Abstract
AIM: To compare the effectiveness of sequential therapy for Helicobacter pylori (H. pylori) infection with that of triple therapy of varying durations.
METHODS: The 460 patients enrolled in this study had H. pylori-associated gastritis or a gastric or duodenal ulcer. After screening, H. pylori-infected patients were randomly assigned to receive either conventional triple therapy for 7, 10 or 14 d, or a new 10-d sequential therapy. Each of the 4 treatment groups included 115 patients. The outcomes of eradication therapy were assessed 4 wk after treatment by the urea breath test and histology.
RESULTS: The overall eradication rate was 81.0%, and eradication rates were 75.7% for 7-d conventional triple therapy, 81.9% for 10-d conventional triple therapy, 84.4% for 14-d conventional triple therapy, and 82.0% for 10-d sequential therapy. Neither intention-to-treat analysis nor per protocol analysis showed significant differences in eradication rates using sequential therapy or the standard triple therapy (P = 0.416 and P = 0.405, respectively).
CONCLUSION: There are no significant differences between 10-d sequential eradication therapy for H. pylori and any duration of standard triple treatment in Korean patients.
Keywords: Helicobacter pylori, Sequential therapy, Triple therapy, Gastric ulcer, Duodenal ulcer, Gastritis
INTRODUCTION
Helicobacter pylori (H. pylori), first identified in 1982, is recognized as a risk factor for gastrointestinal ulcers, chronic gastritis, gastric adenocarcinoma, and mucosa-associated lymphoid tissue lymphoma. The most widely used eradication therapy at this time combines a proton pump inhibitor (PPI) with 2 antibiotics, amoxicillin and clarithromycin. However, the failure rate of this triple therapy is 10%-20%[1-4] and has increased with the emergence of clarithromycin resistance[5-7]. When first-line triple therapy fails, a quadruple regimen of PPI, bismuth, tetracycline, and metronidazole is usually applied. The failure rate of this second-line therapy (20%-30%) is also high, which may stem from metronidazole resistance[6,8,9].
To address an increase in antibiotic resistance in H. pylori in Western countries, a 10-d sequential therapy was proposed. This new regimen includes a PPI plus 1 g of amoxicillin twice daily for the first 5 d followed by a PPI, 500 mg of clarithromycin and 500 mg of tinidazole twice daily for the remaining 5 d. Since Zullo et al[10]. first developed this therapy, it has produced higher eradication rates than triple therapy in several studies[11-15]. However, few studies have reported outcomes of sequential therapy in East Asian countries. Since antibiotic-resistant strains differ in geographical distribution, an H. pylori eradication therapy may vary in effectiveness between regions[16,17].
Furthermore, no universal evidence-based guidelines have been established for the optimal duration of triple therapy. Some countries prefer a 7-d therapy, and in other countries treatment longer than 7-d is deemed mandatory[1,18,19]. Most previous comparative studies tested the sequential regimen against 7-d or 10-d triple therapy.
We conducted this study to prospectively compare the H. pylori eradication rate obtained with a 10-d sequential regimen to the rates achieved with the conventional 7-d, 10-d and 14-d triple regimens in a Korean cohort of H. pylori-infected patients.
MATERIALS AND METHODS
Study population
From March 2008 to August 2011, we interviewed and enrolled patients who visited Korea University Anam Hospital with H. pylori-positive gastritis or peptic ulcer (gastric and/or duodenal ulcer) identified in gastroduodenoscopy. H. pylori infection was detected using a urea breath test, rapid urease test, or histopathological investigation. This was a prospective randomized controlled study.
Four hundred and sixty patients tested positive for H. pylori during this time. Study inclusion criteria were H. pylori-associated gastritis, or gastric or duodenal ulcer, and age 18 years or older. Criteria for exclusion from the study were: (1) serious kidney disease that required drug dosage adjustment; (2) previous treatment with antibiotics within the past 4 wk; (3) PPI treatment during the previous 8 wk; (4) previous H. pylori eradication failure; (5) significant cardiopulmonary, endocrine or hepatic disease, or a hematologic disorder; (6) previous surgery of the upper gastrointestinal tract; (7) history of malignancy; (8) history of drug or alcohol misuse; (9) antiulcer medication treatment within previous 4 wk; (10) systemic glucocorticoid or anticoagulation treatment; (11) severe psychiatric or neurological disease; and (12) current pregnancy or lactation. After screening, participants were randomly assigned to treatment groups using a computer generated list. Patients provided written consent to participate, and the Institutional Review Board of Korea University Hospital approved this study. We conducted the study in agreement with the principles of the Declaration of Helsinki, the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use and Guidelines for Good Clinical Practice.
Treatment protocol
Eligible patients who had no documented contraindications were randomly assigned to one of four treatments: (1) standard triple therapy (rabeprazole 20 mg + amoxicillin 1.0 g + clarithromycin 500 mg twice daily) for 7 d; (2) standard triple therapy for 10 d; (3) standard triple therapy for 14 d; and (4) a 10-d sequential treatment (amoxicillin 1.0 g + rabeprazole 20 mg twice daily for the first 5 d, followed by rabeprazole 20 mg + clarithromycin 500 mg + tinidazole 500 mg twice daily for the remaining 5 d).
Confirmation of eradication
At least 4 wk after completion of treatment, a urea breath test and histopathological diagnosis were performed to determine if H. pylori had been successfully eradicated. Drug side effects were assessed with a questionnaire.
Statistical analysis
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS 18.0 for Windows; SPSS Inc., Chicago, IL, United States). Power calculations to determine sample size based on previously published data[20] showed that 420 patients would be required to detect a treatment difference at the 5% level of significance with a power of 80%. Hence the sample size was set at 460 patients to allow for a possible 10% dropout rate.
Univariate analysis, with age, gender, endoscopic diagnosis, smoking, alcohol habits, and medications (NSAID) as variables, was performed using the χ2 test. The H. pylori eradication rate was determined using intention-to-treat (ITT) and per protocol (PP) analyses using the χ2 test. P < 0.05 was considered statistically significant.
RESULTS
Patient characteristics
The mean age of the 460 patients enrolled in this study was 46.8 years. Two hundred and thirty-nine patients were male and 221 were female. Each of the 4 treatment groups included 115 patients. The groups did not differ significantly in gender, previous disease history, endoscopic diagnosis, smoking, alcohol consumption, NSAID and aspirin use, or use of previous medications (Table 1).
Table 1.
Clinical characteristics of patients with Helicobacter pylori infection
| 7-d eradication | 10-d eradication | 14-d eradication | 10-d sequentialeradication | Total | Pvalue | |
| Number of patients | 115 | 115 | 115 | 115 | 460 | |
| Gender | 0.959 | |||||
| Male | 60 | 58 | 62 | 59 | 239 | |
| Female | 55 | 57 | 53 | 56 | 221 | |
| Endoscopic diagnosis | 0.940 | |||||
| GU | 48 | 52 | 52 | 48 | 200 | |
| DU | 40 | 36 | 32 | 36 | 144 | |
| Gastritis as NUD | 27 | 27 | 31 | 31 | 116 | |
| Smokers | 40 | 36 | 53 | 44 | 176 | 0.117 |
| Alcohol drinkers | 42 | 48 | 58 | 56 | 204 | 0.123 |
| NSAID/ASA | 12 | 16 | 19 | 13 | 60 | 0.513 |
GU: Gastric ulcer; DU: Duodenal ulcer; NUD: Non-ulcer dyspepsia; NSAID: Non-steroidal anti-inflammatory drug; ASA: Acetylsalicylic acid.
Eradication rates
Twenty-five of the 460 patients did not return to hospital for eradication testing and 8 patients discontinued the medication due to side effects. Eradication results were confirmed in 427 patients; eradication was confirmed in 408 of the 427 patients by the urea breath test and in 19 patients by histopathological examination for H. pylori.
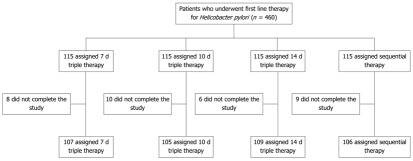
Among the 427 patients, 107 patients received the 7-d conventional triple therapy, 105 received the 10-d conventional triple therapy, 109 received the 14-d conventional triple therapy, and 106 received the 10-d sequential therapy (Figure 1).
Figure 1.
Flow diagram for patients enrolled in the study.
The overall eradication rate among the 427 patients was 81.0%. The eradication rates according to group were as follows: 70.4%/75.7% (ITT/PP) for the 7-d conventional triple therapy group, 74.7%/81.9% for the 10-d conventional triple therapy group, 80.0%/84.4% for the 14-d conventional triple therapy group, and 75.6%/82.0% for the 10-d sequential therapy group. Neither the ITT nor the PP analysis showed a significant difference in eradication rates between the new 10-d sequential therapy and the standard triple therapy (P = 0.416 and P = 0.405, respectively) (Table 2).
Table 2.
Helicobacter pylori eradication rates according to treatment group
| 7-deradication | 10-deradication | 14-deradication | 10-dsequentialeradication | Total | Pvalue | |
| ITT | 70.4% 81/115 | 74.7% 86/115 | 80.0% 92/115 | 75.6% 87/115 | 75.2% 346/460 | 0.416 |
| PP | 75.7% 81/107 | 81.9% 86/105 | 84.4% 92/109 | 82.0% 87/106 | 81.0% 346/427 | 0.405 |
ITT: Intention-to-treat; PP: Per protocol.
Compliance and side-effects
The compliance was greater than 95% for all of the triple conventional groups and the 10-d sequential therapy group. In the latter group, 15 patients showed side effects including problems with taste (n = 1), loose stools (n = 3), nausea/vomiting (n = 4), epigastric discomfort (n = 3), abdominal distention (n = 3) and itching (n = 1) (Table 3). For the triple therapy, reported side effects included problems with taste, loose stools, abdominal discomfort, nausea, vomiting, epigastric discomfort, and itching. Most side effects were not severe (Table 3).
Table 3.
Adverse events
| 7-deradication | 10-deradication | 14-deradication | 10-dsequentialeradication | |
| Taste alterations | 1 | 1 | 1 | 1 |
| Loose stools | 3 | 3 | 2 | 3 |
| Abdominal distention | 2 | 3 | 3 | 3 |
| Nausea/vomiting | 2 | 4 | 3 | 4 |
| Epigastric discomfort | 3 | 2 | 3 | 3 |
| Itching | 0 | 1 | 0 | 1 |
| Total | 11 | 14 | 12 | 15 |
DISCUSSION
We compared the H. pylori eradication rates obtained after 10-d sequential therapy with those achieved after 7-, 10- and 14-d conventional triple therapy and found no significant differences.
Triple therapy, which is currently used most widely, combines a PPI with 2 antibiotics such as clarithromycin and amoxicillin[2]. However, resistance to clarithromycin now stands at 13.8%-16.7%[5,21], and recent reports place the rate of H. pylori eradication at 74%-83.6%[18,22].
The Maastricht III consensus report published in 2005 recommends a quadruple therapy, which includes a PPI, bismuth, tetracycline, and metronidazole, as a second-line eradication therapy[1]. The effectiveness of quadruple therapy is compromised, however, by resistance to metronidazole in H. pylori[8,23,24]. The prevalence of metronidazole resistance in Korea has increased from 33.3% in 1994, to 47.7% in 1999, to 66.2% in 2003[25].
In Western countries, increased resistance to triple therapy fosters much discussion. Notably, an increase in clarithromycin resistance found through large-scale studies in several European countries is linked to extensive use of this antibiotic in both children and adults, and to an increasing rate of failure to eradicate H. pylori using conventional therapy[20,26-28]. Nevertheless, European guidelines for H. pylori treatment specify triple therapy with a PPI, amoxicillin, and clarithromycin or metronidazole for 14 d, or quadruple therapy with a PPI, amoxicillin, clarithromycin, and metronidazole for 10-14 d[1,29].
Zullo et al[10] reported a new 10-d sequential therapy for H. pylori in 2000. This regimen includes a PPI plus 1 g of amoxicillin for the first 5 d followed by a PPI, 500 mg of clarithromycin, and 500 mg of tinidazole for the remaining 5 d. Some studies report that this regimen yields a higher H. pylori eradication rate than triple therapy[11,30,31] and improves the rate in children as well as in adults[32,33]. The mechanisms underlying the effects of the 10-d sequential treatment are not known; however, the early administration of amoxicillin appears to weaken the cell walls of H. pylori, reducing resistance to clarithromycin and enhancing the treatment effects[28,34]. The 5-d PPI and amoxicillin regimen may reduce the numbers of H. pylori by 50%, which when followed by 5 d of triple therapy, is thought to improve the overall effectiveness of the regimen[35,36]. In addition, the use of 3 or more antibiotics improves the treatment effects[20].
In our patient cohort, the 10-d sequential therapy did not achieve a higher eradication rate than the standard triple therapy. This discrepancy may reflect in part genetic differences in H. pylori strains in the East and West[37]. In addition, the high rate of antibiotic resistance in Korea, in particular to metronidazole, may contribute to geographical variations in treatment effectiveness. Factors in addition to location and antibiotic resistance that may affect H. pylori eradication include patient age and compliance, gastric acid concentration, individual response to PPI, and differences in prevalence of H. pylori Cag A genotype[38]. We did not investigate gastric acid concentration, antibiotic resistance (or minimal inhibitory concentrations), or H. pylori genotypes; and as all subjects were recruited at a single center, geographical differences in H. pylori sensitivity and in treatment protocol did not emerge in this study.
The optimal treatment duration using triple therapy is not established. A meta-analysis performed by Fuccio et al[39] concluded that extending triple therapy beyond 7 d does not significantly improve outcome compared to the standard 7-d regimen. However, individual studies report higher eradication rates using 10 or 14 d of triple therapy as compared to 7 d[40,41]. Guidelines in the United States and Europe favor longer durations of triple therapy[1,19]. Previous comparative studies of the sequential regimen involved the 7-d and 10-d triple therapy. The present study aimed to clarify the relationship of treatment duration to the H. pylori eradication rate.
Data from East Asian countries on use of sequential therapy are limited, and further study is needed to determine the optimal duration of treatment for the triple therapy in Asian populations. Several comparative studies of sequential therapy conducted in Korea differed in outcome. Three of these studies produced higher eradication rates for sequential therapy and another failed to detect a significant difference[38,42-45]. This lack of concordance may be related to known regional variations in prevalence of H. pylori resistance to antibiotics, especially clarithromycin, in Korea[17].
In conclusion, we detected no significant differences between the 10-d sequential eradication therapy for H. pylori and any duration of the standard triple treatment tested (7-, 10- and 14-d regimens) in a group of Korean patients. More research is needed to determine whether this finding also holds true for patients from other East Asian countries.
COMMENTS
Background
Helicobacter pylori (H. pylori), first identified in 1982, has been identified as a risk factor for gastrointestinal ulcer, chronic gastritis, gastric adenocarcinoma, and mucosa-associated lymphoid tissue lymphoma. To date, the most widely used eradication therapy is administration of a proton pump inhibitor (PPI) and 2 types of antibiotics; amoxicillin and clarithromycin. However, the success rate of these multiple antibiotics to eradicate H. pylori has decreased in recent years. An increase in the antibiotic resistance of H. pylori has been reported in Western countries, and a 10 d sequential eradication therapy was proposed to address this problem.
Research frontiers
The 10-d sequential therapy includes a PPI plus 1 g amoxicillin for the first 5 d followed by a PPI, clarithromycin 500 mg, and tinidazole 500 mg for the remaining 5 d. There is no established evidence about the duration of triple therapy. Guidelines for the duration of triple therapy varies according to region. So, a Korean research team evaluated whether H. pylori eradication with 10 d of sequential therapy is better than the conventional 7-, 10- and 14-d triple therapy in a Korean cohort.
Applications
The researchers concluded that there was no significant difference between 7-, 10-, 14-d standard triple treatment and 10-d sequential therapy in eradication of H. pylori in a Korean cohort of patients. Data from East Asian countries on use of sequential therapy are limited, and further study is needed to determine the optimal duration of treatment for triple therapy in Asian populations.
Terminology
Sequential therapy is a new regimen to eradicate H. pylori includes a PPI plus 1 g of amoxicillin twice daily for the first 5 d followed by a PPI, 500 mg of clarithromycin and 500 mg of tinidazole twice daily for the remaining 5 d.
Peer review
This is an interesting study aimed at comparing the eradication rate of a 7, 10 and 14 d triple therapy vs sequential therapy in Korean patients. Interestingly, the authors found that the eradication rate provided by sequential therapy is similar to that of standard therapy of the same duration. Therefore, the final message is that the most important factor in the eradication of H. pylori is the duration of the therapy instead of the way of administration of antibiotics and PPI.
Footnotes
Peer reviewers: Angelo Zullo, MD, Gastroenterologia ed Endoscopia Digestiva, Ospedale Nuovo Regina Margherita, Via E. Morosini, 30, 00153 Roma, Italy; Francesco Franceschi, MD, PhD, Assistant Professor, Internal Medicine, Catholic University of Rome, Gemelli Hospital, Largo A Gemelli 8, 00168 Rome, Italy
S- Editor Cheng JX L- Editor Cant MR E- Editor Zheng XM
References
- 1.Malfertheiner P, Megraud F, O’Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ, Lam SK, Xiao SD, Tan HJ, Wu CY, et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009;24:1587–1600. doi: 10.1111/j.1440-1746.2009.05982.x. [DOI] [PubMed] [Google Scholar]
- 3.Kim BW, Choi MG, Moon SB, Kim BK, Chae HS, Kim JK, Chung IS, Sun HS, Park DH. Pooled analysis of antibiotic therapy for helicobacter pylori eradication in Korea. Korean J Gastroenterol. 1999;34:42–49. [Google Scholar]
- 4.Sasaki M, Ogasawara N, Utsumi K, Kawamura N, Kamiya T, Kataoka H, Tanida S, Mizoshita T, Kasugai K, Joh T. Changes in 12-Year First-Line Eradication Rate of Helicobacter pylori Based on Triple Therapy with Proton Pump Inhibitor, Amoxicillin and Clarithromycin. J Clin Biochem Nutr. 2010;47:53–58. doi: 10.3164/jcbn.10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bang SY, Han DS, Eun CS, Kim JE, Ahn SB, Sohn JH, Jeon YC, Kang JO. [Changing patterns of antibiotic resistance of Helicobacter pylori in patients with peptic ulcer disease] Korean J Gastroenterol. 2007;50:356–362. [PubMed] [Google Scholar]
- 6.Mégraud F, Lehours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev. 2007;20:280–322. doi: 10.1128/CMR.00033-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vakil N, Lanza F, Schwartz H, Barth J. Seven-day therapy for Helicobacter pylori in the United States. Aliment Pharmacol Ther. 2004;20:99–107. doi: 10.1111/j.1365-2036.2004.02029.x. [DOI] [PubMed] [Google Scholar]
- 8.Osato MS, Reddy R, Graham DY. Metronidazole and clarithromycin resistance amongst Helicobacter pylori isolates from a large metropolitan hospital in the United States. Int J Antimicrob Agents. 1999;12:341–347. doi: 10.1016/s0924-8579(99)00079-5. [DOI] [PubMed] [Google Scholar]
- 9.Lee H, Kim JJ. [Impact of metronidazole resistance on eradication rate for Helicobacter pylori] Korean J Gastroenterol. 2005;46:142–145. [PubMed] [Google Scholar]
- 10.Zullo A, Rinaldi V, Winn S, Meddi P, Lionetti R, Hassan C, Ripani C, Tomaselli G, Attili AF. A new highly effective short-term therapy schedule for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2000;14:715–718. doi: 10.1046/j.1365-2036.2000.00766.x. [DOI] [PubMed] [Google Scholar]
- 11.Zullo A, Gatta L, De Francesco V, Hassan C, Ricci C, Bernabucci V, Cavina M, Ierardi E, Morini S, Vaira D. High rate of Helicobacter pylori eradication with sequential therapy in elderly patients with peptic ulcer: a prospective controlled study. Aliment Pharmacol Ther. 2005;21:1419–1424. doi: 10.1111/j.1365-2036.2005.02519.x. [DOI] [PubMed] [Google Scholar]
- 12.Hassan C, De Francesco V, Zullo A, Scaccianoce G, Piglionica D, Ierardi E, Panella C, Morini S. Sequential treatment for Helicobacter pylori eradication in duodenal ulcer patients: improving the cost of pharmacotherapy. Aliment Pharmacol Ther. 2003;18:641–646. doi: 10.1046/j.1365-2036.2003.01694.x. [DOI] [PubMed] [Google Scholar]
- 13.Vakil N. New developments in the treatment of Helicobacter pylori: is sequential therapy the answer? Rev Gastroenterol Disord. 2008;8:217–218. [PubMed] [Google Scholar]
- 14.Sánchez-Delgado J, Calvet X, Bujanda L, Gisbert JP, Titó L, Castro M. Ten-day sequential treatment for Helicobacter pylori eradication in clinical practice. Am J Gastroenterol. 2008;103:2220–2223. doi: 10.1111/j.1572-0241.2008.01924.x. [DOI] [PubMed] [Google Scholar]
- 15.Marshall B. Sequential therapy for Helicobacter pylori: a worthwhile effort for your patients. Ann Intern Med. 2008;148:962–963. doi: 10.7326/0003-4819-148-12-200806170-00227. [DOI] [PubMed] [Google Scholar]
- 16.Kim JY, Kim NY, Kim SJ, Baik GH, Kim GH, Kim JM, Nam RH, Kim HB, Lee DH, Jung HC, et al. [Regional difference of antibiotic resistance of helicobacter pylori strains in Korea] Korean J Gastroenterol. 2011;57:221–229. doi: 10.4166/kjg.2011.57.4.221. [DOI] [PubMed] [Google Scholar]
- 17.Kim N, Kim JM, Kim CH, Park YS, Lee DH, Kim JS, Jung HC, Song IS. Institutional difference of antibiotic resistance of Helicobacter pylori strains in Korea. J Clin Gastroenterol. 2006;40:683–687. doi: 10.1097/00004836-200609000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Kim BG, Lee DH, Ye BD, Lee KH, Kim BW, Kim SG, Kim SW, Kim SK, Kim JJ, Kim HY, et al. Comparison of 7-day and 14-day proton pump inhibitor-containing triple therapy for Helicobacter pylori eradication: neither treatment duration provides acceptable eradication rate in Korea. Helicobacter. 2007;12:31–35. doi: 10.1111/j.1523-5378.2007.00468.x. [DOI] [PubMed] [Google Scholar]
- 19.Peterson WL, Fendrick AM, Cave DR, Peura DA, Garabedian-Ruffalo SM, Laine L. Helicobacter pylori-related disease: guidelines for testing and treatment. Arch Intern Med. 2000;160:1285–1291. doi: 10.1001/archinte.160.9.1285. [DOI] [PubMed] [Google Scholar]
- 20.Vaira D, Zullo A, Vakil N, Gatta L, Ricci C, Perna F, Hassan C, Bernabucci V, Tampieri A, Morini S. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a randomized trial. Ann Intern Med. 2007;146:556–563. doi: 10.7326/0003-4819-146-8-200704170-00006. [DOI] [PubMed] [Google Scholar]
- 21.Kim JM, Kim JS, Jung HC, Kim N, Song IS. [Antibiotic resistance of Helicobacter pylori isolated from Korean patients in 2003] Korean J Gastroenterol. 2004;44:126–135. [PubMed] [Google Scholar]
- 22.Lee JH, Hong SP, Kwon CI, Phyun LH, Lee BS, Song HU, Ko KH, Hwang SG, Park PW, Rim KS, et al. [The efficacy of levofloxacin based triple therapy for Helicobacter pylori eradication] Korean J Gastroenterol. 2006;48:19–24. [PubMed] [Google Scholar]
- 23.Kim N, Lim SH, Lee KH, Koo MS, Kim JM, Hwang JH, Kim JW, Lee DH, Jung HC, Song IS. [Retreatment of Helicobacter pylori infection with triple therapy after initial treatment failure] Korean J Gastroenterol. 2003;42:195–203. [PubMed] [Google Scholar]
- 24.Mun GH, Hahm JS, Ryu KH, Lee OY, Han DS, Yoon BC, Choi HS, Lee MH, Lee CS, Park KN, et al. Metronidazole Resistance and the Eradication of Helicobacter pylori. Korean J Gastrointest Endosc. 1998;18:847–852. [Google Scholar]
- 25.Kim JM. [Antibiotic resistance of Helicobacter pylori isolated from Korean patients] Korean J Gastroenterol. 2006;47:337–349. [PubMed] [Google Scholar]
- 26.Koletzko S, Richy F, Bontems P, Crone J, Kalach N, Monteiro ML, Gottrand F, Celinska-Cedro D, Roma-Giannikou E, Orderda G, et al. Prospective multicentre study on antibiotic resistance of Helicobacter pylori strains obtained from children living in Europe. Gut. 2006;55:1711–1716. doi: 10.1136/gut.2006.091272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francavilla R, Lionetti E, Castellaneta S, Margiotta M, Piscitelli D, Lorenzo L, Cavallo L, Ierardi E. Clarithromycin-resistant genotypes and eradication of Helicobacter pylori. J Pediatr. 2010;157:228–232. doi: 10.1016/j.jpeds.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 28.De Francesco V, Margiotta M, Zullo A, Hassan C, Troiani L, Burattini O, Stella F, Di Leo A, Russo F, Marangi S, et al. Clarithromycin-resistant genotypes and eradication of Helicobacter pylori. Ann Intern Med. 2006;144:94–100. doi: 10.7326/0003-4819-144-2-200601170-00006. [DOI] [PubMed] [Google Scholar]
- 29.Gené E, Calvet X, Azagra R, Gisbert JP. Triple vs. quadruple therapy for treating Helicobacter pylori infection: a meta-analysis. Aliment Pharmacol Ther. 2003;17:1137–1143. doi: 10.1046/j.1365-2036.2003.01566.x. [DOI] [PubMed] [Google Scholar]
- 30.Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008;148:923–931. doi: 10.7326/0003-4819-148-12-200806170-00226. [DOI] [PubMed] [Google Scholar]
- 31.Tong JL, Ran ZH, Shen J, Xiao SD. Sequential therapy vs. standard triple therapies for Helicobacter pylori infection: a meta-analysis. J Clin Pharm Ther. 2009;34:41–53. doi: 10.1111/j.1365-2710.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- 32.Gatta L, Vakil N, Leandro G, Di Mario F, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta-analysis of randomized controlled trials in adults and children. Am J Gastroenterol. 2009;104:3069–3079; quiz 1080. doi: 10.1038/ajg.2009.555. [DOI] [PubMed] [Google Scholar]
- 33.Albrecht P, Kotowska M, Szajewska H. Sequential therapy compared with standard triple therapy for Helicobacter pylori eradication in children: a double-blind, randomized, controlled trial. J Pediatr. 2011;159:45–49. doi: 10.1016/j.jpeds.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 34.Webber MA, Piddock LJ. The importance of efflux pumps in bacterial antibiotic resistance. J Antimicrob Chemother. 2003;51:9–11. doi: 10.1093/jac/dkg050. [DOI] [PubMed] [Google Scholar]
- 35.De Francesco V, Zullo A, Margiotta M, Marangi S, Burattini O, Berloco P, Russo F, Barone M, Di Leo A, Minenna MF, et al. Sequential treatment for Helicobacter pylori does not share the risk factors of triple therapy failure. Aliment Pharmacol Ther. 2004;19:407–414. doi: 10.1046/j.1365-2036.2004.01818.x. [DOI] [PubMed] [Google Scholar]
- 36.Moshkowitz M, Konikoff FM, Peled Y, Santo M, Hallak A, Bujanover Y, Tiomny E, Gilat T. High Helicobacter pylori numbers are associated with low eradication rate after triple therapy. Gut. 1995;36:845–847. doi: 10.1136/gut.36.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jang S, Jones KR, Olsen CH, Joo YM, Yoo YJ, Chung IS, Cha JH, Merrell DS. Epidemiological link between gastric disease and polymorphisms in VacA and CagA. J Clin Microbiol. 2010;48:559–567. doi: 10.1128/JCM.01501-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi WH, Park DI, Oh SJ, Baek YH, Hong CH, Hong EJ, Song MJ, Park SK, Park JH, Kim HJ, et al. [Effectiveness of 10 day-sequential therapy for Helicobacter pylori eradication in Korea] Korean J Gastroenterol. 2008;51:280–284. [PubMed] [Google Scholar]
- 39.Fuccio L, Minardi ME, Zagari RM, Grilli D, Magrini N, Bazzoli F. Meta-analysis: duration of first-line proton-pump inhibitor based triple therapy for Helicobacter pylori eradication. Ann Intern Med. 2007;147:553–562. doi: 10.7326/0003-4819-147-8-200710160-00008. [DOI] [PubMed] [Google Scholar]
- 40.Calvet X, García N, López T, Gisbert JP, Gené E, Roque M. A meta-analysis of short versus long therapy with a proton pump inhibitor, clarithromycin and either metronidazole or amoxycillin for treating Helicobacter pylori infection. Aliment Pharmacol Ther. 2000;14:603–609. doi: 10.1046/j.1365-2036.2000.00744.x. [DOI] [PubMed] [Google Scholar]
- 41.Calvet X, López-Lorente M, Cubells M, Barè M, Gálvez E, Molina E. Two-week dual vs. one-week triple therapy for cure of Helicobacter pylori infection in primary care: a multicentre, randomized trial. Aliment Pharmacol Ther. 1999;13:781–786. doi: 10.1046/j.1365-2036.1999.00552.x. [DOI] [PubMed] [Google Scholar]
- 42.Kwon JH, Lee DH, Song BJ, Lee JW, Kim JJ, Park YS, Kim N, Jeong SH, Kim JW, Lee SH, et al. Ten-day sequential therapy as first-line treatment for Helicobacter pylori infection in Korea: a retrospective study. Helicobacter. 2010;15:148–153. doi: 10.1111/j.1523-5378.2010.00748.x. [DOI] [PubMed] [Google Scholar]
- 43.Park HG, Jung MK, Jung JT, Kwon JG, Kim EY, Seo HE, Lee JH, Yang CH, Kim ES, Cho KB, et al. Randomised clinical trial: a comparative study of 10-day sequential therapy with 7-day standard triple therapy for Helicobacter pylori infection in naïve patients. Aliment Pharmacol Ther. 2012;35:56–65. doi: 10.1111/j.1365-2036.2011.04902.x. [DOI] [PubMed] [Google Scholar]
- 44.Kim YS, Kim SJ, Yoon JH, Suk KT, Kim JB, Kim DJ, Kim DY, Min HJ, Park SH, Shin WG, et al. Randomised clinical trial: the efficacy of a 10-day sequential therapy vs. a 14-day standard proton pump inhibitor-based triple therapy for Helicobacter pylori in Korea. Aliment Pharmacol Ther. 2011;34:1098–1105. doi: 10.1111/j.1365-2036.2011.04843.x. [DOI] [PubMed] [Google Scholar]
- 45.Oh HS, Lee DH, Seo JY, Cho YR, Kim N, Jeoung SH, Kim JW, Hwang JH, Park YS, Lee SH, et al. Ten-day sequential therapy is more effective than proton pump inhibitor-based therapy in Korea: a prospective, randomized study. J Gastroenterol Hepatol. 2012;27:504–509. doi: 10.1111/j.1440-1746.2011.06922.x. [DOI] [PubMed] [Google Scholar]