Abstract
AIM: To compare the efficacy of the proton-pump inhibitor, rabeprazole, with that of the H2-receptor antagonist, ranitidine, as on-demand therapy for relieving symptoms associated with non-erosive reflux disease (NERD).
METHODS: This is a single center, prospective, randomized, open-label trial of on-demand therapy with rabeprazole (group A) vs ranitidine (group B) for 4 wk. Eighty-three patients who presented to the American University of Beirut Medical Center with persistent gastroesophageal reflux disease (GERD) symptoms and a normal upper gastrointestinal endoscopy were eligible for the study. Patients in group A (n = 44) were allowed a maximum rabeprazole dose of 20 mg twice daily, while those in group B (n = 39) were allowed a maximum ranitidine dose of 300 mg twice daily. Efficacy was assessed by patient evaluation of global symptom relief, scores of the SF-36 quality of life (QoL) questionnaires, total number of pills used, and number of medication-free days.
RESULTS: Among the 83 patients who were enrolled in the study, 76 patients (40 in the rabeprazole group and 36 in the ranitidine group) completed the 4-wk trial. Baseline characteristics were comparable between both groups. After 4 wk, there was no significant difference in the subjective global symptom relief between the rabeprazole and the ranitidine groups (71.4% vs 65.4%, respectively; P = 0.9). There were no statistically significant differences between mean cumulative scores of the SF-36 QoL questionnaire for the two study groups (rabeprazole 22.40 ± 27.53 vs ranitidine 17.28 ± 37.06; P = 0.582). There was no significant difference in the mean number of pills used (rabeprazole 35.70 ± 29.75 vs ranitidine 32.86 ± 26.98; P = 0.66). There was also no statistically significant difference in the mean number of medication-free days between both groups.
CONCLUSION: Rabeprazole has a comparable efficacy compared to ranitidine when given on-demand for the treatment of NERD. Both medications were associated with improved quality of life.
Keywords: Proton-pump inhibitors, H2-receptor antagonists, Non-erosive reflux disease, Gastroesophageal reflux disease, Quality of life
INTRODUCTION
Gastroesophageal reflux disease (GERD) is a chronic, remitting-relapsing medical condition that involves the reflux of gastric contents into the esophagus causing a multitude of unpleasant symptoms including heartburn, sore throat, chest pain, cough, and regurgitation. GERD has been shown to have a significant negative impact on the quality of life (QoL) of affected patients and may even impair their daily activities[1]. The prevalence of GERD has markedly increased over the past two decades affecting 20%-40% of the western population[2-5]. This prevalence is predicted to rise further with time[6].
Non-erosive reflux disease (NERD) has been defined by the Vevey NERD Consensus Group as a subcategory of GERD that is characterized by reflux-related symptoms with the absence of esophageal mucosal erosions or breaks at conventional endoscopy and without recent acid-suppressive therapy[7]. About two-thirds of patients with typical GERD symptoms, such as heartburn, belching, cough, nausea, sore throat and voice changes, have no erosive changes on upper gastrointestinal endoscopic evaluation[2]. The complex pathophysiology of NERD and the exact mechanisms by which the associated symptoms are caused remain unclear[7]. It is highly evident, however, that the degree of acidity and duration of esophageal acid exposure play an essential role in NERD symptomatology. These factors are not different from the precipitants of moderate erosive reflux disease (ERD)[7-11].
The majority of GERD patients use acid-suppressive medications to control their symptoms. Unlike ERD, symptoms of NERD are more difficult to control, and tend to have a lower response rate, even to the most potent proton-pump inhibitors (PPIs)[12,13]. Nonetheless, initial management of NERD is similar to that of GERD, and includes the use of a PPI or H2-receptor antagonist (H2RA). Around 75% of patients with NERD report a relapse of their symptoms after cessation of the initial therapy. Therefore, long-term management is often needed to maintain symptom control[1]. The preferred maintenance therapeutic strategy is supposed to utilize the least amount of medication. Hence, on-demand therapy should be a reasonable therapeutic mode for managing patients with NERD symptoms. By convention, medications for on-demand therapy should ensure a rapid onset of action in order to give instantaneous relief.
To our knowledge, and after reviewing the literature, no clinical studies have compared a PPI to an H2RA as on-demand therapy for NERD. The objective of our trial is to compare the efficacy of a PPI, rabeprazole, to that of a standard H2RA, ranitidine, as an on-demand therapy for treating patients with NERD. We selected rabeprazole among the various currently available PPIs because it results in a faster and a more prompt acid suppression and has the advantage of a first-dose symptom relief[14-16]. H2RAs including ranitidine are widely used on-demand for the management of GERD symptoms.
MATERIALS AND METHODS
The study was a 4-wk prospective, randomized, single center, open-label trial of on-demand therapy with rabeprazole vs ranitidine for patients with NERD. Patients who were considered eligible for enrollment had to be older than 18 years of age, had persistent GERD symptoms (typical or atypical), and a negative upper gastrointestinal endoscopy exam. Exclusion criteria were age below 18 years and older than 75 years, allergy to rabeprazole or ranitidine, any degree of esophagitis or mucosal damage on upper gastrointestinal endoscopy, pregnancy, and any use of PPI or H2RA within 2 wk of enrollment into the study.
Patients who presented to the American University of Beirut Medical Center with persistent GERD symptoms and a normal upper gastrointestinal endoscopy were considered candidates for the study. After signing a written informed consent, patients were asked to complete a baseline SF-36 QoL questionnaire. They were randomized by an independent investigator using a computer-generated random number table to one of two groups: those to receive rabeprazole (group A) and those to receive ranitidine (group B). Patients in group A were allowed a maximum oral rabeprazole dose of 20 mg (10 mg tablets, twice daily), while those in group B were allowed a maximum oral ranitidine dose of 300 mg (150 mg tablets, twice daily). A research fellow was responsible for contacting patients by phone on a daily basis over the 4-wk study period to assess for the number of pills taken, the need for a rescue medication, as well as the occurrence of any side effects. At the end of the 4 wk, patients were asked about their global symptom relief and were also requested to answer the post-treatment QoL questionnaire.
Primary efficacy endpoints were assessed by the subjective global symptom relief, total number of pills used, number of medication-free days, and the need for rescue medications. Secondary endpoints included the scores of the QoL questionnaires and the occurrence of side effects. The study protocol and informed consent were approved by the Institutional Review Board at the American University of Beirut Medical Center.
Statistical analysis
Analysis of the primary end-point (global symptom relief) was done according to intent-to-treat (ITT) basis. The association between the drug group and response for binary measures was assessed using the Fischer’s Exact test. For continuous measures, either a two sample t-test or Wilcoxon two sample rank sum test were used depending on whether normality held or not. Frequency tables and cross-tabulations were derived in order to depict any associations between the different variables. The paired samples t-test and the independent-samples t-test were used to compare the QoL score before and after treatment. A P-value at or below 0.05 was significant. The data were entered and analyzed using SPSS for Microsoft version 18.0 (SPSS Inc, United States).
Sample size calculation: The sample size was estimated based on an expected response rate of 80% for rabeprazole and 50% for ranitidine. Therefore, we had to include 42 patients in each arm of the study to detect a statistical significance using a power of 80% and a margin of error of 5%.
RESULTS
A total of 83 patients with symptoms consistent with GERD and a negative upper gastrointestinal endoscopy were enrolled in the study. The random assignment of patients into two arms resulted in 44 patients (53%) in group A assigned to receive rabeprazole, and 39 patients (47%) in group B assigned to receive ranitidine. Overall, seven patients dropped out of the study; two patients because of mild medication side effects (both of whom were receiving rabeprazole) and five were lost to follow-up. Seventy-six patients completed the 4-wk trial per-protocol, 40 of whom were assigned to the rabeprazole group and 36 to the ranitidine group. The ITT population consisted of 83 patients.
Baseline characteristics between both groups were comparable (Table 1). The mean age of individuals in group A was 45.43 ± 15.16 years vs 45.08 ± 15.29 years for those in group B. There was a slight female predominance in both groups: 63.6% in group A and 59% in group B. Most patients had been symptomatic for more than 1 year, with duration ranging from 3 mo to 20 years. The majority of patients (85.5%) suffered from extra-esophageal manifestations, with globus sensation, metallic taste, shortness of breath, as well as thick sputum production being the commonest among these manifestations. Esophageal manifestations were encountered in approximately 69% of patients.
Table 1.
Patient baseline characteristics n (%)
| Group A(n= 44) | Group B(n= 39) | Pvalue | |
| Age (mean ± SD) (yr) | 45.4 ± 15.2 | 45.1 ± 15.3 | 0.916 |
| Gender M:F | 16:28 | 16:23 | 0.663 |
| Duration of symptoms (yr) | 4.68 ± 7.79 | 3.85 ± 4.21 | 0.552 |
| Esophageal manifestations | 28 (63.6) | 29 (74.4) | 0.293 |
| Regurgitation and heartburn | 21 (47.7) | 23 (59.0) | 0.306 |
| Epigastric pain | 8 (18.2) | 9 (23.1) | 0.581 |
| Nausea | 7 (15.9) | 6 (15.4) | 0.948 |
| Vomiting | 0 (0) | 1 (2.6) | 0.285 |
| Extra-esophageal manifestations | 38 (86.4) | 33 (84.6) | 0.821 |
| Chest pain | 16 (36.4) | 10 (25.6) | 0.293 |
| Globus | 24 (54.5) | 16 (41.0) | 0.219 |
| Hoarseness | 15 (34.1) | 10 (25.6) | 0.402 |
| Metallic taste | 22 (50.0) | 16 (41.0) | 0.413 |
| Nocturnal cough | 17 (38.6) | 09 (23.1) | 0.127 |
| Dyspnea | 21 (47.7) | 20 (51.3) | 0.746 |
| Sore throat | 20 (45.5) | 13 (33.3) | 0.260 |
| Thick sputum production | 24 (54.5) | 13 (33.3) | 0.052 |
| Mixed symptoms | 25 (56.8) | 24 (61.5) | 0.663 |
Group A, rabeprazole; Group B, ranitidine. M: Male; F: Female.
Global symptom improvement was subjectively assessed at the end of the 4-wk treatment phase. Improvement was reported in 71.4% (20 out of 28) of patients in group A and 65.4% (17 out of 26) of patients in group B, while worsening of symptoms was noted in 3.6% and 3.8 % of patients in group A and B, respectively (Table 2). Of the patients in group A, 25% experienced no change in symptoms compared to 30.8% of group B patients (P = 0.889). There was no difference in gender distribution between responders and non-responders in either group. However, the mean age of responders to rabeprazole was significantly lower than that of patients who did not respond to this drug (38.90 ± 13.15 years vs 53.14 ± 17.68 years; P = 0.033). This difference was not noted in patients who received ranitidine.
Table 2.
Subjective global symptom assessment n (%)
| Better | Same | Worse | Total | |
| Group A | 20 (71.4) | 7 (25.0) | 1 (3.6) | 28 |
| Group B | 17 (65.4) | 8 (30.8) | 1 (3.8) | 26 |
| Total | 37 | 15 | 2 | 54 |
P = 0.9; Group A, rabeprazole; Group B, ranitidine.
Regarding the mean number of pills consumed, patients in group A used a mean number of 35.70 ± 29.75 pills of rabeprazole 10 mg, while group B patients consumed a mean number of 32.86 ± 26.98 pills of ranitidine 150 mg, (P = 0.66). The mean number of medication-free days was 13.08 ± 9.73 in group A, and 10.78 ± 10.14 in group B (P = 0.32).
The SF-36 QoL questionnaire was completed by a total of 65 patients at baseline: 37 in group A (84.1%) and 28 in group B (71.8%). Mean cumulative scores at baseline were comparable between groups A and B, 106.38 ± 30.30 vs 104.43 ± 29.82, respectively (P = 0.797) (Figure 1). A follow-up SF-36 QoL questionnaire was obtained from 50 patients immediately after the 4-wk treatment phase: 25 were in group A (62.5%) and 25 in group B (69.4%). Mean cumulative scores at follow-up were 135.32 ± 32.19 for group A and 122.76 ± 37.48 for group B (P = 0.210). Mean scores of both groups increased significantly (P < 0.01 for group A and P = 0.028 for group B, compared to respective baseline scores). However, the absolute score differences between baseline and 4 wk for groups A and B were (22.40 ± 27.53 vs 17.28 ± 37.06, respectively; P = 0.582), indicating that both drugs improve patient QoL to the same extent.
Figure 1.
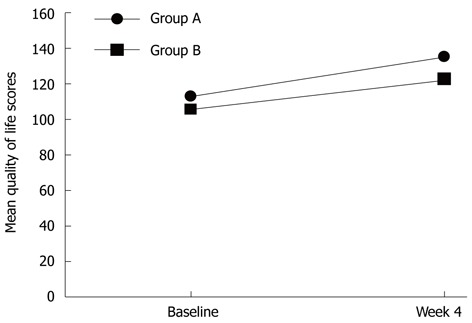
Mean quality of life scores at baseline and immediately after treatment (n = 25 at baseline). Group A, rabeprazole; Group B, ranitidine.
DISCUSSION
The present study compares the efficacy of the PPI rabeprazole to that of the H2RA ranitidine, as an on-demand option in the management of patients with NERD. The impact on QoL was also evaluated as a secondary outcome with both drug regimens.
Analysis of our data showed that rabeprazole is as effective as the routinely and commonly used ranitidine in controlling both the typical and atypical symptoms in patients with NERD. Evidence from several previous studies supports our findings regarding the efficacy of anti-secretory (H2RA and PPI) therapy in patients with NERD[17-24]. However, no head-to-head comparison between any H2RA and PPI had been carried out in the setting of on-demand therapy for NERD.
Our PubMed® search identified a number of clinical trials[14,21-25] that assessed the efficacy of various PPIs (rabeprazole, omeprazole, esomeprazole, lansoprazole) when given as on-demand in patients with NERD. Almost all of these studies were conducted over a period of time ranging from 3 mo to 6 mo and preceded by initial short periods (around 4-8 wk) of daily treatment with the designated PPI to achieve complete symptom resolution. A study by Lind et al[21] randomized 424 patients with NERD to one of three groups: omeprazole 20 mg, omeprazole 10 mg, or placebo. After 6 mo of on-demand therapy, it was concluded that omeprazole was effective in the majority of NERD patients. In another placebo-controlled study, Talley et al[22] assigned 342 patients to either esomeprazole 20 mg or placebo. On-demand therapy with esomeprazole 20 mg was found suitable for the long-term symptom management of NERD patients. In a separate placebo-controlled trial, Talley et al[23] assessed the efficacy of on-demand therapy with esomeprazole 40 mg or 20 mg in patients with NERD and showed that both dosages were superior to placebo in controlling heartburn in those patients. Bytzer et al[24] achieved favorable results in a 6-mo trial of on-demand rabeprazole 10 mg in patients with NERD. Five hundred and twenty-three patients with NERD were given 4 wk of rabeprazole 10 mg once daily. The 432 patients who had complete resolution of their symptoms were then randomized for the on-demand phase of the study to two groups: rabeprazole 10 mg and placebo. Symptom relief was significantly better in the rabeprazole group compared to the placebo group.
Rabeprazole was also investigated as on-demand treatment by Ponce et al[25] in patients with NERD and low-grade esophagitis. Symptom control was maintained in over 85% of patients during six months of on-demand rabeprazole 20 mg therapy, following a 4-wk daily run-in period with rabeprazole 20 mg per day. During the study period, PPI consumption was found to be low and patient satisfaction with the treatment was high.
Rabeprazole appeared to be ideal for our study given its rapid onset of action and powerful acid suppression[14-16]. Studies involving NERD patients have documented its superiority over placebo. In addition, on-demand use of rabeprazole for the management of NERD incurs the least cost in comparison with other PPIs[26].
H2RAs have been widely used on-demand for patients with GERD. Clinical studies have demonstrated that, when given on-demand, they are superior to placebo in controlling heartburn in this group of patients[18,19,27]. These findings may be extrapolated to NERD patients who constitute the majority of patients with GERD. H2RAs are known to have a rapid onset, but a short duration of action. They suppress acid for approximately 4 to 8 h and produce incomplete inhibition of post-prandial gastric acid secretion. They inhibit acid secretion by up to 70% over a 24-h period. A major disadvantage of using these drugs is the development of tolerance that occurs within two weeks of uninterrupted daily intake[28]. Thus, tolerance would be less concerning if they are to be used on an on-demand basis[29]. PPIs have the advantage over H2RA in controlling both basal and food-stimulated acid secretion producing a longer-lasting acid suppression in addition to the fact that tolerance has not been observed with PPIs.
Our pilot study has a few limitations. One is the open-label nature of the study. Although patients were randomized to different arms, they were aware of the arm they were randomized to. This may have created some bias especially if those patients were previously treated with the same medication class that they were assigned to. The sample size was also relatively small and further investigation based on a larger number of patients is necessary to corroborate our data. Our study duration was short, then again the purpose of our study was not to prove the efficacy of either of the two drugs, but rather to compare them.
The advantages of our study include the fact that it is the first to compare an H2RA to a PPI in the setting of on-demand therapy for NERD. We also showed response to on-demand therapy for both typical and atypical reflux symptoms. Finally, this is a “pure” on-demand study, in the sense that it was performed without a preceding continuous anti-secretory treatment period.
In conclusion, rabeprazole and ranitidine have been shown to be comparable in efficacy when given on-demand for the treatment of NERD. Both medications were associated with a statistically significant improved quality of life.
COMMENTS
Background
Non-erosive reflux disease (NERD) is the most prevalent of the subcategories of gastroesophageal reflux diseases (GERD). It is a chronic condition with a significant impact on patients’ quality of life. On-demand acid-suppressive agents constitute the mainstay in the management of NERD. The most favorable of such agents should be fast-acting, long-lasting, potent, and safe, in order to reach an effective and timely symptom control.
Research frontiers
Acid-suppressive medications differ in terms of power of acid inhibition, onset and duration of action, and drug interactions. Identification of the ideal agent in the context of chronic and on-demand therapy is of utmost interest to patients and researchers alike.
Innovations and breakthroughs
The authors compared the efficacy of the proton-pump inhibitor (PPI) rabeprazole, to that of the H2-receptor antagonist ranitidine, as on-demand therapy for relieving symptoms associated with NERD. They concluded that both possessed comparable efficacy and were associated with an improved quality of life. Thus, a fast-acting PPI, rabeprazole, is an effective on-demand option for the management of NERD.
Applications
In conditions where an acid-suppressive medication is to be prescribed for a long period of time, recognizing the agent that has the best efficacy and safety profiles would have a prompt and significant clinical impact.
Terminology
NERD is a non-erosive reflux disease, commonly defined as the presence of classic GERD symptoms in the absence of esophageal mucosal injury during upper endoscopy. The majority of patients with GERD fall into the NERD subcategory.
Peer review
The article is novel and interesting. It answers an important research question, which might result in changing clinical practice of NERD management. The design is appropriate, and the manuscript is well written.
Footnotes
Peer reviewers: Khaled Jadallah, MD, Assistant Professor of Medicine, Consultant, Gastroenterologist and Hepatologist, Department of Internal Medicine, King Abdullah University Hospital, Jordan University of Science and Technology, Irbid 22110, Jordan; Florencia Georgina Que, MD, Department of Surgery, Mayo Clinic, 200 First Street Southwest, Rochester, MN 55905, United States
S- Editor Cheng JX L- Editor Logan S E- Editor Zhang DN
References
- 1.Carlsson R, Dent J, Watts R, Riley S, Sheikh R, Hatlebakk J, Haug K, de Groot G, van Oudvorst A, Dalväg A, et al. Gastro-oesophageal reflux disease in primary care: an international study of different treatment strategies with omeprazole. International GORD Study Group. Eur J Gastroenterol Hepatol. 1998;10:119–124. [PubMed] [Google Scholar]
- 2.Fass R, Fennerty MB, Vakil N. Nonerosive reflux disease--current concepts and dilemmas. Am J Gastroenterol. 2001;96:303–314. doi: 10.1111/j.1572-0241.2001.03511.x. [DOI] [PubMed] [Google Scholar]
- 3.Locke GR, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448–1456. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 4.Ronkainen J, Aro P, Storskrubb T, Johansson SE, Lind T, Bolling-Sternevald E, Graffner H, Vieth M, Stolte M, Engstrand L, Talley NJ, Agréus L. High prevalence of gastroesophageal reflux symptoms and esophagitis with or without symptoms in the general adult Swedish population: a Kalixanda study report. Scand J Gastroenterol. 2005;40:275–285. doi: 10.1080/00365520510011579. [DOI] [PubMed] [Google Scholar]
- 5.Mishima I, Adachi K, Arima N, Amano K, Takashima T, Moritani M, Furuta K, Kinoshita Y. Prevalence of endoscopically negative and positive gastroesophageal reflux disease in the Japanese. Scand J Gastroenterol. 2005;40:1005–1009. doi: 10.1080/00365520510023260. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB. Time trends of gastroesophageal reflux disease: a systematic review. Clin Gastroenterol Hepatol. 2007;5:17–26. doi: 10.1016/j.cgh.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Modlin IM, Hunt RH, Malfertheiner P, Moayyedi P, Quigley EM, Tytgat GN, Tack J, Heading RC, Holtman G, Moss SF. Diagnosis and management of non-erosive reflux disease--the Vevey NERD Consensus Group. Digestion. 2009;80:74–88. doi: 10.1159/000219365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winter JW, Heading RC. The nonerosive reflux disease-gastroesophageal reflux disease controversy. Curr Opin Gastroenterol. 2008;24:509–515. doi: 10.1097/MOG.0b013e3283025c57. [DOI] [PubMed] [Google Scholar]
- 9.Martínek J, Benes M, Hucl T, Drastich P, Stirand P, Spicák J. Non-erosive and erosive gastroesophageal reflux diseases: No difference with regard to reflux pattern and motility abnormalities. Scand J Gastroenterol. 2008;43:794–800. doi: 10.1080/00365520801908928. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro M, Green C, Faybush EM, Esquivel RF, Fass R. The extent of oesophageal acid exposure overlap among the different gastro-oesophageal reflux disease groups. Aliment Pharmacol Ther. 2006;23:321–329. doi: 10.1111/j.1365-2036.2006.02747.x. [DOI] [PubMed] [Google Scholar]
- 11.Martinez SD, Malagon IB, Garewal HS, Cui H, Fass R. Non-erosive reflux disease (NERD)--acid reflux and symptom patterns. Aliment Pharmacol Ther. 2003;17:537–545. doi: 10.1046/j.1365-2036.2003.01423.x. [DOI] [PubMed] [Google Scholar]
- 12.Dean BB, Gano AD, Knight K, Ofman JJ, Fass R. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clin Gastroenterol Hepatol. 2004;2:656–664. doi: 10.1016/s1542-3565(04)00288-5. [DOI] [PubMed] [Google Scholar]
- 13.Galmiche JP. Non-erosive reflux disease and atypical gastro-oesophageal reflux disease manifestations: treatment results. Drugs. 2006;66 Suppl 1:7–13; discussion 29-33. doi: 10.2165/00003495-200666001-00003. [DOI] [PubMed] [Google Scholar]
- 14.Miner P, Orr W, Filippone J, Jokubaitis L, Sloan S. Rabeprazole in nonerosive gastroesophageal reflux disease: a randomized placebo-controlled trial. Am J Gastroenterol. 2002;97:1332–1339. doi: 10.1111/j.1572-0241.2002.05769.x. [DOI] [PubMed] [Google Scholar]
- 15.Inamori M, Togawa J, Takahashi K, Yoneda M, Fujisawa N, Iwasaki T, Ozawa Y, Kikuchi T, Muramatsu K, Chiguchi G, et al. Comparison of the effect on intragastric pH of a single dose of omeprazole or rabeprazole: which is suitable for on-demand therapy? J Gastroenterol Hepatol. 2003;18:1034–1038. doi: 10.1046/j.1440-1746.2003.03126.x. [DOI] [PubMed] [Google Scholar]
- 16.Pace F, Pallotta S, Casalini S, Porro GB. A review of rabeprazole in the treatment of acid-related diseases. Ther Clin Risk Manag. 2007;3:363–379. [PMC free article] [PubMed] [Google Scholar]
- 17.Johannessen T, Petersen H, Kristensen P, Fosstvedt D, Kleveland PM, Dybdahl J, Løge I. Cimetidine on-demand in dyspepsia. Experience with randomized controlled single-subject trials. Scand J Gastroenterol. 1992;27:189–195. doi: 10.3109/00365529208999947. [DOI] [PubMed] [Google Scholar]
- 18.Johannessen T, Kristensen P. On-demand therapy in gastroesophageal reflux disease: a comparison of the early effects of single doses of fast-dissolving famotidine wafers and ranitidine tablets. Clin Ther. 1997;19:73–81. doi: 10.1016/s0149-2918(97)80074-4. [DOI] [PubMed] [Google Scholar]
- 19.Galmiche JP, Shi G, Simon B, Casset-Semanza F, Slama A. On-demand treatment of gastro-oesophageal reflux symptoms: a comparison of ranitidine 75 mg with cimetidine 200 mg or placebo. Aliment Pharmacol Ther. 1998;12:909–917. doi: 10.1046/j.1365-2036.1998.00384.x. [DOI] [PubMed] [Google Scholar]
- 20.Simon TJ, Berlin RG, Gardner AH, Stauffer LA, Gould AL, Getson AJ. Self-Directed Treatment of Intermittent Heartburn: A Randomized, Multicenter, Double-Blind, Placebo-Controlled Evaluation of Antacid and Low Doses of an H(2)-Receptor Antagonist (Famotidine) Am J Ther. 1995;2:304–313. [PubMed] [Google Scholar]
- 21.Lind T, Havelund T, Lundell L, Glise H, Lauritsen K, Pedersen SA, Anker-Hansen O, Stubberöd A, Eriksson G, Carlsson R, et al. On demand therapy with omeprazole for the long-term management of patients with heartburn without oesophagitis--a placebo-controlled randomized trial. Aliment Pharmacol Ther. 1999;13:907–914. doi: 10.1046/j.1365-2036.1999.00564.x. [DOI] [PubMed] [Google Scholar]
- 22.Talley NJ, Venables TL, Green JR, Armstrong D, O’Kane KP, Giaffer M, Bardhan KD, Carlsson RG, Chen S, Hasselgren GS. Esomeprazole 40 mg and 20 mg is efficacious in the long-term management of patients with endoscopy-negative gastro-oesophageal reflux disease: a placebo-controlled trial of on-demand therapy for 6 months. Eur J Gastroenterol Hepatol. 2002;14:857–863. doi: 10.1097/00042737-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Talley NJ, Lauritsen K, Tunturi-Hihnala H, Lind T, Moum B, Bang C, Schulz T, Omland TM, Delle M, Junghard O. Esomeprazole 20 mg maintains symptom control in endoscopy-negative gastro-oesophageal reflux disease: a controlled trial of ‘on-demand’ therapy for 6 months. Aliment Pharmacol Ther. 2001;15:347–354. doi: 10.1046/j.1365-2036.2001.00943.x. [DOI] [PubMed] [Google Scholar]
- 24.Bytzer P, Blum A, De Herdt D, Dubois D. Six-month trial of on-demand rabeprazole 10 mg maintains symptom relief in patients with non-erosive reflux disease. Aliment Pharmacol Ther. 2004;20:181–188. doi: 10.1111/j.1365-2036.2004.01999.x. [DOI] [PubMed] [Google Scholar]
- 25.Ponce J, Argüello L, Bastida G, Ponce M, Ortiz V, Garrigues V. On-demand therapy with rabeprazole in nonerosive and erosive gastroesophageal reflux disease in clinical practice: effectiveness, health-related quality of life, and patient satisfaction. Dig Dis Sci. 2004;49:931–936. doi: 10.1023/b:ddas.0000034551.39324.c3. [DOI] [PubMed] [Google Scholar]
- 26.Hughes DA, Marchetti M, Colombo G. Cost minimization of on-demand maintenance therapy with proton pump inhibitors in nonerosive gastroesophageal reflux disease. Expert Rev Pharmacoecon Outcomes Res. 2005;5:29–38. doi: 10.1586/14737167.5.1.29. [DOI] [PubMed] [Google Scholar]
- 27.Faaij RA, Van Gerven JM, Jolivet-Landreau I, Masclee AA, Vendrig EM, Schoemaker RC, Jacobs LD, Cohen AF. Onset of action during on-demand treatment with maalox suspension or low-dose ranitidine for heartburn. Aliment Pharmacol Ther. 1999;13:1605–1610. doi: 10.1046/j.1365-2036.1999.00654.x. [DOI] [PubMed] [Google Scholar]
- 28.Pettit M. Treatment of gastroesophageal reflux disease. Pharm World Sci. 2005;27:432–435. doi: 10.1007/s11096-005-4798-7. [DOI] [PubMed] [Google Scholar]
- 29.Metz DC, Inadomi JM, Howden CW, van Zanten SJ, Bytzer P. On-demand therapy for gastroesophageal reflux disease. Am J Gastroenterol. 2007;102:642–653. doi: 10.1111/j.1572-0241.2006.00998.x. [DOI] [PubMed] [Google Scholar]


