Abstract
Hepatitis C virus (HCV) hepatitis and other diseases related to HCV, such as cryoglobulinemia, lymphoma and renal failure, impair health-related quality of life (HRQoL). In addition, HCV per se might directly influence HRQoL via colonization of microglia in the brain or, indirectly, via the effect of systemic inflammatory cytokines which, in turn, can trigger brain interleukin production. The treatment of HCV-related disorders with interferon (IFN) has an effect on HRQoL. Initially, IFN causes a transient deterioration of HRQoL, due to the induction of depression and other side effects of treatment. Subsequently, the subjects who obtain a sustained virologic response experience an improvement in HRQoL. Only rarely does interferon treatment causes permanent detrimental effects on HRQoL, due to residual psychiatric or neurologic side effects. Liver transplantation is the only treatment for end-stage HCV-related liver disease. HRQoL generally improves massively a few months after transplantation, except in the case of serious complications of the transplant procedure. Furthermore, high levels of anxiety and neuroticism pre-transplant are associated with lower HRQoL one year after transplant. Additionally, six months after transplant, patients with HCV who experience virologic recurrence show significantly greater depression, anxiety, phobic anxiety, and paranoid ideation than anti-HCV-negative patients. In conclusion, optimal care for the overall well-being of patients with HCV infection requires adequate knowledge of their neurological and psychological status.
Keywords: Hepatitis C virus, Quality of life, Transplantation, Hepatitis, Cirrhosis
INTRODUCTION
The concept of quality of life (QoL) is an attempt to define, in analyzable terms, the effect of functional outcome of a disease and its treatment on the patient. What emerges should be a functional definition that is measurable over time. The questions that are included in the evaluation of QoL are drawn from various domains of the experience of the patient and should, therefore, represent in the most complete way his/her self-perception concerning physical, psychological, relational and working experience. This conceptualization is based on the World Health Organization definition of health as “A state of complete physical, mental, and social well-being and not merely the absence of disease or infirmity”. This definition is so broad that it includes elements which are beyond the traditional domain of medicine and health caring systems. Opportunity, education, spiritual attitudes, social security, working satisfaction, social relationships, goods availability are elements of QoL that are independent of medicine. What we deal with, by using the concept of health-related quality of life (HRQoL), is the functional effect of an illness and of its therapy on an individual.
Four broad domains contribute to HRQoL: (1) somatic sensations; (2) psychological state; (3) social interactions; and (4) physical and occupational functions.
Various questionnaires, if possible self-administered, have been developed to assess HRQoL. They are subdivided into generic tools, which encompass a global overview of the above-mentioned domains, and specific tools which are oriented to the peculiar consequences of a disease or a class of diseases.
The interest of HRQoL assessment in relation to hepatitis C virus (HCV) infection depends firstly on the acknowledgment that HCV infection is a major public health problem. It has been estimated that about 3% of humans in the word are infected by the HCV[1]. In Europe, it is estimated that there are approximately 5 million HCV carriers; the peak prevalence among individuals is in the fifth decade of age[2]. Therefore, HCV infection mainly involves adults in the period of their active life.
About 10%-20% of the individuals who are chronically infected by HCV are likely to develop liver cirrhosis over a median period of about 30 years[3] and, out of the HCV-infected patients with liver cirrhosis, 2%-6% per year develop hepatocellular carcinoma[4]. In addition, a minority of subjects will develop immunological disorders, such as cryoglobulinemia and lymphoma[5]. Obviously all these individuals will suffer from symptoms due to the diseases caused by HCV infection (but that can also have other causes), rather than from the virus infection per se.
Decompensated liver cirrhosis, the final stage of cirrhosis, has an ominous consequence both on survival[6,7] and on HRQoL[8]. This group of subjects display obvious limitations to HRQoL that are mainly related to the consequences of liver insufficiency and portal hypertension (i.e., hepatic encephalopathy, ascites, upper intestinal tract bleeding, sexual dysfunction, lower leg cramps and itch)[8]. However, the number of subjects with these complications of end-stage liver disease is relatively small, compared with the vast majority of patients having HCV in the absence of clinically significant liver disease.
Despite the initial idea that the majority of patients with HCV infection have merely asymptomatic seropositivity, some data suggest that HCV itself may diminish HRQoL even in the absence of advanced liver disease, perhaps due to HCV brain colonization or to systemic activation of cytokines which, in turn, influence brain cytokine production and neurotransmission.
Other important issues concerning HRQoL in HCV-infected patients are those related to the side effects of interferon (IFN) treatment and to liver transplantation, which is the treatment of choice of end-stage liver disease (Figure 1).
Figure 1.
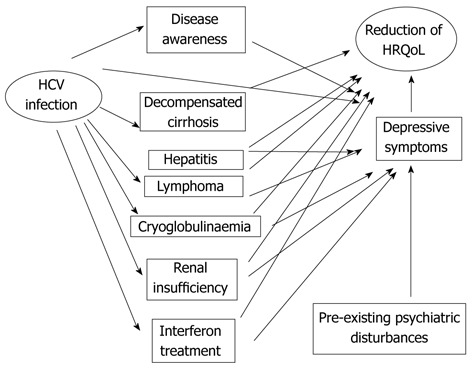
Pathways producing a reduction of health-related quality of life in hepatitis C virus-infected patients: both direct and indirect mechanisms play a role. HCV: Hepatitis C virus; HRQoL: Health-related quality of life.
HCV INFECTION PER SE
The issue of whether HCV infection per se (i.e., independent of the development of liver disease or other severe organic diseases related to HCV infection) may have an effect of HRQoL is a focus of debate. Several studies reported that patients with chronic HCV have a significant reduction in their HRQoL that is not related to the severity of the liver disease[9].
Depression can account for a reduction of HRQoL, since HRQoL depends on a patient’s self-perception and evaluation. Therefore, any change in mood can obviously influence HRQoL. The prevalence of depression in HCV-infected individuals was reported to be higher than that in the general population (59% vs 21%)[10]. It should be noted, however, that depressive symptoms in patients with HCV infection might be due to pre-existing psychiatric disorders, to a reaction to the awareness of being infected, to adverse biological effects of HCV and, finally, to the consequences of liver disease; at least in the individuals who develop severe chronic hepatitis. In addition, coping styles and sources of information are of paramount importance in perceiving disease severity and disability, since they influence patients’ stress levels and/or a pessimistic/optimistic view about the future[11].
An association between HCV infection and depression is therefore likely. However, the role of confounders-such as intravenous substance abuse or promiscuous sexual behaviour-related both to HCV infection and possibly to mood and psychological dimensions may bias statistical assessment. Therefore, further studies are required to determine with certainty the existence of a true association between depression and chronic HCV infection, together with its extent and the mechanisms implicated.
Moreover, strong clues for the existence of a true pathophysiological link between HCV infection and depression derive from the observation that depression has a higher prevalence in drug addicts who are infected with HCV compared to those who are not infected[12]. In addition, HCV-infected patients who report no history of intravenous drug abuse complain of lower HRQoL compared with healthy normal subjects[13].
As mentioned above, the most difficult confounder to be controlled is the effect of the patient’s awareness of his/her infection. In fact, it is reasonable to assume that the awareness of being infected with a virus which potentially is both life-threatening and transmittable by sexual intercourse may have an adverse emotional affect per se, regardless of any biological effect of the virus. However, the observation that patients with chronic HCV infection have reduced HRQoL scores compared to those with hepatitis B virus infection, suggests that HCV infection per se reduces HRQoL, rather than the non-specific patient awareness of a viral infection[13]. Also, HRQoL was found to be reduced even in patients who were not aware of being HCV carriers[14].
Some pathophysiological insight into the possible mechanisms involved in the reduction of HRQoL due to HCV reduction was provided by Weissenborn et al[15] who reported alterations of mood (increased anxiety and depression) and cognition, together with changes in both the midbrain serotoninergic and striatal dopaminergic systems, irrespective of viraemia and normal liver function, in patients with HCV infection. The existence of brain alteration directly caused by HCV is provided by the evidence of deficits of attention, executive function and verbal learning, of electroencephalogram slowing in the absence of liver cirrhosis and/or substance use disorder, and of peculiar alterations on magnetic resonance spectroscopy in HCV-infected patients[16-18]. It has been suggested that monocytes infected by HCV can cross the blood-brain barrier producing a secondary infection of microglia[19-22]. Such an infection is thought to be facilitated by immunosuppression caused by human immunodeficiency virus or by immunosuppressive treatment in transplanted patients[17,21-23].
Microglia infection by HCV may disturb neuronal function due to local cytokine production[16,20,23]. Another hypothesis is that the noxious effect on the brain is due to systemic induction of inflammatory cytokines [interleukin (IL)-1, IL-6 and tumor necrosis factor-α] by HCV which, via the blood-brain barrier, cause microglial activation and in-brain cytokine production[24]. This is the mechanism of the “sickness behaviour” syndrome, which includes non-specific depressive symptoms: fatigue, anhedonia, apathy, emotional lability, irritability, agitation, anorexia, psychomotor retardation, sleep disturbance, social withdrawal, hyperalgesia, decreased libido, and cognitive impairment[25-27].
HCV INFECTION AND IFN TREATMENT
Another approach to solve the problem of the existence, if any, of a direct effect of HCV infection on mood and HRQoL, is to examine the results of trials on HCV treatment. Such studies concern patients with chronic viral hepatitis; therefore, the dimension of liver disease cannot be dissociated from that of HCV infection per se. At any rate, large studies based on the SF36 health questionnaire have shown a significant improvement in quality of life scores in those patients with chronic hepatitis who obtain a sustained viral response[28,29]. However, these kinds of studies cannot remove the bias caused by patient satisfaction due to the awareness of being healed of hepatitis C.
In addition, the interpretation of the change of HRQoL in HCV-infected patients who are treated with IFN is biased by the fact that IFN itself is a proinflammatory cytokine that induces neuropsychiatric symptoms. In fact, 12%-41% of subjects with HCV hepatitis who are treated with IFN develop neuropsychiatric symptoms, even if they did not have a history of previous mental disorder[10]. An even higher percentage of individuals, ranging from 17%-58%, develop neuropsychiatric symptoms, mainly depression, if they had had a history of previous mental disorder[10]. The depressive syndrome induced by IFN has two expressions: (1) a depression-specific syndrome characterized by depressed mood, anhedonia, anxiety, subjective cognitive disturbance and sometimes suicidal ideation; and (2) a neurovegetative syndrome, characterized by fatigue, insomnia, anorexia, pain, psychomotor slowness. Generally, symptoms remit within 2-3 wk of discontinuation of IFN treatment[30]. Nevertheless, some patients may continue to complain of cognitive difficulties 12-24 mo after discontinuation of IFN treatment[31]. Obviously, these neuropsychiatric IFN side effects influence HRQoL, which depends on subjective perception and self-report of personal well-being. Therefore, the cessation of IFN treatment and, thus, of the discomfort caused by treatment itself, might explain a rebound perception of increased HRQoL.
LIVER TRANSPLANTATION
Liver transplantation (LT) is a life-saving intervention for many patients with end-stage liver disease: survival after this intervention ranges between 90%-70% at one and five years, respectively. Many studies have reported significant improvements in HRQoL and satisfactory psychological outcome after LT[32,33]. The topic was recently reviewed by Tome et al[34] who observed that general HRQoL improves dramatically after LT; however, when compared with the general population, the vast majority of LT patients have significant deficits in most HRQoL domains. In addition, while some domains, such as physical functioning and psychosocial adaptation, significantly improve after transplantation, psychological health shows a lower improvement than physical functioning. On the whole, sexual life does not improve significantly; however, results in the literature so far are extremely heterogeneous, suggesting that this effect is essentially unpredictable in individual patients. A relationship between the aetiology of liver disease and HRQoL has been reported: HCV infections impinge on HRQoL in the patients with recurrence of liver disease[35]. The impairment is mainly related to physical functioning and fatigue[35,36]. An intriguing issue is the interplay amongst HRQoL, psychological status and brain function. Interestingly, elevated levels of anxiety and neuroticism on pre-transplantation assessment were associated with poor psychological outcome one year after transplantation. These findings suggest that personality and affective status may be important determinants of long-term psychological health after LT. Accordingly, patients with HCV who experienced virologic recurrence within 6 mo of LT showed significantly greater depression, anxiety, phobic anxiety, and paranoid ideation than anti-HCV-negative patients[33].
In conclusion, independent of the underlying pathophysiologic mechanisms, HCV infection is a signal of vulnerability for poor HRQoL and should be considered in patient assessment.
Footnotes
Peer reviewer: Satoru Kakizaki, MD, PhD, Assistant Professor, Department of Medicine and Molecular Science, Gunma University Graduate School of Medicine, 3-39-15, Showa-machi, Maebashi 371-8511, Japan
S- Editor Gou SX L- Editor Logan S E- Editor Zhang DN
References
- 1.Shepard CW, Finelli L, Alter MJ. Global epidemilogy of hepatitic C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 3.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 4.Sangiovanni A, Del Ninno E, Fasani P, De Fazio C, Ronchi G, Romeo R, Morabito A, De Franchis R, Colombo M. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004;126:1005–1014. doi: 10.1053/j.gastro.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 5.Faustini A, Colais P, Fabrizi E, Bargagli AM, Davoli M, Di Lallo D, Di Napoli A, Pezzotti P, Sorge C, Grillo R, et al. Hepatic and extra-hepatic sequelae, and prevalence of viral hepatitis C infection estimated from routine data in at-risk groups. BMC Infect Dis. 2010;10:97. doi: 10.1186/1471-2334-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Amico G, Morabito A, Pagliaro L, Marubini E. Survival and prognostic indicators in compensated and decompensated cirrhosis. Dig Dis Sci. 1986;31:468–475. doi: 10.1007/BF01320309. [DOI] [PubMed] [Google Scholar]
- 7.Ginés P, Quintero E, Arroyo V, Terés J, Bruguera M, Rimola A, Caballería J, Rodés J, Rozman C. Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987;7:122–128. doi: 10.1002/hep.1840070124. [DOI] [PubMed] [Google Scholar]
- 8.Marchesini G, Bianchi G, Amodio P, Salerno F, Merli M, Panella C, Loguercio C, Apolone G, Niero M, Abbiati R. Factors associated with poor health-related quality of life of patients with cirrhosis. Gastroenterology. 2001;120:170–178. doi: 10.1053/gast.2001.21193. [DOI] [PubMed] [Google Scholar]
- 9.Younossi Z, Kallman J, Kincaid J. The effects of HCV infection and management on health-related quality of life. Hepatology. 2007;45:806–816. doi: 10.1002/hep.21565. [DOI] [PubMed] [Google Scholar]
- 10.Quelhas R, Lopes A. Psychiatric problems in patients infected with hepatitis C before and during antiviral treatment with interferon-alpha: a review. J Psychiatr Pract. 2009;15:262–281. doi: 10.1097/01.pra.0000358313.06858.ea. [DOI] [PubMed] [Google Scholar]
- 11.Constant A, Castera L, Quintard B, Bernard PH, de Ledinghen V, Couzigou P, Bruchon-Schweitzer M. Psychosocial factors associated with perceived disease severity in patients with chronic hepatitis C: relationship with information sources and attentional coping styles. Psychosomatics. 2005;46:25–33. doi: 10.1176/appi.psy.46.1.25. [DOI] [PubMed] [Google Scholar]
- 12.Johnson ME, Fisher DG, Fenaughty A, Theno SA. Hepatitis C virus and depression in drug users. Am J Gastroenterol. 1998;93:785–789. doi: 10.1111/j.1572-0241.1998.225_a.x. [DOI] [PubMed] [Google Scholar]
- 13.Foster GR, Goldin RD, Thomas HC. Chronic hepatitis C virus infection causes a significant reduction in quality of life in the absence of cirrhosis. Hepatology. 1998;27:209–212. doi: 10.1002/hep.510270132. [DOI] [PubMed] [Google Scholar]
- 14.Rodger AJ, Jolley D, Thompson SC, Lanigan A, Crofts N. The impact of diagnosis of hepatitis C virus on quality of life. Hepatology. 1999;30:1299–1301. doi: 10.1002/hep.510300504. [DOI] [PubMed] [Google Scholar]
- 15.Weissenborn K, Ennen JC, Bokemeyer M, Ahl B, Wurster U, Tillmann H, Trebst C, Hecker H, Berding G. Monoaminergic neurotransmission is altered in hepatitis C virus infected patients with chronic fatigue and cognitive impairment. Gut. 2006;55:1624–1630. doi: 10.1136/gut.2005.080267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forton DM, Allsop JM, Main J, Foster GR, Thomas HC, Taylor-Robinson SD. Evidence for a cerebral effect of the hepatitis C virus. Lancet. 2001;358:38–39. doi: 10.1016/S0140-6736(00)05270-3. [DOI] [PubMed] [Google Scholar]
- 17.Forton DM, Hamilton G, Allsop JM, Grover VP, Wesnes K, O’Sullivan C, Thomas HC, Taylor-Robinson SD. Cerebral immune activation in chronic hepatitis C infection: a magnetic resonance spectroscopy study. J Hepatol. 2008;49:316–322. doi: 10.1016/j.jhep.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 18.Weissenborn K, Krause J, Bokemeyer M, Hecker H, Schüler A, Ennen JC, Ahl B, Manns MP, Böker KW. Hepatitis C virus infection affects the brain-evidence from psychometric studies and magnetic resonance spectroscopy. J Hepatol. 2004;41:845–851. doi: 10.1016/j.jhep.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Forton DM, Karayiannis P, Mahmud N, Taylor-Robinson SD, Thomas HC. Identification of unique hepatitis C virus quasispecies in the central nervous system and comparative analysis of internal translational efficiency of brain, liver, and serum variants. J Virol. 2004;78:5170–5183. doi: 10.1128/JVI.78.10.5170-5183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weissenborn K, Tryc AB, Heeren M, Worthmann H, Pflugrad H, Berding G, Bokemeyer M, Tillmann HL, Goldbecker A. Hepatitis C virus infection and the brain. Metab Brain Dis. 2009;24:197–210. doi: 10.1007/s11011-008-9130-5. [DOI] [PubMed] [Google Scholar]
- 21.Laskus T, Radkowski M, Bednarska A, Wilkinson J, Adair D, Nowicki M, Nikolopoulou GB, Vargas H, Rakela J. Detection and analysis of hepatitis C virus sequences in cerebrospinal fluid. J Virol. 2002;76:10064–10068. doi: 10.1128/JVI.76.19.10064-10068.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vargas HE, Laskus T, Radkowski M, Wilkinson J, Balan V, Douglas DD, Harrison ME, Mulligan DC, Olden K, Adair D, et al. Detection of hepatitis C virus sequences in brain tissue obtained in recurrent hepatitis C after liver transplantation. Liver Transpl. 2002;8:1014–1019. doi: 10.1053/jlts.2002.36393. [DOI] [PubMed] [Google Scholar]
- 23.Forton DM, Thomas HC, Murphy CA, Allsop JM, Foster GR, Main J, Wesnes KA, Taylor-Robinson SD. Hepatitis C and cognitive impairment in a cohort of patients with mild liver disease. Hepatology. 2002;35:433–439. doi: 10.1053/jhep.2002.30688. [DOI] [PubMed] [Google Scholar]
- 24.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raison C. The effects of hepatitis C and its treatment on mental health. Focus. 2006;21:4–6. [PubMed] [Google Scholar]
- 27.Raison CL, Miller AH. The neuroimmunology of stress and depression. Semin Clin Neuropsychiatry. 2001;6:277–294. doi: 10.1053/scnp.2001.0060277. [DOI] [PubMed] [Google Scholar]
- 28.Bernstein D, Kleinman L, Barker CM, Revicki DA, Green J. Relationship of health-related quality of life to treatment adherence and sustained response in chronic hepatitis C patients. Hepatology. 2002;35:704–708. doi: 10.1053/jhep.2002.31311. [DOI] [PubMed] [Google Scholar]
- 29.McHutchison JG, Manns M, Patel K, Poynard T, Lindsay KL, Trepo C, Dienstag J, Lee WM, Mak C, Garaud JJ, et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002;123:1061–1069. doi: 10.1053/gast.2002.35950. [DOI] [PubMed] [Google Scholar]
- 30.Valentine AD, Meyers CA, Talpaz M. Treatment of neurotoxic side effects of interferon-alpha with naltrexone. Cancer Invest. 1995;13:561–566. doi: 10.3109/07357909509024923. [DOI] [PubMed] [Google Scholar]
- 31.Lieb K, Engelbrecht MA, Gut O, Fiebich BL, Bauer J, Janssen G, Schaefer M. Cognitive impairment in patients with chronic hepatitis treated with interferon alpha (IFNalpha): results from a prospective study. Eur Psychiatry. 2006;21:204–210. doi: 10.1016/j.eurpsy.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 32.Bryan S, Ratcliffe J, Neuberger JM, Burroughs AK, Gunson BK, Buxton MJ. Health-related quality of life following liver transplantation. Qual Life Res. 1998;7:115–120. doi: 10.1023/a:1008849224815. [DOI] [PubMed] [Google Scholar]
- 33.O’Carroll RE, Couston M, Cossar J, Masterton G, Hayes PC. Psychological outcome and quality of life following liver transplantation: a prospective, national, single-center study. Liver Transpl. 2003;9:712–720. doi: 10.1053/jlts.2003.50138. [DOI] [PubMed] [Google Scholar]
- 34.Tome S, Wells JT, Said A, Lucey MR. Quality of life after liver transplantation. A systematic review. J Hepatol. 2008;48:567–577. doi: 10.1016/j.jhep.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 35.Bona MD, Rupolo G, Ponton P, Iemmolo RM, Boccagni P, Destro C, Ermani M, Naccarato R, Burra P. The effect of recurrence of HCV infection of life after liver transplantation. Transpl Int. 1998;11 Suppl 1:S475–S479. doi: 10.1007/s001470050523. [DOI] [PubMed] [Google Scholar]
- 36.Singh N, Gayowski T, Wagener MM, Marino IR. Quality of life, functional status, and depression in male liver transplant recipients with recurrent viral hepatitis C. Transplantation. 1999;67:69–72. doi: 10.1097/00007890-199901150-00011. [DOI] [PubMed] [Google Scholar]


