Abstract
Soft tissue sarcomas are an uncommon and diverse group of more than 50 mesenchymal malignancies. The pathogenesis of many of these is poorly understood, but others have begun to reveal the secrets of their inner workings. With considerable effort over recent years, soft tissue sarcomas have increasingly been classified on the basis of underlying molecular alterations. In turn, this has allowed the development and application of targeted agents in several specific, molecularly defined, sarcoma subtypes. This review will focus the rationale for targeted therapy in sarcoma, with emphasis on the relevance of specific molecular factors and pathways in both translocation-associated sarcomas and in genetically complex tumors. In addition, we will address some of the early successes in sarcoma targeted therapy as well as a few challenges and disappointments in this field. Finally we will discuss several possible opportunities represented by poorly understood, but potentially promising new therapeutic targets, as well as several novel biologic agents currently in preclinical and early phase I/II trials. This will provide the reader with context for understanding the current state this field and a sense of where it may be headed in the coming years.
Keywords: Soft tissue sarcoma, targeted therapy, molecular mechanisms
Introduction
Soft tissue sarcomas are rare tumors showing mesenchymal differentiation that may arise anywhere in the body. They are comprised of a broadly diverse array of subtypes, each with distinct molecular, clinical and behavioral features.1 Although outcomes vary greatly by sarcoma subtype, current therapies are limited and cure is only attainable by complete surgical resection. Neither radiation nor conventional cytotoxic chemotherapy have had much impact in improving survival, although radiation may prevent local recurrence and chemotherapy may temporarily delay disease progression.2, 3 The need for more effective therapies is highlighted by poor five year sarcoma survivals of approximately 50% -- a rate that has shown little improvement in recent years.4
Fortunately, the past few decades have led to a vastly improved understanding of the molecular underpinnings of sarcomagenesis. It is hoped that these new insights will lead to the development of more effective therapies against driving events in these tumors. In part because of their rarity, sarcomas may represent ideal targets for the discovery of new treatments. Rare, or orphan diseases can be defined as diseases affecting fewer than 0.07% of the population (<200,000 people) in the US and less than 0.05% of the population in Europe.5 While advances in treatments for the most common cancers have occurred only incrementally over the past 20 years, dramatic successes in therapy have been seen disproportionately in orphan diseases, including some sarcomas. The Orphan Drug Act, introduced in 1982, was enacted to provide incentives to encourage research into therapies for rare diseases.6 Since then, over 300 agents have been approved for orphan diseases and nearly a thousand more are in development.7 These rapid advances may not only be due to financial incentives, but rather to the underlying biology of rare tumors. 8 That is, many rare tumors may be driven by a single genetic event, and rely on this aberration to survive (oncogenic addiction), whereas more common malignancies are driven by a wider array of molecular events.8
This theory has been borne out in some sarcomas, most notably gastrointestinal stromal tumor (GIST) and dermatofibrosarcoma protuberans (DFSP), in which targeted agents have had a high degree of treatment success. However, in other sarcoma types, the molecular events underlying sarcomagenesis are more complex, and determining the most effective targeted strategies remains a work in progress. This review will highlight some of the recent advances in understanding and targeting the molecular pathways involved in sarcomagenesis.
Current Diagnosis and Management of Sarcoma
Sarcomas are a diverse group of tumors, not only because of the wide array of histologic subtypes, but also due to their varied clinical courses and expected prognoses. A correct diagnosis is critical, not just in separating the benign from the malignant tumors, but also to predict the behavior of malignant tumors and to select appropriate therapeutic options. Ideally, diagnosis of sarcoma should be performed by a pathologist familiar not only with the natural history and morphologic features of these tumors, but also with their genetics and molecular biology. Molecular findings should always be interpreted within the context of clinical, histologic, and immunohistochemical data. Appropriate treatment then requires a multidisciplinary collaboration between the pathologist, radiologists, medical, surgical, and radiation oncologists, and orthopedic oncologists, among others. While much of current treatment relies on complete surgical resection, with or without adjuvant radiation or conventional chemotherapy, effective options for metastatic disease are limited, and there is now an increasing demand for more effective targeted therapies. As diverse as sarcomas are, so too are the types of molecularly targeted agents under development and investigation. An understanding of the mechanisms and relevance of these agents is grounded in the underlying pathology and molecular pathogenesis of sarcoma. As the custodians and curators of tumor diagnostic specimens buttressed by a thorough appreciation of pathogenesis, pathologists play a critical and central role in the clinical demonstration of the molecular determinates used to inform rational therapeutic decisions. To maintain the traditional value-added role of pathology as the provider of information informing therapeutic approaches, as a discipline pathologists must embrace and become conversant with the relevant molecular pathways involved in sarcomagenesis and the application of this information to clinical management. To ignore this emerging revolution threatens to undermine the central role pathologists in the therapeutic value chain.
Sarcoma Molecular Pathogenesis
Sarcomas may be broadly classified by genomic events underlying their development as 1) those with specific translocations or gene amplification, 2) those with defining oncogenic mutations and 3) those with complex genomic rearrangements. Each class contains diverse tumors with a wide array of clinical, histological and molecular characteristics.
Translocation-Associated Sarcomas
Specific, recurrent translocations have thus far been identified in 22 types of benign and malignant bone and soft tissue neoplasms, including 19 soft tissue sarcomas (including 4 tumors of at least intermediate (rarely metastasizing) biologic potential).9 Overall, translocation sarcomas are currently thought to account for 20-30% of all sarcomas,10, 11 and this number continues to swell with new discoveries of recurrent translocations in additional tumor types. Typically, recurrent translocations in sarcoma result in chimeric fusion genes which function as transcription factors, as is epitomized by the EWSR1-FLI1 fusion gene in Ewing sarcoma. Less frequently, translocation results in overexpression or constitutive activation of a growth factor receptor tyrosine kinase (RTK) or other chimeric growth factor signaling protein, as is seen in DFSP, in which wild types PDGFB is overexpressed under the COL1A1 promoter, and inflammatory myofibroblastic tumor (IMT) in which ALK fusion partners promote dimerization of the ALK tyrosine kinase thereby rendering it constitutively active.12, 13
Amplification-Associated Sarcomas
Recurrent amplifications have only been identified in a few soft tissue sarcomas, most notably well-differentiated/ dedifferentiated liposarcoma, in which amplification of chromosome 12q13-15, including HDM2 (MDM2) and CDK4 is characteristic.14 Hdm2 functions as an inhibitor of p53. Accordingly, amplification and subsequent overexpression of this chromosomal locus results in inhibition of p53-dependent cell-cycle arrest and apoptosis. Cdk4 is a cell cycle regulator, and over expression of this factor promotes proliferation, while other gene loci within this interval may also have pro-oncogenic effects.
Amplification of MYC has been described in secondary (radiation-induced) angiosarcoma,15 and may be seen sporadically in other sarcomas.16-18 Myc is a proto-oncogenic transcription factor, which can act as either a transactivator or repressor, and has been reported to be involved in a variety of human malignancies.19, 20
Targeted Therapy Against Fusion Genes
Translocation sarcomas have not yet proven to be as amenable to targeted therapy as had once been hoped, despite a greatly improved understanding of the mechanisms by which chimeric fusion genes promote sarcomagenesis. The few cases in which fusion genes have been successfully targeted are those in which the transgene results in overexpression or constitutive activation of a growth factor or growth factor receptor. The prototype for this class of sarcomas is DFSP, in which the growth factor PDGFB is fused to the promoter of the constitutively expressed COL1A1 encoding a collagen.21 Here, inhibition of PDGFR by the RTK- inhibitor (TKI) imatinib mesylate has been shown to dramatically reduce tumor size in previously unresectable cases.22
IMT represents another potential target for TKI therapy. About 50% of IMT are characterized by translocations involving the ALK receptor tyrosine kinase, which result in Alk overexpression,23 and/or aberrant localization to the intracellular compartment where receptor dimerization and constitutive activation occur.24, 25Agents currently under investigation for ALK rearrangement-positive IMT include crizotinib, a dual Alk/Met inhibitor, which has shown anecdotal benefits in ALK-positive but not ALK-negative IMT in early phase I trials.26
Another example of successful targeting of a fusion gene is in the locally aggressive diffuse-type tenosynovial giant cell tumor (pigmented villonodular synovitis). Here, constitutive expression of colony stimulating factor-1 (CSF-1) under the control of the COL6A3 collagen promoter in neoplastic cells acts to recruit large numbers of inflammatory and histiocytic cells, including the eponymous multinucleated giant cells.27, 28 Targeted inhibition of CSF1R with imatinib has shown promising results in early studies.29 While it remains unclear if this therapy has autocrine, paracrine or combined effects on the proliferation of the neoplastic cells themselves, the reduction in overall tumor size and cellularity from blocking the recruitment of inflammatory cells may result in improved surgical resectability as well as symptomatic relief.29
Success targeting chimeric transcription factors has been more elusive. Myxoid liposarcomas are characterized by a fusion between FUS and DDIT3. This fusion oncoprotein is reported to both promote proliferation and arrest cells in a primitive pre-adipocyte stage of development.30, 31 While it is not a specific targeted agent in the conventional sense, one study has suggested that trabectedin may directly interact with the FUS-DDIT3 fusion protein and inhibit its ability to bind to DNA promoter sequences.32 Trabectedin has shown some promise as an adjuvant therapy in myxoid liposarcoma.33
Another approach is to inhibit downstream factors upregulated by fusion genes. Several chimeric transcription factors, including PAX3-FOXO1A and those involving EWSR1 or FUS fusion genes, among others, upregulate expression of growth factors, including IGF1R, PDGFR and MET hepatocyte growth factor receptor,34-42 which may be targeted by various TKIs. Early preclinical studies have shown some response to MET-inhibitors in alveolar soft part sarcoma,43 and clear cell sarcomas,44 while inhibition of IGF1R may be beneficial in Ewing sarcoma.45-47 Similarly, the SS18-SSX fusion oncoprotein seen in synovial sarcoma upregulates FGF, which activates the Ras pathway and promotes proliferation.48, 49 Preclinical studies have suggested that targeted therapy against FGFR may inhibit growth of synovial sarcoma.49
It has also been postulated that translocation variants in sarcoma may be predictive of patient outcome, however, the encouraging results of early studies in alveolar rhabdomyosarcoma, Ewing sarcoma and synovial sarcoma have not been clearly validated in more recent reports. 50-57
Sarcomas with Driving Oncogenic Mutations
Several sarcomas have been identified in which tumorigenesis is primarily driven by single activating gene mutations. The exemplar of this class of sarcoma is GIST, which represents one of the earliest great success stories of targeted therapy. GISTs require activating mutations in the KIT receptor tyrosine kinase, or less frequently, in PDGFRA for tumor proliferation.58, 59 The majority of cases have mutations in exon 11 of KIT, and respond dramatically to imatinib mesylate therapy, with up to 80% of patients demonstrating at least partial response, while mutations in exon 9 or PDGFRA render tumors resistant.58, 59 These tumors illustrate the concept of oncogenic addiction, in which activation of a single pathway is required for tumorigenesis. Unfortunately, GIST frequently develop secondary resistance to imatinib, which in many cases is due to additional activating mutations in KIT or to mutations in PDGFRA.60 In these cases, second line therapy with a different TKI, such as sunitinib, have shown some benefits in delaying tumor progression.61 Agents directed against PDGFR-α are also in development.62 Ultimately, however, patients are rarely cured with TKI therapy alone.61
Sarcomas with Complex Karyotypes
The largest category of sarcoma includes those with complex cytogenetic alterations. This class is primarily comprised of higher grade spindle cell and pleomorphic sarcomas, such as leiomyosarcoma, undifferentiated pleomorphic sarcoma/ malignant fibrous histiocytoma (UPS/MFH) and angiosarcoma.63
Complex karyotype sarcomas are thought to occur as a result of genomic instability and failure of DNA repair and maintenance mechanisms. One pathway by which this may occur is by telomere loss. Telomeres are responsible for maintaining the integrity of chromosome ends, however, these sequences themselves shorten within each mitotic cycle and are prone to breakage under stress conditions or uncontrolled proliferation. While in normal cells, telomere shortening results in cellular senescence, in tumor cells which have circumvented normal cell cycle controls, division continues to occur, with disastrous consequences for chromosomes. Telomere loss allows sticky ends of chromosomes to bind to nearby strands of DNA, inducing a cycle of unregulated fusion and breakage, with the end result of bizarre chromosomal inversions, amplifications, duplications, and translocations which characterize high grade malignancies.64 Genomic rearrangements may also occur as a result of a single catastrophic event, known as chromothripsis, which appears to occur in at least 2-3% of all cancers, and is suggested to occur in up to 25% of malignant bone tumors.65
Paradoxically, some sarcomas, including Ewing sarcoma, UPS/MFH and liposarcoma, actually have increased telomerase activity, despite prior genomic rearrangement.66-68 High telomerase activity or the presence of alternative lengthening of telomeres is associated with poor prognosis.66, 67 It is thought, therefore, that targeting of telomere maintenance mechanisms may be an effective therapy in selected mesenchymal tumors.69
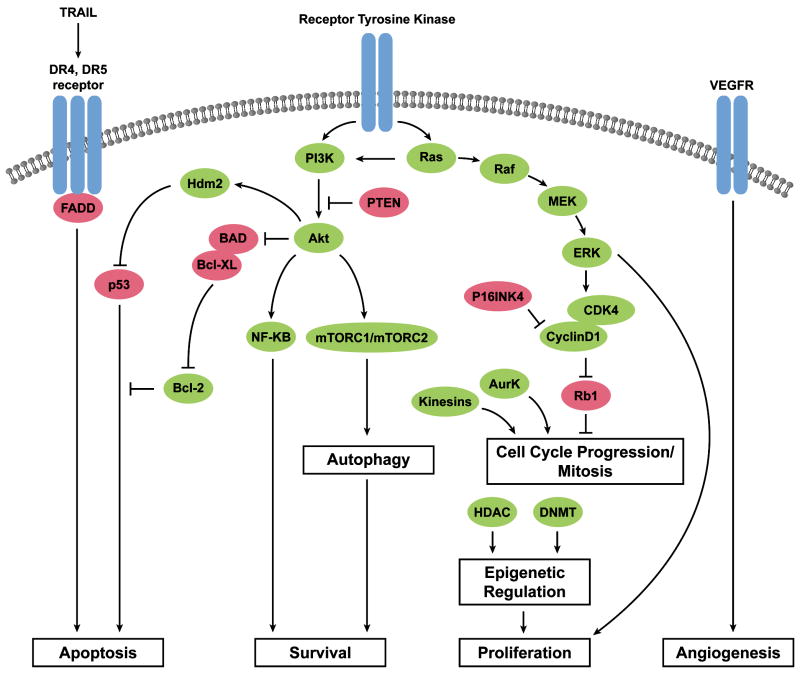
Regardless of the underlying chromosomal events, complex karyotype sarcomas, like carcinomas, seem to develop mutations or activating events in particular cell survival or proliferation pathways, including cell-cycle checkpoints, apoptosis, stress response, and metabolic/proliferative pathways.70, 71 While no single event may be seen in all tumors, understanding which pathways are the most frequently dysregulated, and at what step in signaling, may narrow the options for the best potential drug-targets (Figure 1).
Figure 1.
Molecular pathways involved in sarcomagenesis. Green indicates pro-tumorigenic factors that are activated or overexpressed in sarcoma. Red indicates tumor suppressors that may be inactivated in sarcoma.
Targets in Transcriptional Regulation
Regulation of DNA transcription and replication involves epigenetic modification of both DNA (via promoter methylation) and DNA-associated proteins such as histones (via acetylation and other post-translational modifications). In the acetylated state, chromatin assumes an open configuration which allows transcription factors access to DNA. De-acetylation of histones by histone deacetylases (HDACs) causes histones to form tightly wound spindles, rendering associated DNA into a compact, inactive state unsuitable for transcription.72 Regulation of acetylation and deacetylation of non-histone factors by histone acetylases and HDACs may also play a role in protein stability, cell signaling and protein cellular localization and function.72 While the epigenetic mechanisms by which HDAC overexpression functions in tumorigenesis are not fully elucidated, HDAC inhibitors have shown promise in preclinical studies of MPNST,73, 74 Ewing sarcoma75 synovial sarcoma,76 fibrosarcoma,77 GIST,78 uterine sarcomas, 79 and dedifferentiated liposarcoma.80 Currently, HDACis are in phase I/II trials for sarcoma and other diseases as single agents and in combinations.
Direct epigenetic regulation of DNA involves transcriptional silencing via promoter hypermethylation. Hypermethylation of regulatory genes has been identified in a variety of sarcomas compared to non-neoplastic tissue.81 In particular, methylation of MGMT, a DNA repair factor, has been reported to be associated with aggressive tumors.81 In addition, hypermethylation of CEBPA, a transcription factor involved in adipocytic differentiation, has been recently identified in 24% of dedifferentiated liposarcoma. Moreover, demethylation of CEBPA in dedifferentiated liposarcoma cell lines and xenografts resulted in growth inhibition.80 Thus, DNA methyltransferases (DNMT), which are required to maintain the hypermethylated state, may represent therapeutic targets in selected sarcomas. As with HDACs, however, we do not know if arbitrary alterations in DNA or DNA binding proteins will be helpful or harmful until human clinical trials are performed.
Targets in Mitotic Machinery
Aurora kinases (AurKs) are a family of serine/threonine kinases required for progressive stages of mitosis and cell division. AurKs are involved in centrosome duplication, spindle formation, alignment of chromosomes on the mitotic spindle, progress through mitotic checkpoints and cytokinesis.82 Dysregulation of AurKs has been reported in a variety of carcinomas,82 but little data is available on their role in mesenchymal neoplasms. Nevertheless, targeted therapy against AurK A and B has shown encouraging anti-tumor effects in in vitro and xenograft studies using Ewing sarcoma-derived cell lines, which appear to overexpress both AurK A and B.83-85 These preclinical results suggest that aurora kinases may be candidate targets for directed therapy in some sarcomas.
Kinesins are another critical component of the mitotic spindle. Kinesins are microtubule dependent motor proteins with ATPase activity, which function in cell division and cellular transport. Kinesins are involved in nearly all aspects of cell division, from chromosome condensation and segregation, to spindle assembly and chromosome positioning, to cytokinesis.86 Altered expression of kinesins in seen in a variety of cancers, and has been associated with tumor progression.86 Although little is understood about the role and regulation of kinesins in sarcoma, kinesin inhibitors have shown activity in some sarcoma xenografts,87 and are currently in phase I trials.88
Targets in Cell Survival and Stress Response
Apoptosis and Cell Death
Several mechanisms exist by which sarcoma cells escape programmed cell death. The intrinsic p53 pathway is one of the most frequently inactivated pathways in sarcoma, TP53 is commonly mutated (inactivated) in complex karyotype sarcomas, including pleomorphic liposarcoma, leiomyosarcoma, and UPS/MFH.89 Alternatively, p53 activity, stability or subcellular localization may be dysregulated. HDM2, whose protein product binds to and inactivates p53 protein, is amplified in some soft tissue sarcoma, including atypical lipomatous tumor/well differentiated liposarcoma and in some bone tumors such as low grade parosteal and intramedullary osteosarcoma.90-92 Effects of p53 on the intrinsic cell-death pathway may also be negated by antiapoptotic regulatory factors, including bcl-2, which is overexpressed in up to 60% of sarcomas.63 Inhibition of p53-mediated apoptosis may protect tumor cells against chemotherapy and radiotherapy-mediated cell death. Accordingly, preliminary studies targeting this pathway using antisense mRNA-mediated knockdown of bcl-2 have shown that loss of bcl-2 may induce apoptosis or sensitize cells to death from conventional chemotherapy.93 Another tactic, which may be useful in well-differentiated/dedifferentiated liposarcoma, involves inhibiting Hdm2-p53 interactions, with agents such as RG7112.62
While the intrinsic cell death pathway responds to internal cellular stimuli, the extrinsic apoptotic pathway is triggered by death receptors located in the cell membrane, and is independent of p53 activation. Binding of Apo2L/TRAIL to the death receptors DR4/TRAIL-R1 or DR5/TRAIL-R2 triggers apoptosis via activation of caspase 8.94 Notably, Ewing sarcoma and rhabdomyosarcoma have both been shown to express TRAIL-receptors, potentially rendering them susceptible to TRAIL-mediated cell death.95 Moreover, recent studies with recombinant TRAIL, both in the laboratory and in phase I trials, have indicated a possible role for addition of TRAIL to chemotherapy regimens in some tumors, including chondrosarcoma, which is otherwise notoriously chemoresistant.96
Autophagy is another survival process known to play a role in sarcomagenesis. Autophagy is a cellular mechanism used to dispose of damaged organelles and reprocess proteins,97 which may either promote apoptosis or rescue of damaged cells, depending on cellular circumstances. In damaged cells or those which are rapidly proliferating, autophagy enables cells to optimize nutrient usage and streamline cellular machinery, and may also degrade depolarized mitochondria that might activate apoptosis.97 In in vitro studies of malignant peripheral nerve sheath tumors (MPNSTs) treated with HDACis, autophagy has been shown to promote tumor survival,73, 74 and inhibition of autophagy results in cell death.73 In GIST, which are normally resistant to apoptosis, inhibition of autophagy potentiates the effects of imatinib, leading to increased cell death.98 Autophagy blockade, in concert with conventional or targeted therapy, may therefore be a useful adjunct therapy.
Cellular response to stress is also mediated by a variety of chaperone and stress-response factors known as heat shock proteins (HSPs). HSPs act as key regulatory factors under conditions of cell stress, and may be pro or anti-apoptotic. For instance, HSP27 and HSP70 are both thought to serve antiapoptotic functions and may play a role in tumorigenesis.99 HSPs, in their role as intracellular chaperones may protect other factors from degradation, and may also interact with both HDACs and non-histone deacetylases.72 HSP90, in particular, has been reported to regulate stability of oncogenic KIT,100 and agents targeting HSP90 (IPI-504) showed promise in early studies.78, 100 However, in phase III studies, IPI-504 did not show a clinical effect in TKI-resistant GISTs. 101 Another HSP90 inhibitor, STA-9090, is currently under investigation.101
Targetable Proliferation Pathways
Sarcomas appear to be heavily reliant upon growth factor signaling for proliferation and survival. Despite this, high grade sarcomas have thus far shown little response to single agent therapy with TKIs,93, 102 although, as discussed above, a larger role may be seen for TKI therapy in translocation-associated sarcomas. Ultimately, because of redundancy and interconnectedness of survival and proliferation pathways, effective targeting may require simultaneous targeting of multiple pathways.
Cell Cycle Regulation
Dysregulated tumor proliferation requires abolition of normal cell-cycle checkpoints. The RB1 tumor suppressor gene functions as a G1/S phase checkpoint. Both inactivation of Rb or loss of p16INK4a, required to maintain activation of Rb, are frequently observed in UPS/MFH, while loss of RB1 is seen in leiomyosarcoma, malignant peripheral nerve sheath tumor (MPNST), and osteosarcoma.63, 91 Loss of p16INK4a is also associated with tumor progression. Attempts to target this pathway via inhibition of the pro-cell-cycle progression factors CDK4 and 6, have thus far been unsuccessful.103
Proliferation and Survival
Cellular proliferation and survival often involve signaling through the interconnected Ras and Akt pathways. The Akt pathway is normally activated by a growth factor binding to a RTK, but may also be activated by downstream events, including activating mutations in PIK3CA, as is seen in myxoid/round cell liposarcoma,104, 105 or by loss of the inhibitor PTEN, which has been reported in leiomyosarcoma, and is associated with aggressive behavior.63, 91 PI3K activation in turn leads to activation of Akt, and subsequently, the mTOR complexes (mTORC) 1 and 2, which regulates protein translation and other cellular processes.
A variety of RTK are overexpressed or constitutively activated in sarcoma, both in translocation associated sarcomas, as discussed above, and in karyotypically complex tumors. Because RTKs and their ligand growth factors are so frequently overexpressed in sarcomas, they are thought to represent attractive therapeutic targets. A wide array of TKIs with varying receptor specificity have been developed in recent years, although, thus far, the response rates in non-translocation sarcomas have been mixed.93, 102 For example, while EGFR is overexpressed in approximately 60% of soft tissue sarcomas, especially in sarcomas with complex karyotypes (eg, UPS/MFH, myxofibrosarcoma, MPNST, and leiomyosarcoma), as well as in synovial sarcoma,106 one phase II study of gefitinib, a specific TKI against EGFR, had poor results, with best response being stable disease only in 10/46 patients.107 In contrast, in preclinical studies of epithelioid sarcoma, which have been shown to overexpress EGFR,108 erlotinib-induced inhibition of EGFR alone was cytostatic, while combined inhibition of EGFR with mTOR had synergistic effects on growth inhibition.109
IGF-1R is also frequently overexpressed in both bone and soft tissue sarcomas, and has been reported to be associated with aggressive behavior in synovial sarcoma and alveolar rhabdomyosarcoma.110 Nevertheless, in sarcomas other than Ewing sarcoma, IGF-1R inhibition has not yet shown promising results.111 The RTK MET has been shown to be activated in MPNST, and in preclinical studies, inhibition of MET with the targeted agent XL184 led to reduction of metastatic potential.112 However, as yet, this agent has not entered the clinical testing stage for sarcoma. Minor activity of MET inhibitor ARQ197 was reported in abstract form in 2009 and it is unclear if future studies will be pursued examining this agent.113
One difficulty in targeting the Akt pathway via RTKs is that one tumor may express multiple RTKs and growth factors, and PI3K may also be activated by Ras signaling. Agents have been developed against the downstream effector mTOR with varying specificities against mTORC1 and mTORC2. One difficulty with mTORC1-inhibitors such as sirolimus and temsirolimus is that blockade of mTOR often leads to paradoxical increase in PI3K and Akt activity.114, 115 This homeostatic mechanism may be one reason there have not been more overt responses in cancer, including sarcomas, to mTOR inhibitors, with the largest phase II study of mTOR inhibitors in metastatic or unresectable soft tissue sarcoma demonstrating only a 2% RECIST response rate.116 To circumvent these homeostatic effects, newer agents in development include dual mTOR and PI3K inhibitors, as well as Akt-inhibitors. It is hoped that elucidation of the mechanisms by which mTOR inhibitors function in sensitive tumors, such as PEComas,117 will lead to improved usage of this family of agents.
The Ras pathway is involved in cell proliferation, survival, differentiation and angiogenesis as well as in motility and invasion, and may cross-activate the Akt pathway via PI3K. Activating RAS mutations have been found in leiomyosarcoma and UPS/MFH,91 and activation of downstream factors MEK and ERK have been described in UPS/MFH,118 and osteosarcoma,119 among others. BRAF, an intermediary in the Ras pathway, has been shown to be mutated in a minority of GIST lacking KIT or PDGFRA mutations.120 V600E mutant B-raf, as found in a tiny subset of GIST, may be targeted by vemurafenib, which has not been well-studied in sarcoma. B-raf is at least partially inhibited by sorafenib, which also has effects on multiple RTKs. Sorafenib has shown antitumor effects in vitro in MPNST, synovial sarcoma and chondrosarcoma via the Ras pathway,121-123 as well as in desmoid tumors,124 and showed minor activity against angiosarcoma in a phase II trial.93, 125 The data with vemurafenib in V600E mutant melanoma indicate the importance of the specificity of the inhibitor for the driving mutant kinase.126 However, the relatively short time to resistance to RAF inhibitors has led many researchers to pursue other inhibitors of the Ras pathway, including agents targeting MEK and ERK. MEK inhibitors have shown promise in preclinical studies,119, 127 and several are in phase I/II trials.
Angiogenesis
Recruitment of new vessels is required for tumors to both grow and metastasize. Thus, targeting of proangiogenic growth factors has long been heralded as a potential therapy for sarcoma, and other tumor types. In addition to their roles in autocrine and paracrine proliferation pathways, VEGF and VEGFR have specific proangiogenic effects and are thought to be required for recruitment of new vessels into a growing tumor.128, 129 VEGF is overexpressed in about a quarter of all soft tissue sarcoma, including epithelioid sarcoma, alveolar soft part sarcoma, UPS/MFH, DFSP, and leiomyosarcoma,128 and has been associated with high metastatic potential.91, 128 VEGF and VEGFR may be targeted by a variety of drugs, including bevacizumab, sunitinib, and pazopanib. Unfortunately, in a number of phase I and II trials, responses to these agents have proven mixed, at best, even in vascular malignancies such as angiosarcoma and hemangioendothelioma.102, 125 Others have reported that bevacizumab in combination with doxorubicin provided at least stable disease for 11/17 patients with metastatic sarcoma, but with significant cardiac toxicity for some.130 Thus far, single agent therapy targeting VEFGR does not appear promising, likely due to the complex, multistep nature of angiogenesis induction.
Other pro-angiogenic factors that may prove targetable include basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), and transforming growth factor alpha (TGF-α).91 Conversely, thrombospondin-1, an inhibitor of VEGF, VEGFR and IL-8, has been reported to be decreased in some sarcomas. 131, 132 ABT-510, a thrombospondin-1 mimetic, has been assessed in a phase II trial, with about half of the treated tumors showing stabilization as best response.131
Other targets
The Notch family of receptors has been implicated in control of differentiation. Preclinical studies have suggested that inhibition of Notch may reduce the invasiveness of both osteosarcoma and rhabdomyosarcoma,133, 134 and promote differentiation of rhabdomyosarcoma.135, 136 However, the effects of Notch signaling appear to be tumor type-specific, as Notch family members may act as either oncogenes or tumor suppressors, and activation of Notch in Ewing sarcoma cell lines led to growth inhibition.137 The gamma secretase inhibitor RO4929097 which blocks notch signaling by preventing cleavage of the activated intracellular domain of Notch from the transmembrane domain is currently in phase I/II trials. It remains to be seen, however if this therapy will have pro- or anti- proliferative effects in a population of unselected tumors.
Another pathway reported to be upregulated in some sarcomas is the hedgehog pathway, which normally plays a critical role in embryogenesis. Activation of downstream targets of the hedgehog pathway has been reported in embryonal rhabdomyosarcomas.138, 139 Moreover, inhibition of hedgehog pathway signaling reduced proliferation of embryonal rhabdomyosarcoma cell lines.140 These findings are the basis of a phase I/II trial combining the hedgehog signaling inhibitor GDC-0449 with a notch signaling inhibitor in metastatic sarcoma.
Conclusion
Targeted therapy, i.e. agents beyond standard cytotoxic chemotherapy agents, offers the hope for improved treatment of this heterogeneous and difficult to manage group of malignancies. While some sarcoma subtypes have responded brilliantly, other common diagnoses such as leiomyosarcoma and UPS have largely failed to respond to the available portfolio of agents to date. Fortunately, an increasing number of agents are under investigation, primarily in phase I/II trials (Table 1), inhibiting any number of cellular kinases and other processes responsible for tumor cell survival. As our understanding of the mechanisms of tumorigenesis and the pathways required for sarcoma survival and metastasis increases, it is hoped that so too will our ability to correctly identify therapeutic targets and develop effective drugs. Future clinical trials will need to more specifically select patients with appropriate molecular alterations for the therapy tested, and examine groups of specific agents to better achieve the goal of truly personalized treatment for sarcoma.
Table 1.
Selected approved and investigational targeted agents in sarcoma
| Agent | Status | Specificity |
|---|---|---|
| Tyrosine Kinase Inhibitors | ||
| Imatinib mesylate | FDA approved for GIST, DFSP | kit, abl, PDGFR |
| Sunitinib | FDA approved GIST (2nd line) | Multiple tyrosine kinases: PDGFR, kit, RET, CSF-1R, Flt3, VEGFR |
| Sorafenib | Phase II125, 141 | Multiple kinases: kit, VEGFR, PDGFR, raf |
| Gefitinib | Phase II107 | EGFR |
| R1507 | Phase I/II142 | IGF-1R |
| Figitumumab | Phase I143 | IGF-1R |
| Crizotinib | Phase I26 | Alk/ Met |
| ARQ197 | Phase II113 | Met |
| mTORC1 Inhibitors | ||
| Sirolimus | Phase II144 | mTORC1 |
| Temsirolimus | Phase II145 | mTORC1 |
| Ridaforolomus (deferolimus) | Phase II/III116 | mTORC1 |
| Everolimus | Phase I/II143 | mTORC1 |
| mTORC catalytic domain inhibitors | ||
| AZD8055 | Preclinical/Phase I146 | mTORC1/mTORC2 |
| PI3K Inhibitors | ||
| GSK1059615 | Phase I147 | PI3K |
| Dual mTOR/PI3K Inhibitors | ||
| BEZ235 | Phase I148 | PI3K class I, mTOR |
| AKT Inhibitors | ||
| MK2206 | Phase I149 | Akt |
| Ras Pathway Inhibitors | ||
| Selumetinib | Phase II150 | Mek |
| Anti-Angiogenic Agents | ||
| Bevacizumab | Phase II130, 151 | VEGFR |
| Pazopanib | Phase II152 | VEGFR, PDGFR, kit, |
| Cediranib | Phase I153 | VEGFR |
| Brivanib | Phase II154 | VEGFR, FGFR |
| ABT-510 | Phase II131 | Thrombospondin mimetic |
| Pro-Apoptotic Agents | ||
| RG7112 (RO5045337) | Phase I/II155 | Hdm2-p53 interactions |
| Dulamnermin (r-hu anti-Apo2/TRAIL) | Phase I96 | TRAIL-R |
| Oblimersen (G3139) | Phase I156 | Bcl-2 (antisense oligonucleotide) |
| Cell Cycle Progression/Proliferation Inhibitors | ||
| PD0332991 | Phase I/II157 | Cdk4 |
| Ispinesib | Phase I87, 88 | Kinesin |
| MLN8054, MLN8237 | Preclinical/Phase I126 | AurkA |
| Epigenetic Modifier Inhibitors | ||
| Panobinostat | Phase II158 | HDAC |
| Vorinostat | Phase II159 | HDAC |
| Azacytidine | Phase I/II160 | DNA methyltransferase |
Footnotes
Disclosures: None
References
- 1.Fletcher CDM, Rydholm A, Singer S, et al. Soft tissue tumours: Epidemiology, clinical features, histopathological typing and grading. In: Fletcher CDM, Unni KK, Mertens F, editors. World Health Organization classification of tumours Pathology and genetics of tumours of soft tissue and bone. IARC Press; Lyon: 2002. pp. 9–18. [Google Scholar]
- 2.Steen S, Stephenson G. Current treatment of soft tissue sarcoma. Proc (Bayl Univ Med Cent) 2008;21:392–396. doi: 10.1080/08998280.2008.11928435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demetri GD, Antonia S, Benjamin RS, et al. Soft tissue sarcoma. J Natl Compr Canc Netw. 2010;8:630–674. doi: 10.6004/jnccn.2010.0049. [DOI] [PubMed] [Google Scholar]
- 4.Clark MA, Fisher C, Judson I, et al. Soft-tissue sarcomas in adults. N Engl J Med. 2005;353:701–711. doi: 10.1056/NEJMra041866. [DOI] [PubMed] [Google Scholar]
- 5.Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 6.Orphan Drug Act, Pub L No 97-414. 1983 [Google Scholar]
- 7.Haffner ME. Adopting orphan drugs--two dozen years of treating rare diseases. N Engl J Med. 2006;354:445–447. doi: 10.1056/NEJMp058317. [DOI] [PubMed] [Google Scholar]
- 8.Braiteh F, Kurzrock R. Uncommon tumors and exceptional therapies: paradox or paradigm? Mol Cancer Ther. 2007;6:1175–1179. doi: 10.1158/1535-7163.MCT-06-0674. [DOI] [PubMed] [Google Scholar]
- 9.Demicco EG, Lazar AJ. Clinicopathologic considerations: how can we fine tune our approach to sarcoma. Seminars in Oncology. 2011;38:S3–S18. doi: 10.1053/j.seminoncol.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Ducimetiere F, Lurkin A, Ranchere-Vince D, et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One. 2011;6:e20294. doi: 10.1371/journal.pone.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitelman F. Recurrent chromosome aberrations in cancer. Mutat Res. 2000;462:247–253. doi: 10.1016/s1383-5742(00)00006-5. [DOI] [PubMed] [Google Scholar]
- 12.Fisher C. Soft tissue sarcomas with non-EWS translocations: molecular genetic features and pathologic and clinical correlations. Virchows Arch. 2010;456:153–166. doi: 10.1007/s00428-009-0776-0. [DOI] [PubMed] [Google Scholar]
- 13.Romeo S, Dei Tos AP. Soft tissue tumors associated with EWSR1 translocation. Virchows Arch. 2010;456:219–234. doi: 10.1007/s00428-009-0854-3. [DOI] [PubMed] [Google Scholar]
- 14.Berner JM, Forus A, Elkahloun A, et al. Separate amplified regions encompassing CDK4 and MDM2 in human sarcomas. Genes Chromosomes Cancer. 1996;17:254–259. doi: 10.1002/(SICI)1098-2264(199612)17:4<254::AID-GCC7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Guo T, Zhang L, Chang NE, et al. Consistent MYC and FLT4 gene amplification in radiation-induced angiosarcoma but not in other radiation-associated atypical vascular lesions. Genes Chromosomes Cancer. 2011;50:25–33. doi: 10.1002/gcc.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hachitanda Y, Toyoshima S, Akazawa K, et al. N-myc gene amplification in rhabdomyosarcoma detected by fluorescence in situ hybridization: its correlation with histologic features. Mod Pathol. 1998;11:1222–1227. [PubMed] [Google Scholar]
- 17.Ueda T, Healey JH, Huvos AG, et al. Amplification of the MYC Gene in Osteosarcoma Secondary to Paget’s Disease of Bone. Sarcoma. 1997;1:131–134. doi: 10.1080/13577149778209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrios C, Castresana JS, Ruiz J, et al. Amplification of the c-myc proto-oncogene in soft tissue sarcomas. Oncology. 1994;51:13–17. doi: 10.1159/000227302. [DOI] [PubMed] [Google Scholar]
- 19.Gustafson WC, Weiss WA. Myc proteins as therapeutic targets. Oncogene. 2010;29:1249–1259. doi: 10.1038/onc.2009.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nesbit CE, Tersak JM, Prochownik EV. MYC oncogenes and human neoplastic disease. Oncogene. 1999;18:3004–3016. doi: 10.1038/sj.onc.1202746. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Hisaoka M, Shimajiri S, et al. Detection of COL1A1-PDGFB fusion transcripts in dermatofibrosarcoma protuberans by reverse transcription-polymerase chain reaction using archival formalin-fixed, paraffin-embedded tissues. Diagn Mol Pathol. 1999;8:113–119. doi: 10.1097/00019606-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Rutkowski P, Debiec-Rychter M, Nowecki Z, et al. Treatment of advanced dermatofibrosarcoma protuberans with imatinib mesylate with or without surgical resection. J Eur Acad Dermatol Venereol. 2011;25:264–270. doi: 10.1111/j.1468-3083.2010.03774.x. [DOI] [PubMed] [Google Scholar]
- 23.Cook JR, Dehner LP, Collins MH, et al. Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumor: a comparative immunohistochemical study. Am J Surg Pathol. 2001;25:1364–1371. doi: 10.1097/00000478-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Coffin CM, Patel A, Perkins S, et al. ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Pathol. 2001;14:569–576. doi: 10.1038/modpathol.3880352. [DOI] [PubMed] [Google Scholar]
- 25.Griffin CA, Hawkins AL, Dvorak C, et al. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res. 1999;59:2776–2780. [PubMed] [Google Scholar]
- 26.Butrynski JE, D’Adamo DR, Hornick JL, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med. 2010;363:1727–1733. doi: 10.1056/NEJMoa1007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dal Cin P, Sciot R, Samson I, et al. Cytogenetic characterization of tenosynovial giant cell tumors (nodular tenosynovitis) Cancer Res. 1994;54:3986–3987. [PubMed] [Google Scholar]
- 28.West RB, Rubin BP, Miller MA, et al. A landscape effect in tenosynovial giant-cell tumor from activation of CSF1 expression by a translocation in a minority of tumor cells. Proc Natl Acad Sci U S A. 2006;103:690–695. doi: 10.1073/pnas.0507321103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravi V, Wang WL, Lewis VO. Treatment of tenosynovial giant cell tumor and pigmented villonodular synovitis. Curr Opin Oncol. 2011;23:361–366. doi: 10.1097/CCO.0b013e328347e1e3. [DOI] [PubMed] [Google Scholar]
- 30.Kuroda M, Ishida T, Takanashi M, et al. Oncogenic transformation and inhibition of adipocytic conversion of preadipocytes by TLS/FUS-CHOP type II chimeric protein. Am J Pathol. 1997;151:735–744. [PMC free article] [PubMed] [Google Scholar]
- 31.Bento C, Andersson MK, Aman P. DDIT3/CHOP and the sarcoma fusion oncoprotein FUS-DDIT3/TLS-CHOP bind cyclin-dependent kinase 2. BMC Cell Biol. 2009;10:89. doi: 10.1186/1471-2121-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forni C, Minuzzo M, Virdis E, et al. Trabectedin (ET-743) promotes differentiation in myxoid liposarcoma tumors. Mol Cancer Ther. 2009;8:449–457. doi: 10.1158/1535-7163.MCT-08-0848. [DOI] [PubMed] [Google Scholar]
- 33.Grosso F, Sanfilippo R, Virdis E, et al. Trabectedin in myxoid liposarcomas (MLS): a long-term analysis of a single-institution series. Ann Oncol. 2009;20:1439–1444. doi: 10.1093/annonc/mdp004. [DOI] [PubMed] [Google Scholar]
- 34.Toretsky JA, Kalebic T, Blakesley V, et al. The insulin-like growth factor-I receptor is required for EWS/FLI-1 transformation of fibroblasts. J Biol Chem. 1997;272:30822–30827. doi: 10.1074/jbc.272.49.30822. [DOI] [PubMed] [Google Scholar]
- 35.El-Badry OM, Minniti C, Kohn EC, et al. Insulin-like growth factor II acts as an autocrine growth and motility factor in human rhabdomyosarcoma tumors. Cell Growth Differ. 1990;1:325–331. [PubMed] [Google Scholar]
- 36.Yee D, Favoni RE, Lebovic GS, et al. Insulin-like growth factor I expression by tumors of neuroectodermal origin with the t(11;22) chromosomal translocation. A potential autocrine growth factor. J Clin Invest. 1990;86:1806–1814. doi: 10.1172/JCI114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prieur A, Tirode F, Cohen P, et al. EWS/FLI-1 silencing and gene profiling of Ewing cells reveal downstream oncogenic pathways and a crucial role for repression of insulin-like growth factor binding protein 3. Mol Cell Biol. 2004;24:7275–7283. doi: 10.1128/MCB.24.16.7275-7283.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zwerner JP, May WA. PDGF-C is an EWS/FLI induced transforming growth factor in Ewing family tumors. Oncogene. 2001;20:626–633. doi: 10.1038/sj.onc.1204133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ayalon D, Glaser T, Werner H. Transcriptional regulation of IGF-I receptor gene expression by the PAX3-FKHR oncoprotein. Growth Horm IGF Res. 2001;11:289–297. doi: 10.1054/ghir.2001.0244. [DOI] [PubMed] [Google Scholar]
- 40.Ginsberg JP, Davis RJ, Bennicelli JL, et al. Up-regulation of MET but not neural cell adhesion molecule expression by the PAX3-FKHR fusion protein in alveolar rhabdomyosarcoma. Cancer Res. 1998;58:3542–3546. [PubMed] [Google Scholar]
- 41.Mercado GE, Xia SJ, Zhang C, et al. Identification of PAX3-FKHR-regulated genes differentially expressed between alveolar and embryonal rhabdomyosarcoma: focus on MYCN as a biologically relevant target. Genes Chromosomes Cancer. 2008;47:510–520. doi: 10.1002/gcc.20554. [DOI] [PubMed] [Google Scholar]
- 42.Lazar AJ, Lahat G, Myers SE, et al. Validation of potential therapeutic targets in alveolar soft part sarcoma: an immunohistochemical study utilizing tissue microarray. Histopathology. 2009;55:750–755. doi: 10.1111/j.1365-2559.2009.03436.x. [DOI] [PubMed] [Google Scholar]
- 43.Tsuda M, Davis IJ, Argani P, et al. TFE3 fusions activate MET signaling by transcriptional up-regulation, defining another class of tumors as candidates for therapeutic MET inhibition. Cancer Res. 2007;67:919–929. doi: 10.1158/0008-5472.CAN-06-2855. [DOI] [PubMed] [Google Scholar]
- 44.Davis IJ, McFadden AW, Zhang Y, et al. Identification of the receptor tyrosine kinase c-Met and its ligand, hepatocyte growth factor, as therapeutic targets in clear cell sarcoma. Cancer Res. 2010;70:639–645. doi: 10.1158/0008-5472.CAN-09-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurzrock R, Patnaik A, Aisner J, et al. A phase I study of weekly R1507, a human monoclonal antibody insulin-like growth factor-I receptor antagonist, in patients with advanced solid tumors. Clin Cancer Res. 2010;16:2458–2465. doi: 10.1158/1078-0432.CCR-09-3220. [DOI] [PubMed] [Google Scholar]
- 46.Tolcher AW, Sarantopoulos J, Patnaik A, et al. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J Clin Oncol. 2009;27:5800–5807. doi: 10.1200/JCO.2009.23.6745. [DOI] [PubMed] [Google Scholar]
- 47.Olmos D, Postel-Vinay S, Molife LR, et al. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing’s sarcoma: a phase 1 expansion cohort study. Lancet Oncol. 2010;11:129–135. doi: 10.1016/S1470-2045(09)70354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagayama S, Katagiri T, Tsunoda T, et al. Genome-wide analysis of gene expression in synovial sarcomas using a cDNA microarray. Cancer Res. 2002;62:5859–5866. [PubMed] [Google Scholar]
- 49.Ishibe T, Nakayama T, Okamoto T, et al. Disruption of fibroblast growth factor signal pathway inhibits the growth of synovial sarcomas: potential application of signal inhibitors to molecular target therapy. Clin Cancer Res. 2005;11:2702–2712. doi: 10.1158/1078-0432.CCR-04-2057. [DOI] [PubMed] [Google Scholar]
- 50.Kazanowska B, Reich A, Stegmaier S, et al. Pax3-fkhr and pax7-fkhr fusion genes impact outcome of alveolar rhabdomyosarcoma in children. Fetal Pediatr Pathol. 2007;26:17–31. doi: 10.1080/15513810701394702. [DOI] [PubMed] [Google Scholar]
- 51.Sorensen PH, Lynch JC, Qualman SJ, et al. PAX3-FKHR and PAX7-FKHR gene fusions are prognostic indicators in alveolar rhabdomyosarcoma: a report from the children’s oncology group. J Clin Oncol. 2002;20:2672–2679. doi: 10.1200/JCO.2002.03.137. [DOI] [PubMed] [Google Scholar]
- 52.de Alava E, Kawai A, Healey JH, et al. EWS-FLI1 fusion transcript structure is an independent determinant of prognosis in Ewing’s sarcoma. J Clin Oncol. 1998;16:1248–1255. doi: 10.1200/JCO.1998.16.4.1248. [DOI] [PubMed] [Google Scholar]
- 53.Zoubek A, Dockhorn-Dworniczak B, Delattre O, et al. Does expression of different EWS chimeric transcripts define clinically distinct risk groups of Ewing tumor patients? J Clin Oncol. 1996;14:1245–1251. doi: 10.1200/JCO.1996.14.4.1245. [DOI] [PubMed] [Google Scholar]
- 54.van Doorninck JA, Ji L, Schaub B, et al. Current treatment protocols have eliminated the prognostic advantage of type 1 fusions in Ewing sarcoma: a report from the Children’s Oncology Group. J Clin Oncol. 2010;28:1989–1994. doi: 10.1200/JCO.2009.24.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Panagopoulos I, Mertens F, Isaksson M, et al. Clinical impact of molecular and cytogenetic findings in synovial sarcoma. Genes Chromosomes Cancer. 2001;31:362–372. doi: 10.1002/gcc.1155. [DOI] [PubMed] [Google Scholar]
- 56.Ladanyi M, Antonescu CR, Leung DH, et al. Impact of SYT-SSX fusion type on the clinical behavior of synovial sarcoma: a multi-institutional retrospective study of 243 patients. Cancer Res. 2002;62:135–140. [PubMed] [Google Scholar]
- 57.Stegmaier S, Poremba C, Schaefer KL, et al. Prognostic value of PAX-FKHR fusion status in alveolar rhabdomyosarcoma: a report from the cooperative soft tissue sarcoma study group (CWS) Pediatr Blood Cancer. 2011;57:406–414. doi: 10.1002/pbc.22958. [DOI] [PubMed] [Google Scholar]
- 58.Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 59.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 60.Wang CM, Huang K, Zhou Y, et al. Molecular mechanisms of secondary imatinib resistance in patients with gastrointestinal stromal tumors. J Cancer Res Clin Oncol. 2010;136:1065–1071. doi: 10.1007/s00432-009-0753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 62.Cassier PA, Labidi-Galy SI, Heudel P, et al. Therapeutic pipeline for soft-tissue sarcoma. Expert Opin Pharmacother. 2011;12:2479–2491. doi: 10.1517/14656566.2011.604633. [DOI] [PubMed] [Google Scholar]
- 63.Guillou L, Aurias A. Soft tissue sarcomas with complex genomic profiles. Virchows Arch. 2010;456:201–217. doi: 10.1007/s00428-009-0853-4. [DOI] [PubMed] [Google Scholar]
- 64.Murnane JP. Telomere loss as a mechanism for chromosome instability in human cancer. Cancer Res. 2010;70:4255–4259. doi: 10.1158/0008-5472.CAN-09-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stephens PJ, Greenman CD, Fu B, et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell. 2011;144:27–40. doi: 10.1016/j.cell.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matsuo T, Shimose S, Kubo T, et al. Telomeres and telomerase in sarcomas. Anticancer Res. 2009;29:3833–3836. [PubMed] [Google Scholar]
- 67.Venturini L, Motta R, Gronchi A, et al. Prognostic relevance of ALT-associated markers in liposarcoma: a comparative analysis. BMC Cancer. 2010;10:254. doi: 10.1186/1471-2407-10-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lafferty-Whyte K, Cairney CJ, Will MB, et al. A gene expression signature classifying telomerase and ALT immortalization reveals an hTERT regulatory network and suggests a mesenchymal stem cell origin for ALT. Oncogene. 2009;28:3765–3774. doi: 10.1038/onc.2009.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sasaki T, Tazawa H, Hasei J, et al. Preclinical evaluation of telomerase-specific oncolytic virotherapy for human bone and soft tissue sarcomas. Clin Cancer Res. 2011;17:1828–1838. doi: 10.1158/1078-0432.CCR-10-2066. [DOI] [PubMed] [Google Scholar]
- 70.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 71.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 72.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 73.Lopez G, Torres K, Lev D. Autophagy blockade enhances HDAC inhibitors’ pro-apoptotic effects: potential implications for the treatment of a therapeutic-resistant malignancy. Autophagy. 2011;7:440–441. doi: 10.4161/auto.7.4.14680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lopez G, Torres K, Liu J, et al. Autophagic survival in resistance to histone deacetylase inhibitors: novel strategies to treat malignant peripheral nerve sheath tumors. Cancer Res. 2011;71:185–196. doi: 10.1158/0008-5472.CAN-10-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sakimura R, Tanaka K, Nakatani F, et al. Antitumor effects of histone deacetylase inhibitor on Ewing’s family tumors. Int J Cancer. 2005;116:784–792. doi: 10.1002/ijc.21069. [DOI] [PubMed] [Google Scholar]
- 76.Ito T, Ouchida M, Morimoto Y, et al. Significant growth suppression of synovial sarcomas by the histone deacetylase inhibitor FK228 in vitro and in vivo. Cancer Lett. 2005;224:311–319. doi: 10.1016/j.canlet.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 77.Sampson ER, Amin V, Schwarz EM, et al. The histone deacetylase inhibitor vorinostat selectively sensitizes fibrosarcoma cells to chemotherapy. J Orthop Res. 2011;29:623–632. doi: 10.1002/jor.21274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Muhlenberg T, Zhang Y, Wagner AJ, et al. Inhibitors of deacetylases suppress oncogenic KIT signaling, acetylate HSP90, and induce apoptosis in gastrointestinal stromal tumors. Cancer Res. 2009;69:6941–6950. doi: 10.1158/0008-5472.CAN-08-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hrzenjak A, Moinfar F, Kremser ML, et al. Histone deacetylase inhibitor vorinostat suppresses the growth of uterine sarcomas in vitro and in vivo. Mol Cancer. 2010;9:49. doi: 10.1186/1476-4598-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taylor BS, DeCarolis PL, Angeles CV, et al. Frequent alterations and epigenetic silencing of differentiation pathway genes in structurally rearranged liposarcomas. Cancer Discovery. 2011;1:587–597. doi: 10.1158/2159-8290.CD-11-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kawaguchi K, Oda Y, Saito T, et al. DNA hypermethylation status of multiple genes in soft tissue sarcomas. Mod Pathol. 2006;19:106–114. doi: 10.1038/modpathol.3800502. [DOI] [PubMed] [Google Scholar]
- 82.Carvajal RD, Tse A, Schwartz GK. Aurora kinases: new targets for cancer therapy. Clin Cancer Res. 2006;12:6869–6875. doi: 10.1158/1078-0432.CCR-06-1405. [DOI] [PubMed] [Google Scholar]
- 83.Wakahara K, Ohno T, Kimura M, et al. EWS-Fli1 up-regulates expression of the Aurora A and Aurora B kinases. Mol Cancer Res. 2008;6:1937–1945. doi: 10.1158/1541-7786.MCR-08-0054. [DOI] [PubMed] [Google Scholar]
- 84.Winter GE, Rix U, Lissat A, et al. An integrated chemical biology approach identifies specific vulnerability of Ewing’s sarcoma to combined inhibition of Aurora kinases A and B. Mol Cancer Ther. 2011;10:1846–1856. doi: 10.1158/1535-7163.MCT-11-0100. [DOI] [PubMed] [Google Scholar]
- 85.Carol H, Boehm I, Reynolds CP, et al. Efficacy and pharmacokinetic/pharmacodynamic evaluation of the Aurora kinase A inhibitor MLN8237 against preclinical models of pediatric cancer. Cancer Chemother Pharmacol. 2011;68:1291–1304. doi: 10.1007/s00280-011-1618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu Y, Feng YM. The role of kinesin family proteins in tumorigenesis and progression: potential biomarkers and molecular targets for cancer therapy. Cancer. 2010;116:5150–5160. doi: 10.1002/cncr.25461. [DOI] [PubMed] [Google Scholar]
- 87.Carol H, Lock R, Houghton PJ, et al. Initial testing (stage 1) of the kinesin spindle protein inhibitor ispinesib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2009;53:1255–1263. doi: 10.1002/pbc.22056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Souid AK, Dubowy RL, Ingle AM, et al. A pediatric phase I trial and pharmacokinetic study of ispinesib: a Children’s Oncology Group phase I consortium study. Pediatr Blood Cancer. 2010;55:1323–1328. doi: 10.1002/pbc.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guillou L, Hogendoorn PC, Bosman FT. Soft tissue sarcomas: introduction to the Virchows Archiv review issue. Virchows Arch. 2010;456:107–109. doi: 10.1007/s00428-009-0875-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mejia-Guerrero S, Quejada M, Gokgoz N, et al. Characterization of the 12q15 MDM2 and 12q13-14 CDK4 amplicons and clinical correlations in osteosarcoma. Genes Chromosomes Cancer. 2010;49:518–525. doi: 10.1002/gcc.20761. [DOI] [PubMed] [Google Scholar]
- 91.Mahalingam D, Mita A, Sankhala K, et al. Targeting sarcomas: novel biological agents and future perspectives. Curr Drug Targets. 2009;10:937–949. doi: 10.2174/138945009789577990. [DOI] [PubMed] [Google Scholar]
- 92.Wunder JS, Eppert K, Burrow SR, et al. Co-amplification and overexpression of CDK4, SAS and MDM2 occurs frequently in human parosteal osteosarcomas. Oncogene. 1999;18:783–788. doi: 10.1038/sj.onc.1202346. [DOI] [PubMed] [Google Scholar]
- 93.Kasper B, Gil T, Awada A. Treatment of patients with advanced soft tissue sarcoma: disappointment or challenge? Curr Opin Oncol. 2007;19:336–340. doi: 10.1097/CCO.0b013e32812143ef. [DOI] [PubMed] [Google Scholar]
- 94.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 95.Wang Y, Mandal D, Wang S, et al. Platelet-derived growth factor receptor beta inhibition increases tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) sensitivity: imatinib and TRAIL dual therapy. Cancer. 2010;116:3892–3902. doi: 10.1002/cncr.25107. [DOI] [PubMed] [Google Scholar]
- 96.Herbst RS, Eckhardt SG, Kurzrock R, et al. Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. J Clin Oncol. 2010;28:2839–2846. doi: 10.1200/JCO.2009.25.1991. [DOI] [PubMed] [Google Scholar]
- 97.Codogno P, Meijer AJ. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ. 2005;12(Suppl 2):1509–1518. doi: 10.1038/sj.cdd.4401751. [DOI] [PubMed] [Google Scholar]
- 98.Gupta A, Roy S, Lazar AJ, et al. Autophagy inhibition and antimalarials promote cell death in gastrointestinal stromal tumor (GIST) Proc Natl Acad Sci U S A. 2010;107:14333–14338. doi: 10.1073/pnas.1000248107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Garrido C, Brunet M, Didelot C, et al. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5:2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- 100.Bauer S, Yu LK, Demetri GD, et al. Heat shock protein 90 inhibition in imatinib-resistant gastrointestinal stromal tumor. Cancer Res. 2006;66:9153–9161. doi: 10.1158/0008-5472.CAN-06-0165. [DOI] [PubMed] [Google Scholar]
- 101.Montemurro M, Bauer S. Treatment of gastrointestinal stromal tumor after imatinib and sunitinib. Curr Opin Oncol. 2011;23:367–372. doi: 10.1097/CCO.0b013e3283477ac2. [DOI] [PubMed] [Google Scholar]
- 102.Park MS, Ravi V, Araujo DM. Inhibiting the VEGF-VEGFR pathway in angiosarcoma, epithelioid hemangioendothelioma, and hemangiopericytoma/solitary fibrous tumor. Curr Opin Oncol. 2010;22:351–355. doi: 10.1097/CCO.0b013e32833aaad4. [DOI] [PubMed] [Google Scholar]
- 103.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 104.Barretina J, Taylor BS, Banerji S, et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet. 2010;42:715–721. doi: 10.1038/ng.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Demicco EG, Torres KE, Ghadimi MP, et al. Involvement of the PI3K/Akt pathway in myxoid/round cell liposarcoma. Mod Pathol. 2011 [Google Scholar]
- 106.Sato O, Wada T, Kawai A, et al. Expression of epidermal growth factor receptor, ERBB2 and KIT in adult soft tissue sarcomas: a clinicopathologic study of 281 cases. Cancer. 2005;103:1881–1890. doi: 10.1002/cncr.20986. [DOI] [PubMed] [Google Scholar]
- 107.Ray-Coquard I, Le Cesne A, Whelan JS, et al. A phase II study of gefitinib for patients with advanced HER-1 expressing synovial sarcoma refractory to doxorubicin-containing regimens. Oncologist. 2008;13:467–473. doi: 10.1634/theoncologist.2008-0065. [DOI] [PubMed] [Google Scholar]
- 108.Cascio MJ, O’Donnell RJ, Horvai AE. Epithelioid sarcoma expresses epidermal growth factor receptor but gene amplification and kinase domain mutations are rare. Mod Pathol. 2010;23:574–580. doi: 10.1038/modpathol.2010.2. [DOI] [PubMed] [Google Scholar]
- 109.Xie X, Ghadimi MP, Young ED, et al. Combining EGFR and mTOR blockade for the treatment of epithelioid sarcoma. Clin Cancer Res. 2011;17:5901–5912. doi: 10.1158/1078-0432.CCR-11-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rikhof B, de Jong S, Suurmeijer AJ, et al. The insulin-like growth factor system and sarcomas. J Pathol. 2009;217:469–482. doi: 10.1002/path.2499. [DOI] [PubMed] [Google Scholar]
- 111.Subbiah V, Kurzrock R. Phase 1 clinical trials for sarcomas: the cutting edge. Curr Opin Oncol. 2011;23:352–360. doi: 10.1097/CCO.0b013e3283477a94. [DOI] [PubMed] [Google Scholar]
- 112.Torres KE, Zhu QS, Bill K, et al. Activated MET is a molecular prognosticator and potential therapeutic target for malignant peripheral nerve sheath tumors. Clin Cancer Res. 2011;17:3943–3955. doi: 10.1158/1078-0432.CCR-11-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Goldberg J, Demetri GD, Choy E, et al. Preliminary results from a phase II study of ARQ 197 in patients with microphthalmia transcription factor family (MiT)-associated tumors. Journal of Clinical Oncology. 2009;27(suppl) abstr 10502. [Google Scholar]
- 114.Zou CY, Smith KD, Zhu QS, et al. Dual targeting of AKT and mammalian target of rapamycin: a potential therapeutic approach for malignant peripheral nerve sheath tumor. Mol Cancer Ther. 2009;8:1157–1168. doi: 10.1158/1535-7163.MCT-08-1008. [DOI] [PubMed] [Google Scholar]
- 115.Wan X, Harkavy B, Shen N, et al. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 116.Chawla SP, Staddon AP, Baker LH, et al. Phase II Study of the Mammalian Target of Rapamycin Inhibitor Ridaforolimus in Patients With Advanced Bone and Soft Tissue Sarcomas. J Clin Oncol. 2012;30:78–84. doi: 10.1200/JCO.2011.35.6329. [DOI] [PubMed] [Google Scholar]
- 117.Wagner AJ, Malinowska-Kolodziej I, Morgan JA, et al. Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol. 2010;28:835–840. doi: 10.1200/JCO.2009.25.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lahat G, Zhang P, Zhu QS, et al. The expression of c-Met pathway components in unclassified pleomorphic sarcoma/malignant fibrous histiocytoma (UPS/MFH): a tissue microarray study. Histopathology. 2011;59:556–561. doi: 10.1111/j.1365-2559.2011.03946.x. [DOI] [PubMed] [Google Scholar]
- 119.Sasaki K, Hitora T, Nakamura O, et al. The role of MAPK pathway in bone and soft tissue tumors. Anticancer Res. 2011;31:549–553. [PubMed] [Google Scholar]
- 120.Agaram NP, Wong GC, Guo T, et al. Novel V600E BRAF mutations in imatinib-naive and imatinib-resistant gastrointestinal stromal tumors. Genes Chromosomes Cancer. 2008;47:853–859. doi: 10.1002/gcc.20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lu X, Tang X, Guo W, et al. Sorafenib induces growth inhibition and apoptosis of human chondrosarcoma cells by blocking the RAF/ERK/MEK pathway. J Surg Oncol. 2010;102:821–826. doi: 10.1002/jso.21661. [DOI] [PubMed] [Google Scholar]
- 122.Ambrosini G, Cheema HS, Seelman S, et al. Sorafenib inhibits growth and mitogen-activated protein kinase signaling in malignant peripheral nerve sheath cells. Mol Cancer Ther. 2008;7:890–896. doi: 10.1158/1535-7163.MCT-07-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Peng CL, Guo W, Ji T, et al. Sorafenib induces growth inhibition and apoptosis in human synovial sarcoma cells via inhibiting the RAF/MEK/ERK signaling pathway. Cancer Biol Ther. 2009;8:1729–1736. doi: 10.4161/cbt.8.18.9208. [DOI] [PubMed] [Google Scholar]
- 124.Gounder MM, Lefkowitz RA, Keohan ML, et al. Activity of Sorafenib against desmoid tumor/deep fibromatosis. Clin Cancer Res. 2011;17:4082–4090. doi: 10.1158/1078-0432.CCR-10-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maki RG, D’Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133–3140. doi: 10.1200/JCO.2008.20.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Madhunapantula SV, Robertson GP. Is B-Raf a good therapeutic target for melanoma and other malignancies? Cancer Res. 2008;68:5–8. doi: 10.1158/0008-5472.CAN-07-2038. [DOI] [PubMed] [Google Scholar]
- 127.Marampon F, Bossi G, Ciccarelli C, et al. MEK/ERK inhibitor U0126 affects in vitro and in vivo growth of embryonal rhabdomyosarcoma. Mol Cancer Ther. 2009;8:543–551. doi: 10.1158/1535-7163.MCT-08-0570. [DOI] [PubMed] [Google Scholar]
- 128.Potti A, Ganti AK, Tendulkar K, et al. Determination of vascular endothelial growth factor (VEGF) overexpression in soft tissue sarcomas and the role of overexpression in leiomyosarcoma. J Cancer Res Clin Oncol. 2004;130:52–56. doi: 10.1007/s00432-003-0504-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.West CC, Brown NJ, Mangham DC, et al. Microvessel density does not predict outcome in high grade soft tissue sarcoma. Eur J Surg Oncol. 2005;31:1198–1205. doi: 10.1016/j.ejso.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 130.D’Adamo DR, Anderson SE, Albritton K, et al. Phase II study of doxorubicin and bevacizumab for patients with metastatic soft-tissue sarcomas. J Clin Oncol. 2005;23:7135–7142. doi: 10.1200/JCO.2005.16.139. [DOI] [PubMed] [Google Scholar]
- 131.Baker LH, Rowinsky EK, Mendelson D, et al. Randomized, phase II study of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 in patients with advanced soft tissue sarcoma. J Clin Oncol. 2008;26:5583–5588. doi: 10.1200/JCO.2008.17.4706. [DOI] [PubMed] [Google Scholar]
- 132.Mentzel T, Brown LF, Dvorak HF, et al. The association between tumour progression and vascularity in myxofibrosarcoma and myxoid/round cell liposarcoma. Virchows Arch. 2001;438:13–22. doi: 10.1007/s004280000327. [DOI] [PubMed] [Google Scholar]
- 133.Roma J, Masia A, Reventos J, et al. Notch pathway inhibition significantly reduces rhabdomyosarcoma invasiveness and mobility in vitro. Clin Cancer Res. 2011;17:505–513. doi: 10.1158/1078-0432.CCR-10-0166. [DOI] [PubMed] [Google Scholar]
- 134.Zhang P, Yang Y, Zweidler-McKay PA, et al. Critical role of notch signaling in osteosarcoma invasion and metastasis. Clin Cancer Res. 2008;14:2962–2969. doi: 10.1158/1078-0432.CCR-07-1992. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 135.Raimondi L, Ciarapica R, De Salvo M, et al. Inhibition of Notch3 signalling induces rhabdomyosarcoma cell differentiation promoting p38 phosphorylation and p21(Cip1) expression and hampers tumour cell growth in vitro and in vivo. Cell Death Differ. 2011 doi: 10.1038/cdd.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Belyea BC, Naini S, Bentley RC, et al. Inhibition of the notch-hey1 axis blocks embryonal rhabdomyosarcoma tumorigenesis. Clin Cancer Res. 17:7324–7336. doi: 10.1158/1078-0432.CCR-11-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bennani-Baiti IM, Aryee DN, Ban J, et al. Notch signalling is off and is uncoupled from HES1 expression in Ewing’s sarcoma. J Pathol. 2011;225:353–363. doi: 10.1002/path.2966. [DOI] [PubMed] [Google Scholar]
- 138.Tostar U, Malm CJ, Meis-Kindblom JM, et al. Deregulation of the hedgehog signalling pathway: a possible role for the PTCH and SUFU genes in human rhabdomyoma and rhabdomyosarcoma development. J Pathol. 2006;208:17–25. doi: 10.1002/path.1882. [DOI] [PubMed] [Google Scholar]
- 139.Pressey JG, Anderson JR, Crossman DK, et al. Hedgehog pathway activity in pediatric embryonal rhabdomyosarcoma and undifferentiated sarcoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2011;57:930–938. doi: 10.1002/pbc.23174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tostar U, Toftgard R, Zaphiropoulos PG, et al. Reduction of human embryonal rhabdomyosarcoma tumor growth by inhibition of the hedgehog signaling pathway. Genes Cancer. 2010;1:941–951. doi: 10.1177/1947601910385449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pacey S, Ratain MJ, Flaherty KT, et al. Efficacy and safety of sorafenib in a subset of patients with advanced soft tissue sarcoma from a Phase II randomized discontinuation trial. Invest New Drugs. 2011;29:481–488. doi: 10.1007/s10637-009-9367-9. [DOI] [PubMed] [Google Scholar]
- 142.Pappo AS, Patel SR, Crowley J, et al. R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: results of a phase II Sarcoma Alliance for Research through Collaboration study. J Clin Oncol. 2011;29:4541–4547. doi: 10.1200/JCO.2010.34.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Quek R, Wang Q, Morgan JA, et al. Combination mTOR and IGF-1R inhibition: phase I trial of everolimus and figitumumab in patients with advanced sarcomas and other solid tumors. Clin Cancer Res. 2010;17:871–879. doi: 10.1158/1078-0432.CCR-10-2621. [DOI] [PubMed] [Google Scholar]
- 144.Schuetze S, Zhao L, Chugh R, et al. Results of a phase II trial of sirolimus (S) and cyclophosphamide (C) in advanced sarcoma. Journal of Clinical Oncology. 2011;29 abstr 10003. [Google Scholar]
- 145.Okuno S, Bailey H, Mahoney MR, et al. A phase 2 study of temsirolimus (CCI-779) in patients with soft tissue sarcomas: a study of the Mayo phase 2 consortium (P2C) Cancer. 2011;117:3468–3475. doi: 10.1002/cncr.25928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Houghton PJ, Gorlick R, Kolb EA, et al. Initial testing (stage 1) of the mTOR kinase inhibitor AZD8055 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2011;58:191–199. doi: 10.1002/pbc.22935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu P, Cheng H, Roberts TM, et al. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Manara MC, Nicoletti G, Zambelli D, et al. NVP-BEZ235 as a new therapeutic option for sarcomas. Clin Cancer Res. 2010;16:530–540. doi: 10.1158/1078-0432.CCR-09-0816. [DOI] [PubMed] [Google Scholar]
- 149.Gorlick R, Maris JM, Houghton PJ, et al. Testing of the Akt/PKB inhibitor MK-2206 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2011 doi: 10.1002/pbc.23412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.MEK inhibitor AZD6244 with or without temsirolimus in treating patients with metastatic, recurrent, or locally advanced soft tissue sarcoma that cannot be removed by surgery. [Jan 21, 2012];2011 Mar 5; [ClinicalTrials.gov website]. Available at: http://clinicaltrials.gov/ct2/show/NCT01206140.
- 151.Yoon SS, Duda DG, Karl DL, et al. Phase II study of neoadjuvant bevacizumab and radiotherapy for resectable soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 2011;81:1081–1090. doi: 10.1016/j.ijrobp.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043) J Clin Oncol. 2009;27:3126–3132. doi: 10.1200/JCO.2008.21.3223. [DOI] [PubMed] [Google Scholar]
- 153.Fox E, Aplenc R, Bagatell R, et al. A phase 1 trial and pharmacokinetic study of cediranib, an orally bioavailable pan-vascular endothelial growth factor receptor inhibitor, in children and adolescents with refractory solid tumors. J Clin Oncol. 2010;28:5174–5181. doi: 10.1200/JCO.2010.30.9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schwartz GK, Maki RG, Ratain MJ, et al. Brivanib (BMS-582664) in advanced soft-tissue sarcoma (STS): Biomarker and subset results of a phase II randomized discontinuation trial. Journal of Clinical Oncology. 2011;29 abstr 10000. [Google Scholar]
- 155.Ray-Coquard IL, Blay J, Italiano A, et al. Neoadjuvant MDM2 antagonist RG7112 for well-differentiated and dedifferentiated liposarcomas (WD/DD LPS): A pharmacodynamic (PD) biomarker study. Journal of Clinical Oncology. 2011;29 abstr 10007b. [Google Scholar]
- 156.Rheingold SR, Hogarty MD, Blaney SM, et al. Phase I Trial of G3139, a bcl-2 antisense oligonucleotide, combined with doxorubicin and cyclophosphamide in children with relapsed solid tumors: a Children’s Oncology Group Study. J Clin Oncol. 2007;25:1512–1518. doi: 10.1200/JCO.2006.09.5125. [DOI] [PubMed] [Google Scholar]
- 157.PD0332991 in patients with advanced or metastatic liposarcoma. [Jan 21, 2012];2011 Oct 25; [ClinicalTrials.gov website]. Available at: http://clinicaltrials.gov/ct2/show/NCT01209598.
- 158.Efficacy and safety assessment of oral LBH589 in adult patients with advanced soft tissue sarcoma after pre-treatment failure (ESTIM-LBH) [Jan 21, 2012];2011 Jul 12; [ClinicalTrials.gov website]. Available at: http://clinicaltrials.gov/ct2/show/NCT01136499.
- 159.Attia S, Mahoney MR, Okuno SH, et al. A phase II consortium trial of vorinostat and bortezomib for advanced soft tissue sarcomas. Journal of Clinical Oncology. 2011;29 abstr 10075. [Google Scholar]
- 160.Matushansky I, Coakley KE, Uldrick TS, et al. A phase I dose-escalation study of azacitidine in combination with temozolamide in patients with soft tissue sarcomas. Journal of Clinical Oncology. 2011;29 Abstract 10073. [Google Scholar]