstb.1998.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabate A, Baldet T, Chandre F, Akogbeto M, Guiguemde TR, Darriet F, Brengues C, et al. The role of agricultural use of insecticides in resistance to pyrethroids in Anopheles gambiae s.l. in Burkina Faso. American Journal of Tropical Medicine and Hygiene. 2002;67:617–622. doi: 10.4269/ajtmh.2002.67.617. [DOI] [PubMed] [Google Scholar]
- Farenhorst M, Mouatcho JC, Kikankie CK, Brooke BD, Hunt RH, Thomas MB, Koekemoer LL, et al. Fungal infection counters insecticide resistance in African malaria mosquitoes. Proceedings of the National Academy of Sciences. 2009;106:17443–17447. doi: 10.1073/pnas.0908530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellous S, Koella JC. Infectious dose affects the outcome of the within-host competition between parasites. The American Naturalist. 2009;173:E177–E184. doi: 10.1086/598490. [DOI] [PubMed] [Google Scholar]
- Godfrey LD, Fuson KJ. Environmental and host plant effects on insecticide susceptibility of the cotton aphid (Homoptera: Aphididae) Journal of Cotton Science. 2001;5:22–29. [Google Scholar]
- Gu W, Müller G, Schlein Y, Novak RJ, Beier JC. Natural plant sugar sources of Anopheles mosquitoes strongly impact malaria transmission potential. PLoS One. 2011;6:e15996. doi: 10.1371/journal.pone.0015996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MHH, Koella JC. Evolution of tolerance: the genetic basis of a host's resistance against parasite manipulation. Oikos. 2003;102:309–317. [Google Scholar]
- Hardstone MC, Lazzaro BP, Scott JG. The effect of three environmental conditions on the fitness of cytochrome P450 monooxygenase-mediated permethrin resistance in Culex pipiens quinquefasciatus. BMC Evolutionary Biology. 2009;9:42. doi: 10.1186/1471-2148-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway J, Field L, Vontas J. An overview of insecticide resistance. Science. 2002;298:96–97. doi: 10.1126/science.1078052. [DOI] [PubMed] [Google Scholar]
- Hemingway J, Hawkes NJ, McCarroll L, Ranson H. The molecular basis of insecticide resistance in mosquitoes. Insect Biochemistry and Molecular Biology. 2004;34:653–665. doi: 10.1016/j.ibmb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Kelly JX, Smilkstein MJ, Brun R, Wittlin S, Cooper RA, Lane KD, Janowsky A, et al. Discovery of dual function acridones as a new antimalarial chemotype. Nature. 2009;459:270–273. doi: 10.1038/nature07937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen G, McKenzie F, Foy B, Schieffelin C, Billingsley P, Beier J. The potential impact of integrated malaria transmission control on entomologic inoculation rate in highly endemic areas. The American Journal of Tropical Medicine and Hygiene. 2000;62:545–551. doi: 10.4269/ajtmh.2000.62.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koella JC, Lorenz L, Bargielowski I. Microsporidians as evolution-proof agents of malaria control? Advances in Parasitology. 2009a;68:315–327. doi: 10.1016/S0065-308X(08)00612-X. [DOI] [PubMed] [Google Scholar]
- Koella JC, Lynch PA, Thomas MB, Read AF. Towards evolution-proof malaria control with insecticides. Evolutionary Applications. 2009b;2:469–480. doi: 10.1111/j.1752-4571.2009.00072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczinski JH, Fanello C, Herve JP, Conway DJ, Carnevale P, Curtis CF. Experimental and molecular genetic analysis of the impact of pyrethroid and non-pyrethroid insecticide impregnated bednets for mosquito control in an area of pyrethroid resistance. Bulletin of Entomological Research. 2000;90:125–132. doi: 10.1017/s0007485300000237. [DOI] [PubMed] [Google Scholar]
- Lambrechts L, Chavatte J-M, Snounou G, Koella JC. Environmental influence on the genetic basis of mosquito resistance to malaria parasites. Proceedings of the Royal Society B. 2006;273:1501–1506. doi: 10.1098/rspb.2006.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Menach A, Takala S, McKenzie FE, Perisse A, Harris A, Flahault A, Smith DL. An elaborated feeding cycle model for reductions in vectorial capacity of night-biting mosquitoes by insecticide-treated nets. Malaria Journal. 2007;6:10. doi: 10.1186/1475-2875-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZM, Liu SS, Liu YQ, Ye GY. Temperature-related fitness costs of resistance to spinosad in the diamondback moth, Plutella xylostella (Lepidoptera: Plutelidae) Bulletin of Entomological Research. 2007;97:627–635. doi: 10.1017/S0007485307005366. [DOI] [PubMed] [Google Scholar]
- Lines JD. Do agricultural insecticides select for insecticide resistance in mosquitoes? A look at the evidence. Parasitology Today. 1988;4:S17–S21. doi: 10.1016/0169-4758(88)90083-x. [DOI] [PubMed] [Google Scholar]
- Lorenz L, Koella JC. The microsporidian parasite Vavraia culicis as a potential late-life acting control agent of malaria. Evolutionary Applications. 2011;4:783–790. doi: 10.1111/j.1752-4571.2011.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynd A, Weetman D, Barbosa S, Egyir-Yawson A, Mitchell S, Pinto J, Hastings I, et al. Field, genetic and modelling approaches show strong positive selection acting upon an insecticide resistance mutation in Anopheles gambiae s.s. Molecular Biology and Evolution. 2010;27:1117–1125. doi: 10.1093/molbev/msq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias DK, Ochomo EO, Atieli FK, Ombok M, Bayoh MN, Olang G, Muhia D, et al. Spatial and temporal variation in the kdr allele L1014S in Anopheles gambiae s.s. and phenotypic variability in susceptibility to insecticides in Western Kenya. Malaria Journal. 2011;10:10. doi: 10.1186/1475-2875-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midega JT, Mbogo CM, Mwambi H, Wilson MD, Ojwang G, Mwangangi JM, Nzovu JG, et al. Estimating dispersal and survival of Anopheles gambiae and Anopheles funestus along the Kenyan coast by using mark-release-recapture methods. Journal of Medical Entomology. 2007;44:923–929. doi: 10.1603/0022-2585(2007)44[923:edasoa]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okech BA, Gouagna LC, Knols B, Kabiru EW. Influence of indoor microclimate and diet on survival of Anopheles gambiae ss (Diptera: Culicidae) in village house conditions in western Kenya. International Journal of Tropical Insect Science. 2007;24:207–212. [Google Scholar]
- Penilla R, Rodriguez A, Hemingway J, Trejo A, Lopez A, Rodriguez M. Cytochrome P450-based resistance mechanism and pyrethroid resistance in the field Anopheles albimanus resistance management trial. Pesticide Biochemistry and Physiology. 2007;89:111–117. [Google Scholar]
- Pennetier C, Costantini C, Corbel V, Licciardi S, Dabiré RK, Lapied B, Chandre F, et al. Mixture for controlling insecticide-resistant malaria vectors. Emerging Infectious Diseases. 2008;14:1707–1714. doi: 10.3201/eid1411.071575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones ML, Lines JD, Thomson MC, Jawara M. Anopheles gambiae gonotrophic cycle duration, biting and exiting behaviour unaffected by permethrin-impregnated bednets in The Gambia. Medical and Veterinary Entomology. 1997;11:71–78. doi: 10.1111/j.1365-2915.1997.tb00292.x. [DOI] [PubMed] [Google Scholar]
- Ranson H, Abdallah H, Badolo A, Moussa W, Karah-Hinzoumbe C, Yangalbe-Kalnone E, Sagnon N'Fale, et al. Insecticide resistance in Anopheles gambiae: data from the first year of a multi-country study highlight the extent of the problem. Malaria Journal. 2009;8:299. doi: 10.1186/1475-2875-8-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson H, Jensen B, Wang X, Prapanthadara L, Hemingway J, Collins FH. Genetic mapping of two loci affecting DDT resistance in the malaria vector Anopheles gambiae. Insect Molecular Biology. 2000;9:499–507. doi: 10.1046/j.1365-2583.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- Raymond B, Sayyed AH, Wright DJ. Genes and environment interact to determine the fitness costs of resistance to Bacillus thuringiensis. Proceedings of the Royal Society B. 2005;272:1519–1524. doi: 10.1098/rspb.2005.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy MR, Overgaard HJ, Abaga S, Reddy VP, Caccone A, Kiszewski AE, Slotman MA. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malaria Journal. 2011;10:184. doi: 10.1186/1475-2875-10-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D, Tren R, Bate R, Zambone J. The Excellent Powder. DDT's Political and Scientific History. Indianapolis: Dog Ear Publishing; 2010. [Google Scholar]
- Takken W, Charlwood JD, Billingsley PF, Gort G. Dispersal and survival of Anopheles funestus and A. gambiae s.l. (Diptera: Culicidae) during the rainy season in southeast Tanzania. Bulletin of Entomological Research. 1998;88:561–566. [Google Scholar]
- Tungu P, Magesa S, Maxwell C, Malima R, Masue D, Sudi W, Myamba J, et al. Evaluation of PermaNet 3.0 a deltamethrin-PBO combination net against Anopheles gambiae and pyrethroid resistant Culex quinquefasciatus mosquitoes: an experimental hut trial in Tanzania. Malaria Journal. 2010;9:21. doi: 10.1186/1475-2875-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijendravarma RK, Kraaijeveld AR, Godfray HCJ. Experimental evolution shows Drosophila melanogaster resistance to a microsporidian pathogen has fitness costs. Evolution. 2008;63:104–114. doi: 10.1111/j.1558-5646.2008.00516.x. [DOI] [PubMed] [Google Scholar]
- Vulule JM, Beach RF, Atieli FK, Roberts JM, Mount DL, Mwangi RW. Reduced susceptibility of Anopheles gambiae to permethrin associated with the use of permethrin-impregnated bednets and curtains in Kenya. Medical and Veterinary Entomology. 1994;8:71–75. doi: 10.1111/j.1365-2915.1994.tb00389.x. [DOI] [PubMed] [Google Scholar]
- Vulule JM, Beach RF, Atieli FK, McAllister JC, Brogdon WG, Roberts JM, Mwange RW, et al. Elevated oxidase and esterase levels associated with permethrin tolerance in Anopheles gambiae from Kenyan villages using permethrin-impregnated nets. Medical and Veterinary Entomology. 1999;13:239–244. doi: 10.1046/j.1365-2915.1999.00177.x. [DOI] [PubMed] [Google Scholar]
- Weiser J, Zizka Z. Brachiola gambiae sp. n. the microsporidian parasite of Anopheles gambiae and A. melas in Liberia. Acta Protozoologica. 2004;43:73–80. [Google Scholar]
- WHO. Test Procedures for Insecticide Resistance Monitoring in Malaria Vectors, Bio-Efficacy and Persistence of Insecticides on Treated Surfaces. Geneva: WHO; 1998. [Google Scholar]
- WHO. Global Malaria Program. Surveillance, Monitoring and Evaluation Unit. WHO, Geneva; 2008. Impact of long-lasting insecticidal-treated nets (LLINs) and artemisin-based combination therapies (ACTs) measured using surveillance data, in four African countries. [Google Scholar]
- Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- Wood RJ, Cook LM. A note on estimating selection pressures on insecticide-resistance genes. Bulletin of the World Health Organization. 1983;61:129–134. [PMC free article] [PubMed] [Google Scholar]
- Zbinden M, Haag CR, Ebert D. Experimental evolution of field populations of Daphnia magna in response to parasite treatment. Journal of evolutionary Biology. 2008;21:1068–1078. doi: 10.1111/j.1420-9101.2008.01541.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data deposited in the Dryad repository: doi:10.5061/dryad.m6g5286d.


