Abstract
AIM: To investigate the expression of Popeye domain containing 3 (Popdc3) and its correlation with clinicopathological features and prognosis of gastric cancer.
METHODS: The method of immunohistochemistry was used to investigate the expression of Popdc3 in 306 cases of human gastric cancer and 84 noncancerous gastric tissues. Simultaneously, the relationship between Popdc3 expression and the survival of the patients was retrospectively analyzed.
RESULTS: Popdc3 was detected in 72 (85.71%) of 84 human nontumor mucosa. High expression of Popdc3 protein was detected in 78 (25.49%) of 306 human gastric cancer cases, and low expression was detected in 228 (74.51%). Low expression of Popdc3 correlated with depth of invasion (P < 0.0001), regional lymph nodes (P < 0.0001) and distant metastasis (P = 0.02), and tumor, nodes, metastasis (TNM) stages (P < 0.0001). On multivariate analysis, only the patient’s gender, regional lymph node metastasis, distant metastasis, TNM stages, and the expression of Popdc3 were independent prognostic factors in patients with gastric cancer. The Kaplan-Meier plot showed that low Popdc3 expression had a much more significant effect on the survival of those patients with early-stage tumors (χ2 = 104.741, P < 0.0001), with a > 51.9% reduction in the three-year survival compared with high Popdc3 expression. In late stages, the difference was also significant (χ2 = 5.930, P = 0.015), with a 32.6% reduction in the three-year survival.
CONCLUSION: Reduced expression of Popdc3 may play a significant role in the carcinogenesis and progression of gastric cancer. Popdc3 may be an independent prognostic factor.
Keywords: Popeye domain containing 3, Gastric cancer, Cell adhesion molecules, Metastasis, Prognosis
INTRODUCTION
Gastric cancer is the second leading cause of cancer-related deaths worldwide because advanced or metastatic gastric cancer constitutes the majority of patients in clinical practice[1]. The incidence of gastric cancer has been falling globally in terms of both incidence and mortality rates since World War II. In the United States in 2009, 21 130 new diagnoses of gastric cancer were estimated and 10 620 deaths expected[2]. Although there has been a noticeable decrease in gastric cancer mortality rate, there is a higher prevalence of gastric cancer in China than in the Western countries[3-7]. The prognosis of advanced gastric cancer is apparently poor, and the 5-year survival rate is less than 30% in the patients after surgery[8,9]. Therefore, it is of great clinical value to further understand the molecular mechanisms involved in gastric cancer and to find valuable diagnostic markers as well as novel therapeutic strategies.
The Popeye domain containing (Popdc) gene family consists of Bves/Popdc1, Popdc2 and Popdc3[10]. They were discovered in 1999 by two independent laboratories using screens to identify novel genes that were highly expressed in the developing heart[11,12]. Several studies have illuminated that within one species, Bves was 24% and 28% identical to Popdc2 and Popdc3, respectively, while Popdc2 and Popdc3 were approximately 50% identical[13-15]. In 2004, the expression pattern of Popdc2 in the heart of chick was reported, but no study had been conducted to test the function of this protein[14]. Until now, Bves is still the most studied member of the Popdc family[10]. After that, Kim et al[16] found that frequent silencing of Popdc3 was associated with promoter hypermethylation in gastric cancer. Although questions about Popdc3 remain unanswered, general trends are beginning to emerge. Previous studies have led us to the hypothesis that Popdc3 may play a role in cell adhesion, cell motility, DNA methylation and tumorigenesis. Nevertheless, further work is required to elucidate its molecular mechanisms in the biology of cancer.
As far as we know, no report is available on the actual expression level of Popdc3 and the correlation between clinicopathologic features and prognosis of gastric cancer patients. Therefore, in this study, we investigated the Popdc3 protein expression profile in 306 primary gastric cancer patients, and found that Popdc3 was lowly expressed in 228 (74.51%) of 306 human gastric cancer cases, suggesting that the low expression level of Popdc3 may be a reliable indicator for the poor prognosis of gastric cancer patients.
MATERIALS AND METHODS
Patients and tissue samples
A total of 306 patients with primary gastric cancer, who underwent routine surgery at the Department of Surgery, the Second Affiliated Hospital of Kunming Medical University from February 1996 to March 2007, were enrolled in this study. The study was approved by the hospital’s ethics committee. Patient diagnosis was established pathologically, and no patient had received any treatment before admission. All patients had follow-up records for over 5 years. The follow-up deadline was April 2011. The survival time was counted from the date of surgery to the end of the follow-up or date of death, which was mostly caused by recurrence or metastasis. According to the tumor, nodes, metastasis (TNM)-7th edition 2009 (UICC/AJCC) and Japanese Classification 2010 in Gastric Cancer[17,18], there were 8 papillary adenocarcinomas, 209 tubular adenocarcinomas, 52 mucinous adenocarcinomas, 37 signet ring cell carcinomas, and 17 highly differentiated adenocarcinomas; 100 were classified as well or moderately differentiated adenocarcinomas, 177 as poorly differentiated adenocarcinomas, and 12 as undifferentiated adenocarcinomas or others. Seventy-three cases were categorized as stage I, 109 were stage II, 92 were stage III, and 32 were stage IV. Eighty-four cases of noncancerous human gastric tissues were obtained from gastrectomies of adjacent gastric cancer margins greater than 5 cm and served as controls.
Immunohistochemistry
Immunohistochemical analysis was undertaken to study altered protein expression in 84 cases of noncancerous human gastric tissues and 306 cases of human gastric cancer tissues[19,20]. According to the protocol for immunohistochemistry on paraffin-embedded tissue sections, slides were baked at 60 °C for 2 h followed by deparaffinization with xylene and rehydrated. The sections were submerged into ethylene diamine tetraacetic acid antigenic retrieval buffer and microwaved for antigenic retrieval, after which they were treated with 3% hydrogen peroxide in methanol to block endogenous peroxidase activity, followed by incubation with 1% bovine serum albumin to block nonspecific binding. Sections were incubated with rabbit anti-Popdc3 polyclonal antibody (ProteinTech Group, Chicago IL) overnight at 4 °C. Normal goat serum was used as a negative control. After rinsing twice for 5 min with Tris buffered saline tween-20, tissue sections were treated with secondary antibody in Tris buffered saline solution for 1 h at room temperature, developed with chromogen at room temperature, and observed under microscope. After that, all tissue sections were counterstained with hematoxylin, dehydrated and mounted. The cytoplasm with strong Popdc3 expression was stained as buffy, whereas weak expression was associated with cell membranes.
Assessment of Popdc3 staining in the tissue sections
The degree of immunostaining was reviewed and scored independently by at least two observers based on the proportion of positively stained tumor cells and intensity of staining[21,22]. Tumor cell proportion was scored as follows: 0 (≤ 5% positive tumor cells), 1 (6%-25% positive tumor cells), 2 (26%-50% positive tumor cells), and 3 (> 51% positive tumor cells). Staining intensity was graded according to the following criteria: 0 (no staining), 1 (weak staining, light yellow), 2 (moderate staining, yellow brown), and 3 (strong staining, brown). Staining index was calculated as the staining intensity score and the proportion of positive tumor cells. Using this method of assessment, we evaluated Popdc3 expression in benign gastric epithelia and malignant lesions by determining the staining index with scores of 0, 1, 2, 3, 4, 6, or 9. The cutoff value for high and low expression levels was chosen based on the heterogeneity measured using the log-rank test with respect to overall survival. An optimal cutoff value was identified as follows: a staining index score of ≥ 4 was used to define tumors with high Popdc3 expression, and a staining index score of ≤ 3 was used to indicate low expression.
Statistical analysis
All statistical analyses were performed using the SPSS 17.0 software. Correlation of Popdc3 expression with immunohistochemistry and clinicopathologic parameters was evaluated by χ2 test or Fisher’s exact probability test. Overall survival rate was calculated by the Kaplan-Meier method and the difference in survival curves was analyzed by the log-rank test. The follow-up time was calculated from the date of surgery to the date of death, or the last known follow-up. Independent prognostic factors were analyzed by the Cox proportional hazards regression model. P < 0.05 was considered statistically significant.
RESULTS
Expression of Popdc3 in gastric cancer and noncancerous mucosa
In our current study, immunohistochemical analysis was done to examine the Popdc3 expression in 306 gastric cancer lesions and 84 noncancerous tissues. Popdc3 was detected in 72 (85.71%) cases of 84 human nontumor mucosa. High expression of Popdc3 protein was detected in 78 (25.49%) cases of 306 human gastric cancer, and low expression was detected in 228 (74.51%). Popdc3 staining was detected mainly in the majority of normal cells, especially in chief cells and smooth muscle. Besides, we also found Popdc3 expression in intestinal metaplasia. However, Popdc3 was mainly localized in the cytoplasm of tumor cells and its weak staining in cell membranes. The differences of Popdc3 expression between gastric cancer and noncancerous mucosa were also statistically significant (χ2 = 1.010E2, P < 0.0001, Figure 1).
Figure 1.
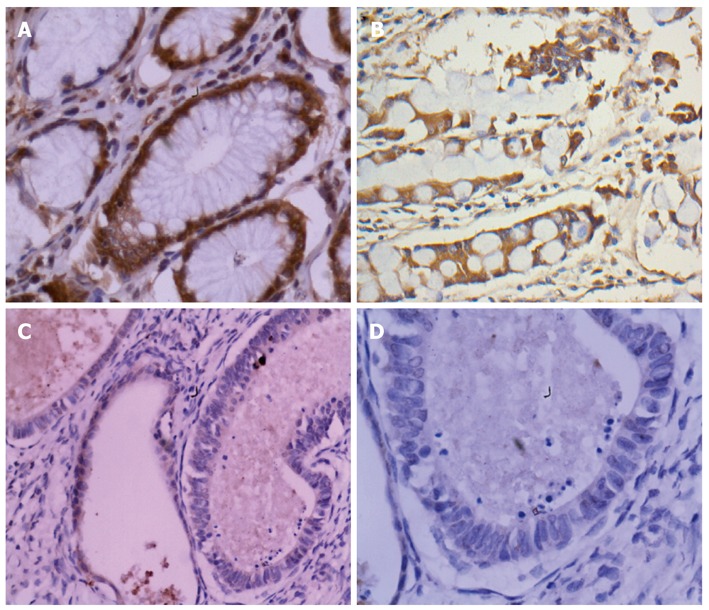
Immunohistochemical staining for Popeye domain containing 3 in gastric cancer lesions and noncancerous tissues. A: Popeye domain containing 3 (Popdc3) was highly expressed in noncancerous tissues, × 400; B: Popdc3 was highly expressed in intestinal metaplasia cells, × 400; C, D: Popdc3 was lowly expressed in tubular adenocarcinoma, × 200 and × 400, respectively.
Reduced Popdc3 expression and clinicopathologic features
Reduced expression of Popdc3 correlated with depth of invasion, regional lymph node and distant metastasis, and TNM stages (P < 0.05). Popdc3 expression did not correlate with age, gender, location of tumor, size of tumor, histologic type and histologic differentiation (P > 0.05, Table 1). The factors for possible prognostic effects in gastric cancer were analyzed by Cox regression analysis. The multivariate analysis suggested that the patient’s gender (P = 0.016), regional lymph node metastasis (P < 0.0001), distant metastasis (P < 0.0001), TNM stages (P = 0.002), and the expression of Popdc3 (P < 0.0001) were independent prognostic factors in patients with gastric carcinoma. However, patient’s age, tumor location, size of tumor, histologic type, histologic differentiation and depth of invasion had no prognostic value (Table 2).
Table 1.
Patient characteristics and Popeye domain containing 3 expression in gastric cancer n (%)
| Clinical parameters |
Popdc3 |
||||
| n | Low | High | χ2 | Pvalue | |
| Age (yr) | |||||
| < 50 | 105 | 72 (68.6) | 33 (31.4) | ||
| ≥ 50 | 201 | 156 (77.6) | 45 (22.4) | 2.968 | 0.085 |
| Gender | |||||
| Male | 199 | 148 (74.4) | 51 (25.6) | ||
| Female | 107 | 80 (74.8) | 27 (25.2) | 0.006 | 0.940 |
| Size (cm) | |||||
| < 5 | 154 | 120 (77.9) | 34 (22.1) | ||
| ≥ 5 | 152 | 108 (71.1) | 44 (28.9) | 1.901 | 0.168 |
| Histology | |||||
| Papillary adenocarcinoma | 8 | 5 (62.5) | 3 (37.5) | ||
| Tubular adenocarcinoma | 209 | 151 (72.2) | 58 (27.8) | ||
| Mucinous adenocarcinoma | 52 | 42 (80.8) | 10 (19.2) | ||
| Signet ring cell carcinoma | 37 | 30 (81.1) | 7 (18.9) | 3.084 | 0.379 |
| Histologic differentiation | |||||
| Well | 17 | 13 (76.5) | 4 (23.5) | ||
| Moderate | 100 | 67 (67) | 33 (33) | ||
| Poor | 177 | 140 (79.1) | 37 (20.9) | ||
| Undifferentiated | 12 | 8 (66.7) | 4 (33.3) | 4.439 | 0.109 |
| Invasion depth | |||||
| T1 | 24 | 10 (41.7) | 14 (58.3) | ||
| T2 | 63 | 32 (50.8) | 31 (49.2) | ||
| T3 | 181 | 149 (82.3) | 32 (17.7) | ||
| T4a | 28 | 24 (85.7) | 4 (14.3) | ||
| T4b | 10 | 7 (70.0) | 3 (30.0) | 48.566 | < 0.0001 |
| Regional lymph nodes | |||||
| N0 | 163 | 94 (57.7) | 69 (42.3) | ||
| N1 | 41 | 39 (95.1) | 2 (4.9) | ||
| N2 | 54 | 49 (90.7) | 5 (9.3) | ||
| N3a | 20 | 14 (70.0) | 6 (30.0) | ||
| N3b | 27 | 19 (70.4) | 8 (29.6) | 52.504 | < 0.0001 |
| Distant metastasis | |||||
| M0 | 274 | 197 (71.9) | 77 (28.1) | ||
| M1 | 32 | 31 (96.9) | 1 (3.1) | 9.412 | 0.002 |
| TNM stages | |||||
| I | 73 | 30 (41.1) | 43 (58.9) | ||
| II | 109 | 81 (74.3) | 28 (25.7) | ||
| III | 92 | 86 (93.5) | 6 (6.5) | ||
| IV | 32 | 31 (96.9) | 1 (3.1) | 70.624 | < 0.0001 |
TNM: Tumor, nodes, metastasis; Popdc3: Popeye domain containing 3.
Table 2.
Multivariate analysis for disease-related deaths (Cox regression model)
| Variables | Pvalue | Hazard ratio | 95% CI |
| Popdc3 | < 0.0001 | 0.193 | 0.133-0.282 |
| Gender | 0.016 | 1.388 | 1.063-1.814 |
| Regional lymph nodes | < 0.0001 | 1.344 | 1.156-1.564 |
| Distant metastasis | < 0.0001 | 11.591 | 6.131-21.915 |
| TNM stages | 0.002 | 1.486 | 1.159-1.905 |
CI: Confidence interval; TNM: Tumor, nodes, metastasis; Popdc3: Popeye domain containing 3.
Correlation between Popdc3 expression and patient prognosis
In stages I and II, the 3-year survival rate of patients with a low expression of Popdc3 was significantly lower than in patients with high Popdc3 expression. The survival estimates showed a dramatic difference in median survival between the high and low Popdc3 expression: the former averaged 55 mo [95% confidence interval (CI): 53.515-56.485], whereas the latter 23 mo (95% CI: 21.201-24.799). For patients with low Popdc3 protein expression, 1- and 3-year survival rates were 76.75% and 16.7%, respectively, which were significantly lower than in patients with high Popdc3 expression (88.46%, 84.6%, respectively, χ2 = 145.095, P < 0.0001). Based on this result, we suggested that diminished expression of Popdc3 is a prognostic indicator of poor survival for patients with gastric cancer. In addition, we further compared the survival between the patients in early TNM stage (stages I and II) or late stages (stages III and IV) with different Popdc3 expression. The results showed that low Popdc3 expression had a much more significant effect on the survival of the patients with early stage tumors (χ2 = 104.741, P < 0.0001), with a > 51.9% reduction in the 3-year survival compared with that of patients with high Popdc3 expression. In late stages, the difference was also significant (χ2 = 5.930, P = 0.015), with a 32.6% reduction in the 3-year survival. Therefore, these data suggested that Popdc3 expression was as an independent prognostic variable for gastric cancer in early stage and late stage (Figure 2 and Table 3).
Figure 2.
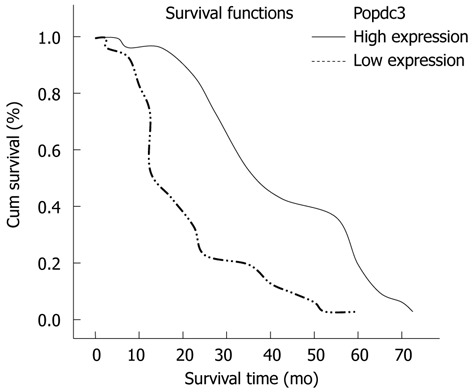
Kaplan-Meier curves with univariate analyses (log-rank) for patients with low Popeye domain containing 3 expression vs high Popeye domain containing 3 expression tumors in all gastric cancer. The cumulative and 3-year survival rates were 88.46% and 84.6%, respectively in the high Popeye domain containing 3 (Popdc3) protein expression group, but was only 76.75% and 16.7% in the low expression group (χ2 = 145.095, P < 0.0001).
Table 3.
Survival estimates and 95% confidence intervals for disease-related deaths
| Groups | Median survival (mo) | 1-yr survival rate (%) | 3-yr survival rate (%) | χ2 | Pvalue |
| Low Popdc3 expression | 23 (21.20-24.79) | 175 (76.75) | 38 (16.7) | ||
| High Popdc3 expression | 55 (53.51-56.48) | 69 (88.46) | 66 (84.6) | 145.095 | < 0.0001 |
| Stage I-II with low Popdc3 expression | 29 (21.00-38.00) | 97 (87.39) | 44 (39.64) | ||
| Stage I-II with high Popdc3 expression | 55 (45.00-61.00) | 65 (91.55) | 65 (91.55) | 104.741 | < 0.0001 |
| Stage III-IV with low Popdc3 expression | 15 (10.00-21.50) | 78 (66.67) | 12 (10.26) | ||
| Stage III-IV with high Popdc3 expression | 30 (12.00-44.00) | 4 (57.14) | 3 (42.86) | 5.93 | 0.015 |
Popdc3: Popeye domain containing 3.
DISCUSSION
The cause of cancer remains elusive and may be multifactorial[23,24]. Work by many researchers has illuminated that epigenetic alterations, like mutations and chromosomal abnormalities, can be causally involved in carcinogenesis[25,26]. Previous publications discovered that aberrant DNA methylation was more frequently present in cancers than mutations[27,28].
As a prototypical member of the Popdc family, Bves is mostly studied over the past several decades. Many reports have shown that Bves is highly conserved and has been identified in a wide variety of vertebrates and invertebrates[29,30]. Both mRNA and protein of Bves are highly expressed in striated and smooth muscles and in various forms of epithelial cell types in the embryo and adult[31,32]. It seems that Bves plays a critical role in cell-cell adhesion and in maintaining epithelial integrity, suggesting that loss of Bves function could result in abnormal cell behavior and disease[33-35]. In 2008, Feng et al[36] used MethyLight assays to analyze DNA methylation status of 27 genes on 49 paired cancerous and noncancerous tissue samples from non-small cell lung cancer (NSCLC) patients and found that Bves were methylated significantly more frequently in tumor tissues than in noncancerous tissues. Methylation of Bves was present in 80% of NSCLC tissues but only in 14% of noncancerous tissues. It is, so far, the first report of a modification of Bves in cancer.
In 2010, Kim et al[16] conducted the first detailed research on the Popdc family and discovered that frequent silencing of Bves and Popdc3 was associated with promoter hypermethylation in gastric cancer. Expression of Bves and Popdc3 was downregulated in 73% of the gastric cancer cell lines and in 69% (Bves) and 87% (Popdc3) of the gastric cancer tissues. Bves and Popdc3 were hypermethylated in 69% (Bves) and 64% (Popdc3) of the gastric cancer tissues. They also found that combined treatment with a DNA methylation inhibitor and a histone deacetylase inhibitor strongly induced Bves and Popdc3 expression. These observations suggested that frequent methylation and inactivation of Bves and Popdc3 in early-stage gastric cancer might predispose cells to other critical changes that cause cancer metastasis.
These results are similar to our study. However, the clinical impact of Popdc3 expression or the prognostic value for gastric cancer was not completely clarified because the number of gastric cancer patients was too small. Actually, it is the first study to explore the correlation between Popdc3 expression and clinical and prognostic factors in gastric cancer. Our study demonstrated that Popdc3 was frequently downregulated in gastric cancer tissues in comparison with those in normal gastric tissues. We examined the relationship between Popdc3 expression and clinicopathological factors in gastric cancer. As a result, reduced level of Popdc3 protein expression in gastric cancer lesions was found mainly associated with depth of invasion, regional lymph node and distant metastasis, and TNM stages. Bves is required for maintenance of E-cadherin in the membrane and plays an important role in cell adhesion and in maintaining epithelial integrity[32]. In development of tumor tissues or diseases, downregulation or mislocalization of E-cadherin is associated with epithelial-mesenchymal transition (EMT)[37]. EMT is considered to be essential for proper development and underlies embryonic processes such as chick gastrulation and coronary vasculature formation[38]. When spontaneously or aberrantly induced in the adult, however, EMT as a hallmark of cancer, may result in loss of epithelial organization and cell tumor tissues invasion of previously normal tissues[39]. Therefore, interfering with E-cadherin function, loss of Bves could result in abnormal cell behavior and disease by promoting EMT programs[40]. Bves was 28% identical to Popdc3 among Popeye family members[12], indicating that Popdc3 may play a role in tumor suppression and interact with Bves.
In this study, multivariate analysis revealed that patient’s gender, regional lymph node and distant metastasis, TNM stages, and the expression of Popdc3 were independent prognostic factors for the disease. Although abnormal expression of Popdc3 in gastric cancer might play an important role in the process of tumorigenesis, its biochemical mechanism and potential impact on patient’s survival is unknown. So we further analyzed and assessed the impact of expression of Popdc3 on patient’s survival. The result indicated that low levels of Popdc3 protein were closely correlated with the prognosis of gastric cancer. A survival curve plotted by the Kaplan-Meier method showed that in patients with low Popdc3 protein expression, the 1- and 3-year survival rates were 76.75% and 16.7%, respectively, which were significantly lower than in patients with high Popdc3 expression (88.46% and 84.6%, respectively). Besides, we further compared the survival between the patients with Popdc3 expression in early TNM stage (stages I and II) or late stage (stages III and IV). We found that low Popdc3 expression had a much more significant effect on the survival of those patients with early stage tumors, with a > 51.9% reduction in the 3-year survival as compared with high Popdc3 expression. In late stages, the difference was also significant, with a 32.6% reduction in 3-year survival. These results suggested Popdc3 expression is an independent prognostic variable for gastric cancer in early stage and late stage. In this regard, routine detection of methylation of Popdc3 in blood might be useful in monitoring and detecting tumor recurrence in early-stage gastric cancer after curative surgical resection.
In conclusion, our study suggests that degradation of Popdc3 is a common feature in gastric cancer that might play an important role in the progression and metastases of gastric cancer. In addition, the potentially important consequence of our work is that Popdc3 may be an attractive therapeutic candidate for gastric cancer. Thus, we believe that more researches on Popdc3 will further provide a basis for the development of potential biomarkers for the diagnosis and prognosis of gastric cancer.
COMMENTS
Background
Although many molecular and biological studies have shown risk factors for gastric cancer, the exact molecular mechanism of gastric cancer has not been clarified completely. There is an urgent need to find special markers closely related to tumor outcome and therapy.
Research frontiers
The Popeye domain containing (Popdc) gene family consists of Bves/Popdc1, Popdc2 and Popdc3. As a prototypical member of the Popdc family, Bves is mostly studied over the past decades. However, the clinical impact of Popdc3 expression on cancers is still unknown. This study was carried out to investigate the alterations in the expression of Popdc3 in surgical specimens of gastric cancer, to explore the possible correlation between Popdc3 expression and clinicopathologic variables, to correlate expression of Popdc3 with lymph node metastasis and distant metastasis.
Innovations and breakthroughs
This is the first study attempting to elucidate the role of Popdc3 in gastric cancer and its prognostic significance. The findings suggest that Popdc3 is downregulated in gastric cancer tissues in comparison with those in normal gastric tissues. This study further demonstrates that degradation of Popdc3 is associated with gastric cancer progression and survival of the patients.
Applications
The results will open an avenue for further research to evaluate the role of Popdc3 in gastric cancer. Popdc3 expression may represent a potential prognostic marker and therapeutic target for gastric cancer.
Terminology
Popdc3, as a member of the Popdc family, was firstly detected in developing and adult striated muscle in vertebrates. Chromosomal mapping indicates that Popdc3 gene is clustered on mouse chromosome 10. Frequent methylation and inactivation of Popdc3 are observed in early-stage gastric cancer might predispose cells to other critical changes that cause cancer metastasis.
Peer review
This is a very well written article about the clinical significance of Popdc3 protein expression observed by IHC in gastric cancer. The message of this article was very clear but the author only did IHC to see the expression of Popdc3. This weakened the strength of this article.
Footnotes
Supported by Health Technology Fund of Yunnan Province, China, No. 2010NS066
Peer reviewer: Sang Kil Lee, MD, Assistant Professor, Department of Gastroenterology, Yonsei University College of Medicine, No. 134 Shinchon-dong, Seodaemun-gu, Seoul 120-752, South Korea
S- Editor Shi ZF L- Editor Ma JY E- Editor Zhang DN
References
- 1.Ajani JA, Barthel JS, Bekaii-Saab T, Bentrem DJ, D’Amico TA, Das P, Denlinger C, Fuchs CS, Gerdes H, Hayman JA, et al. Gastric cancer. J Natl Compr Canc Netw. 2010;8:378–409. doi: 10.6004/jnccn.2010.0030. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Ye YW, Dong RZ, Zhou Y, Du CY, Wang CM, Fu H, Shi YQ. Prognostic analysis of familial gastric cancer in Chinese population. J Surg Oncol. 2011;104:76–82. doi: 10.1002/jso.21896. [DOI] [PubMed] [Google Scholar]
- 4.Chen JG, Zhu J, Zhang YH, Lu JH. Cancer survival in Qidong, China, 1992-2000. IARC Sci Publ. 2011;(162):43–53. [PubMed] [Google Scholar]
- 5.Law SC, Mang OW. Cancer survival in Hong Kong SAR, China, 1996-2001. IARC Sci Publ. 2011;(162):33–41. [PubMed] [Google Scholar]
- 6.Xishan H, Chen K, Min H, Shufen D, Jifang W. Cancer survival in Tianjin, China, 1991-1999. IARC Sci Publ. 2011;(162):69–84. [PubMed] [Google Scholar]
- 7.Xiang YB, Jin F, Gao YT. Cancer survival in Shanghai, China, 1992-1995. IARC Sci Publ. 2011;(162):55–68. [PubMed] [Google Scholar]
- 8.Thun M, Jemal A, Desantis C, Blackard B, Ward E. An overview of the cancer burden for primary care physicians. Prim Care. 2009;36:439–454. doi: 10.1016/j.pop.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Yu JC, Kang WM, Ma ZQ. Treatment strategy for early gastric cancer. Surg Oncol. 2011:Epub ahead of print. doi: 10.1016/j.suronc.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Hager HA, Bader DM. Bves: ten years after. Histol Histopathol. 2009;24:777–787. doi: 10.14670/hh-24.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reese DE, Zavaljevski M, Streiff NL, Bader D. bves: A novel gene expressed during coronary blood vessel development. Dev Biol. 1999;209:159–171. doi: 10.1006/dbio.1999.9246. [DOI] [PubMed] [Google Scholar]
- 12.Andrée B, Hillemann T, Kessler-Icekson G, Schmitt-John T, Jockusch H, Arnold HH, Brand T. Isolation and characterization of the novel popeye gene family expressed in skeletal muscle and heart. Dev Biol. 2000;223:371–382. doi: 10.1006/dbio.2000.9751. [DOI] [PubMed] [Google Scholar]
- 13.Wada AM, Reese DE, Bader DM. Bves: prototype of a new class of cell adhesion molecules expressed during coronary artery development. Development. 2001;128:2085–2093. doi: 10.1242/dev.128.11.2085. [DOI] [PubMed] [Google Scholar]
- 14.Breher SS, Mavridou E, Brenneis C, Froese A, Arnold HH, Brand T. Popeye domain containing gene 2 (Popdc2) is a myocyte-specific differentiation marker during chick heart development. Dev Dyn. 2004;229:695–702. doi: 10.1002/dvdy.20015. [DOI] [PubMed] [Google Scholar]
- 15.Vasavada TK, DiAngelo JR, Duncan MK. Developmental expression of Pop1/Bves. J Histochem Cytochem. 2004;52:371–377. doi: 10.1177/002215540405200308. [DOI] [PubMed] [Google Scholar]
- 16.Kim M, Jang HR, Haam K, Kang TW, Kim JH, Kim SY, Noh SM, Song KS, Cho JS, Jeong HY, et al. Frequent silencing of popeye domain-containing genes, BVES and POPDC3, is associated with promoter hypermethylation in gastric cancer. Carcinogenesis. 2010;31:1685–1693. doi: 10.1093/carcin/bgq144. [DOI] [PubMed] [Google Scholar]
- 17.Santiago JM, Sasako M, Osorio J. [TNM-7th edition 2009 (UICC/AJCC) and Japanese Classification 2010 in Gastric Cancer. Towards simplicity and standardisation in the management of gastric cancer] Cir Esp. 2011;89:275–281. doi: 10.1016/j.ciresp.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 19.Zeng Q, Zhao Y, Yang Y, Chen XX, Wang G, Zhang P, Cui Y, Su S, Li K. Expression of Cystatin C in human stomach neoplasms. Mol Med Report. 2010;3:607–611. doi: 10.3892/mmr_00000304. [DOI] [PubMed] [Google Scholar]
- 20.Ishigami S, Ueno S, Nishizono Y, Matsumoto M, Kurahara H, Arigami T, Uchikado Y, Setoyama T, Arima H, Yoshiaki K, et al. Prognostic impact of CD168 expression in gastric cancer. BMC Cancer. 2011;11:106. doi: 10.1186/1471-2407-11-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, Ochiai A, Rüschoff J, Henkel T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 22.Geller SA, Dhall D, Alsabeh R. Application of immunohistochemistry to liver and gastrointestinal neoplasms: liver, stomach, colon, and pancreas. Arch Pathol Lab Med. 2008;132:490–499. doi: 10.5858/2008-132-490-AOITLA. [DOI] [PubMed] [Google Scholar]
- 23.Nobili S, Bruno L, Landini I, Napoli C, Bechi P, Tonelli F, Rubio CA, Mini E, Nesi G. Genomic and genetic alterations influence the progression of gastric cancer. World J Gastroenterol. 2011;17:290–299. doi: 10.3748/wjg.v17.i3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandey R, Misra V, Misra SP, Dwivedi M, Kumar A, Tiwari BK. Helicobacter pylori and gastric cancer. Asian Pac J Cancer Prev. 2010;11:583–588. [PubMed] [Google Scholar]
- 25.Hatziapostolou M, Iliopoulos D. Epigenetic aberrations during oncogenesis. Cell Mol Life Sci. 2011;68:1681–1702. doi: 10.1007/s00018-010-0624-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodríguez-Paredes M, Esteller M. Cancer epigenetics reaches mainstream oncology. Nat Med. 2011;17:330–339. doi: 10.1038/nm.2305. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe Y, Maekawa M. Methylation of DNA in cancer. Adv Clin Chem. 2010;52:145–167. doi: 10.1016/s0065-2423(10)52006-7. [DOI] [PubMed] [Google Scholar]
- 28.Corvalan AH, Maturana MJ. Recent patents of DNA methylation biomarkers in gastrointestinal oncology. Recent Pat DNA Gene Seq. 2010;4:202–209. doi: 10.2174/187221510794751695. [DOI] [PubMed] [Google Scholar]
- 29.Knight RF, Bader DM, Backstrom JR. Membrane topology of Bves/Pop1A, a cell adhesion molecule that displays dynamic changes in cellular distribution during development. J Biol Chem. 2003;278:32872–32879. doi: 10.1074/jbc.M301961200. [DOI] [PubMed] [Google Scholar]
- 30.Ripley AN, Osler ME, Wright CV, Bader D. Xbves is a regulator of epithelial movement during early Xenopus laevis development. Proc Natl Acad Sci USA. 2006;103:614–619. doi: 10.1073/pnas.0506095103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ripley AN, Chang MS, Bader DM. Bves is expressed in the epithelial components of the retina, lens, and cornea. Invest Ophthalmol Vis Sci. 2004;45:2475–2483. doi: 10.1167/iovs.04-0013. [DOI] [PubMed] [Google Scholar]
- 32.Osler ME, Chang MS, Bader DM. Bves modulates epithelial integrity through an interaction at the tight junction. J Cell Sci. 2005;118:4667–4678. doi: 10.1242/jcs.02588. [DOI] [PubMed] [Google Scholar]
- 33.Hager HA, Roberts RJ, Cross EE, Proux-Gillardeaux V, Bader DM. Identification of a novel Bves function: regulation of vesicular transport. EMBO J. 2010;29:532–545. doi: 10.1038/emboj.2009.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gingold-Belfer R, Bergman M, Alcalay Y, Schlesinger H, Aravot D, Berman M, Salman H, Brand T, Kessler-Icekson G. Popeye domain-containing 1 is down-regulated in failing human hearts. Int J Mol Med. 2011;27:25–31. doi: 10.3892/ijmm.2010.558. [DOI] [PubMed] [Google Scholar]
- 35.Jayagopal A, Yang JL, Haselton FR, Chang MS. Tight junction-associated signaling pathways modulate cell proliferation in uveal melanoma. Invest Ophthalmol Vis Sci. 2011;52:588–593. doi: 10.1167/iovs.10-5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng Q, Hawes SE, Stern JE, Wiens L, Lu H, Dong ZM, Jordan CD, Kiviat NB, Vesselle H. DNA methylation in tumor and matched normal tissues from non-small cell lung cancer patients. Cancer Epidemiol Biomarkers Prev. 2008;17:645–654. doi: 10.1158/1055-9965.EPI-07-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wells A, Yates C, Shepard CR. E-cadherin as an indicator of mesenchymal to epithelial reverting transitions during the metastatic seeding of disseminated carcinomas. Clin Exp Metastasis. 2008;25:621–628. doi: 10.1007/s10585-008-9167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 39.Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15:117–134. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams CS, Zhang B, Smith JJ, Jayagopal A, Barrett CW, Pino C, Russ P, Presley SH, Peng D, Rosenblatt DO, et al. BVES regulates EMT in human corneal and colon cancer cells and is silenced via promoter methylation in human colorectal carcinoma. J Clin Invest. 2011;121:4056–4069. doi: 10.1172/JCI44228. [DOI] [PMC free article] [PubMed] [Google Scholar]


