Abstract
Intraspecific adaptation in Abies sachalinensis was examined using models based on long-term monitoring data gathered during a reciprocal transplant experiment with eight seed source populations and six transplantation sites along an altitudinal gradient. The consequence of local adaptation was evaluated by testing the home-site advantage for upslope and downslope transplants at five ages. The populations’ fitness-linked trait was set as their productivity (tree height × survival rate) at each age. The effects of global warming were evaluated on the basis of the 36-year performance of downslope transplants. Evidence was found for adaptive genetic variation affecting both height and survival from an early age. Increasing the distance between seed source and planting site significantly reduced productivity for both upslope and downslope transplantation, demonstrating the existence of a significant home-site advantage. The decrease in productivity was most distinct for upslope transplantations, indicating strong local adaptation to high altitudes. Global warming is predicted to increase the productivity of high-altitude populations. However, owing to their existing local adaptation, all tested populations exhibited lower productivity under warming than demes that were optimal for the new climate. These negative predictions should be considered when planning the management of locally adapted plant species such as A. sachalinensis.
Keywords: Abies sachalinensis, altitudinal gradient, global warming, home-site advantage, local adaptation, long-term data, productivity, reciprocal transplant experiment
Introduction
Modern plant taxa have survived long periods of climate variation associated with glacial–interglacial cycles. Plant species have been exposed to wide fluctuations in temperature during these cycles, and the fossil pollen record suggests that most of them have at one time or another migrated in response to climate change events (Davis and Shaw 2001). It has been suggested that both forest herbs and woody plants have undergone or are undergoing similar range shifts in response to the current global warming (Lenoir et al. 2008, 2009). However, the current warming is occurring so rapidly that many plant species will be unable to respond with sufficient speed through geographic range shifts alone (Davis and Shaw 2001; Jump and Penuelas 2005). In particular, woody plants with long life cycles need to respond to such rapid warming by either short-term phenotypic plasticity or long-term microevolutionary processes (Jump and Penuelas 2005; Aitken et al. 2008; Kuparinen et al. 2010; Hoffmann and Sgro 2011). Tree species that have already undergone local adaptation, that is, that have become genetically adapted to the currently prevailing climatic conditions of their habitats (Kawecki and Ebert 2004), may not be able to respond adequately to rapid warming because of their relatively low plasticity. As such, it is important to study the extent of plants’ local adaptation and to be able to predict their responses to global warming in the near future, especially for woody plants (Aitken et al. 2008; Hoffmann and Sgro 2011).
Reciprocal transplant experiments are powerful tools for detecting local adaptation, because they compare the fitness of different populations within specific habitats using a ‘local deme vs foreign demes’ criterion (Kawecki and Ebert 2004). If the local deme of a particular site exhibits greater fitness within that site than demes from other habitats, it is considered to have undergone local adaptation (Kawecki and Ebert 2004). The ‘home-site advantage’ concept can be used to quantify the extent of local adaptation (Galloway and Fenster 2000; Montalvo and Ellstrand 2000); it is important because it assesses the superiority of fitness of the local deme (i.e. home-site plants) compared to that of foreign demes in a fashion that accounts for the physical distance between the planting site and the stand from which the seeds of the foreign deme were collected (Montalvo and Ellstrand 2000). The observation of a home-site advantage for every deme tested provides robust evidence for local adaptation (Galloway and Fenster 2000). Conversely, the detection of home-site advantages for some but not all of the reciprocal transplants (e.g. if local adaptation was detected for transplants from a lowland to a mountain site, but not for transplants from a montane stand to the lowland site) suggests the occurrence of ‘asymmetrical local adaptation’ (Mercer et al. 2008). Because of the long life cycles for woody plant species, it is necessary to conduct long-term studies to identify their (asymmetrical) local adaptation using reciprocal transplant experiments. Thus, previous studies in which intraspecific adaptation has been demonstrated in woody plants were conducted over periods of 12–20 years (Eriksson et al. 1980; Rehfeldt et al. 1999; Wu and Ying 2004; Savolainen et al. 2007). These studies examined the genetic traits of populations over wide geographic scales. Adaptive divergence would perhaps be expected at the extremes of wide geographic ranges because it will be accelerated or maintained by strong selective forces and restrictions on gene flow to other populations owing to distance and differences in the timing of flowering (Howe et al. 2003; Premoli 2003; Savolainen et al. 2007; Chuine 2010). Conversely, within smaller and more confined regions, intraspecific adaptation might be expected to be comparatively rare in woody species because these promoting factors would be absent. However, previous studies have reported evidence of intraspecific adaptation across an altitudinal gradient within a small area in woody conifers (Conkle 1973; Rehfeldt 1983; Kitzmiller 2005) and also in herb/grass species (Byars et al. 2007; Mercer et al. 2008). These intraspecific adaptations are presumably due to the substantial environmental changes experienced when moving up the altitudinal gradient, even in such small areas (Körner 2007); in contrast, there is little environmental variation on moving latitudinally over the same geographic scale. However, because of the lack of long-term data on woody plant species, it is still unclear whether and/or to what extent the effects of intraspecific adaptation are visible at different stages of such plants’ lives (but see Conkle 1973).
Long-term reciprocal transplant experiments are also useful for estimating populations’ responses to environmental change because they make it possible to compare fitness in different environments using a ‘home vs away’ criterion (Kawecki and Ebert 2004). A deme's performance across a range of climatic conditions can be evaluated using this approach; therefore, previous studies involving long-term reciprocal transplant experiments with woody plants have successfully predicted the performance of the population they examined in the face of climate change (Rehfeldt et al. 2001, 2002). These studies indicate that future performance is influenced by both the extent of a population's genetic adaptation to its current climate and its plasticity. For a population that has already undergone intraspecific adaptation to the current climate of its habitat, climate change (and especially global warming) would be expected to have a significant and negative impact because of strong genetic constraints that would restrict its ability to respond to the changing conditions (Rehfeldt et al. 1999; Savolainen et al. 2004; Aitken et al. 2008). In such cases, it is best to evaluate the future response of each stand by focusing on two different approaches: the extent of the change in the performance of the current population in response to the predicted climatic change (Rehfeldt et al. 2002), and the difference between the performance of the current population and that of an ‘optimal’ population under global warming. The former can be examined using a ‘home vs away’ approach, while the latter can be estimated using a ‘local vs foreign’ approach. In the ‘local vs foreign’ approach, a population that is not native to the site but native to the warmer climatic condition is considered to display an optimal response and is regarded as the ‘local’ population after global warming: the population that is currently native to the transplantation site is regarded as the ‘foreign’ population. As such, the experiment effectively compares the performance of a ‘local’ population that is optimally adapted to the warmer conditions to that of the current population after a global warming event. As the experimental sites differ primarily in terms of air temperature alone (Körner 2007), reciprocal transplant experiments across an altitudinal gradient are ideally suited for evaluating the impact of global warming. However, reciprocal transplant experiments of this kind have not previously been performed with woody species owing to the long timescales required.
In the study reported herein, we performed a reciprocal transplant experiment using Sakhalin fir (Abies sachalinensis) populations from various positions across a steep altitudinal gradient (230–1200 m a.s.l.) on a regional scale. This experiment has been conducted under the auspices of the University of Tokyo Hokkaido Forest (UTHF) since the 1970s (Kurahashi and Hamaya 1981); long-term time-series data going back between 10 and 36 years from 2009 have been collected. Abies sachalinensis is a sub-boreal conifer that plays an important role in timber production in northern Japan; the experiment was originally undertaken to provide information for the forestry industry. Genetic variation along the altitudinal gradient was detected for growth and phenological traits in the early stages of the experiment (Kurahashi and Hamaya 1981), but the long-term data have not previously been examined with the intent of studying local adaptation.
The study reported herein initially examined the extent of altitude-dependent adaptation in populations of this species in a small region using time-series data spanning periods of 10–36 years. On the basis of these initial results, changes in the productivity of the populations in response to global warming were predicted. In light of these predictions, some consideration was devoted to the types of forest management strategies that will be required to maintain productivity in the face of global warming in a fashion that accounts for evolutionary adaptation (Hoffmann and Sgro 2011). Ultimately, our aim was to answer the following three questions:
How do genetic and environmental factors affect growth and survival at different altitudes?
What is the magnitude of the home-site advantage across the altitudinal range and what trends are apparent in the time-series?
How is A. sachalinensis expected to respond to the predicted global warming?
Materials and methods
The studied species
Sakhalin fir (A. sachalinensis) is a coniferous wind-pollinated and wind-dispersed climax species with a geographic range that extends from eastern Siberia to Hokkaido, the northernmost island of the Japanese archipelago. It is one of the dominant species in natural sub-boreal forests of the Far East and is an important component of forest ecosystems (Kato 1952). Its current altitudinal distribution ranges from 200 to 1600 m a.s.l., with the center of the population growing on mountainous land at 400–600 m a.s.l. Its demographic history suggests that it had become a dominant species by the time of the last glacial period 20 000 years ago (Igarashi 1996), that it survived in a glacial refugium in the eastern part of Hokkaido, and that it has not had any recent contact with congeners (Tsumura and Suyama 1998). Genetic variation in several traits has previously been observed in populations across its altitudinal range (Kurahashi and Hamaya 1981; Eiga and Sakai 1984; Saho et al. 1994; Goto et al. 2011).
Study site
Our study was conducted on a regional scale, in a site covering an area of 150 km2 with a linear range of <25 km in any given direction. The site is on the southwestern slope of Mt. Dairoku (1459 m a.s.l.) in the UTHF, which covers the region from 43°10′35″N, 142°22′48″E to 43°20′59″N, 142°40′51″E in central Hokkaido. A reciprocal transplant experiment across the site's altitudinal gradient was conducted using eight natural stands as seed sources; the altitudes of the collection stands were 230, 340, 420, 530, 730, 930, 1100, and 1200 m. Five of these altitudes (i.e. all of them but those at 340, 420 and 1200 m) were also used as transplantation test sites, with the transplants being cultivated next to the seed source populations. In addition, a sixth transplantation site at 420 m was used, located approximately 10 km away from the seed source at this altitude. Six altitudes were thus examined in the reciprocal transplant experiment; the corresponding sites are henceforth referred to as site-230, site-420, site-530, site-730, site-930, and site-1100. It has been reported that both the soil and the forest vegetation types within the UTHF at altitudes of <700–800 m differ substantially from those in higher altitudinal zones (Kato 1952; Nakata et al. 1994). Thus, below 700–800 m, the soil is of the brown forest type; above 700–800 m, it gradually transitions to the black mountain type (Nakata et al. 1994). At around the same point, the bamboo species that is dominant at lower altitudes (Sasa senanensis) is replaced by Sasa kurilensis (Kato 1952). Climate data for 2009 and 2010 for all of the monitored sites were collected using digital loggers (HOBO Pro RH/Temp; Onset Computer Corporation); information on the snow depth at the sites in 1974–1975 was obtained from the records of the UTHF (Table 1).
Table 1.
Study sites and their meteorological conditions in 2009 and 2010
| Average temperature (°C) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Location* | Altitude (m) | Annual | Summer | Winter | Maximum (°C) | Minimum (°C) | First frost date | Snow depth† (cm) |
| Mt. top | 1459 | −0.05 | 10.80 | −9.65 | 26.88 | −21.49 | 6 October | – |
| Population | 1200 | 1.75 | 9.88 | −6.43 | 24.82 | −20.21 | 8 October | – |
| Population and site | 1100 | 1.99 | 10.40 | −6.46 | 27.06 | −21.72 | 9 October | 280 |
| Population and site | 930 | 2.57 | 11.01 | −5.92 | 26.16 | −20.64 | 9 October | 248 |
| Population and site | 730 | 3.81 | 12.11 | −4.53 | 26.99 | −20.17 | 10 October | 123 |
| Population and site | 530 | 4.80 | 12.98 | −3.41 | 28.10 | −19.33 | 16 October | 112 |
| Population and site | 420 | 5.55 | 13.72 | −2.66 | 28.57 | −18.52 | 31 October | – |
| Population and site | 230 | 6.53 | 14.79 | −1.78 | 32.25 | −20.26 | 10 November | 42 |
Winter and summer average temperatures were calculated from continuous data collected from October 2009 to March 2010 and from April to September 2010, respectively. The first frost date indicates the first date with a temperature <0°C in autumn 2009.
Population, site, and Mt. top refer to the seed source population, the reciprocal transplantation site at a given altitude, and the crest of Mt. Dairoku, respectively.
Data refers to the maximum snow depth derived from the monitoring during 1974–1975 by the University of Tokyo Hokkaido Forest (UTHF).
The reciprocal transplant experiment
In 1973, open-pollinated seeds were collected from five mother trees in each of the eight populations (i.e. 40 mother trees in total). In early May 1974, all seeds derived from each mother tree were sown at a density of 60 g/m2 in a nursery seed bed (230 m a.s.l.). Two growing seasons after their germination, the offspring were randomly selected and transferred to Jiffy™ pots. At the end of a third growing season, in September 1976, they were planted at the six sites of the reciprocal transplant experiment. Prior to their planting, all vegetation (including dwarf bamboo) was removed from the planting sites and the soil was scarified to create a uniform soil surface. Five offspring plants from each mother tree were planted at each site, which was duplicated in every sites. The seedlings were transplanted in grids, with one family per row at a spacing of 1.2 m, and a separate column for the descendents of each mother tree (giving a total of 40 columns). In total, 2400 trees (five transplants × five half-sib families × eight seed sources × six planting sites × two replicates) were transplanted. The planting rule, that is, the order in which the families were planted, was the same in all six sites. After 20 years, two of the five trees in each family were evenly thinned to thin the lines in the lower three sites (i.e. site-230, -420, and -530). The identities and heights of the surviving trees were recorded at seven points in time, when the trees were 5, 6, 10, 13, 18, 31, and 36 years old. Transplants were identified on the basis of their location within the plot; data were collected for both living transplants and those that died without missing, because the woody parts of the dead transplants persisted for several years.
Statistical analysis
Height and survival
It was assumed that the phenotype of the transplants was a consequence of their genotype, their living environment, and the interaction between these two factors (genotype × environment). The two measured traits, tree height and survival, were examined using analysis of variance (anova) to determine the significance and contribution of genetic and environmental effects at each age; these analyses were conducted using the SAS 9.2 software package (SAS Institute Inc., Cary, NC, USA). For each trait at each age, the SAS program PROC GLM was used with fixed effects for the eight seed source populations, the six planting sites, and the interactions between them (population × planting site), and random effects associated with the family variables for each population and replicate at each planting site. Survival data for the transplants aged 5 and 6 years were excluded from the analyses because mortality so soon after transplantation was extremely rare. The survival data were analyzed by calculating the survival rate for plants from a particular treatment. The variance components for tree height and survival were then analyzed using the SAS program PROC Varcomp.
All subsequent statistical analyses were performed using R 2.10.1 (R Development Core Team 2009). We quantified the effects of genotype and environment on both height and survival traits at five ages (10, 13, 18, 31, and 36 years). The fixed effects were estimated using generalized linear mixed models (GLMMs) of the form (Rehfeldt 1983):
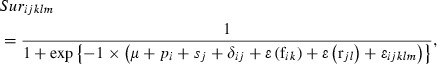 |
(1) |
where i is an index running over the different seed source populations (i = 1–8), j is an index running over the different planting sites (j = 1–6), k is the family within the ith seed source population (k = 1–5), l is the number of the replicate at the jth planting site (l = I/II), and m is the individual within a particular treatment (m = 1–5). Hijklm and Surijklm are the height and chance of survival, respectively, for the mth plant from the kth family of the ith population within the lth replicate of the jth site. μ is an intercept, pi is the contribution of the ith population, sj is the contribution of the jth planting site, δij is the effect of the interaction between the ith population and the jth site, ε(fik) is the effect of the kth family in the ith population, ε(rjl) is the effect of the lth replicate at the jth site, and εijklm is the error. The first model, in which the response variable is Hijklm, is the ‘height model’; it has a normally distributed error structure and an identity link function. The model with the response variable Surijklm is the ‘survival model’; it has a binomial error structure and a logit-link function, and its outputs are binary (alive/dead). In both models, pi, sj, and δij, are fixed effects that are treated as categorical variables, while ε(fik) and ε(rjl) are random effects with which we were not concerned but did not ignore. The fitting of all models was checked using likelihood-ratio tests, comparing the new models’ predictions to those of a null model. The behavior of the coefficients pi and sj for both models at different altitudes was evaluated by plotting them against the seed source and planting site altitudes. We used the height and survival models for the 36-year-old plants to assess the nature of the genetic cline (if any) within the tested area and the impact of the environmental gradient across which the transplantation sites were distributed.
Testing the home-site advantage
The productivity of each population under the various conditions examined was assessed using a parameter calculated by multiplying the average height of the relevant transplants by their survival rate at the age of evaluation; this provides a measure of the population's overall fitness (Savolainen et al. 2007). The outputs of the height and survival models were used to calculate the productivity with controlling errors related to family-related factors and site replication. The productivity of the ith population at the jth planting site (Pij) was calculated by multiplying the estimated Hij and Surij values: Hij × Surij = Pij. The significance and magnitude of the home-site advantage were tested by comparing the Pij values for local (i.e. indigenous, from the same altitude as the planting site, i = j) and foreign (i.e. non-indigenous, from different altitudes to the planting site, i≠j) demes in a way that accounted for the environmental distance between the planting site and the ‘native’ site of the foreign deme (Montalvo and Ellstrand 2000). The relationship between the relative productivity (relative-Pij) and the altitudinal distance (DISTij) was examined for every tree age considered (10, 13, 18, 31, and 36 years). In the following discussion, relative-Pij refers to the performance of transplants at the jth planting site relative to the local plants, and DISTij refers to the vertical distance between the ‘native’ site of the foreign deme and the planting site (i.e. the difference between the altitude at which the mother tree was growing and that of the planting site). At each site, the relative-P value and DIST of local plants were defined as being 1 and 0, respectively. Transplants from a higher to a lower altitude were assigned positive DISTij values, while transplants to a higher altitude resulted in negative values. The relationship between the change in relative performance following transplantation and DISTij was examined using the following GLM:
| (2) |
where yij is the response variable, that is, the relative-Pij of the ith population at the jth site, and α and β are the parameters estimated by the GLM; note that z indicates the transplanting direction (z = upslope/downslope). Although β has two independent parameters associated with the direction, we estimated both from a single equation in order to obtain a common intercept (the value when DIST = 0). The model was defined assuming a normally distributed error structure and a log link function in order to avoid negative yij values. A home-site advantage was considered to have been identified if there was a significant decrease in relative-P as DIST increased; it was necessary to assess the significance of β. Differences in the magnitudes of the home-site advantage between upslope/downslope transplantations and the associated changes over time were examined by examining the trends in the populations’β values as they aged.
Predicted performance in the event of global warming
To estimate the long-term change in population productivities caused by rapid global warming, estimated Pij values for 36-year-old transplants in the downslope direction were considered, because downslope movements simulate the effects of warming. Pij indicates the nature of the population's response (in terms of productivity) to the warmer conditions experienced following transplantation from the ‘native’ith stand to the jth site. The impact of warming was assessed using two different approaches. First, the overall-Pij of the transplanted population was compared to that of the same population in its ‘native’ site (i.e. the ith site; Pii). Because this approach provides a ‘before and after’ type comparison of the change in the population's behavior in response to warming, it can be used to predict differences between the population's future performance and that achieved under current conditions in its native stand. This is called a ‘home vs away’ test in the terminology used to describe reciprocal transplant experiments (Kawecki and Ebert 2004); such tests have been used to describe species’ niches and the way they are affected by global warming (Rehfeldt et al. 2002). However, this approach only examines one aspect of the impact on performance because it does not provide information on how the change in each population's productivity tracks the change in the environment. To test this, we need to determine how close the productivity of a certain population will be to the maximum level that could potentially be achieved under the new climatic conditions created by global warming. It is reasonable to assume that the current populations are (at least) well adapted to their native site and that their performance within that site is essential as good as can possibly be achieved under the currently prevailing climatic conditions. We therefore compared the productivity of the ith deme after being transplanted downwards to site j (Pij) to that of the deme native to site j (Pjj). This is the ‘local vs foreign’ approach as described by Kawecki and Ebert (2004) for evaluating home-site advantages in terms of the performance of a transplanted population relative to that of the local population, using the model presented in eqn (2). By identifying home-site advantages, the likely negative effects of global warming can be estimated because the transplanted deme will be maladapted (in genetic terms) to the climate at the transplantation site and its performance will decline in proportion to the magnitude of the difference between its native environment and that of the transplantation site.
For the first approach, we constructed a ‘home-away model’ based on the niche breadth model (Rehfeldt et al. 2002). A GLM based on the following equation was used:
| (3) |
where yij is the response variable, that is, the overall-Pij of the ith population at the jth site; yhome is the home productivity of the ith population (Pii); α’, β’, γ, and ω are estimated parameters; and Altj is the altitude of the jth site. In the GLM, yhome is set as an offset and is not associated with any parameter; the log link function is used with a normally distributed error structure. Equation (3) estimates two effects: (1) the effect of warming, using the DIST variable, and (2) the negative impact on performance at the edge of the tree's niche (Rehfeldt et al. 2002), using the quadratic term that reflects the site condition (Altj). The model's goodness of fit was checked using likelihood-ratio tests to compare its deviances to those of a null model. From the output of the home-away model, the change in overall-P of the ith population was projected along the DIST. The DIST values were then converted to temperature increases (ΔTemp) using meteorological data collected at all of the sites during 2009 and 2010 (Table 1), and the model's output was analyzed as a function of ΔTemp. For the second approach, we reused the model described by eqn (2) but considered only the 36-year downslope transplants. As before, the DIST values were converted to ΔTemp to facilitate analysis of the changes in relative performance.
The degree of warming was analyzed as discussed in previous publications. A high-resolution projection using the most extreme model of the Meteorological Research Institute [Coupled General Circulation Model version 2 (MRI-CGCM2); Yukimoto et al. 2001] forecasts a temperature increase of 4°C or more within the next 80 years in the area examined in this work (Kurihara et al. 2005). Four potential warming scenarios were considered, including the most extreme scenario discussed above; thus, ΔTemp values of +0.5, 1.0, 2.0 and 4.0°C relative to current levels were considered. The impact of the different warming scenarios on A. sachalinensis was considered in the context of local adaptation.
Results
Variation in measured traits
The average heights and survival rates of the various 36-year-old transplants were measured and multiplied to calculate their productivity (Fig. 1). Overall, the trees at the lower three sites, especially at site-420 and site-530, seemed to exhibit good growth and to retain high survivability. In contrast, at site-1100, most plants were <300 cm tall even after 36 years, and the survival rates for all transplants were below 40%. It was noteworthy that the populations from higher altitudes tended to be stunted at the lowest sites (site-230 and site-420), while the populations from lower altitudes exhibited particularly low survival rates at the highest sites (site-1100 and site-930; Fig. 1).
Figure 1.
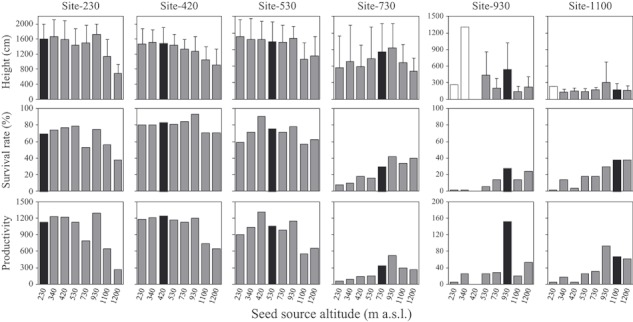
Average tree height data, showing the SD (upper), survival rate (middle), and productivity (average height × survival rate) (lower) of 36-year-old transplants for all seed source populations at each of the planting sites. Values for transplants are arranged according to seed source altitude. Each site code (e.g. site-230) refers to a different planting site and its altitude; these are also arranged by altitude. The ‘local deme’ (i.e. the deme native to the site in question) is indicated by black bars. The open bars in the charts for site-930 and site-1100 indicate the height of the sole survivor out of fifty initial transplants, not an average tree height.
Various patterns were apparent in the 36-year productivities for the different seed source populations and planting sites (Fig. 1). When examining the effects of the seed source altitude, a single peak was identified for the three highest sites, with the highest productivities being observed for local plants (i.e. the home-site populations) or neighbor populations (Fig. 1). For the lower three sites, transplants whose site of origin was a long way from the planting site tended to exhibit low productivities: transplants originated from 1100 and 1200 m a.s.l. performed poorly in all three sites, and those from 230 m a.s.l. performed poorly at site-530 (Fig. 1).
The anovas for tree height and survival at 36 years indicated the existence of highly significant differences in seed source populations, planting sites, and their interactions (Table 2). That is to say both of these traits were affected by the identity of the seed source population and the planting site. For tree height, significant results were observed in all seven age classes examined, from 5 to 36 years (Table 2). However, the relative influence of the seed source population and planting site differed according to tree age: for young trees, the seed source population had a greater effect on height than did the planting site, but the opposite pattern was seen as the trees grew older. The effect of the seed source population on survival was only marginally significant (P = 0.088) at 10 years of age; subsequently, from the age of 13 onwards, both the population and the site consistently exhibited significant effects (Table 2). The seed source population had a greater relative impact on tree height than it did on the trees’ survival rates.
Table 2.
Results of analyses of variance of the height and survival of Abies sachalinensis in reciprocal transplants at several ages from 5 to 36 years
| Height | Survival | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Effect | df | F-value | P-value | Variance | df | F-value | P-value | Variance |
| 5 | Population | 7 | 110.9 | <0.001 | 30.1 | ||||
| Planting site | 5 | 37.9 | <0.001 | 6.7 | |||||
| Population × site | 35 | 2.8 | <0.001 | 2.3 | |||||
| 6 | Population | 7 | 105.6 | <0.001 | 29.2 | ||||
| Planting site | 5 | 34.6 | <0.001 | 7.2 | |||||
| Population × site | 35 | 3.1 | <0.001 | 2.8 | |||||
| 10 | Population | 7 | 118.0 | <0.001 | 20.7 | 7 | 1.8 | 0.088 | 2.9 |
| Planting site | 5 | 228.4 | <0.001 | 31.1 | 5 | 27.4 | <0.001 | 22.5 | |
| Population × site | 35 | 7.6 | <0.001 | 7.2 | 35 | 2.2 | <0.001 | 7.1 | |
| 13 | Population | 7 | 83.8 | <0.001 | 9.3 | 7 | 3.7 | <0.001 | 1.9 |
| Planting site | 5 | 476.7 | <0.001 | 49.7 | 5 | 42.9 | <0.001 | 29.4 | |
| Population × site | 35 | 12.8 | <0.001 | 10.0 | 35 | 3.1 | <0.001 | 12.0 | |
| 18 | Population | 7 | 88.9 | <0.001 | 6.7 | 7 | 7.7 | <0.001 | 2.5 |
| Planting site | 5 | 809.4 | <0.001 | 64.3 | 5 | 116.8 | <0.001 | 50.6 | |
| Population × site | 35 | 12.7 | <0.001 | 7.8 | 35 | 4.2 | <0.001 | 11.3 | |
| 31 | Population | 7 | 23.2 | <0.001 | 4.8 | 7 | 7.3 | <0.001 | 5.0 |
| Planting site | 5 | 401.8 | <0.001 | 68.0 | 5 | 113.2 | <0.001 | 49.9 | |
| Population × site | 35 | 3.5 | <0.001 | 3.5 | 35 | 3.5 | <0.001 | 9.1 | |
| 36 | Population | 7 | 20.8 | <0.001 | 7.1 | 7 | 4.7 | <0.001 | 3.0 |
| Planting site | 5 | 168.0 | <0.001 | 56.1 | 5 | 120.0 | <0.001 | 52.6 | |
| Population × site | 34 | 2.1 | <0.001 | 2.2 | 35 | 3.4 | <0.001 | 8.6 | |
The fixed effects in all models were the seed source population, planting site, and their interaction. For each of the tests, variance components of the fixed and random effects are represented as well as F values and statistical probabilities (those of the random effects are not shown). Entries in bold type indicate significant effects.
Parameter estimation
The GLMMs for height and survival were found to be adequately well fit at all five ages considered, as judged by likelihood-ratio tests (P < 0.001 for all 10 models). To examine the patterns associated with changes along the altitudinal gradient, we focused on the two models for the traits of plants at 36 years of age (Fig. 2). This was carried out because in both the height and the survival models, the estimated values for the coefficients associated with the fixed effects increased with age (data not shown). The heights and survival rates for the seed source population and planting site at the lowest altitude (230 m) were used as reference values when examining altitude-related genetic and environmental effects (Fig. 2). For the 36-year height model (Fig. 2A), the environmental gradient was analyzed in terms of the trend in the coefficients of the planting site (sj) with increasing altitude; the negative effects on tree height became increasingly severe as site altitude increased. A particularly large decline was observed when the site altitude exceeded 730 m a.s.l. The genetic cline was analyzed by examining the decrease in the source population coefficients (pj) in response to changes in altitude; these were especially pronounced if populations from intermediate altitudes, particularly that from 930 m a.s.l., were not considered (Fig. 2A). The environmental gradient observed for the 36-year survival model (Fig. 2B) was similar to that for the tree height; in both cases, a substantial decrease was observed at around 730 m a.s.l. The genetic cline was distinct in that there was an inverse relationship between the altitude of the seed source population and the survival trait, with only the 930 m stand defying this trend (Fig. 2B).
Figure 2.
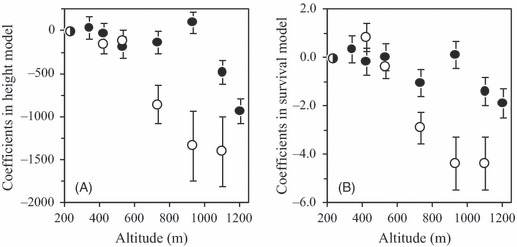
Coefficients for the population and planting site variables obtained from two types of GLMM (generalized linear mixed model). GLMMs were constructed to assess the influence of the seed source population (closed circles), the planting site (open circles), and their interaction on the 36-year tree height (A) and 36-year survival (B). The results for the seed source population at 230 m a.s.l. and site-230 were used as reference values (half-closed circles) to facilitate the graphical visualization of the genetic and environmental effects across the altitudinal gradient.
Home-site advantage
In general, the productivity of the foreign plants did not exceed that of the local plants, that is, the relative-Pij values for foreign plants were typically <1.0. Specifically, 33 foreign population samples out of 42 exhibited a relative 36-year productivity of <1.0 (Fig. 3E). By plotting the relative-Pij against the upslope/downslope DISTij at 10, 13, 18, 31, and 36 years, it was possible to observe the effects of altitudinal distance that were apparent throughout ages considered (Fig. 3A–E). The GLM regressions in all ages yielded significant results for both upslope and downslope transplantations. The results consistently indicated that the relative-Pij of foreign plants decreased as the altitudinal distance between their site of origin and the site of planting increased, irrespective of the direction, indicating the existence of a significant home-site advantage. However, the behavior of these effects as the trees aged differed depending on the direction of transplantation (Fig. 3F). For trees transplanted downwards, there was little age-related variation in the coefficients of DISTij. In contrast, the coefficients of DISTij for upwardly transplanted trees increased gradually with age (Fig. 3F). Thus, at 10 years of age, trees transplanted 500 m upwards outperformed those transplanted 500 m downwards, with relative productivity values of 0.93 and 0.67, respectively. By 36 years of age, the corresponding relative productivity values for trees transplanted up- and downslope were 0.31 and 0.70, respectively.
Figure 3.
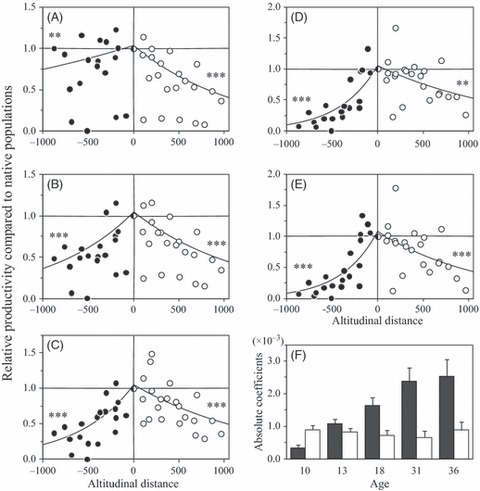
The magnitude of the home-site advantage across the altitudinal range for trees of different ages (A–E) and its behavior over time (F). Relative productivities were calculated on the basis of the performance of the transplanted population relative to that of the local population at a given planting site. The plots are arranged in order of the altitudinal distance, DIST, that is, the difference between the altitude of the seed source and the altitude of the planting site, to determine whether the local plants (DIST = 0) outperformed the transplants. The letters A to E refer to the different testing ages, that is, 10, 13, 18, 31, and 36 years, respectively. A negative DIST value (closed circle) indicates upward transplantation, while a positive DIST value (open circle) indicates downward transplantation. Lines indicate the results of regression analyses (eqn 2 in Materials and methods) with the associated significance level, ***P < 0.001, **P < 0.01. The variation in the magnitude of the home-site advantage over time (F) is shown in terms of the absolute coefficient of DIST, that is, the β term from eqn 2, for trees transplanted upslope (closed bar) and downslope (open bar); the error bars indicate the standard error. Higher values of β reflect more severe transfer effects.
Predicted effects of global warming
Predictions using the home-away model
The home-away model constructed using the data for overall-Pij in 36-year-old trees planted downward exhibited a good statistical fit (likelihood-ratio test, P < 0.001; Fig. 4). Of the parameters in this model (eqn 3), DISTij and Altj had significantly positive values: 3.29 × 10−3, P < 0.001; 9.39 × 10−3, P = 0.001, respectively. The value of 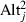 was significantly negative: −7.40 × 10−6, P = 0.021. The DISTij effect suggests that productivity could be increased by further downslope transplantation. However, this effect is counterbalanced by the quadratic term of Altj; as a result, the altitude that yielded the optimal overall-Pij was 412 m. That is to say, the home-away model suggests that productivity decreases when trees are transplanted downslope to positions below this altitude (Fig. 4). Different seed source populations exhibited different trends in overall-Pij as a function of the downslope DIST, depending on the seed source altitude. For populations sourced from lower altitudes, the productivity values (overall-Pij) increased for small values of DISTij but then decreased for larger values. Conversely, the productivities of populations sourced from higher altitudes continued to increase even for transplantations of several hundred meters (in the case of the highest, 1200 m a.s.l. population, the overall-P only began to decrease when the distance of downslope transitions excessed 788 m, Fig. 4). Notably, the difference in the magnitude of the populations’ overall-Pij values was dependent on the difference in their offset values.
was significantly negative: −7.40 × 10−6, P = 0.021. The DISTij effect suggests that productivity could be increased by further downslope transplantation. However, this effect is counterbalanced by the quadratic term of Altj; as a result, the altitude that yielded the optimal overall-Pij was 412 m. That is to say, the home-away model suggests that productivity decreases when trees are transplanted downslope to positions below this altitude (Fig. 4). Different seed source populations exhibited different trends in overall-Pij as a function of the downslope DIST, depending on the seed source altitude. For populations sourced from lower altitudes, the productivity values (overall-Pij) increased for small values of DISTij but then decreased for larger values. Conversely, the productivities of populations sourced from higher altitudes continued to increase even for transplantations of several hundred meters (in the case of the highest, 1200 m a.s.l. population, the overall-P only began to decrease when the distance of downslope transitions excessed 788 m, Fig. 4). Notably, the difference in the magnitude of the populations’ overall-Pij values was dependent on the difference in their offset values.
Figure 4.
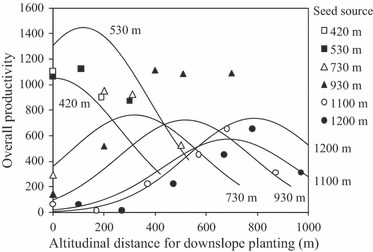
Changes in 36-year productivity for each seed source population when transplanted downwards. Overall 36-year productivities were estimated from downward transplants and are arranged according to altitudinal distance. Data for each seed source population are shown using different symbols (the corresponding altitudes are shown in the legend). The six lines represent the regression lines according to the different originating altitudes, based on a home-away model (eqn 3 in Materials and methods).
By transforming the DIST scale to ΔTemp, the outputs of the home-away model, including the current and maximum productivities, were used to identify relationships between temperature and productivity, and to assess the impact of warming on the productivity of different populations (Table 3). According to our meteorological data, a downslope shift of 100 m was associated with a temperature increase of 0.509°C (R2 = 0.991, Table 1); the temperature thus increased by 1.0°C for every 196 m move downslope. This linear relationship was used to convert DIST values to ΔTemp. The productivity data for populations subjected to downslope shifts indicate that in the event of warming, populations that currently live at higher altitudes will experience an increase in overall-P under all scenarios investigated (Table 3). For the most extreme scenario (ΔTemp of +4.0°C), the 1200 m a.s.l. population is predicted to offer the highest productivity of all the tested populations; its productivity would be expected to increase by 100 times relative to the current value. In contrast, the overall-P for the 420 m a.s.l. population is predicted to decrease in all scenarios; in the most extreme scenario, it declines to 1% of its current value (Table 3). The impact of global warming is thus dependent on the population's altitude of origin: populations from lower altitudes may be impacted more severely than those from higher places.
Table 3.
The outputs of the home-away model and the predicted overall productivities as a result of temperature increases
| (ii) Best condition | (iii) Temperature warming scenario | |||||||
|---|---|---|---|---|---|---|---|---|
| Altitude of seed source population (m) | (i) Current condition | Productivity | ΔAltitude (m) | ΔTemperature (°C) | Δ+0.5°C | Δ+1.0°C | Δ+2.0°C | Δ+4.0°C |
| 420 | 1049.4 (100) | 1049.9 (100) | −8 | +0.04 | 988.0 (94) | 806.5 (77) | 350.1 (33) | 11.9 (1) |
| 530 | 1308.6 (100) | 1449.7 (111) | −118 | +0.06 | 1445.6 (110) | 1384.6 (106) | 827.7 (63) | 53.3 (4) |
| 730 | 362.3 (100) | 764.3 (211) | −318 | +1.62 | 535.2 (148) | 685.6 (189) | 733.0 (202) | 151.1 (42) |
| 930 | 99.7 (100) | 723.9 (726) | −518 | +2.64 | 196.9 (198) | 337.3 (338) | 645.1 (647) | 425.4 (472) |
| 1100 | 17.3 (100) | 572.3 (3309) | −688 | +3.50 | 43.8 (253) | 96.0 (555) | 300.8 (1739) | 533.1 (3082) |
| 1200 | 7.5 (100) | 736.1 (9859) | −788 | +4.01 | 21.8 (293) | 55.4 (742) | 232.2 (3111) | 736.1 (9859) |
The productivities of each contemporary population, (i) in the contemporary climate (altitudinal distance = 0 in Fig. 4), (ii) under conditions where the maximum value would be realized, and (iii) under the global warming scenarios, were projected from the home-away model illustrated in Fig. 4. Values in parentheses beside the productivities indicate the predicted activity as a percentage of that achieved under current climatic conditions.
Deviation from optimal productivity
As the 36-year productivity data indicated the existence of significant home-site advantages (Fig. 3E), comparisons of the performance of local plants and foreign plants transferred downslope could be used to predict the gap between the optimum and predicted performance under new climatic conditions. After revising the DIST in Fig. 3E (but only for downslope) to ΔTemp, the future decline of the predicted productivity relative to the optimum productivity was demonstrated along the warming scenario (Fig. 5). The optimum value was not realized in any warming scenario. In fact, the difference between the optimal and predicted productivities was found to increase in tandem with the temperature increase (Fig. 5). Thus, for temperature increases of +0.5, 1.0, 2.0, and 4.0°C, respectively, the predicted productivities would be less than the optimal values by 8.3, 15.9, 29.3, and 50.1% on average, respectively.
Figure 5.
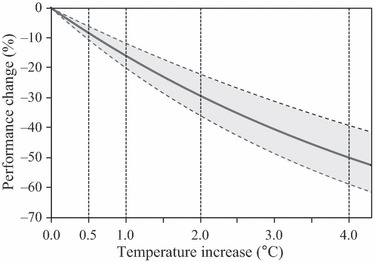
Differences between the 36-year relative productivity of transplants and that of ‘optimal’ local species, used to simulate the effects of warming. This regression curve was estimated from the productivities of downslope transplants relative to that of local plants and was redrawn from the model output shown in Fig. 3E, with the x-axis converted from altitudinal distance to temperature change.
Discussion
Altitudinal gradient pattern
The 36-year reciprocal transplant experiment demonstrated that both tree height and survival were significantly affected by the altitude of the seed source population, the altitude of origin of the planting site, and the interaction between these variables (Table 2). It is assumed that the influence of the altitude of origin is because of genetic factors, while that of the site altitude is because of environmental factors. Our results thus demonstrate that the two traits studied were both subject to genetic control and also sensitive to the living environment. Although both genetic and environmental effects were significant during the testing period, the two traits differed in terms of their behavior over time. The height of young trees was primarily sensitive to genetic factors, with environmental effects becoming more significant as the trees aged; similar findings have previously been reported (Franklin 1979). It is possible that the decrease in the relative importance of genetic factors compared to the environment associated with the onset of the growth stage may be due to abiotic factors such as site quality or to competition within the stand (Franklin 1979). This behavior has also been explained as a consequence of increased interaction between genetic and environmental effects (Ying et al. 1989). However, the environmental influence on height observed in this work was greater than that reported in previous studies (Franklin 1979; Ying et al. 1989); it is possible that this is simply due to the wider range of climatic conditions examined across the six planting sites in this work (Table 1). The used planting sites spanned most of the natural altitudinal range of the studied species, A. sachalinensis. It is therefore reasonable to assume that all of the environmental factors that are active in natural stands would have been operative during the experiments. In contrast, most common-garden trials are conducted under relatively mild climatic conditions compared to those encountered in the target species’ natural habitats (c.f. Franklin 1979). While the trend in the relative influence of genetic and environmental factors on survival over time was less unambiguous than that for height, it was nevertheless apparent that the importance of environmental factors increased with age in this case as well (Table 2). As tree death would be caused by cumulative sum of various environmental effects, it is perhaps to be expected that genetic factors would play a lesser role than the environment.
The effects of the genotype × environment interaction on both height and survival were found to be most significant at the sapling stage, accounting for 10.0% of the variation in tree height and 12.0% of that in survival at 13 years. Conceptually, interaction effects are indicated when certain environmental effects are observed only when paired with specific genotypes (Ying et al. 1989; Howe et al. 2003). In this work, interactions were found to have relatively light impact on either of the studied traits (Table 2). This could be a consequence of examining a series of connected sites whose environments transitioned smoothly from one to another along a gradient rather than a discrete set of sites with substantially different environments. The absence of strikingly different site conditions could thus account for the modest impact of genotype–environment interactions in the model (Table 2).
Analysis of the coefficients for the seed source populations and planting sites estimated using eqn (1) suggests that the genetic influence on both tree height and survival exhibited clinal patterns along the altitudinal gradient, although that for survival was more distinct than that for height (Fig. 2). These clinal patterns basically paralleled the estimated environmental gradient: for both factors, increasing the altitude caused a decrease in both height and survival (Fig. 2). This kind of parallelism in the effects of genetic and environmental factors is referred to as a ‘co-gradient’ pattern and is suggestive of the action of selective forces that drive the adaptive response of plants to their native climate (Byars et al. 2007). The observed intraspecific variation of A. sachalinensis across local altitudes might thus reflect adaptive responses that evolved by locally driven selection (Byars et al. 2007; Savolainen et al. 2007).
The intraspecific variation in tree height with planting site altitude was very similar to that observed in a previous study using data from the same reciprocal transplant experiment (Kurahashi and Hamaya 1981). In both our study and its predecessor, populations with lower altitudes of origin exhibited a higher genetic property for rapid growth. Similar trends in intraspecific behavior have been noted in several studies on different conifer species (Hermann and Lavender 1968; Rehfeldt 1983, 1989; Oleksyn et al. 1998; Green 2005). In contrast, the intraspecific variation in survival as a function of local altitude has rarely been discussed owing to a lack of empirical data. Vitasse et al. (2009) observed that populations of Abies alba growing at lower altitudes exhibited increased survival. In wide-range provenance trials, Savolainen et al. (2007) detected a negative relationship between latitude and survival rate in Pinus sylvestris, while Chang-Geng (1989) demonstrated a positive relationship between local latitude and survival in Pinus armandii. As these findings indicate, it can be difficult to draw general conclusions from observed trends in survival; this is probable due to the heterogeneity of the tested environments, limitations in the ability to control environmental variables, and/or species-specific response to given environment. As such, more data will be required for a rigorous discussion of factors affecting survival.
In addition to the genetic cline related to height and survival, it is worth noting the presence of flexion points for environmental effects at around 700 m a.s.l. in the outputs of the height and survival models (Fig. 2). At around this altitude on this mountain, dramatic changes are observed in the forest vegetation, soil type, and snow depth; above 700 m, soil nutrients become less abundant, the depth of snowfalls becomes greater, and S. kurilensis becomes more abundant than S. senanensis (Kato 1952; Nakata et al. 1994; and Table 1). A similar flexion point was indicated in the timing of first frosts based on the meteorological data for 2009–2010; the autumn frost occurred almost simultaneously at sites above 730 m a.s.l. but was delayed in an altitude-dependent fashion at sites below 530 m a.s.l. (Table 1). These abiotic flexion points were probably due to geographic factors. GIS projections (created using Arc GIS 9.3; ESRI, Inc., Redlands, CA, USA data not shown) for the studied region indicate that the eastern, southern, and western faces of the mountain are exposed, that is, not shielded from the wind by other mountain ridges. It is thus likely that geographic factors indirectly affected the living conditions for plants at the high-altitude sites, that is, their responses were subject to abiotic modification. The relative abundance of the two Sasa species is known to be dependent on the soil type (Nakata et al. 1994) and snow depth (Toyooka et al. 1983). The observed flexion points for A. sachalinensis are presumably due to abiotic factors, the seasonality and severity of which are determined by geographic features.
Detection of local adaptation on the basis of observed home-site advantages
Throughout the testing periods, significant negative relationships between upslope and/or downslope DIST and the relative productivity (relative-P) were detected (Fig. 3). This supports the home-site advantage hypothesis that the fitness-linked traits for the foreign population declined relative to the local population as the environmental distance between the site of origin and that of transplanted increases (Galloway and Fenster 2000; Montalvo and Ellstrand 2000). At each site, the best performance was achieved by the local, indigenous population. This result provides direct evidence for local adaptation (Kawecki and Ebert 2004). The evidence for local adaptation in A. sachalinensis is robust because it became apparent at an early age and remained so for more than 25 years, for both the upslope and downslope directions. To our knowledge, this is the first demonstration of consistent local adaptation in conifers from youth to maturity across an entire region.
The disadvantage of upslope transplants relative to local plants increased with age, indicating that the negative effects on productivity gradually increased as trees grew older (Fig. 3F). Conversely, the productivity of the downslope transplants decreased constantly in line with the altitudinal distance between the local site and the transplant's site of origin, independently of the trees’ ages. The observation of increasing negative effects with age is consistent with the report of Montalvo and Ellstrand (2000). We observed that in the long term, upslope transplantation had a more severe negative impact on survival and productivity than downslope transplantation. Situations in which local adaptation is only observed to have significant consequences in a certain range of altitudes are discussed in terms of ‘asymmetrical local adaptation’, which has been observed in crop species (Mercer et al. 2008). In the strict sense, our observations are not consistent with the definition of asymmetrical local adaptation because local adaptation was observed for all altitudes examined in this work. However, in a broader sense, the concept seems relevant to our findings because asymmetry was observed in either the magnitude of adaptation or the nature of the adaptation itself. Similar asymmetries in which the effects of local adaptation are more pronounced at higher altitudes have previously been reported for other conifers (Conkle 1973; Rehfeldt 1983). The observed asymmetry could potentially be attributed to two different factors. First, selective pressures act cumulatively, as explained by Montalvo and Ellstrand (2000). Consequently, for plants brought from a lower altitude to a higher one, maladaptation might become gradually apparent with age (Rehfeldt et al. 1999; Wu and Ying 2004). Second, the home-site advantage might be masked by genetically fixed tendencies for more rapid growth with lower mortality in younger stages (shown in Fig. 2). As a result, the maladaptation of low-altitude populations to high-altitude sites might be underestimated for younger trees. The results of this long-term study thus provide evidence for a different type of asymmetric local adaptation to that reported by Mercer et al. (2008), as indicated by the strong impact of the maladaptation of foreign transplants into high-altitude environments. Asymmetric local adaptation of this kind would be consistent with classical findings from studies on wide-range (e.g. latitudinally wide distributed) conifers (Eriksson et al. 1980; Savolainen et al. 2007).
Our finding that A. sachalinensis has undergone genetic adaptation to the conditions encountered at a specific altitude within a narrow area (<25 km range horizontally) is also notable because of the geographic scale over which it was observed. Selective forces and evolutionary potential have previously been suggested to drive intraspecific adaptation on similarly fine scales: in the herbaceous alpine plants, Poa hiemata, the observation that local adaptation had significant effects even within a very small altitudinal range (<150 m) was taken to be indicative of very high evolutionary potential (Byars et al. 2007). The long life cycles and substantial gene flow of woody plants tend to restrict such genetic divergence. However, intraspecific adaptation (especially to high altitudes) has been observed in several species in situations involving steep environmental gradients in restricted mountain areas (Conkle 1973; Rehfeldt 1983; Kitzmiller 2005; Savolainen et al. 2007). These genetic divergences have been attributed to strong selective forces or barriers to gene flow imposed by phenological discordance (Howe et al. 2003; Premoli 2003; Savolainen et al. 2007; Chuine 2010). Our results regarding local adaptation on a single mountainous slope seem to indicate that the evolutionary potential of the studied species was relatively high and that the species has been subjected to strong selective forces. However, the observed strong local adaptation within a restricted area also suggests that rapid changes in environmental conditions could potentially have significant deleterious effects, as discussed in the following section (shown in Figs 3 and 5).
In previous publications describing common-garden studies on A. sachalinensis, potential selective forces affecting growth and phenological or ecophysiological traits have been suggested (Kurahashi and Hamaya 1981; Eiga and Sakai 1984; Kurahashi et al. 1993; Saho et al. 1994; Goto et al. 2011). Thus, high-altitude populations have been found to undergo growth cessation and subsequent cold acclimation at an earlier timing in autumn than those from lower altitudes (Kurahashi and Hamaya 1981; W. Ishizuka and S. Goto unpublished data) and exhibited greater cold-hardiness in winter (Eiga and Sakai 1984). Moreover, high-altitude populations had an earlier reproductive onset and physiological traits that confer stress tolerance, that is, low susceptibility to disease and thick defensive leaf (Kurahashi et al. 1993; Saho et al. 1994; Goto et al. 2011). These genetic traits would favor their survival under the harsh conditions encountered at high altitudes, in which trees are exposed to long winters, deep snows, and high risks of fungal infection (Eiga and Sakai 1984). On the other hand, these traits also have negative effects on the trees’ growth potential (and thus on their growth rate compared to low-altitude populations) (Rehfeldt 1983; Sakai et al. 2003; Green 2005). Such traits would be less advantageous than those that favor strong growth in the milder climates encountered at lower altitudes. The trade-off between growth and hardiness is thus expected to have a significant impact on local adaptation, as demonstrated in this study.
Evaluation of warming effects
Previous studies on climate change in Japan (MRI-CGCM2; Yukimoto et al. 2001) have suggested that the air temperature in our study area will increase by 4°C during the current century (Kurihara et al. 2005). As long-lived tree species are unlikely to track such a rapid change by means of range shifts (Davis and Shaw 2001), climate change will give rise to pronounced discrepancies between the optimal climate for populations that have undergone extensive local adaptation and the climate they actually experience (Rehfeldt et al. 2002; Savolainen et al. 2004). Although resident plants will respond to new climatic conditions through phenotypic plasticity, our 36 years of long-term monitoring data suggest that this will not be sufficient to enable them to achieve optimal performance (Table 3, Fig. 5). On the basis of the first approach, in which the performance of a single deme was compared in different sites (using the home-away model described by eqn 3), temperature increases were predicted to initially increase the population's overall productivity but to cause a decline if they increased beyond those encountered in the species’ distribution zone (Table 3). This result can be attributed to the species having a niche limitation at low altitudes (200 m a.s.l.) and requiring a moderate climate for optimum productivity, such as that found at 400–600 m a.s.l. (which is the its current dominant zone). Under the most extreme warming scenario considered, our model predicts that the overall productivity will increase at least 30 times for populations at altitudes above 1100 m a.s.l. In contrast, overall the productivity of populations inhabiting lower altitudes (<530 m a.s.l.) will decrease to <5% of the current productivity (Table 3). We conclude that most of the population (except that inhabiting sites below 230 m a.s.l.) will exhibit an increase in overall productivity, but the extent of the increase will depend on the level of global warming. This finding is similar to that presented in a longitudinal analysis of P. sylvestris, which showed that climate change would increase the fitness of several populations (Rehfeldt et al. 2002).
The second approach, in which the response of the transplanted population was compared to that of the local deme, generated more pessimistic predictions (Fig. 5). A home-site advantage was observed under all conditions examined, indicating that the local demes consistently exhibited superior performance to the transplants. Consequently, we were able to use this approach to quantify the differences between the performance of current population in a changed climate and that of a population that has adapted optimally to the new climate. For the warmest scenario (+4°C), the model predicted that the relative performance of the current generation of this species would be up to 50% below the optimum level (Fig. 5), because there would be a significant lag between onset of climate change and the genetic adaptation of the local population to the new climatic conditions (Savolainen et al. 2004; Aitken et al. 2008). Thus, current populations will not adapt to the changes induced by global warming over the short timescales, and there will be a substantial delay in the onset of adaptation to yield optimal performance under the new climates, even though some of the existing populations (notably those living at high altitudes) will exhibit better productivity than they do at present. It should be noted that this discussion of the potential adverse effects of global warming on the current and future performance of A. sachalinensis takes no account of other warming-influenced factors, such as changes in the amount of precipitation or the biotic relationships within the stand that might affect populations’ productivity.
From a practical standpoint, our results are alarming because they suggest that populations that have undergone local adaptation to their current circumstances will exhibit reduced ecological resilience as warming proceeds because of the genetic constraint on their adaptation potential (Savolainen et al. 2004; Aitken et al. 2008). This could be especially problematic for local communities that feature other woody species. In natural stands where A. sachalinensis coexists with other species, such as most of those found on the studied mountain slope (Kato 1952), A. sachalinensis inevitably competes with those other species. If those coexisting trees exhibited greater phenotypic plasticity, as demonstrated by Cordell et al. (1998), the performance of A. sachalinensis after global warming could end up being inferior to that of its competitor, which might adapt to the new conditions more effectively. Such changes in the balance of interspecific competition could alter the stand's species composition or even the whole forest ecosystem (Reyer et al. 2010). It has been suggested that it may be possible to counteract such effects and maintain or improve the performance of specific target species by redistributing genes that confer optimal performance (Rehfeldt et al. 2002; Aitken et al. 2008).
In terms of redistributing optimal genes, we recommend a priori upslope transplantation for mitigating the impact of global warming. This approach is likely to be effective in maintaining the productivity of A. sachalinensis for two reasons. First, because considerable time is required for woody transplants to grow, it would be necessary to establish plantations in milder climatic conditions than in conditions that reflect the current climate. Second, the negative effects of a priori upslope transplantation may be relatively small for young trees (Fig. 3F). Because of the poor predicted performance of the middle–lower montane populations under global warming (Table 3), the value of upslope transplantation would be particularly high on the middle–lower slopes of the mountain. A priori transplantation would result in the effective redistribution of genotypes within this altitude. Consequently, in the case of species that have already undergone local adaptation, it will be essential to strike a balance between minimizing the adverse effects of upslope transplantation and reducing productivity losses by maladaptation to future warming.
Conclusions
The study reported herein was conducted to address the lack of long-term monitoring data with which to evaluate the magnitude of local, altitude-dependent intraspecific adaptations that affect the growth and survival of woody plant species. In this paper, we report a long-term reciprocal transplant experiment in which the height and survival of A. sachalinensis trees were monitored over periods of 5–36 years. Robust evidence that A. sachalinensis demes have undergone significant intraspecific adaptation to their local altitude (and thus to the local climate) on a regional scale was obtained, because a home-site advantage was consistently detected both for upslope and downslope transplanting regimes. This strong adaptation may restrict the ability of the current population to respond effectively to rapid climatic change, and A. sachalinensis may suffer from competition with other tree species that have greater phenotypic plasticity. Consequently, we recommend the redistribution of optimal genotypes to mitigate the negative impact of global warming. In order to mitigate the impact of ongoing climate change, it will be important to quantify the extent of intraspecific adaptation in dominant woody species and devise optimized reforestation strategies that take this information into account.
Data deposited in the Dryad repository: doi:10.5061/dryad.hh2g4s48.
Acknowledgments
The authors thank Y. Takahashi, K. Ohya, A. Michigami, S. Shibano, N. Kimura, M. Matsui, H. Ogawa, Y. Ando, Y. Nakatsubo, Y. Sato, K. Uchishiba, and the staff of the UTHF for field measurements. We are also grateful to A. Kurahashi, M. Mimura, and K. Fukaya for helpful comments on analysis, Y. Tsumura and H. Iwata for critical reading of the manuscript, and O. Savolainen and three anonymous reviewers for useful comments. This study was supported by grants-in-aid from the Japan Society for the Promotion of Science (JSPS).
Literature cited
- Aitken SN, Yeaman S, Holliday JA, Wang TL, Curtis-McLane S. Adaptation, migration or extirpation: climate change outcomes for tree populations. Evolutionary Applications. 2008;1:95–111. doi: 10.1111/j.1752-4571.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byars SG, Papst W, Hoffmann AA. Local adaptation and cogradient selection in the alpine plant, Poa hiemata, along a narrow altitudinal gradient. Evolution. 2007;61:2925–2941. doi: 10.1111/j.1558-5646.2007.00248.x. [DOI] [PubMed] [Google Scholar]
- Chang-Geng M. Geographic variation in Pinus armandii Franch. Silvae Genetica. 1989;38:81–90. [Google Scholar]
- Chuine I. Why does phenology drive species distribution? Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2010;365:3149–3160. doi: 10.1098/rstb.2010.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conkle MT. Growth data for 29 years from California elevational transect study of ponderosa pine. Forest Science. 1973;19:31–40. [Google Scholar]
- Cordell S, Goldstein G, Mueller-Dombois D, Webb D, Vitousek PM. Physiological and morphological variation in Metrosideros polymorpha, a dominant Hawaiian tree species, along an altitudinal gradient: the role of phenotypic plasticity. Oecologia. 1998;113:188–196. doi: 10.1007/s004420050367. [DOI] [PubMed] [Google Scholar]
- Davis MB, Shaw RG. Range shifts and adaptive responses to quaternary climate change. Science. 2001;292:673–679. doi: 10.1126/science.292.5517.673. [DOI] [PubMed] [Google Scholar]
- Eiga S, Sakai A. Altitudinal variation in freezing resistance of Saghalien fir (Abies sachalinensis. Canadian Journal of Botany. 1984;62:156–160. [Google Scholar]
- Eriksson G, Andersson S, Eiche V, Ifver J, Persson A. Severity index and transfer effects on survival and volume production of Pinus sylvestris in northern Sweden. Studia forestalia Suecica. 1980;156:1–32. [Google Scholar]
- Franklin EC. Model relating levels of genetic variance to stand development of four north American conifers. Silvae Genetica. 1979;28:207–212. [Google Scholar]
- Galloway LF, Fenster CB. Population differentiation in an annual legume: local adaptation. Evolution. 2000;54:1173–1181. doi: 10.1111/j.0014-3820.2000.tb00552.x. [DOI] [PubMed] [Google Scholar]
- Goto S, Iijima H, Ogawa H, Ohya K. Outbreeding depression caused by intraspecific hybridization between local and nonlocal genotypes in Abies sachalinensis. Restoration Ecology. 2011;19:243–250. [Google Scholar]
- Green DS. Adaptive strategies in seedlings of three co-occurring, ecologically distinct northern coniferous tree species across an elevational gradient. Canadian Journal of Forest Research. 2005;35:910–917. [Google Scholar]
- Hermann RK, Lavender DP. Early growth of Douglas-fir from various altitudes and aspects in southern Oregon. Silvae Genetica. 1968;17:143–150. [Google Scholar]
- Hoffmann AA, Sgro CM. Climate change and evolutionary adaptation. Nature. 2011;470:479–485. doi: 10.1038/nature09670. [DOI] [PubMed] [Google Scholar]
- Howe GT, Aitken SN, Neale DB, Jermstad KD, Wheeler NC, Chen THH. From genotype to phenotype: unraveling the complexities of cold adaptation in forest trees. Canadian Journal of Botany. 2003;81:1247–1266. [Google Scholar]
- Igarashi Y. A late glacial climatic reversion in Hokkaido, northeast Asia, inferred from the Larix pollen record. Quaternary Science Reviews. 1996;15:989–995. [Google Scholar]
- Jump AS, Penuelas J. Running to stand still: adaptation and the response of plants to rapid climate change. Ecology Letters. 2005;8:1010–1020. doi: 10.1111/j.1461-0248.2005.00796.x. [DOI] [PubMed] [Google Scholar]
- Kato R. The vegetation of the Tokyo University Forest in Hokkaido (in Japanese with English summary) Bulletin of the Tokyo University Forests. 1952;43:1–18. [Google Scholar]
- Kawecki TJ, Ebert D. Conceptual issues in local adaptation. Ecology Letters. 2004;7:1225–1241. [Google Scholar]
- Kitzmiller JH. Provenance trials of ponderosa pine in northern California. Forest Science. 2005;51:595–607. [Google Scholar]
- Körner C. The use of ‘altitude’ in ecological research. Trends in Ecology & Evolution. 2007;22:569–574. doi: 10.1016/j.tree.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Kuparinen A, Savolainen O, Schurr FM. Increased mortality can promote evolutionary adaptation of forest trees to climate change. Forest Ecology and Management. 2010;259:1003–1008. [Google Scholar]
- Kurahashi A, Hamaya T. Variation of morphological characters and growth response of Saghalien fir (Abies sachalinensis) in different altitude (in Japanese with English summary) Bulletin of the Tokyo University Forests. 1981;71:101–151. [Google Scholar]
- Kurahashi A, Ogasawara S, Iguchi K, Hamaya T. 1993. pp. 417–420. Altitudinal variation in Abies sachalinensis– growth and reproduction trait of 19-years individual in reciprocal transplant examination – (in Japanese). Summary of the 104th Meeting of Forest Society in Japan.
- Kurihara K, Ishihara K, Sasaki H, Fukuyama Y, Saitou H, Takayabu I, Murazaki K, et al. Projection of climatic change over Japan due to global warming by high-resolution regional climate model in MRI. SOLA. 2005;1:97–100. [Google Scholar]
- Lenoir J, Gegout JC, Marquet PA, de Ruffray P, Brisse H. A significant upward shift in plant species optimum elevation during the 20th century. Science. 2008;320:1768–1771. doi: 10.1126/science.1156831. [DOI] [PubMed] [Google Scholar]
- Lenoir J, Gegout JC, Pierrat JC, Bontemps JD, Dhote JF. Differences between tree species seedling and adult altitudinal distribution in mountain forests during the recent warm period (1986–2006) Ecography. 2009;32:765–777. [Google Scholar]
- Mercer K, Martinez-Vasquez A, Perales HR. Asymmetrical local adaptation of maize landraces along an altitudinal gradient. Evolutionary Applications. 2008;1:489–500. doi: 10.1111/j.1752-4571.2008.00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalvo AM, Ellstrand NC. Transplantation of the subshrub Lotus scoparius: testing the home-site advantage hypothesis. Conservation Biology. 2000;14:1034–1045. [Google Scholar]
- Nakata M, Tanaka H, Yagi H. Altitudinal changes in vegetation and soils on Mt. Dairoku, central Hokkaido, Japan (in Japanese with English summary) Japanese Journal of Ecology. 1994;44:33–47. [Google Scholar]
- Oleksyn J, Modrzynski J, Tjoelker MG, Zytkowiak R, Reich PB, Karolewski P. Growth and physiology of Picea abies populations from elevational transects: common garden evidence for altitudinal ecotypes and cold adaptation. Functional Ecology. 1998;12:573–590. [Google Scholar]
- Premoli AC. Isozyme polymorphisms provide evidence of clinal variation with elevation in Nothofagus pumilio. Journal of Heredity. 2003;94:218–226. doi: 10.1093/jhered/esg052. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. [Google Scholar]
- Rehfeldt GE. Adaptation of Pinus contorta populations to heterogeneous environments in northern Idaho. Canadian Journal of Forest Research. 1983;13:405–411. [Google Scholar]
- Rehfeldt GE. Ecological adaptations in Douglas-fir (Pseudotsuga menziesii var Glauca) – a synthesis. Forest Ecology and Management. 1989;28:203–215. [Google Scholar]
- Rehfeldt GE, Ying CC, Spittlehouse DL, Hamilton DA. Genetic responses to climate in Pinus contorta: niche breadth, climate change, and reforestation. Ecological Monographs. 1999;69:375–407. [Google Scholar]
- Rehfeldt GE, Wykoff WR, Ying CC. Physiologic plasticity, evolution, and impacts of a changing climate on Pinus contorta. Climatic Change. 2001;50:355–376. [Google Scholar]
- Rehfeldt GE, Tchebakova NM, Parfenova YI, Wykoff WR, Kuzmina NA, Milyutin LI. Intraspecific responses to climate in Pinus sylvestris. Global Change Biology. 2002;8:912–929. [Google Scholar]
- Reyer C, Lasch P, Mohren GMJ, Sterck FJ. Inter-specific competition in mixed forests of Douglas-fir (Pseudotsuga menziesii) and common beech (Fagus sylvatica) under climate change, a model-based analysis. Annals of Forest Science. 2010;67:805 p1–11. [Google Scholar]
- Saho H, Takahashi I, Kurahashi A. 1994. pp. 224–247. Relationship between the elevation of seed collecting sites and the susceptibility of Abies sachalinensis to Scleroderris lagerbergii in Hokkaido, Japan. Meeting of the IUFRO Working Party, Canker and shoot blight of conifers. Vallombrosa, Italy.
- Sakai A, Matsui K, Kabeya D, Sakai S. Altitudinal variation in lifetime growth trajectory and reproductive schedule of a sub-alpine conifer, Abies mariesii. Evolutionary Ecology Research. 2003;5:671–689. [Google Scholar]
- Savolainen O, Bokma F, Garcia-Gil R, Komulainen P, Repo T. Genetic variation in cessation of growth and frost hardiness and consequences for adaptation of Pinus sylvestris to climatic changes. Forest Ecology and Management. 2004;197:79–89. [Google Scholar]
- Savolainen O, Pyhajarvi T, Knurr T. Gene flow and local adaptation in trees. Annual Review of Ecology Evolution and Systematics. 2007;38:595–619. [Google Scholar]
- Toyooka H, Sato M, Ishizuka S. Distribution Map of the Sasa Group in Hokkaido, Explanatory Note (in Japanese) Hokkaido Branch, Sapporo, Japan: Forestry and Forest Products Research Institute; 1983. p. 36. [Google Scholar]
- Tsumura Y, Suyama Y. Differentiation of mitochondrial DNA polymorphisms in populations of five Japanese Abies species. Evolution. 1998;52:1031–1042. doi: 10.1111/j.1558-5646.1998.tb01831.x. [DOI] [PubMed] [Google Scholar]
- Vitasse Y, Delzon S, Bresson CC, Michalet R, Kremer A. Altitudinal differentiation in growth and phenology among populations of temperate-zone tree species growing in a common garden. Canadian Journal of Forest Research. 2009;39:1259–1269. [Google Scholar]
- Wu HX, Ying CC. Geographic pattern of local optimality in natural populations of lodgepole pine. Forest Ecology and Management. 2004;194:177–198. [Google Scholar]
- Ying CC, Thompson C, Herring L. Geographic variation, nursery effects, and early selection in lodgepole pine. Canadian Journal of Forest Research. 1989;19:832–841. [Google Scholar]
- Yukimoto S, Noda A, Kitoh A, Sugi M, Kitamura Y, Hosaka M, Shibata K, et al. The new Meteorological Research Institute coupled GCM (MRI-CGCM 2) -Model climate and variability. Papers in Meteorology and Geophysics. 2001;51:47–88. [Google Scholar]


