Abstract
The vaccinia virus A56 protein was one of the earliest-described poxvirus proteins with an identifiable activity. While originally characterized as a haemagglutinin protein, A56 has other functions as well. The A56 protein is capable of binding two viral proteins, a serine protease inhibitor (K2) and the vaccinia virus complement control protein (VCP), and anchoring them to the surface of infected cells. This is important; while both proteins have biologically relevant functions at the cell surface, neither one can locate there on its own. The A56–K2 complex reduces the amount of virus superinfecting an infected cell and also prevents the formation of syncytia by infected cells; the A56–VCP complex can protect infected cells from complement attack. Deletion of the A56R gene results in varying effects on vaccinia virus virulence. In addition, since the gene encoding the A56 protein is non-essential, it can be used as an insertion point for foreign genes and has been deleted in some viruses that are in clinical development as oncolytic agents.
Introduction – the A56 protein
Orthopoxviruses are some of the most complex viruses infecting humans and include variola virus, the causative agent of smallpox, and vaccinia virus (VACV), which is used as a live vaccine. Although smallpox has been eradicated, VACV is still studied as a model organism to understand basic aspects of pox virology, as a vaccine vector for immunizations against other infectious agents and for oncolytic cancer therapy. It is also studied because human infections with zoonotic poxviruses like monkeypox virus still occur. While VACV is a large virus, containing a genome of nearly 200 kbp that encodes more than 200 proteins, the genome is still small compared with that of the host cell. For this reason, VACV encodes a number of multi-functional proteins, one of which is A56. The A56 protein is able to bind two other viral proteins, a serine protease inhibitor (K2) and the vaccinia virus complement-control protein (VCP), and express them at the surface of the infected cell. The A56–K2 complex binds to the entry–fusion machinery of VACV; this reduces superinfection and prevents cell–cell fusion of infected cells. The A56–VCP complex protects infected cells from complement attack and contributes to viral virulence. These complexes were only discovered recently, but the history of A56 protein extends much farther back in time.
The A56 protein was one of the first VACV genes to be identified and studied (Nagler, 1942). This was because what is now called the A56 protein had haemagglutination activity; it was called VACV haemagglutinin (HA) from the 1940s until near the end of the 20th century. The presence of a VACV protein with HA activity was believed to be important because several other viruses such as influenza, measles and mumps viruses contained proteins with HA activity. However, unlike these other viral envelope proteins, which were important for viral entry, a biologically relevant function could not ultimately be ascribed to the VACV HA activity. Nevertheless, the HA activity of poxviruses allowed the classification of poxviruses into those that had HA activity and those that did not. Members of the orthopoxvirus genus were the only ones that had HA activity (Fenner et al., 1988). Furthermore, haemabsorption and HA-inhibition assays were early methods used to differentiate between various orthopoxviruses and variola virus isolates from various parts of the world (Fenner et al., 1988). Later, when the protein (now called ‘A56’) and the gene (designated ‘A56R’ but also designated VACWR181 in VACV strain WR) responsible for this activity were first identified (Ichihashi, 1977; Shida, 1986a), the A56R ORF was used to build phylogenetic trees that showed the genetic relationships between the members of the genus Orthopoxvirus (Hutin et al., 2001).
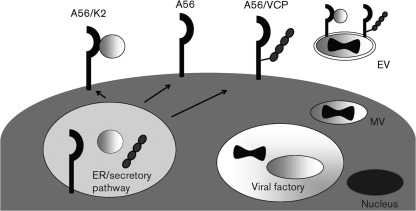
The ability to track the protein by its haemagglutinating activity also provided a means to show that the protein is found on the cell membrane of the infected cell (Blackman & Bubel, 1972), but not on mature virus (MV), the most abundant form of virus made during an infection (Fig. 1). While the protein is not found on MV, it is present on another infectious form of virus called the extracellular virus (EV) (Payne & Norrby, 1976). The EV is critical for the efficient spread of the virus in vitro and in vivo. However, unlike the other glycoproteins found on EV, the A56 protein has considerably more sequence variability between different orthopoxviruses. This may be because, unlike the other EV glycoproteins, A56 is not essential for formation of the EV. As mentioned earlier, this sequence variability, initially recognized in HA-inhibition assays, made A56R a useful ORF for building phylogenetic trees.
Fig. 1.
Diagram of the location of the A56 protein in a VACV-infected cell. A56, K2 and VCP all have signal sequences that result in their being trafficked through the endoplasmic reticulum (ER). Presumably, initial protein–protein interactions take place here, followed by movement through the Golgi apparatus and to the plasma membrane. As a transmembrane protein, A56 is found on the surface of the infected cell, where it is present as a monomer, as well as in complex with the K2 protein and with VCP. Since A56 is also one of the glycoproteins found on the extracellular virus (EV), the A56–K2 complex (and probably the A56–VCP complex) is also expressed on the EV outer membrane. EV is formed by a small portion of MV interacting with the Golgi apparatus or endosomal membranes, where the MV picks up an additional membrane that contains an additional set of membrane proteins, including A56.
Based on the A56 protein sequence, the calculated molecular mass of the protein is ~35 kDa, but the protein contains putative sites for both N- and O-glycosylation and runs at an apparent molecular mass of 85 kDa when measured by SDS-PAGE (Brown et al., 1991; Payne, 1992; Shida & Dales, 1981). Thus, A56 is heavily glycosylated and this glycosylation is linked to its HA activity. The positions of the five predicted N-linked glycosylation sites are shown in Fig. 2. Blocking N-linked glycosylation with tunicamycin results in an apparent molecular mass of 62 kDa and this moiety retains its HA activity. In the presence of tunicamycin, A56 is still able to incorporate labelled glucosamine, confirming that the protein also undergoes O-linked glycosylation. Because HA activity is lost when the protein is completely deglycosylated, this implies that O-linked glycans are involved in the HA activity (Shida & Dales, 1981). A prior review on A56 by Hisatoshi Shida (Shida, 1989) pointed out that A56 was the first viral protein shown to be O-glycosylated, and that this protein provided additional evidence that O-linked glycosylation occurs as a protein transits through the Golgi apparatus (Shida & Dales, 1981; Spiro, 1966). There are predicted to be as many as 23 O-linked glycosylation sites, which all fall between residues 149 and 255 (with 20 falling between residues 175 and 230). The heavy glycosylation of the A56 protein may be the reason why anti-A56 antibodies cannot neutralize EV or protect from infection (Galmiche et al., 1999; Pulford et al., 2004). Interestingly, completely unglycosylated A56 protein migrates with an apparent molecular mass of 58 kDa; this is different from the 35 kDa calculated from the sequence of the ORF (Shida, 1986b). Shida pointed out that this discrepancy is not because of other post-translational modifications, because in vitro translation of the A56 ORF resulted in a protein that migrated at ~60 kDa (Shida, 1989). Thus, this is a clear example of a difference between predicted and observed molecular masses, which are known to occur. Additionally, the A56R gene has two separate promoters for early and late gene expression. Interestingly, late expression also produces a smaller, 68 kDa form of the protein (Brown et al., 1991), although the biological significance of this smaller protein is not known. Both the 85 and 68 kDa forms of the protein can be seen by using Western blotting at late time points.
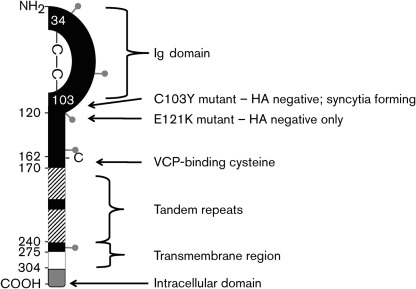
Fig. 2.
Schematic map of the domains of the A56 protein. After a cleaved signal sequence (aa 1–16), there is an Ig domain (aa 17–105), which includes a predicted intramolecular disulfide bridge (between cysteines 34 and 103), a stalk region (aa 121–275) containing tandem repeats (aa 170–240, striped boxes) that are highly modified by O-linked glycosylation, a transmembrane domain (aa 276–303, white box), and a short cytoplasmic tail (aa 304–315, grey box). The Ig domain is believed to be involved in binding the K2 protein. Cysteine 162 forms a disulfide bridge with the free N-terminal cysteine of VCP. Also shown are the approximate locations of the predicted N-linked glycosylation sites (grey lollipops) at aa 37, 69, 112, 161 and 254.
A schematic diagram of the A56 protein is shown in Fig. 2. The ~100 aa N-terminal region of A56 (after the signal sequence) has sequence similarities with the immunoglobulin (Ig) superfamily. This region of the protein is more highly conserved among orthopoxviruses than the rest of the A56 ectodomain (Aguado et al., 1992; Cavallaro & Esposito, 1992). In this Ig domain, it was hypothesized that the two cysteines in the ectodomain form an intramolecular disulfide bridge (Jin et al., 1989). Following on from this, modern computer modelling of this portion of the A56 protein supports formation of an Ig domain structure, shows the first two cysteines of A56 to be in close proximity and predicts that these cysteines form an intramolecular bond (Fig. 3). While not experimentally proven, but based on phenotypes of mutated viruses, the Ig domain may be involved in the observed cell–cell fusion regulatory activity. An analysis of VACV mutants produced by chemical mutagenesis (Shida & Matsumoto, 1983) revealed that the cell–cell fusion regulatory properties and haemabsorption properties of the A56 protein are separate (Seki et al., 1990). While A56R gene-knockout viruses cannot perform either function, one point mutant (Glu121 to Lys) was found to be HA negative but could still prevent syncytia formation. Since the exact mechanism of haemagglutination is not known, it is difficult to speculate as to why this mutation causes a loss of activity, especially when the O-linked glycosylation was linked to HA activity. Additionally, a virus containing a Cys103 to Tyr mutation did not have HA activity or the ability to prevent syncytia formation. As discussed earlier, this cysteine is predicted to form an intramolecular disulfide bond (Figs 2 and 3), so this mutation is likely to destabilize the entire domain, resulting in an abnormally folded protein that has been reported to make it to the cell surface, but which does not have HA activity or prevent syncytia formation (Seki et al., 1990). As discussed later, a third cysteine at position 162 (just before the first tandem repeat) has recently been shown to form a disulfide bridge with VCP (DeHaven et al., 2010). Between the Ig domain and the transmembrane domain there are two tandem-repeat motifs, which start near residue 170 and continue until residue 240 (Jin et al., 1989). These repeats, which are predicted to be heavily modified by O-glycosylation, do not share sequence similarity with other proteins. While the functions of the tandem repeats in the A56 protein are unknown, tandem repeats are often important in protein structure and function. The transmembrane domain, which anchors the protein in membranes, has been implicated as an important domain that results in A56 interacting with the F13 protein (also called VP37) (Oie et al., 1990). Since the F13 protein is a key protein in the formation of EV (Blasco & Moss, 1991), F13 may help direct the incorporation of A56 into the EV envelope. A short intracellular domain of ~12 aa that is present at the C terminus of the protein may be responsible for trafficking A56 protein out of the endoplasmic reticulum (ER) and into the Golgi apparatus (Shida & Matsumoto, 1983). Analysis of a panel of viruses with various HA and syncytia-inducing phenotypes revealed that an A56 protein lacking the cytoplasmic tail was transported from the ER to the Golgi apparatus more slowly but could still make it to the plasma membrane, while a protein with an aberrant cytoplasmic tail [owing to an upstream nucleotide insertion(s)] interfered with transport of the protein out of the ER.
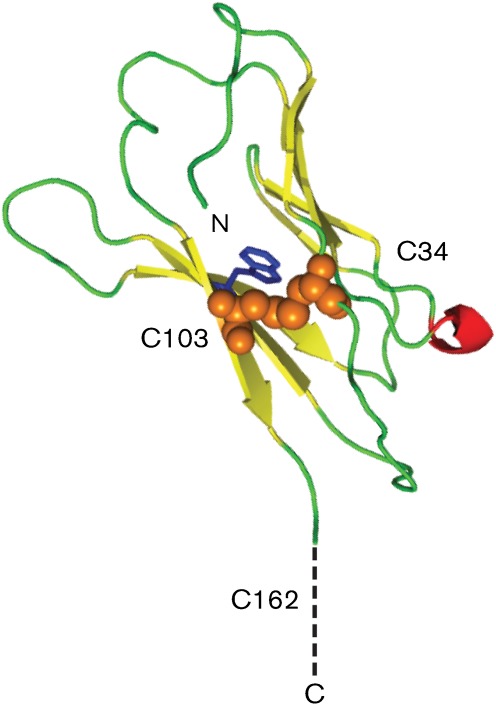
Fig. 3.
Predicted structure of the N terminal region of the A56 protein and homology modelling of the Ig domain of A56. Predicted beta sheets are coloured yellow and predicted alpha helices are coloured red; random coils are coloured green. The predicted disulfide bridge between cysteine residues 34 and 103 is indicated by orange spheres. A buried tryptophan residue at aa 29 that is conserved among IgG-like domains is coloured blue. Outside of the model of the Ig domain, the unpaired cysteine at aa 162 is shown. The IgG-like domain of A56 was used as a query sequence for five iterations of psi–blast (Altschul et al., 1997) searching of the National Center for Biotechnology Information (NCBI) RefSeq database (Pruitt et al., 2007). A large number of proteins with sequence homology were identified; the best scoring protein from this query for which an atomic structure has been determined is the T-cell-receptor alpha-chain IgG domain from CF34 [PDB 3FFC, chain D (Gras et al., 2009)], with an E score of 9.02×10−27 and 22 % sequence identity. The A56 sequence was threaded onto this three-dimensional structure using the homology modelling program modeller 9v8 (Eswar et al., 2006). The resulting model was then energy minimized by using the program cns version 1.3 (Brünger et al., 1998). The overall quality of this minimized model was evaluated using the molprobity server (Chen et al., 2010). It was found that 98.9 % of the residues resided in the allowable regions of a Ramachandran plot and had overall root-mean-square deviations for bonds and angles of 0.004 and 0.671, respectively. The model was rendered using the program pymol.
A56 protein inhibits spontaneous cell–cell fusion of VACV infected cells by forming a complex with K2
The ability to haemagglutinate was not the only early function ascribed to A56. Cells infected with certain strains of VACV can result in cell–cell fusion, and in 1971 this was directly linked to a lack of A56 protein (Ichihashi & Dales, 1971). Interestingly, the loss of another viral protein, K2, also causes infected cells to fuse. This was reported by three different groups in 1992 (Law & Smith, 1992; Turner & Moyer, 1992; Zhou et al., 1992). Recently, it has been shown that the A56 and K2 proteins form a complex on the surface of infected cells, and that this complex is responsible for preventing syncytia formation (Turner & Moyer, 2006). The K2 protein is also found on EV particles, as observed by electron microscopy and proteomics studies (Brum et al., 2003; Manes et al., 2008). K2, also known as serine protease inhibitor (SPI)-3, is one of a family of poxvirus proteins that share sequence similarities with serpin proteins (Boursnell et al., 1988). K2 protein (SPI-3) has in vitro protease-inhibition activity (Turner et al., 2000; Wang et al., 2000), but this activity is not related to its ability to prevent syncytia formation (Turner & Moyer, 1995). While regions of K2 that are important for its interaction with A56 have been identified (Turner & Moyer, 1995), the domain of A56 that interacts with K2 has not been identified. In a transient transfection system, A56 mutated at either Cys34 or Cys103 was able to be expressed on the cell surface (and bind VCP) (DeHaven et al., 2010) but could not bind K2 (B. C. DeHaven & S. N. Isaacs, unpublished), again implying that the Ig domain is involved in interacting with K2.
The mechanism by which the A56–K2 complex inhibits spontaneous fusion of infected cells was not clear until the discovery that the virus encoded a multi-subunit entry fusion complex (EFC), which was found on the MV membrane (Senkevich et al., 2005), interacted with the A56–K2 complex (Wagenaar & Moss, 2007). It was further shown that the A56–K2 complex interacts directly with the A16 and G9 proteins, two of the proteins that are part of the EFC (Wagenaar et al., 2008). This finding led to the hypothesis that the A56–K2 complex on infected cells could prevent reinfection of already infected cells (Moss, 2006). Support for this hypothesis was obtained experimentally when recombinant VACVs with deletions of one of the genes encoding the A56–K2 complex were used to infect cells, followed by superinfection with VACV carrying a luciferase reporter gene. Superinfection of cells that were infected by wild-type virus expressing the A56–K2 complex had significantly lower luciferase levels than cells infected with a virus expressing a mutated A56–K2 complex (Turner & Moyer, 2008). It has also been shown that transfecting the A56R and K2L genes into cells is sufficient to diminish both infection and virion induced cell–cell fusion (Wagenaar & Moss, 2009). Antibodies against either the K2 (Turner & Moyer, 2006, 2008) or A56 proteins (Wagenaar & Moss, 2009) increase the amount of superinfection and cell fusion seen. This may be because of the antibodies either blocking the A56–K2 complex from interacting with the MV EFC or perhaps by causing the displacement of K2 from A56, rendering the complex non-functional. Since syncytia formation of infected cells is mediated by EV, it appears that the A56–K2 complex is inhibiting syncytia formation by ‘inhibiting’ EV re-entry into an infected cell. This would occur first by the non-fusogenic dissolution of the EV outer envelope (Law et al., 2006) that exposes the EFC machinery on the MV, and then entry into an already infected cell is inhibited by engagement with A56–K2. Many other viruses have evolved mechanisms to prevent superinfection prior to entry (Berngruber et al., 2010). For example, the influenza neuraminidase protein cleaves the entry receptor as new virus is released, preventing superinfection (Huang et al., 2008), while retroviruses can downregulate entry receptors (Lindwasser et al., 2007; Wildum et al., 2006). Other viruses, such as vesicular stomatitis virus, prevent superinfection by slowing endocytosis of new virus (Simon et al., 1990). Thus, while other viruses prevent superinfection prior to entry, none uses a mechanism that actually interferes with their own entry proteins, as in the case of orthopoxviruses.
The A56–K2 complex on the surface of infected cells prevents superinfection by incoming MV particles. This should not be confused with the ability of another set of poxvirus proteins (A33 and A36) on infected cells that repel EV away from newly infected cells (Doceul et al., 2010). By repelling EV away from a newly infected cell, virus spread to distant uninfected cells is enhanced. Thus, in VACV a means has evolved to prevent or at least reduce superinfection by MV and EV prior to virus entry. Given the vital role that EV plays in the spread of virus in vitro and in vivo, we postulate that the repulsion of EV away from newly infected cells has a more important function than the A56–K2 complex preventing superinfection by MV. However, the A56–K2 complex may play a valuable role early in the initial spread of virus away from the very first cells infected.
The interaction of A56 and K2 has been shown to occur in VACV and cowpox virus (CPXV), and homologues of both proteins are present in all sequenced members of the genus Orthopoxvirus. There was a curious report of an ectromelia virus (ECTV) that caused syncytia formation despite the presence of the ECTV homologues of A56 and K2 (Erez et al., 2009). However, cell–cell fusion is a complicated process, and other factors may be at work. The phenotype described for this ECTV might be explained by the presence of mutations in other viral genes. Also of note is that Chang et al. have recently shown that MV surface proteins A25 and A26 are fusion suppressors (Chang et al., 2010). They found that mutated VACVs that contain deletions of these proteins cause spontaneous fusion at neutral pH. This may explain the syncytia-forming phenotype reported by Erez et al. (2009).
Cell surface expression of VCP through interactions with A56
K2 is not the only viral protein that directly interacts with the ectodomain of the A56 protein. Recently, a new interaction was discovered between A56 and VCP. VCP is a 35 kDa soluble protein that was previously characterized as being the major secreted protein from VACV-infected cells (Isaacs et al., 1992; Kotwal & Moss, 1988; Kotwal et al., 1990). VCP has the ability to inhibit several steps of the complement cascade (Bernet et al., 2004; Kotwal et al., 1990; Liszewski et al., 2006, 2009; McKenzie et al., 1992; Miller et al., 1997; Mullick et al., 2005; Rosengard et al., 1999, 2002; Sahu et al., 1998; Sfyroera et al., 2005). Research has shown that VCP-deletion viruses are mildly attenuated in vivo (DeHaven et al., 2010; Isaacs et al., 1992), possibly owing to an improved adaptive immune response in the absence of complement inhibition (Girgis et al., 2011). Besides being secreted, VCP was found to be expressed on the surface of infected cells (Girgis et al., 2008). Surface expression required the presence of the free N-terminal cysteine of VCP and the A56 protein (Girgis et al., 2008). Furthermore, it was shown recently that the N-terminal free cysteine of VCP forms a covalent bond with the unpaired cysteine of the ectodomain of the A56 protein (Cys162) (DeHaven et al., 2010). This results in expression of VCP on the surface of infected cells. It is not yet known whether the K2 protein and VCP can interact with the same A56 molecule. Based on the domains of A56 that K2 and VCP interact with (Figs 2 and 3), it is possible that a heterotrimer could form. However, we favour a model in which the A56 protein is expressed at early and late time points as a monomer and some A56 molecules interact with K2, while other molecules interact with VCP. We base this model on the temporal organization of VACV protein synthesis. The K2 protein is expressed from an early promoter (Turner & Moyer, 1995) while VCP is expressed from a late promoter (B. C. DeHaven & S. N. Isaacs, unpublished; Moulton et al., 2010). Thus, this would keep the K2 protein and VCP from competing for A56 molecules.
In contrast to the apparently unique function of the A56–K2 complex for preventing superinfection by interacting with the viral EFC, surface expression of a complement-control protein is seen in other viruses. Several herpesviruses and flaviviruses express complement regulators that are found on the surface of infected cells (Bernet et al., 2003; Chung et al., 2006; Friedman et al., 1984; Harris et al., 1990). This A56–VCP complex can protect cells from complement-mediated lysis of infected cells, and surface-bound VCP may be important for full virulence in vivo (DeHaven et al., 2010; Girgis et al., 2008). Proteomics data on MV and EV particles from VACV indicate that VCP is found on EV (Manes et al., 2008) (Fig. 1). While this has not been confirmed experimentally, it is intriguing to speculate that VCP trafficked to the EV envelope provides additional protection from the host complement attack.
The interaction between VCP and A56 does not seem to be limited to VACV. The variola virus homologue of VCP, called SPICE (for smallpox inhibitor of complement enzymes), can also bind to the VACV A56 protein when plasmids expressing both proteins are transfected into cells. Also, immunofluorescence staining of ECTV-infected cells shows surface expression of its complement-control protein (called EMICE). Interestingly, in transfection studies, EMICE does not interact with the VACV A56 protein, but is able to bind to the ECTV A56 protein (DeHaven et al., 2010). This suggests co-evolution of these proteins might have occurred. It is unclear which protein might be driving this co-evolution, but, given the greater differences between orthopoxvirus A56 proteins in the domain where the complement control protein binds, we hypothesize that A56 may be driving this co-evolution. It is unclear whether there is evidence of similar co-evolution between A56 and K2. Despite only 83 % identity between the CPXV and VACV A56 proteins, the CPXV K2 protein can interact with the VACV A56 protein and inhibit syncytia formation (Turner & Moyer, 1995). If the K2 protein interacts with the Ig domain of A56 this region has less sequence divergence (Aguado et al., 1992; Cavallaro & Esposito, 1992) and thus little co-evolution has been required.
A56 and VACV virulence
The A56 protein is not needed for viral replication in cell culture. However, the contributions of A56 to VACV virulence have not been fully elucidated and the data are inconsistent (see Table 1). While work with a plaque-purified virus (NYCBH strain) showed significant attenuation with an A56R gene-knockout virus in intracranial and intranasal challenge models (Lee et al., 1992), it was not compared with a gene-rescue virus and the knockout contained the β-galactosidase marker gene. Similarly, work with Western Reserve (WR)-strain-based VACV virus, in which the A56R gene was deleted, showed a small amount of attenuation when given by the intracranial route in young mice (Flexner et al., 1987). An additional study using WR showed an increase in LD50 levels in both intracranial and intraperitoneal models when A56 is knocked out (Shida et al., 1988). However, in this same study, no attenuation was seen when a foreign gene [the human T-cell leukemia virus (HTLV) glycoprotein] was inserted in place of A56R in the LC16mO or LO strains; deleting A56 resulted in little-to-no attenuation in both intracranial and intraperitoneal models (Shida et al., 1988). It should be noted that the LO and LC16mO strains are more attenuated than the WR strain. Based on these imperfect studies, it appears that deletion of the A56R gene does attenuate the virus in some cases, and this attenuation is more readily seen when starting with a virulent virus.
Table 1. Summary of in vivo work with A56 and/or VCP and K2 mutant viruses.
| Virus strain or gene mutation* | Route† | Attenuation‡ | Notes§ | References |
| A56-knockout virus | ||||
| NYCBH | IC | ++ | LD50 = 1.6×105 p.f.u. versus 1.9×102 p.f.u. for WT | Lee et al. (1992) |
| NYCBH | IN | +++ | LD50>1×108 p.f.u. versus 2.5×104 p.f.u. for WT | Lee et al. (1992) |
| WR | IC | + | 100 % death at 17 days; 100 % death for WT was 8 days | Flexner et al. (1987) |
| WR | IP | ++ | LD50 = 7.8×107 p.f.u. versus 9.3×105 p.f.u. for WT (A56R ORF was replaced with HTLV glycoprotein) | Shida et al. (1988) |
| WR | IC | ++ | LD50 = 1.5×102 p.f.u. versus <10 p.f.u. for WT (A56R ORF was replaced with HTLV glycoprotein) | Shida et al. (1988) |
| WR | IC, ID | + | In rabbits, lower mortality after IC and smaller lesions after ID infection. (A56R ORF was replaced with HTLV glycoprotein) | Shida et al. (1988) |
| LC16mO, LO | IC, IP | No | A56R ORF was replaced with HTLV glycoprotein | Shida et al. (1988) |
| A56 binding-partner mutant | ||||
| K2ko (WR) | IN | No | Identical death rates versus WT | Law & Smith (1992) |
| K2ko (CPXV) | IN | No | LD50 = 5.3×105 p.f.u. versus 5.1×105 p.f.u. for WT | Thompson et al. (1993) |
| VCPko (WR) | ID | ++ | Smaller lesions formed in rabbits, guinea pigs compared with parental virus | Isaacs et al. (1992) |
| VCPko (WR) | IN, ID | ++ | For ID, smaller lesions formed in mice; for IN, VCPko mice survived 104 p.f.u. challenge while rescue virus-infected mice died. | DeHaven et al. (2010); Girgis et al. (2011) |
| VCPmut (WR) | IN, ID | ++ | For ID, lesions formed that were intermediate in size between VCPwt and VCPko; for IN, four of five mice survived 104 p.f.u. challenge while none of the mice challenged with a VCPko-rescue virus survived. | DeHaven et al. (2010) |
K2ko, K2L ORF deletion; VCPko, VCP ORF deletion; VCPmut, mutated N-terminal cysteine that results in VCP that does not form a homodimer and does not interact with A56.
IC, Intracranial; IN, intranasal; ID, intradermal; IP, intraperitoneal.
The relative degree of attenuation observed is indicated by the number of plus (+) signs.
WT, Wild type; HTLV, human T-cell leukaemia virus.
Deletion of the VACV proteins that bind the A56 protein provides a clearer, if unfinished picture of the contribution of A56 to VACV virulence, as these mutants appear to result in different levels of virulence in vivo (Table 1). The deletion of the K2L ORF does not appear to markedly attenuate vaccinia. No differences were seen when comparing a wild-type VACV with a K2L-knockout VACV, which were given to mice by the intranasal route of infection (Law & Smith, 1992). Similarly, intranasal infection of mice with a K2L-knockout or wild-type CPXV showed nearly identical LD50 (Thompson et al., 1993). Since deletion of the K2L gene results in the same syncytia-producing phenotype as deletion of the A56R gene, these findings appear to indicate that the viruses that do not form a functional A56–K2 complex remain virulent in mice. These viruses may even compensate for the loss of superinfection control by increased pathogenesis because of syncytia formation.
As previously mentioned, VCP is needed for full VACV (strain WR) virulence, as knockout viruses are attenuated in an intradermal rabbit model (Isaacs et al., 1992), and both intradermal and intranasal mouse models (DeHaven et al., 2010; Girgis et al., 2011). Importantly, a VCP mutant (VCPmut) that cannot form the A56–VCP complex (owing to mutation of the unpaired N-terminal cysteine of VCP) is also attenuated after intranasal and intradermal challenge (DeHaven et al., 2010). Taken as a whole, these experiments seem to suggest that the A56–VCP complex is more important to virulence in mouse models than the A56–K2 complex. However, direct comparisons of mutations that affect these two complexes with appropriate rescue viruses have not been carried out.
Gene screening using A56R-knockout viruses and oncolytic vectors
Because the A56R gene is non-essential for virus replication, it has been used as a region of the VACV genome suitable for insertion and expression of foreign genes (Shida, 1989). The HA properties of the A56 protein allow for easy identification of recombinant plaques with foreign genes inserted into the A56R locus, even in the absence of a selection marker. That is, the addition of chicken erythrocytes to an infected cell monolayer will cause wild-type plaques to appear red, while recombinant viruses with A56 deleted will remain white (Oda, 1965; Shida & Matsumoto, 1983). Another application of A56 mutants has been in the development of VACVs as oncolytic cancer vectors. Recently, a new vector was developed, GLV-1h68, which includes the deletion of A56 along with a series of other gene deletions [thymidine kinase (J2R) and F14.5L, a non-essential MV protein] (Izmailyan & Chang, 2008). When compared with the parental virus in which only J2R and F14.5 were deleted, the additional A56 knockout resulted in a virus that was further attenuated and able to reduce the size of breast cancer tumours in a nude mouse model. Why including an A56R gene deletion in this oncolytic virus improved the survival of tumour-bearing mice is unknown, but it is intriguing to speculate upon the potential contribution of infected tumour cells that did not express the K2 protein or VCP on their surfaces. GLV-1h68 has since been successfully used to shrink tumours in several xenograft mouse models (Gentschev et al., 2010; Lin et al., 2008; Worschech et al., 2009; Yu et al., 2009a, b).
Conclusions
The history of A56 can be divided into two acts. In the first act, the protein was discovered and many basic characteristics of the protein were described. After a number of years of diminished work on A56, the last decade has seen a burst of studies that focus on the A56 protein. During this second act, important discoveries about interactions with other viral proteins and their relevant biological functions have been made. The A56 protein is found in several locations during an infection (Fig. 1), and its interactions with other viral proteins produce multiple effects. While A56 is a ‘non-essential’ protein, it is an important one: through its interaction with the K2 protein it is involved in preventing reinfection of already-infected cells, which may promote viral spread as well as inhibiting syncytia formation. Through its interactions with VCP, it is involved with defending infected cells from the immune response of the host. Research on A56 has also been incorporated into new oncolytic vectors, and new panels of mutant viruses are in development that may assist in further elucidating the role A56 plays in pathogenesis.
Acknowledgements
The authors would like to thank Joe Esposito for helpful discussions and the reviewers of the submitted manuscript whose comments helped improve this mini-review. S. N. I. is supported in part by Public Health Service grants U01-AI077913, U01-AI066333 and U54-AI057168 (Middle Atlantic Regional Center of Excellence in Biodefense and Emerging Infectious Diseases) from the National Institute of Allergy and Infectious Disease. B. C. D. is supported by institutional training grants T32 AI07324 and U01-AI066333.
References
- Aguado B., Selmes I. P., Smith G. L. (1992). Nucleotide sequence of 21.8 kbp of variola major virus strain Harvey and comparison with vaccinia virus. J Gen Virol 73, 2887–2902 10.1099/0022-1317-73-11-2887 [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25, 3389–3402 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernet J., Mullick J., Singh A. K., Sahu A. (2003). Viral mimicry of the complement system. J Biosci 28, 249–264 10.1007/BF02970145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernet J., Mullick J., Panse Y., Parab P. B., Sahu A. (2004). Kinetic analysis of the interactions between vaccinia virus complement control protein and human complement proteins C3b and C4b. J Virol 78, 9446–9457 10.1128/JVI.78.17.9446-9457.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berngruber T. W., Weissing F. J., Gandon S. (2010). Inhibition of superinfection and the evolution of viral latency. J Virol 84, 10200–10208 10.1128/JVI.00865-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman K. E., Bubel H. C. (1972). Origin of the vaccinia virus hemagglutinin. J Virol 9, 290–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco R., Moss B. (1991). Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-Dalton outer envelope protein. J Virol 65, 5910–5920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursnell M. E., Foulds I. J., Campbell J. I., Binns M. M. (1988). Non-essential genes in the vaccinia virus HindIII K fragment: a gene related to serine protease inhibitors and a gene related to the 37K vaccinia virus major envelope antigen. J Gen Virol 69, 2995–3003 10.1099/0022-1317-69-12-2995 [DOI] [PubMed] [Google Scholar]
- Brown C. K., Turner P. C., Moyer R. W. (1991). Molecular characterization of the vaccinia virus hemagglutinin gene. J Virol 65, 3598–3606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum L. M., Turner P. C., Devick H., Baquero M. T., Moyer R. W. (2003). Plasma membrane localization and fusion inhibitory activity of the cowpox virus serpin SPI-3 require a functional signal sequence and the virus encoded hemagglutinin. Virology 306, 289–302 10.1016/S0042-6822(02)00017-X [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Adams P. D., Clore G. M., DeLano W. L., Gros P., Grosse-Kunstleve R. W., Jiang J. S., Kuszewski J., Nilges M., et al. (1998). Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr 54, 905–921 10.1107/S0907444998003254 [DOI] [PubMed] [Google Scholar]
- Cavallaro K. F., Esposito J. J. (1992). Sequences of the raccoon poxvirus hemagglutinin protein. Virology 190, 434–439 10.1016/0042-6822(92)91229-N [DOI] [PubMed] [Google Scholar]
- Chang S. J., Chang Y. X., Izmailyan R., Tang Y. L., Chang W. (2010). Vaccinia virus A25 and A26 proteins are fusion suppressors for mature virions and determine strain-specific virus entry pathways into HeLa, CHO-K1, and L cells. J Virol 84, 8422–8432 10.1128/JVI.00599-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen V. B., Arendall W. B., III, Headd J. J., Keedy D. A., Immormino R. M., Kapral G. J., Murray L. W., Richardson J. S., Richardson D. C. (2010). MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr 66, 12–21 10.1107/S0907444909042073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K. M., Liszewski M. K., Nybakken G., Davis A. E., Townsend R. R., Fremont D. H., Atkinson J. P., Diamond M. S. (2006). West Nile virus nonstructural protein NS1 inhibits complement activation by binding the regulatory protein factor H. Proc Natl Acad Sci U S A 103, 19111–19116 10.1073/pnas.0605668103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHaven B. C., Girgis N. M., Xiao Y., Hudson P. N., Olson V. A., Damon I. K., Isaacs S. N. (2010). Poxvirus complement control proteins are expressed on the cell surface through an intermolecular disulfide bridge with the viral A56 protein. J Virol 84, 11245–11254 10.1128/JVI.00372-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doceul V., Hollinshead M., van der Linden L., Smith G. L. (2010). Repulsion of superinfecting virions: a mechanism for rapid virus spread. Science 327, 873–876 10.1126/science.1183173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez N., Paran N., Maik-Rachline G., Politi B., Israely T., Schnider P., Fuchs P., Melamed S., Lustig S. (2009). Induction of cell-cell fusion by ectromelia virus is not inhibited by its fusion inhibitory complex. Virol J 6, 151 10.1186/1743-422X-6-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eswar N., Webb B., Marti-Renom M. A., Madhusudhan M. S., Eramian D., Shen M. Y., Pieper U., Sali A. (2006). Comparative protein structure modeling using Modeller. Curr Protoc Bioinformatics 5, Unit 5.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner F., Henderson D. A., Arita I., Jezek Z., Ladnyi D. (1988). Smallpox and Its Eradication. Geneva: WHO [Google Scholar]
- Flexner C., Hügin A., Moss B. (1987). Prevention of vaccinia virus infection in immunodeficient mice by vector-directed IL-2 expression. Nature 330, 259–262 10.1038/330259a0 [DOI] [PubMed] [Google Scholar]
- Friedman H. M., Cohen G. H., Eisenberg R. J., Seidel C. A., Cines D. B. (1984). Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature 309, 633–635 10.1038/309633a0 [DOI] [PubMed] [Google Scholar]
- Galmiche M. C., Goenaga J., Wittek R., Rindisbacher L. (1999). Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology 254, 71–80 10.1006/viro.1998.9516 [DOI] [PubMed] [Google Scholar]
- Gentschev I., Ehrig K., Donat U., Hess M., Rudolph S., Chen N., Yu Y. A., Zhang Q., Bullerdiek J., et al. (2010). Significant growth inhibition of canine mammary carcinoma xenografts following treatment with oncolytic vaccinia virus GLV-1h68. J Oncol 2010, 736907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis N. M., Dehaven B. C., Fan X., Viner K. M., Shamim M., Isaacs S. N. (2008). Cell surface expression of the vaccinia virus complement control protein is mediated by interaction with the viral A56 protein and protects infected cells from complement attack. J Virol 82, 4205–4214 10.1128/JVI.02426-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis N. M., Dehaven B. C., Xiao Y., Alexander E., Viner K. M., Isaacs S. N. (2011). The vaccinia virus complement control protein modulates adaptive immune responses during infection. J Virol 85, 2547–2556 10.1128/JVI.01474-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras S., Burrows S. R., Kjer-Nielsen L., Clements C. S., Liu Y. C., Sullivan L. C., Bell M. J., Brooks A. G., Purcell A. W., McCluskey J. (2009). The shaping of T cell receptor recognition by self-tolerance. Immunity 30, 193–203 10.1016/j.immuni.2008.11.011 [DOI] [PubMed] [Google Scholar]
- Harris S. L., Frank I., Yee A., Cohen G. H., Eisenberg R. J., Friedman H. M. (1990). Glycoprotein C of herpes simplex virus type 1 prevents complement-mediated cell lysis and virus neutralization. J Infect Dis 162, 331–337 10.1093/infdis/162.2.331 [DOI] [PubMed] [Google Scholar]
- Huang I. C., Li W., Sui J., Marasco W., Choe H., Farzan M. (2008). Influenza A virus neuraminidase limits viral superinfection. J Virol 82, 4834–4843 10.1128/JVI.00079-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutin Y. J., Williams R. J., Malfait P., Pebody R., Loparev V. N., Ropp S. L., Rodriguez M., Knight J. C., Tshioko F. K., et al. (2001). Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg Infect Dis 7, 434–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihashi Y. (1977). Vaccinia-specific hemagglutinin. Virology 76, 527–538 10.1016/0042-6822(77)90235-5 [DOI] [PubMed] [Google Scholar]
- Ichihashi Y., Dales S. (1971). Biogenesis of poxviruses: interrelationship between hemagglutinin production and polykaryocytosis. Virology 46, 533–543 10.1016/0042-6822(71)90057-2 [DOI] [PubMed] [Google Scholar]
- Isaacs S. N., Kotwal G. J., Moss B. (1992). Vaccinia virus complement-control protein prevents antibody-dependent complement-enhanced neutralization of infectivity and contributes to virulence. Proc Natl Acad Sci U S A 89, 628–632 10.1073/pnas.89.2.628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izmailyan R., Chang W. (2008). Vaccinia virus WR53.5/F14.5 protein is a new component of intracellular mature virus and is important for calcium-independent cell adhesion and vaccinia virus virulence in mice. J Virol 82, 10079–10087 10.1128/JVI.00816-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D. Y., Li Z. L., Jin Q., Hao Y. W., Hou Y. D. (1989). Vaccinia virus hemagglutinin. A novel member of the immunoglobulin superfamily. J Exp Med 170, 571–576 10.1084/jem.170.2.571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotwal G. J., Moss B. (1988). Vaccinia virus encodes a secretory polypeptide structurally related to complement control proteins. Nature 335, 176–178 10.1038/335176a0 [DOI] [PubMed] [Google Scholar]
- Kotwal G. J., Isaacs S. N., McKenzie R., Frank M. M., Moss B. (1990). Inhibition of the complement cascade by the major secretory protein of vaccinia virus. Science 250, 827–830 10.1126/science.2237434 [DOI] [PubMed] [Google Scholar]
- Law K. M., Smith G. L. (1992). A vaccinia serine protease inhibitor which prevents virus-induced cell fusion. J Gen Virol 73, 549–557 10.1099/0022-1317-73-3-549 [DOI] [PubMed] [Google Scholar]
- Law M., Carter G. C., Roberts K. L., Hollinshead M., Smith G. L. (2006). Ligand-induced and nonfusogenic dissolution of a viral membrane. Proc Natl Acad Sci U S A 103, 5989–5994 10.1073/pnas.0601025103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. S., Roos J. M., McGuigan L. C., Smith K. A., Cormier N., Cohen L. K., Roberts B. E., Payne L. G. (1992). Molecular attenuation of vaccinia virus: mutant generation and animal characterization. J Virol 66, 2617–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. F., Price D. L., Chen C. H., Brader P., Li S., Gonzalez L., Zhang Q., Yu Y. A., Chen N., et al. (2008). Oncolytic vaccinia virotherapy of anaplastic thyroid cancer in vivo. J Clin Endocrinol Metab 93, 4403–4407 10.1210/jc.2008-0316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindwasser O. W., Chaudhuri R., Bonifacino J. S. (2007). Mechanisms of CD4 downregulation by the Nef and Vpu proteins of primate immunodeficiency viruses. Curr Mol Med 7, 171–184 10.2174/156652407780059177 [DOI] [PubMed] [Google Scholar]
- Liszewski M. K., Leung M. K., Hauhart R., Buller R. M., Bertram P., Wang X., Rosengard A. M., Kotwal G. J., Atkinson J. P. (2006). Structure and regulatory profile of the monkeypox inhibitor of complement: comparison to homologs in vaccinia and variola and evidence for dimer formation. J Immunol 176, 3725–3734 [DOI] [PubMed] [Google Scholar]
- Liszewski M. K., Leung M. K., Hauhart R., Fang C. J., Bertram P., Atkinson J. P. (2009). Smallpox inhibitor of complement enzymes (SPICE): dissecting functional sites and abrogating activity. J Immunol 183, 3150–3159 10.4049/jimmunol.0901366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes N. P., Estep R. D., Mottaz H. M., Moore R. J., Clauss T. R., Monroe M. E., Du X., Adkins J. N., Wong S. W., Smith R. D. (2008). Comparative proteomics of human monkeypox and vaccinia intracellular mature and extracellular enveloped virions. J Proteome Res 7, 960–968 10.1021/pr070432+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie R., Kotwal G. J., Moss B., Hammer C. H., Frank M. M. (1992). Regulation of complement activity by vaccinia virus complement-control protein. J Infect Dis 166, 1245–1250 10.1093/infdis/166.6.1245 [DOI] [PubMed] [Google Scholar]
- Miller C. G., Shchelkunov S. N., Kotwal G. J. (1997). The cowpox virus-encoded homolog of the vaccinia virus complement control protein is an inflammation modulatory protein. Virology 229, 126–133 10.1006/viro.1996.8396 [DOI] [PubMed] [Google Scholar]
- Moss B. (2006). Poxvirus entry and membrane fusion. Virology 344, 48–54 10.1016/j.virol.2005.09.037 [DOI] [PubMed] [Google Scholar]
- Moulton E. A., Bertram P., Chen N., Buller R. M., Atkinson J. P. (2010). Ectromelia virus inhibitor of complement enzymes protects intracellular mature virus and infected cells from mouse complement. J Virol 84, 9128–9139 10.1128/JVI.02677-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullick J., Bernet J., Panse Y., Hallihosur S., Singh A. K., Sahu A. (2005). Identification of complement regulatory domains in vaccinia virus complement control protein. J Virol 79, 12382–12393 10.1128/JVI.79.19.12382-12393.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagler F. P. O. (1942). Application of Hirst's phenomenon to the titration of vaccinia virus and vaccinia immune serum. Med J Aust 1, 281–283 [Google Scholar]
- Oda M. (1965). Rescue of dermovaccinia abortive infection by neurovaccinia virus in L cells. Virology 25, 664–666 10.1016/0042-6822(65)90096-6 [DOI] [PubMed] [Google Scholar]
- Oie M., Shida H., Ichihashi Y. (1990). The function of the vaccinia hemagglutinin in the proteolytic activation of infectivity. Virology 176, 494–504 10.1016/0042-6822(90)90019-N [DOI] [PubMed] [Google Scholar]
- Payne L. G. (1992). Characterization of vaccinia virus glycoproteins by monoclonal antibody precipitation. Virology 187, 251–260 10.1016/0042-6822(92)90313-E [DOI] [PubMed] [Google Scholar]
- Payne L. G., Norrby E. (1976). Presence of haemagglutinin in the envelope of extracellular vaccinia virus particles. J Gen Virol 32, 63–72 10.1099/0022-1317-32-1-63 [DOI] [PubMed] [Google Scholar]
- Pruitt K. D., Tatusova T., Maglott D. R. (2007). NCBI reference sequences (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res 35 Database issueD61–D65 10.1093/nar/gkl842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulford D. J., Gates A., Bridge S. H., Robinson J. H., Ulaeto D. (2004). Differential efficacy of vaccinia virus envelope proteins administered by DNA immunisation in protection of BALB/c mice from a lethal intranasal poxvirus challenge. Vaccine 22, 3358–3366 10.1016/j.vaccine.2004.02.034 [DOI] [PubMed] [Google Scholar]
- Rosengard A. M., Alonso L. C., Korb L. C., Baldwin W. M., III, Sanfilippo F., Turka L. A., Ahearn J. M. (1999). Functional characterization of soluble and membrane-bound forms of vaccinia virus complement control protein (VCP). Mol Immunol 36, 685–697 10.1016/S0161-5890(99)00081-4 [DOI] [PubMed] [Google Scholar]
- Rosengard A. M., Liu Y., Nie Z., Jimenez R. (2002). Variola virus immune evasion design: expression of a highly efficient inhibitor of human complement. Proc Natl Acad Sci U S A 99, 8808–8813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu A., Isaacs S. N., Soulika A. M., Lambris J. D. (1998). Interaction of vaccinia virus complement control protein with human complement proteins: factor I-mediated degradation of C3b to iC3b1 inactivates the alternative complement pathway. J Immunol 160, 5596–5604 [PubMed] [Google Scholar]
- Seki M., Oie M., Ichihashi Y., Shida H. (1990). Hemadsorption and fusion inhibition activities of hemagglutinin analyzed by vaccinia virus mutants. Virology 175, 372–384 10.1016/0042-6822(90)90422-N [DOI] [PubMed] [Google Scholar]
- Senkevich T. G., Ojeda S., Townsley A., Nelson G. E., Moss B. (2005). Poxvirus multiprotein entry-fusion complex. Proc Natl Acad Sci U S A 102, 18572–18577 10.1073/pnas.0509239102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfyroera G., Katragadda M., Morikis D., Isaacs S. N., Lambris J. D. (2005). Electrostatic modeling predicts the activities of orthopoxvirus complement control proteins. J Immunol 174, 2143–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida H. (1986a). Nucleotide sequence of the vaccinia virus hemagglutinin gene. Virology 150, 451–462 10.1016/0042-6822(86)90309-0 [DOI] [PubMed] [Google Scholar]
- Shida H. (1986b ). Variants of vaccinia virus hemagglutinin altered in intracellular transport. Mol Cell Biol 6, 3734–3745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida H. (1989). Vaccinia virus hemagglutinin. Subcell Biochem 15, 405–440 [DOI] [PubMed] [Google Scholar]
- Shida H., Dales S. (1981). Biogenesis of vaccinia: carbohydrate of the hemagglutinin molecules. Virology 111, 56–72 10.1016/0042-6822(81)90653-X [DOI] [PubMed] [Google Scholar]
- Shida H., Matsumoto S. (1983). Analysis of the hemagglutinin glycoprotein from mutants of vaccinia virus that accumulates on the nuclear envelope. Cell 33, 423–434 10.1016/0092-8674(83)90424-5 [DOI] [PubMed] [Google Scholar]
- Shida H., Hinuma Y., Hatanaka M., Morita M., Kidokoro M., Suzuki K., Maruyama T., Takahashi-Nishimaki F., Sugimoto M., et al. (1988). Effects and virulences of recombinant vaccinia viruses derived from attenuated strains that express the human T-cell leukemia virus type I envelope gene. J Virol 62, 4474–4480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon K. O., Cardamone J. J., Jr, Whitaker-Dowling P. A., Youngner J. S., Widnell C. C. (1990). Cellular mechanisms in the superinfection exclusion of vesicular stomatitis virus. Virology 177, 375–379 10.1016/0042-6822(90)90494-C [DOI] [PubMed] [Google Scholar]
- Spiro R. G. (1966). Characterization of carbohydrate units of glycoproteins. Methods Enzymol 8, 26–52 10.1016/0076-6879(66)08006-6 [DOI] [Google Scholar]
- Thompson J. P., Turner P. C., Ali A. N., Crenshaw B. C., Moyer R. W. (1993). The effects of serpin gene mutations on the distinctive pathobiology of cowpox and rabbitpox virus following intranasal inoculation of Balb/c mice. Virology 197, 328–338 10.1006/viro.1993.1594 [DOI] [PubMed] [Google Scholar]
- Turner P. C., Moyer R. W. (1992). An orthopoxvirus serpinlike gene controls the ability of infected cells to fuse. J Virol 66, 2076–2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner P. C., Moyer R. W. (1995). Orthopoxvirus fusion inhibitor glycoprotein SPI-3 (open reading frame K2L) contains motifs characteristic of serine proteinase inhibitors that are not required for control of cell fusion. J Virol 69, 5978–5987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner P. C., Moyer R. W. (2006). The cowpox virus fusion regulator proteins SPI-3 and hemagglutinin interact in infected and uninfected cells. Virology 347, 88–99 10.1016/j.virol.2005.11.012 [DOI] [PubMed] [Google Scholar]
- Turner P. C., Moyer R. W. (2008). The vaccinia virus fusion inhibitor proteins SPI-3 (K2) and HA (A56) expressed by infected cells reduce the entry of superinfecting virus. Virology 380, 226–233 10.1016/j.virol.2008.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner P. C., Baquero M. T., Yuan S., Thoennes S. R., Moyer R. W. (2000). The cowpox virus serpin SPI-3 complexes with and inhibits urokinase-type and tissue-type plasminogen activators and plasmin. Virology 272, 267–280 10.1006/viro.2000.0377 [DOI] [PubMed] [Google Scholar]
- Wagenaar T. R., Moss B. (2007). Association of vaccinia virus fusion regulatory proteins with the multicomponent entry/fusion complex. J Virol 81, 6286–6293 10.1128/JVI.00274-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenaar T. R., Moss B. (2009). Expression of the A56 and K2 proteins is sufficient to inhibit vaccinia virus entry and cell fusion. J Virol 83, 1546–1554 10.1128/JVI.01684-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenaar T. R., Ojeda S., Moss B. (2008). Vaccinia virus A56/K2 fusion regulatory protein interacts with the A16 and G9 subunits of the entry fusion complex. J Virol 82, 5153–5160 10.1128/JVI.00162-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. X., Turner P. C., Ness T. L., Moon K. B., Schoeb T. R., Moyer R. W. (2000). The cowpox virus SPI-3 and myxoma virus SERP1 serpins are not functionally interchangeable despite their similar proteinase inhibition profiles in vitro. Virology 272, 281–292 10.1006/viro.2000.0378 [DOI] [PubMed] [Google Scholar]
- Wildum S., Schindler M., Münch J., Kirchhoff F. (2006). Contribution of Vpu, Env, and Nef to CD4 down-modulation and resistance of human immunodeficiency virus type 1-infected T cells to superinfection. J Virol 80, 8047–8059 10.1128/JVI.00252-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worschech A., Chen N., Yu Y. A., Zhang Q., Pos Z., Weibel S., Raab V., Sabatino M., Monaco A., et al. (2009). Systemic treatment of xenografts with vaccinia virus GLV-1h68 reveals the immunologic facet of oncolytic therapy. BMC Genomics 10, 301 10.1186/1471-2164-10-301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. A., Galanis C., Woo Y., Chen N., Zhang Q., Fong Y., Szalay A. A. (2009a). Regression of human pancreatic tumor xenografts in mice after a single systemic injection of recombinant vaccinia virus GLV-1h68. Mol Cancer Ther 8, 141–151 10.1158/1535-7163.MCT-08-0533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z., Li S., Brader P., Chen N., Yu Y. A., Zhang Q., Szalay A. A., Fong Y., Wong R. J. (2009b ). Oncolytic vaccinia therapy of squamous cell carcinoma. Mol Cancer 8, 45 10.1186/1476-4598-8-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Sun X. Y., Fernando G. J., Frazer I. H. (1992). The vaccinia virus K2L gene encodes a serine protease inhibitor which inhibits cell-cell fusion. Virology 189, 678–686 10.1016/0042-6822(92)90591-C [DOI] [PubMed] [Google Scholar]