Abstract
Secondary activation of the endothelin system is thought to be involved in toxic liver injury. This study tested the hypothesis that dual endothelin-converting enzyme / neutral endopeptidase blockade might be able to attenuate acute toxic liver injury.
Male Sprague-Dawley rats were implanted with subcutaneous minipumps to deliver the novel compound SLV338 (10 mg/kg*d) or vehicle. Four days later they received two intraperitoneal injections of D-galactosamine (1.3 g/kg each) or vehicle at an interval of 12 hours. The animals were sacrificed 48 hours after the first injection.
Injection of D-galactosamine resulted in very severe liver injury, reflected by strongly elevated plasma liver enzymes, hepatic necrosis and inflammation, and a mortality rate of 42.9 %. SLV338 treatment did not show any significant effect on the extent of acute liver injury as judged from plasma parameters, hepatic histology and mortality. Plasma measurements of SLV338 confirmed adequate drug delivery. Plasma concentrations of big endothelin-1 and endothelin-1 were significantly elevated in animals with liver injury (5-fold and 62-fold, respectively). Plasma endothelin-1 was significantly correlated with several markers of liver injury. SLV338 completely prevented the rise of plasma big endothelin-1 (p < 0.05) and markedly attenuated the rise of endothelin-1 (p = 0.055).
In conclusion, dual endothelin-converting enzyme / neutral endopeptidase blockade by SLV338 did not significantly attenuate D-galactosamine-induced acute liver injury, although it largely prevented the activation of the endothelin system. An evaluation of SLV338 in a less severe model of liver injury would be of interest, since very severe intoxication might not be relevantly amenable to pharmacological interventions.
Keywords: endothelin, endothelin-converting enzyme, neutral endopeptidase, D-galactosamine, acute liver failure
Introduction
The liver cell damage seen in acute liver failure is not only due to direct effects of the precipitating drug, toxin, viral or other cause, but also due to a secondary release of proinflammatory and cytotoxic mediators from activated Kupffer, stellate, and sinusoidal endothelial cells, thus creating a vicious circle [1,2]. Endothelin (ET) seems to be one of those mediators. As recently published in this journal by our group and also reported by others, the ET system is typically activated in acute liver failure [3-6]. ET-1 might play a key role in the pathogenesis of the microcirculatory disorders associated with acute liver injury by mediating sinusoidal vasoconstriction, lowering the perfusion rate and promoting leukocyte adhesion [7,8]. Blockade of the activated ET system might provide a therapeutic option for acute toxic liver injury. ET A receptor antagonists have been shown to be beneficial in experimental models of acute liver injury [5,6]. Neutral endopeptidase (NEP) blockade has beneficial effects in toxic liver cirrhosis [9]. The metalloprotease endothelin-converting enzyme (ECE)-1 is important for the production of active ET-1 by cleaving its precursor big-ET-1 [10]. An upregulation of ECE-1 was observed in the early phase of toxic liver injury [11]. The present study set out to investigate the therapeutic potential of a dual ECE/NEP blockade, using the novel compound SLV338, in a rat model of D-galactosamine (GalN)-induced acute liver injury. GalN-induced acute liver injury is an established model for the investigation of hepatotoxic pathomechanisms [3,4,12].
Material and Methods
Animals and drug administration
The animal experiments were conducted in accordance with local institutional guidelines for the care and use of laboratory animals. Male Sprague-Dawley rats (250-300 g; Crl:CD(SD), Charles River, Sulzfeld, Germany) were maintained under controlled conditions (20 ± 2°C, 12 h light/dark cycle) with free access to food and water. Animals were divided into 4 groups: controls (Con; n = 9), controls with SLV338 treatment (Con+SLV; n = 9), D-galactosamine (GalN; n = 20) and D-galactosamine with SLV338 treatment (GalN+SLV; n = 20). SLV338 (or vehicle) was administered by subcutaneous osmotic minipumps (Alzet, Cupertino, CA, USA) at a rate of 10 mg/kg*d based on previous experience. The osmotic minipumps were implanted under anesthesia with isoflurane 4 days before the application of GalN. SLV338 was released until the end of the experiment. Baseline blood samples were taken in all animals from the retro-orbital plexus 2 days before the application of GalN. GalN (or vehicle) was given twice (1.3 g/kg) as an intraperitoneal injection at an interval of 12 hours. Food and water intake were monitored after the injection of GalN. The animals were sacrificed 48 hours after the first injection of GalN. Blood samples were obtained, liver and kidneys were excised, washed in ice-cold saline, blotted dry and weighed. A slice of the right liver lobe and the right kidney were immediately frozen in liquid nitrogen. The remainder of the liver and the left kidney were fixed in formalin.
Plasma analyses
Aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transferase bilirubin, cholinesterase, total protein, glucose, creatinine, urea, lipase, creatine kinase and sodium were measured using an automated analyzer (Cobas Integra 800, Roche, Grenzach, Germany). Quantification of SLV338 plasma concentrations was performed after solid phase extraction using a validated reversed phase high-pressure liquid chromatography method with MS/MS detection (Sciex Api 3000, Perkin Elmer, Waltham, MA, USA).
Tissue and plasma ET-1 and Big-ET-1
Tissue samples were prepared as described recently [13]. Big-ET-1, ET-1 and protein were determined by a commercially available enzyme immunoassay according to the manufacturer's instructions (Immundiagnostik AG, Bensheim, Germany). Plasma samples were analyzed with the same assays.
Histology
Formalin-fixed liver specimens were embedded in paraffin, cut into 3 μm sections and subjected to hematoxylin-eosin staining for morphological evaluation as described previously [3,12]. In brief, histological assessment was performed semi-quantitatively in relation to the extent of necrosis and neutrophil infiltration. A score from 0 to 3 was assigned to each specimen. Necrosis: 0, normal histology; 1, minor necrosis; 2, widely distributed patchy necrosis of hepatocytes; and 3, complete lobular disruption and diffuse hepatocyte necrosis. Neutrophil infiltration: 0, normal histology; 1, minimal perilobular inflammatory reaction; 2, marked inflammatory reaction; 3, panlobular inflammatory reaction. All samples were evaluated blinded and independently by two investigators.
Statistical analysis
Data was analyzed with SPSS 17.0 (SSPS Inc., Chicago, IL, USA). Results are expressed as mean ± standard error of the mean. The nonparametric Mann-Whitney U test was used to detect significant differences between groups of interest. Bivariate correlation was assessed by correlation analysis. Pearson's correlation coefficient was used. Mortality was compared using Pearson's chi-square test.
Results
Injection of GalN resulted in severe liver injury reflected by strongly elevated plasma liver enzymes and bilirubin, reduced total plasma protein and glucose (Table 1), histological signs of hepatic necrosis and inflammation, reduced food and water intake and a considerable mortality rate of 42.9 % (Table 2). There was no significant change of plasma cholinesterase activity, probably because of its half-life of about 14 days. Baseline plasma values were not different between the groups (data not shown), except for urea (Con 36 ± 3 vs. GalN+SLV 29 ± 1 mg/dl; p < 0.05). Indicators of renal (creatinine, urea, sodium) and pancreatic function (lipase) were unaffected by the injection of GalN, which confirms that a selective liver injury had occurred.
Table 1.
Plasma parameters in rats with D-galactosamine-induced acute liver failure with or without SLV338 treatment
| Con | Con+SLV | GalN | GalN+SLV | |
|---|---|---|---|---|
| N | 9 | 9 | 19 | 22 |
| Aspartate aminotransferase (U/I) | 266 ± 27 | 185 ± 11* | 5802 ± 1299** | 5743 ± 1217** |
| Alanine aminotransferase (U/I) | 46 ± 7 | 40 ± 2 | 2937 ± 700*** | 2970 ± 592*** |
| Gamma-glutamyl transferase (U/I) | 2.0 ± 0.01 | 4.3 ± 2 | 8.4 ± 2** | 9.2 ± 1** |
| Bilirubin (mg/dl) | 0.1 ± 0.01 | 0.1 ± 0.01 | 3.2 ± 0.6** | 3.7 ± 0.6*** |
| Cholinesterase (U/l) | 96 ± 14 | 105 ± 14 | 94 ± 8 | 87 ± 9 |
| Total protein (g/dl) | 5.0 ± 0.1 | 5.0 ± 0.1 | 4.2 ± 0.1*** | 4.2 ± 0.2** |
| Glucose (mg/dl) | 159 ± 7 | 168 ± 7 | 89 ± 13*** | 94 ± 12** |
| Creatinine (mg/dl) | 0.27 ± 0.02 | 0.40 ± 0.13 | 0.33 ± 0.02 | 0.35 ± 0.03* |
| Urea (mg/dl) | 37 ± 3 | 36 ± 6 | 43 ± 2 | 44 ± 5 |
| Sodium (mmol/l) | 143 ± 0.9 | 142 ± 0.6 | 141 ± 0.4 | 141 ± 0.6 |
| Lipase (U/I) | 8.3 ± 0.5 | 9.1 ± 0.2 | 18.2 ± 7.6 | 11.0 ± 1.4 |
| Creatine kinase (U/I) | 1891 ± 169 | 1135 ± 90** | 2687 ± 1483* | 866 ± 108*** |
| Big endothelin-1 / Endothelin-1 | 13.7 ± 7.0 | 12.5 ± 7.4 | 8.4 ± 3.5 | 18.0 ± 14 |
| SLV338 (μg/l) | 0 ± 0 | 703 ± 230 | 0 ± 0 | 545 ± 88 |
Con, controls; SLV, SLV338; GalN, D-galactosamine.
Data are means ± standard error of the mean.
*p < 0.05, **p < 0.01, ***p < 0.001 vs. Con.
Table 2.
Mortality, weight, food and water intake, liver weight and histology in rats with D-galactosamine-induced acute liver failure with or without SLV338 treatment
| Con | Con+SLV | GalN | GalN+SLV | |
|---|---|---|---|---|
| N | 9 | 9 | 19 | 22 |
| Mortality (%) | 0 | 0 | 42.9 | 37.1 |
| Weight at injection of GalN (g) | 304 ± 7 | 292 ± 3 | 300 ± 4 | 297 ± 3 |
| Weight 48 h after GalN (g) | 298 ± 8 | 298 ± 3 | 280 ± 6 | 283 ± 4 |
| Food intake within 48 h after GalN (ml) | 47 ± 0.2 | 49 ± 0.5* | 20 ± 1.4*** | 26 ± 2.0***# |
| Water intake within 48 h after GalN (ml) | 69 ± 1.9 | 89 ± 2.8*** | 55 ± 2.4** | 65 ± 1.2*## |
| Liver weight (g) | 10.5 ± 0.4 | 10.6 ± 0.2 | 9.5 ± 0.5 | 8.5 ± 0.4* |
| Liver necrosis score (0-3) | 0.61 ± 0.3 | 0.22 ± 0.1 | 1.95 ± 0.2** | 2.02 ± 0.2** |
| Liver inflammation score (0-3) | 0 ± 0 | 0 ± 0 | 0.97 ± 0.2** | 1.09 ± 0.2*** |
Con, controls; SLV, SLV338; GalN, D-galactosamine.
Data are means ± standard error of the mean.
*p < 0.05, **p < 0.01, ***p < 0.001 vs. Con. #p < 0.05, ##p < 0.01 vs. GalN.
Plasma concentrations of SLV338 at study end confirm correct drug delivery (Table 1). As shown in Tables 1 and 2, treatment with SLV338, starting 4 days before the injection of GalN, did not significantly improve any of the mentioned parameters of liver injury. Neither was there a significant reduction of mortality. SLV338 significantly increased food and water intake in rats with liver injury. However, this effect occurred in control animals as well (Table 2).
At study end, plasma big-ET-1 and ET-1 concentrations were significantly elevated in GalN-treated animals (5-fold and 62-fold, respectively; Figure 1), as described previously [3]. SLV338 completely prevented the rise of plasma big-ET-1 (p < 0.05) and markedly attenuated the GalN-induced rise of ET-1 (p = 0.055; Figure 1). Plasma ET-1 was significantly correlated with several markers of liver injury as shown in Table 3. This was not the case for plasma big-ET-1. Neither was there a significant correlation between the SLV338 plasma concentrations and markers of liver injury in the GalN+SLV group (data not shown). Because of large inter-individual variations, the ratio of plasma big-ET-1/ET-1, which is indicative of the conversion rate of big-ET-1 to ET-1, was not significantly different between the groups. However, SLV338-treatment more than doubled the mean ratio of plasma big-ET-1/ET-1 in rats with liver injury (Table 1).
Figure 1.
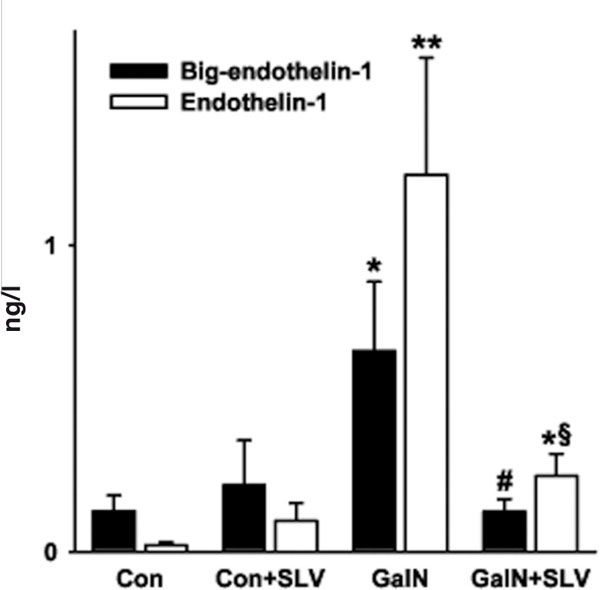
Plasma big endothelin-1 and endothelin-1 in rats with D-galactosamine-induced acute liver failure with or without SLV338 treatment. Con, controls; SLV, SLV338; GalN, D-galactosamine. Data are means ± standard error of the mean. *p = 0.05, **p < 0.001 vs. Con. #p < 0.05 vs. GalN. §p = 0.055 vs. GalN.
Table 3.
Bivariate correlation between plasma endothelin-1 and markers of liver injury in all rats that received D-galactosamine (with and without SLV338 treatment)
| Endothelin-1 | |
|---|---|
| Aspartate aminotransferase | 0.43** |
| Alanine aminotransferase | 0.44** |
| Plasma glucose | -0.31* |
| Food intake within 48 h after GalN | -0.35* |
| Water intake within 48 h after GalN | -0.42** |
| Liver necrosis score | 0.38* |
| Liver inflammation score | 0.35* |
GalN, D-galactosamine.
Pearson's correlation coefficient is given. Only statistically significant results are shown. There was no significant correlation between plasma big endothelin-1 and any markers of liver injury. n = 41. *p < 0.05, **p < 0.01.
The hepatic ET-1 content was altered neither by the injection of GalN nor by SLV338 treatment (Table 4). The renal ET-1 content was significantly decreased in animals that received GalN. SLV338 treatment tended to decrease renal ET-1 concentrations in rats with and without GalN-induced liver injury. Big-ET-1 was lowest in the GalN+SLV group (Table 4).
Table 4.
Big endothelin-1 and endothelin-1 tissue concentrations in rats with D-galactosamine-induced acute liver failure with or without SLV338 treatment
| Con | Con+SLV | GalN | GalN+SLV | |
|---|---|---|---|---|
| N | 9 | 9 | 19 | 22 |
| Liver | ||||
| Big Endothelin-1 (pg/100mg protein) | 29 ± 9 | 25 ± 2 | 38 ± 4 | 21 ± 4# |
| Endothelin-1 (pg/100mg protein) | 40 ± 16 | 45 ± 13 | 19 ± 3 | 25 ± 6 |
| Kidney | ||||
| Big Endothelin-1 (pg/100mg protein) | 18 ± 3 | 16 ± 4 | 18 ± 4 | 13 ± 2* |
| Endothelin-1 (pg/100mg protein) | 122 ± 22 | 67 ± 19 | 59 ± 11* | 43 ± 6** |
Con, controls; SLV, SLV338; GalN, D-galactosamine.
Data are means ± standard error of the mean.
*p < 0.05, **p < 0.01 vs. Con. #p < 0.01 vs. GalN.
Livers were slightly lighter in rats with liver injury compared to controls (Table 2). Kidney weights were not different between the groups (data not shown).
Discussion
The present study tested the hypothesis that dual ECE/NEP blockade might be able to attenuate acute toxic liver injury in a rat model of GalN-induced acute liver failure. Endpoints of the study were manifestations of liver failure such as elevated liver enzymes, hepatic necrosis and inflammation, and mortality. In addition, big-ET-1 and ET-1 plasma and tissue concentrations were determined to evaluate the involvement of the ET system.
Treatment with the novel dual ECE/NEP inhibitor SLV338 did not have any significant effect on the extent of acute liver injury as judged from plasma parameters, hepatic histology and mortality. This was not due to insufficient treatment, since plasma measurements of SLV338 confirmed adequate drug delivery. There was no toxic effect of SLV338, as far as plasma parameters of liver, kidney, pancreas and muscle are concerned.
This study reproduced some of the findings that led to its initial hypothesis. As previously described in similar models [3-5], the ET system was strongly activated, and a significant correlation between plasma ET-1 concentrations and markers of liver damage was found. This seems to be in line with a previous finding that exogenous perfusion with ET-1 aggravates acute liver injury in perfused livers of rats treated with GalN [14]. As hypothesized, the activation of the ET system was markedly reduced by SLV338 treatment in the present study. It remains unclear why this effect does not translate into improved clinical outcome. Plasma parameters, histological alterations and the high mortality within 48 hours after GalN administration indicate that the present protocol produced a very severe form of acute liver failure. It is conceivable that SLV338 provides protection against acute liver injury under less severe conditions. ECE inhibition has for example been reported to be protective in a rat model of hepatic ischemia/reperfusion injury which was less severe, thus allowing a follow-up of 14 days [15].
As described previously [3], the ET system was not upregulated at the hepatic and renal level in the present study. In contrast, ET-1 tissue contents were even decreased in the kidneys of the animals that received GalN. Those findings suggest that the local ET system of liver and kidneys is not responsible for the strong increase of plasma ET-1 concentrations. We previously speculated that an activated vascular ET system might be the source of the increased plasma ET-1, since the endothelium is the most probable structure able to liberate large amounts of ET-1 [3,16]. However, the unchanged overall amount of ET-1 in the liver does not exclude locally elevated concentrations at crucial localizations, e.g. around the hepatic sinusoids.
In conclusion, SLV338 did not significantly attenuate acute toxic liver injury in this very severe rat model of GalN-induced acute liver failure, although it largely prevented the activation of the ET system. An evaluation of SLV338 in a less severe model of liver injury would be of interest, since very severe intoxication might not be relevantly amenable to pharmacological interventions.
Conflicts of interest
This study was supported by Solvay Pharmaceuticals (now Abbott Products GmbH).
B. Hocher was an employee of Solvay Pharmaceuticals until recently. Y. Fischer is an employee of Abbott Products GmbH.
Abbreviations
ECE: endothelin-converting enzyme; ET: endothelin; GalN: D-galactosamine; NEP: neutral endopeptidase.
References
- Rosser BG, Gores GJ. Liver cell necrosis: cellular mechanisms and clinical implications. Gastroenterology. 1995;108:252–75. doi: 10.1016/0016-5085(95)90032-2. [DOI] [PubMed] [Google Scholar]
- Andus T, Bauer J, Gerok W. Effects of cytokines on the liver. Hepatology. 1991;13:364–75. [PubMed] [Google Scholar]
- Heiden S, Pfab T, Websky K Von, Vignon-Zellweger N, Godes M, Relle K. et al. Tissue specific activation of the endothelin system in severe acute liver failure. European Journal of Medical Research. 2008;13:327–9. [PubMed] [Google Scholar]
- Anand R, Harry D, Holt S, Milner P, Dashwood M, Goodier D. et al. Endothelin is an important determinant of renal function in a rat model of acute liver and renal failure. Gut. 2002;50:111–7. doi: 10.1136/gut.50.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmes D, Skawran S, Stratmann U, Armann B, Minin E, Herbst H, Spiegel HU. Amelioration of microcirculatory damage by an endothelin A receptor antagonist in a rat model of reversible acute liver failure. J Hepatol. 2005;42:350–7. doi: 10.1016/j.jhep.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Hocher B, Zart R, Diekmann F, Slowinski T, Thone-Reineke C, Lutz J, Bauer C. Protective effects of the mixed endothelin receptor antagonist bosentan in ratswith CCL4-induced liver injury. J Cardiovasc Pharmacol. 1995;26(Suppl 3):S130–S131. [PubMed] [Google Scholar]
- Zhang JX, Pegoli W Jr. Clemens MG. Endothelin-1 induces direct constriction of hepatic sinusoids. Am J Physiol. 1994;266:G624–G632. doi: 10.1152/ajpgi.1994.266.4.G624. [DOI] [PubMed] [Google Scholar]
- Spiegel HU, Scommotau S, Uhlmann D, Giersch B. Effect of the endothelin receptor antagonist bosentan on postischemic liver microcirculation. Zentralbl Chir. 1996;121:788–93. [PubMed] [Google Scholar]
- Sansoe G, Aragno M, Mastrocola R, Restivo F, Mengozzi G, Smedile A. et al. Neutral endopeptidase (EC 3.4.24.11) in cirrhotic liver: a new target to treat portal hypertension? J Hepatol. 2005;43:791–8. doi: 10.1016/j.jhep.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Xu D, Emoto N, Giaid A, Slaughter C, Kaw S, deWit D, Yanagisawa M. ECE-1: a membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell. 1994. pp. 473–85. [DOI] [PubMed]
- Nagata T, Kudo H, Nishino T, Doi Y, Itoh H, Fujimoto S. Increased immunoreactivities against endothelin-converting enzyme-1 and monocyte chemotactic protein-1 in hepatic stellate cells of rat fibrous liver induced by thioacetamide. Med Mol Morphol. 2005;38:161–72. doi: 10.1007/s00795-005-0292-5. [DOI] [PubMed] [Google Scholar]
- Shito M, Balis UJ, Tompkins RG, Yarmush ML, Toner M. A fulminant hepatic failure model in the rat: involvement of interleukin-1beta and tumor necrosis factor-alpha. Dig Dis Sci. 2001;46:1700–8. doi: 10.1023/A:1010653504568. [DOI] [PubMed] [Google Scholar]
- Quaschning T, Voss F, Relle K, Kalk P, Vignon-Zellweger N, Pfab T. et al. Lack of endothelial nitric oxide synthase promotes endothelin-induced hypertension: lessons from endothelin-1 transgenic/endothelial nitric oxide synthase knockout mice. J Am Soc Nephrol. 2007;18:730–40. doi: 10.1681/ASN.2006050541. [DOI] [PubMed] [Google Scholar]
- Iwai M, Yamauchi T, Shimazu T. Endothelin 1 aggravates acute liver injury in perfused livers of rats after treatment with D-galactosamine. Hepatology. 1998. pp. 503–9. [DOI] [PubMed]
- Uhlmann D, Witzigmann H, Senninger N, Hauss J, Spiegel HU. Protective role of an endothelin-converting enzyme inhibitor (FR901533) in hepatic ischemia/reperfusion injury. Microvasc Res. 2001;62:43–54. doi: 10.1006/mvre.2001.2309. [DOI] [PubMed] [Google Scholar]
- Goraca A. New views on the role of endothelin (minireview) Endocr Regul. 2002;36:161–7. [PubMed] [Google Scholar]


