Abstract
Background
Pulmonary hypertension (PH) is driven by diverse pathogenic etiologies. Owing to their pleiotropic actions, microRNA (miRNA) are potential candidates for coordinated regulation of these disease stimuli.
Methods and Results
Using a network biology approach, we identify miRNA associated with multiple pathogenic pathways central to PH. Specifically, microRNA-21 (miR-21) is predicted as a PH-modifying miRNA, regulating targets integral to bone morphogenetic protein (BMP) and Rho/Rho kinase signaling as well as functional pathways associated with hypoxia, inflammation, and genetic haplo insufficiency of the BMP Receptor Type 2 (BMPRII). To validate these predictions, we have found that hypoxia and BMPRII signaling independently up-regulate miR-21 in cultured pulmonary arterial endothelial cells. In a reciprocal feedback loop, miR-21 down-regulates BMPRII expression. Furthermore, miR-21 directly represses RhoB expression and Rho kinase activity, inducing molecular changes consistent with decreased angiogenesis and vasodilation. In vivo, miR-21 is up-regulated in pulmonary tissue from several rodent models of PH and in humans with PH. Upon induction of disease in miR-21-null mice, RhoB expression and Rho-kinase activity are increased, accompanied by exaggerated manifestations of PH.
Conclusions
A network-based bioinformatic approach coupled with confirmatory in vivo data delineates a central regulatory role for miR-21 in PH. Furthermore, this study highlights the unique utility of network biology for identifying disease-modifying miRNA in PH.
Keywords: Pulmonary Heart Disease, microRNA, Network Biology, Molecular Biology, Vasculature
Introduction
Pulmonary hypertension (PH) is a complex vascular disease that is clinically defined as a maladaptive increase in pulmonary arterial pressure. Etiologies are varied and are classified in five sub-categories 1. Later stages of disease are dominated by a dysregulated balance of vascular effectors controlling vascular tone, cellular proliferation, and thrombosis. Currently, overarching pathogenic pathways, such as Rho kinase signaling, are implicated as regulators of these effectors in PH 2. Nevertheless, it remains unclear how such diverse upstream triggers of PH cause a common pathophenotype.
Notably, transforming growth factor (TGF)/bone morphogenetic protein (BMP) signaling, inflammatory signaling, and hypoxic stress represent distinct triggers of PH which are active across etiologies [as reviewed in 1, 2]. The underpinnings of cross-talk between these pathways in the pulmonary vasculature are only beginning to be understood 3, 4. Identification of integrating factors that functionally link these pathways to PH may be especially amenable to in silico predictive approaches.
Recently, microRNA molecules (miRNA), which are conserved, non-protein-coding RNA molecules, have been identified as essential mediators of a variety of genes and cellular processes. Their expression can be regulated in a transcriptional or post-transcriptional fashion. Inside the cell, miRNA negatively regulate gene expression by primarily binding to the 3' untranslated regions of messenger RNA (mRNA) transcripts to repress translation and/or degrade mRNA. Efficient binding relies upon Watson-Crick base-pairing between the 7 nucleotide "seed sequence" of a given miRNA and its mRNA target, and several algorithms have accordingly been developed to predict mRNA targets of each miRNA 5.
Owing to their pleiotropic vascular functions 6, miRNA may coordinately regulate multiple disease pathways in the pulmonary vasculature, but their importance in PH is just beginning to emerge 7. Initial attempts to identify miRNA involved in complex diseases such as PH by using existing predictive algorithms have been reported but remain unproven 8, 9. Here, we have used a network-based bioinformatics approach to identify miRNA that regulate multiple interacting targets in the same functional network to generate robust actions in PH in vivo. This concept has been proposed previously 10, but it has yet to be utilized as a method by which to rank miRNA with the highest likelihood of critically affecting disease. Encompassed in this set, microRNA-21 (miR-21) is linked to hypoxia, BMP signaling, and inflammatory signaling and, thus, is predicted to regulate downstream pathways relevant to PH. However, current data are sparse regarding the specific function of miR-21 in the pulmonary vasculature in vivo. Driven by these network-based predictions, we demonstrate the direct relationship of miR-21 to multiple pathogenic stimuli, its dynamic regulation in PH, and its critical role in the control of PH in vivo. As a result, this study offers a rational mechanism-based strategy for ascertaining the integrative role of miRNA in PH and perhaps other complex pathophenotypes.
Methods
Network-based analyses
Using Medline (PubMed) and the search term "pulmonary hypertension," we compiled a list of 131 genes that are directly implicated in the development of PH. We refer to this group of PH-relevant genes as the “PH-module” (Supplemental Table 1). Analysis of interactions between PH-relevant genes (“PH-network”) was performed using consolidated databases cataloguing a variety of molecular interactions, referred to as the "consolidated interactome." The TargetScan 5 (Conserved) algorithm was used for miRNA target prediction 11. Further details regarding the methods of network-based bioinformatics, including hypergeometric analysis 12, are in Supplemental Methods.
Cells, animals, and human reagents
Primary human pulmonary arterial endothelial cells (HPAECs), VHL flox/flox;Cre-ER mice, IL-6 transgenic mice, BMPR2-heterozygous (+/−) mice, Sprague-Dawley rats, and mmu-miR-21-null (−/−) mice are described in Supplemental Methods. The Harvard Center for Comparative Medicine approved the use of animals in these experiments. Formalin-fixed paraffin-embedded human PAH lung specimens were collected from unused and discarded surgical samples; non-diseased human lung specimens have been previously described 13. The Partners Healthcare IRB and the New England Organ Bank approved the use of these human specimens.
Induction of PH in mice
8-week-old miR-21-null (−/−) and miR-21-wildtype (+/+) littermate mice were injected with SU5416 (20 mg/kg, Sigma-Aldrich), followed by exposure to normobaric hypoxia (10% O2, OxyCycler chamber, Biospherix Ltd, Redfield, NY) or normoxia (21% O2) for 1–3 weeks, as previously described 14.
Right heart catheterization and physiological measurements
Systemic blood pressure was determined in unanesthetized mice by tail-cuff plethysmography (Visitech Systems) 15. Repeated values (10–20) were averaged at each determination. Right heart catheterization and measurement of right ventricular systolic pressure (RVSP) were performed as previously described for mice 16 and for rats 17.
Statistical analysis
Unless otherwise indicated, all numerical quantifications represent mean ± standard error of the mean for three or more independent experiments, each performed in triplicate (N=number of independent experimental repetitions). Images are representative of experiments that have been repeated at least three times. Paired samples were compared by Student’s t test. Comparison of multiple samples was performed by one-way ANOVA followed by Student Newman-Keuls post hoc tests (and confirmed by Tukey post hoc tests) to calculate p-values. Values of p ≤ 0.05 are considered significant.
Additional information
See Supplemental Methods for a detailed description of manipulation of miRNA and mRNA expression in cultured cells, F-actin labeling, measurement of protein expression, and tissue analyses.
Results
A network biology-based approach predicts disease-modifying miRNA in PH
To identify potential disease-modifying miRNA in PH, a list was derived of regulatory factors that are strongly suspected to influence this disease (the “PH-module,” Supplemental Table 1). Based on a highly sensitive and specific in silico miRNA target prediction algorithm, TargetScan 5 (Conserved) 11, of the 153 conserved "groups" of miRNA defined by identical seed sequences, a great majority (129) are predicted to target at least one member of the PH-module (Supplemental Figure 2A). Thus, simply cross-referencing known PH-relevant genes with miRNA target lists offers little insight into which miRNA exert the most powerful influence on disease-relevant pathways.
To specifically identify miRNA that may robustly regulate disease phenotype by targeting multiple related genes in a functionally integrated pathways, network analysis was employed to determine the functional interconnectivity among the PH-relevant target genes. Using the “consolidated interactome” (see Methods), mapping of known interactions among genes in the PH-module revealed a dense network (i.e., the "PH-network," Supplemental Figure 1). This network includes 115 genes (of the 131 genes in the PH-module, 115 were found in the consolidated interactome) with 255 direct interconnections (edges) between them and a largest connected component (LCC) size of 82 nodes. Notably, both of these parameters are substantially larger than those generated from random gene associations (Figure 1A, Left graph: LCC, Right graph: edges). Thus, the size and dense interconnections of the PH-network reflect its tendency to act in a functionally coordinated fashion, creating an ideal substrate with which to identify miRNA that preferentially target functionally-related genes.
Figure 1.
A network biology approach identifies PH-modifying miRNA. (A) The PH-network displays substantial functional interconnections. Left graph: The mean LCC size derived from 100,000 randomly chosen modules of 115 genes from the consolidated interactome (4.5 ± 2.5, mean ± standard deviation) is significantly smaller than the LCC of the PH-network (82 nodes). The maximum LCC size (max size) from randomly selected gene modules is 31. (** signifies p<<10−5). Right graph: The mean number of direct interconnections (edges) within 100,000 randomly chosen modules of 115 genes from the consolidated interactome (9.4 ± 5.6, mean ± standard deviation) is significantly smaller than the number of edges in the PH-network (255 edges). The maximum number of edges (max edges) within randomly selected gene modules is 53. (** signifies p<<10−5). (B) MiRNA that associate with the PH-network (29 miRNA groups) target a subset of pathways related to hypoxia, inflammation, and/or TGF-β. (C) A subset of miRNA previously associated with hypoxia, inflammation and TGF-β is predicted to target the PH-network. MiRNA groups predicted by enrichment analysis (Table 1) are bolded. (D) Predicted target network of 7 miRNA groups identified both by enrichment analysis and literature review reveals genes that may represent points of coordinated miRNA regulation in PH. Circles: predicted gene targets. Triangles: miRNA. Blue lines: predicted associations of miRNA and targets. Dotted gray lines: gene interactions documented in the consolidated interactome. Circle size is proportional to the number of miRNA groups (among these 7) predicted to target that particular gene.
Having established the specific interconnectedness of the PH-network, a hypergeometric analysis was then used to rank miRNA according to the proportion of their targets that interact with the PH-network, while adjusting for the variation in the total targets predicted for each miRNA within the entire consolidated interactome 12. The use of the hypergeometric analysis allows for ranking of miRNA by the proportion of their predicted targets within the interconnected network, thus increasing the likelihood that a predicted miRNA will target functional networks of PH-relevant genes. This enrichment analysis identified 29 miRNA groups for which there exists a 5% or lower probability (p≤0.05) that the overlap of their predicted target list with the PH-network occurred by chance (Table 1). We chose to further analyze only these miRNA, since they represent the most likely candidates that regulate multiple genes and pathways to coordinate pathogenic effects within the PH-network. Importantly, when the same hypergeometric analysis was performed on a group of poorly connected genes of the same size (e.g., an alternative module of 115 randomly sampled genes with an LCC of 4 and 8 edges), no miRNA were identified that recognize multiple targets and carry p≤0.05 (Supplemental Figure 2B). This finding reinforces the importance of the interconnectedness of the PH-network in its ability to facilitate predictions of miRNA with functional significance in PH.
Table 1.
A subset of microRNA predicted to target the PH-network. MiRNA are ranked by p-value (the lowest value indicating the least likelihood that the targets of a given miRNA are encompassed in the PH-network by random chance).
| MicroRNA | MiRNA targets in PH- network |
MiRNA targets outside PH - network |
PH-network genes that are not targets |
Remaining genes in consolidated interactome |
p-value |
|---|---|---|---|---|---|
| miR-130/301 | 14 | 502 | 101 | 11026 | 0.0004 |
| miR-21/590-5p | 7 | 150 | 108 | 11378 | 0.0008 |
| miR-361/361-5p | 5 | 91 | 110 | 11437 | 0.0022 |
| miR-135 | 10 | 357 | 105 | 11171 | 0.0024 |
| miR-375 | 5 | 102 | 110 | 11426 | 0.0035 |
| miR-204/211 | 9 | 320 | 106 | 11208 | 0.0037 |
| miR-873 | 5 | 108 | 110 | 11420 | 0.0043 |
| miR-17-5p/20/93.mr/106/519.d | 14 | 671 | 101 | 10857 | 0.0045 |
| miR-216/216a | 5 | 111 | 110 | 11417 | 0.0048 |
| miR-27ab | 13 | 622 | 102 | 10906 | 0.0059 |
| miR-200bc/429 | 12 | 569 | 103 | 10959 | 0.0074 |
| miR-149 | 6 | 182 | 109 | 11346 | 0.0082 |
| miR-205 | 6 | 200 | 109 | 11328 | 0.0120 |
| miR-455/455-5p | 4 | 94 | 111 | 11434 | 0.0132 |
| miR-224 | 5 | 161 | 110 | 11367 | 0.0183 |
| miR-153 | 8 | 370 | 107 | 11158 | 0.0211 |
| miR-145 | 8 | 372 | 107 | 11156 | 0.0216 |
| miR-410 | 7 | 303 | 108 | 11225 | 0.0222 |
| miR-383 | 3 | 63 | 112 | 11465 | 0.0233 |
| miR-148/152 | 8 | 381 | 107 | 11147 | 0.0239 |
| miR-219/219-5p | 5 | 190 | 110 | 11338 | 0.0312 |
| miR-182 | 10 | 580 | 105 | 10948 | 0.0351 |
| miR-1/206 | 8 | 419 | 107 | 11109 | 0.0352 |
| miR-340/340-5p | 11 | 672 | 104 | 10856 | 0.0372 |
| miR-290-5p/292-5p/371-5p | 4 | 139 | 111 | 11389 | 0.0395 |
| miR-96/1271 | 9 | 527 | 106 | 11001 | 0.0440 |
| miR-24 | 6 | 288 | 109 | 11240 | 0.0446 |
| miR-190 | 3 | 85 | 112 | 11443 | 0.0452 |
| miR-221/222 | 5 | 217 | 110 | 11311 | 0.0462 |
Notably, the PH-relevant targets of these 29 miRNA groups encompass pathways (Supplemental Table 2) that are associated, by varying degrees, with hypoxia, inflammation, and TGF/BMP signaling (Figure 1B). When compared to a list of miRNA previously implicated in these signaling categories (Supplemental Table 3), there is significant overlap. This latter list identifies over 100 miRNA, but only 17 of which relate to all three functional categories (Supplemental Table 3, Figure 1C), a relatively high percentage of which (7 of 17 miRNA groups, Fig. 1C, bolded miRNA) is also predicted by the hypergeometric enrichment analysis (Table 1). A map of the interactions in the PH-network among the predicted targets of these 7 miRNA groups (Figure 1D) reveals a group of genes preferentially targeted by multiple miRNA that may be subject to coordinated regulation. Furthermore, included in these 7 miRNA groups are miR-204 7, which has been previously implicated in PH pathogenesis, as well as the miR-17–92 family 18 and miR-21 19, 20, both of which have been previously associated with cellular phenotypes relevant to PH pathology. Thus, these multiple corroborating lines of evidence strongly indicate the importance of these 7 miRNA groups at the crucial intersection of hypoxia, inflammation, and TGF/BMP signaling in PH.
As a predicted PH-modifying miRNA, miR-21 is up-regulated by hypoxia and BMPRII signaling and reciprocally down-regulates BMPRII expression
Driven by this network-based approach, miR-21 was chosen for validation as a central regulatory factor in PH given its high ranking and the availability of reagents to test this hypothesis. To confirm these predictions, the regulation of miR-21 by upstream triggers of PH was assessed in cultured human pulmonary arterial endothelial cells (HPAECs), which contribute substantially to dysregulated pathophenotypes seen in PH 1.
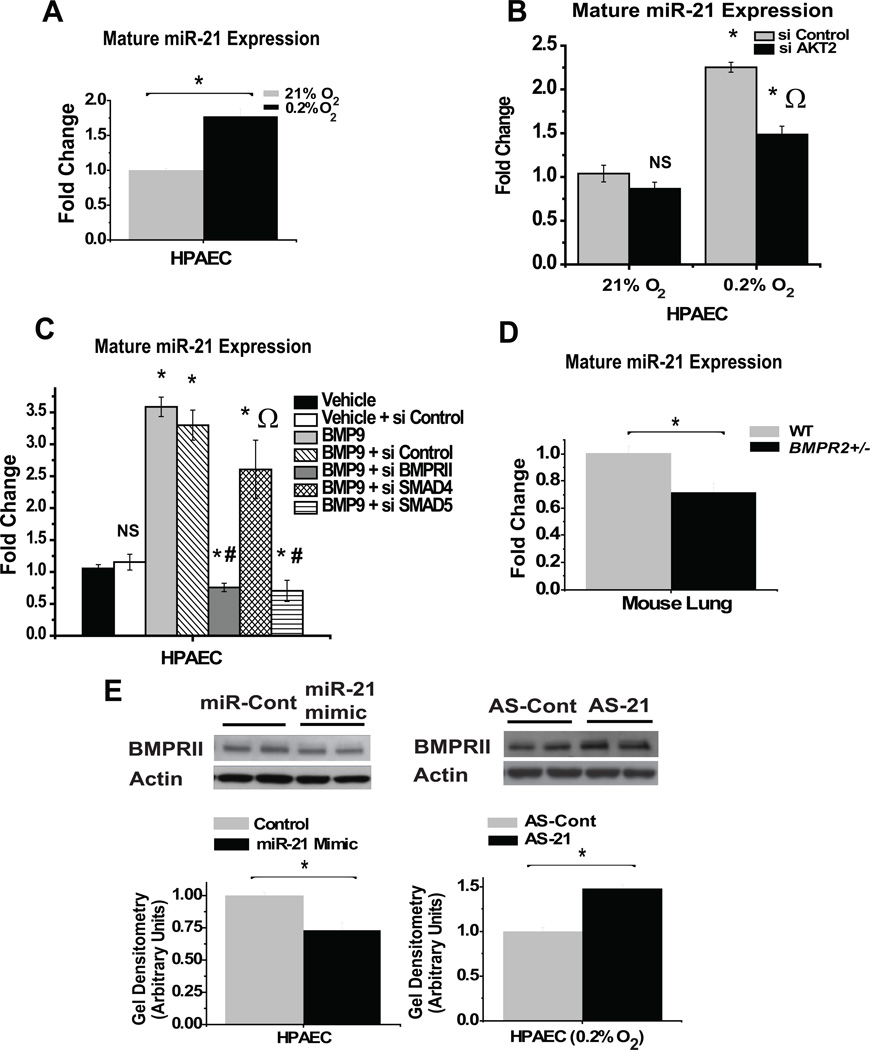
In regard to hypoxia, expression of mature miR-21 (p=0.002; Figure 2A), and its precursor, pri-miR-21 (p=0.003, Supplemental Figure 3A), are increased by approximately 2-fold in HPAECs after chronic exposure to 0.2% O2, corroborating prior findings in transformed cells 21. Moreover, during normoxia, forced expression of a master transcription factor of hypoxia, HIF-1α, increases miR-21 expression (p=0.03, Supplemental Figure 3B). However, because HIF-1α does not appear to bind the miR-21 promoter for direct transcriptional induction 22, hypoxic up-regulation of miR-21 may instead rely indirectly on HIF-dependent and/or HIF-independent factors. Recently, it was demonstrated that the activity of AKT2 is essential for the transcriptional up-regulation of miR-21 in hypoxic transformed cells 23. Correspondingly, in hypoxic human PAECs, inhibitory RNA knockdown of AKT2 (>80% transcript knockdown, Supplemental Figure 4A) partially represses the up-regulation of miR-21, indicating its essential role in the control of this miRNA in hypoxic pulmonary vascular cell types (p=0.03, Figure 2B).
Figure 2.
MiR-21 is up-regulated by hypoxia- and BMPRII-dependent signaling and reciprocally down-regulates BMPRII expression. (A) As measured by RT-PCR, hypoxic HPAECs display increased levels of mature miR-21. (B) Knockdown of AKT2 (si AKT2) negligibly affects miR-21 expression during normoxia but partially inhibits the specific up-regulation of miR-21 during hypoxia. (* signifies p≤0.05 compared with si Control samples at 21% O2; Ω signifies p≤0.05 compared with si Control samples at 0.2% O2). (C) Exposure to BMP9 increases mature miR-21 expression in HPAECs; BMP9-dependent up-regulation of miR-21 is abrogated by knockdown of BMPR2 or SMAD5 but not SMAD4. (* signifies p≤0.05, NS signifies p>0.05 as compared with vehicle control; Ω signifies p>0.05 as compared with BMP9 + si Control; # signifies p≤0.05 as compared with vehicle + si Control). (D) Lungs of BMPR2 (+/−) mice display reduced expression of miR-21 compared to wildtype littermates (WT). (E) (Left blots) As measured by immunoblot densitometry, BMPRII expression is decreased in HPAECs previously transfected with miR-21 oligonucleotide mimics. (Right blots) BMPRII expression is increased in hypoxic HPAECs after inhibition of miR-21 (AS-21). In (A–D), control miR-21 expression is assigned a fold change of 1, to which other conditions are compared. In (E), immunoblots are representative of experiments performed at least in triplicate; gel densitometry is normalized to actin levels and compared as arbitrary units. In all panels, error bars reflect SEM; (*) denotes p≤ 0.05 (N≥3), NS signifies p>0.05 (N≥3). For all experiments, N≥3.
Similarly, we explored whether BMPRII specifically regulates miR-21. In HPAECs, BMP9 up-regulates miR-21 by over 3-fold (p= 0.008, Figure 2C). To determine if BMPRII and its downstream SMAD partners mediate this process, specific inhibitory RNA were used which down-regulate transcript expression of BMPR2, SMAD4, or SMAD5 by at least 80% (Supplemental Figure 4B–D). Up-regulation of miR-21 is abrogated by knockdown of BMPRII (Figure 2C). In correlation, lungs of mice that carry a heterozygous genetic deficiency in BMPR2 24 display a 30% decrease in expression of miR-21 (p = 0.03, Figure 2D). Consistent with prior reports 19, 25, in HPAECs, knockdown of SMAD5, an intracellular receptor-SMAD factor (R-SMAD) that interacts with BMPRII, also inhibits up-regulation of miR-21, but the same response is not observed after knockdown of SMAD4, a downstream partner of R-SMADs (Figure 2C). Thus, BMPRII is essential for the up-regulation of miR-21 in response to BMP stimulation, and this process depends on certain, but not all, canonical SMAD factors.
In addition to these upstream processes, miR-21 can recognize a target sequence in the 3’ untranslated region of BMPR2 transcripts, 26 but endogenous regulation has not been demonstrated. As assessed by immunoblotting, forced expression of miR-21 decreases BMPRII expression by approximately 25% in HPAECs (p = 0.02, Figure 2E, left blot), and inhibition of miR-21 increases BMPRII protein expression by 40% during hypoxia (p = 0.004, Figure 2E, right blot), when endogenous miR-21 levels are augmented. Taken together with the up-regulation of miR-21 by hypoxia and BMPRII activity, these data validate the predicted functional connection between miR-21 and two major pathogenic triggers of PH, hypoxia and BMPRII-dependent signaling.
MiR-21 represses RhoB to suppress Rho/Rho kinase activity and induce molecular changes consistent with decreased angiogenesis and vasodilation in HPAECs
Given this upstream control of miR-21 expression, the targets of miR-21 coinciding directly with the PH-module (Supplemental Table 2) were analyzed for their known regulation of vasoactive phenotypes. The small GTPase RhoB, a previously validated target of miR-21 in tumor cells 27, emerged as a key candidate, given its function in activating Rho kinase, which in turn can drive pulmonary vascular remodeling and PH in rodents and humans [as reviewed in 28].
Furthermore, among the several candidate PH-modifying miRNA, only miR-21 is predicted to regulate RhoB (Figure 1D). Thus, other miRNA are unlikely to compensate for changes in RhoB if miR-21 levels are altered in PH. In support of this hypothesis, in HPAECs, antisense inhibition of activated, endogenous miR-21 during hypoxia increases RhoB protein expression by nearly 60% (p=0.02, Figure 3A). Conversely, forced over-expression of miR-21 in hypoxic cultured HPAECs decreases RhoB protein expression by 30% (p=0.01, Figure 3B). Notably, this decrease is modest, perhaps reflecting the high baseline levels of endogenous miR-21 in HPAECs 29, and likely indicating that loss of function experiments in this context are more useful than forced expression to determine miR-21 function. Accordingly, in response to knockdown of miR-21, Rho kinase activity increases, as demonstrated by a nearly 50% elevation of Rho kinase-dependent phosphorylation of threonine-853 in myosin phosphatase 30 (Figure 3C). Thus, endogenous miR-21 directly represses RhoB and Rho kinase activity in hypoxic HPAECs.
Figure 3.
MiR-21 inhibits RhoB expression to suppress Rho kinase activity in HPAECs. (A–B) As measured by immunoblot densitometry, RhoB expression is increased in hypoxic HPAECs after inhibition of miR-21 (A) and is decreased in hypoxic HPAECs transfected with miR-21 mimic (B). (C) Rho kinase-dependent phosphorylation of threonine-853 (MP853) in myosin phosphatase (MP) is increased in hypoxic HPAECs after inhibition of miR-21. (D) NOS3 expression is decreased in hypoxic HPAECs after inhibition of miR-21. (E) Intensity of staining with phalloidin-FITC (green) to detect F-actin formation is increased in hypoxic HPAECs after inhibition of miR-21. Furthermore, as demonstrated by more fibers traversing nuclei, increased complexity of F-actin network formation is evident in AS-21-transfected cells (asterisk) as compared with AS-Cont-transfected cells (double asterisk). In (A–D), error bars reflect SEM; (*) denotes p≤ 0.05 (N≥3), NS signifies p>0.05 (N≥3). Immunoblots and micrographic images are representative of experiments performed at least in triplicate; gel densitometry is normalized to actin levels and compared as arbitrary units. For all experiments, N≥3.
In response to elevated Rho activity, HPAECs typically display phenotypic responses that facilitate angiogenesis and vasoconstriction, which drive vascular remodeling and dysfunction in PH 1. Among its several vasoconstrictive mechanisms, Rho kinase activation represses endothelial nitric oxide synthase (NOS3) 31. Correspondingly, inhibition of miR-21, which induces RhoB and Rho kinase activity, decreases hypoxic NOS3 expression by nearly 40% (Figure 3D). Rho activation also induces formation of actin stress fibers (F-actin) to control angiogenesis32. Correspondingly, inhibition of miR-21 during hypoxia increases F-actin, as assessed by increased phalloidin-FITC immunofluorescence and increased complexity of the F-actin network (Figure 3E). As a result, reflecting its repressive actions on Rho/Rho kinase activity, miR-21 induces molecular changes consistent with decreased angiogenesis and vasodilation in hypoxic HPAECs, which may counteract the pathogenic mechanisms critical to PH 1.
MiR-21 expression is increased in remodeled pulmonary vessels of animal models of PH and human PH
To validate these findings in vivo, regulation of miR-21 was assessed in distinct rodent models of PH as well as human lung obtained from PH patients. First, in lung tissue of transgenic mice over-expressing human interleukin-6 (IL-6), an inflammatory model of severe PH 33, miR-21 is up-regulated 4-fold (p=0.03, Figure 4A). Second, a hypoxia-induced model of PH was studied whereby conditional homozygous deficiency of the Von Hippel Lindau protein (VHL-null) leads to constitutive expression of master transcriptional regulators of hypoxia, HIF-1α and HIF-2α34. Similar to mice carrying the naturally occurring Chuvash loss-of-function VHL mutation 35, VHL-null mice manifest increased right ventricular systolic pressure (RVSP), consistent with hypoxia-induced PH (p<0.001, Supplemental Figure 5A). Correspondingly, miR-21 is up-regulated 3-fold in lung tissue of VHL-null mice (p=0.007, Figure 4B). MiR-21 expression in whole lung homogenate of mice is not consistently altered after exposure to 10% oxygen for 3 weeks (Supplemental Figure 5B). However, when chronic hypoxia is combined with administration of the vascular endothelial growth factor receptor 2 (VEGFR2)-antagonist SU5416 in mice, a well-established set of exposures that can induce severe PH in rats 36 and mice 37, an approximately 2-fold increase (compared to time 0) in miR-21 is observed. This elevation persists over time (p<0.03 when comparing time 0 to all subsequent time points (Figure 4C) and correlates with increased right ventricular systolic pressure and PH (p<0.01 when comparing time 0 to all subsequent time points, Supplemental Figure 5C, left graph). Finally, in the lung tissue of rats exposed to monocrotaline, a well-established rodent model of PH resulting from pulmonary endothelial injury and intense inflammatory reaction 38, pulmonary expression of miR-21 increases over time (1–4 weeks post-monocrotaline exposure) (p<0.02 when comparing time 0 to all subsequent time points, Figure 4D), which correlates with increased right ventricular systolic pressure and PH (p<0.005 for time points 2–4 weeks post-exposure compared with time 0, Supplemental Figure 5D).
Figure 4.
MiR-21 expression is increased in in vivo models of PH. (A) MiR-21 expression is increased in mouse lung over-expressing IL-6 (N=5 mice per group). (B) RT-PCR reveals that miR-21 is up-regulated in the lungs of VHL-null mice as compared with wildtype littermates (N=6 mice per group). (C) MiR-21 is up-regulated in the lungs of mice exposed to a combination of SU5416 and 10% O2 for 1–3 weeks compared to time 0 (N=3 mice per group). (D) MiR-21 steadily increases over time in lungs of rats after 1–4 weeks post-monocrotaline exposure (N=3 rats per treatment group) compared to time 0. Control miR-21 expression is assigned a fold change of 1, to which other conditions are compared. In all panels, error bars reflect SEM; (*) denotes p≤ 0.05 (N≥3), NS signifies p>0.05 (N≥3).
In correlation with previous reports of ubiquitous miR-21 expression39, in situ histological staining for miR-21 in mouse lung specimens reveals uniform but modest staining throughout the parenchyma (alveoli and small pulmonary vessels, Supplemental Figure 5E). Importantly, corresponding with the RT-PCR data from whole lung homogenate (Figure 4), increased in situ staining for miR-21 is particularly evident in the small (<100 µm) pulmonary vessels in VHL-null mice and IL-6 transgenic mice (p<0.02 in both models, Figure 5A) as well as monocrotaline-treated rat (p<0.01, Figure 5B). Increased staining for miR-21 is also evident in diseased pulmonary vessels (<200 µm) derived from 3 human patients (Table 2) with pulmonary arterial hypertension (PAH), a class of PH defined by characteristic histopathology and the absence of left heart failure, as compared with 3 previously described non-diseased human lung samples 13 (p<0.0001, Figure 5C). Thus, increased pulmonary vascular expression of miR-21 is common to a variety of etiologies of PH in both rodents and humans.
Figure 5.
MiR-21 expression is increased in remodeled pulmonary vessels in animal models of PH and human PAH patients. (A–B) In situ staining and quantitation reveal increased expression of miR-21 in <100 µm pulmonary vessels of VHL-null mice and IL-6 transgenic mice as compared with wildtype control mice (N=3 mice per group) (A) and in <100 µm pulmonary vessels of monocrotaline-exposed rats as compared with vehicle (N= 3 rats per group) (B). (C) Increased expression of miR-21 in <200 µm pulmonary vessels from lungs of 3 human patients with PAH (Patients A, B, C) as compared with 3 non-diseased human lung specimens (Patients 1, 2, 3). In all panels, error bars reflect SEM; (*) denotes p≤ 0.05 (N≥3), NS signifies p>0.05 (N≥3).
Table 2.
Clinical characteristics of patients with PAH.
| ID | Age (years) |
Gender | Mean PA (right heart catheterization) |
RVSP (echocardiography) |
Clinical Description |
|---|---|---|---|---|---|
| A | 34 | Female | 50 mm Hg | 107 mm Hg | Cardiopulmonary arrest (autopsy) |
| B | 64 | Female | 55 mm Hg | 106 mm Hg | Cardiopulmonary arrest (autopsy) |
| C | 68 | Female | 44 mm Hg | 83 mm Hg | Scleroderma |
Targeted deletion of miR-21 increases Rho kinase activity and alters the development of PH in vivo
To assess definitively the function of miR-21 in regulating the development of PH, disease severity was compared in mice genetically replete or deficient in miR-21 (+/+ or −/−). Construction and validation of a miR-21-null mouse have been described elsewhere 40. At baseline, they are deficient in pulmonary expression of miR-21 (Supplemental Figure 6A). They demonstrate no differences from wildtype littermates in pulmonary arterial histology (Supplemental Figure 6B) or right ventricular systolic pressure (RVSP) (Supplemental Figure 6C).
Given its specific induction of miR-21 in wildtype mice (Figure 4C), injury with SU5416 and chronic (3 week) exposure to normobaric 10% oxygen was employed to discern better a role for miR-21 in controlling PH. As compared with normoxia, both hypoxia (p=0.02) and hypoxia with SU5416 (p=0.001) induce similar increases in RVSP in wildtype mice, but these conditions do not demonstrate dramatic differences in RVSP when compared to each other (p>0.05, Supplemental Figure 5C, right graph). However, after exposure to SU5416 and chronic hypoxia, RVSP is significantly and consistently elevated in miR-21-null mice, as compared with wildtype littermate control mice (p=0.0007, Figure 6A). Such differences are not the result of varying effects on polycythemia or systemic blood pressure, as hematocrit levels and systolic arterial blood pressures are similar in treated miR-21-null and wildtype mice (Supplemental Figure 6D–E). Right ventricular remodeling is also evident in miR-21-null mice, as assessed by an increase in the mass ratio of the right ventricle to left ventricle and septum (RV/LV+S) (p=0.01, Figure 6B). Moreover, immunohistochemical staining of α-smooth muscle actin in the small pulmonary vessels (≤ 100 µm diameter) of miR-21-null mice demonstrates exaggerated pulmonary vascular remodeling, as quantified by an increase in the overall ratio of muscularized vessel wall thickness to vessel diameter (p<0.001, Figure 6C). Consistent with the repression of RhoB and Rho kinase activity by miR-21 in HPAECs (Figure 3), RhoB expression is elevated in miR-21-null mice in both the vascular intima and media of small pulmonary vessels (<100 µm diameter) as assessed by immunohistochemistry (p<0.001, Figure 6D). Correspondingly, these vessels display increased Rho kinase-dependent levels of phosphorylated myosin phosphatase in miR-21-null mice (p=0.01, Figure 6E). Furthermore, pulmonary tissue derived from treated miR-21-null mice exhibits a substantial increase in the transcriptional expression of at least one Rho-dependent vasoconstrictive effector of PH 1, endothelin-1 (p=0.02) (Figure 6F). Thus, endogenous miR-21 is necessary for repression of both RhoB and Rho kinase activity in the hypoxic pulmonary vasculature in vivo. In response, as demonstrated by these independent physiological and histological parameters, miR-21-null mice display exaggerated manifestations of PH, thus identifying a central regulatory role for miR-21 in this disease.
Figure 6.
miR-21-null (−/−) mice display exaggerated manifestations of PH when exposed to SU5416 and chronic hypoxia. (A–C) After exposure to SU5416 and 10% O2, miR-21-null mice display increased RVSP (A), increased relative right ventricular mass (RV/LV+S) (B), and increased pulmonary vascular remodeling (C). In (C), remodeling is evident in miR-21-null mice (right panels, arrows), with increased medial thickening and cellularity, as compared with pulmonary vessels (left panels, arrowheads) in wildtype littermates (α-smooth muscle actin stain). A significant increase in medial wall thickness relative to vessel diameter in <100 µm pulmonary vessels (right graph) is evident in miR-21-null mice. (N=8 mice per treatment group in A–C). (D) Immunohistochemistry (arrows) and quantitation (graph) reveal increased expression of RhoB in <100 µm pulmonary vessels of treated miR-21-null (−/−) mice (N=5 mice per group). (E) Rho kinase-dependent expression of phosphorylated myosin phosphatase [p-MP (T853), arrows, left panels] is increased in <100 µm pulmonary vessels of treated miR-21-null mice. Stain for total myosin phosphatase in consecutive sections is shown (MP, arrows, right panels) (N=5 mice per group). (F) Lung tissue from treated miR-21-null mice display increased transcript levels of endothelin-1. Wildtype expression is assigned a fold change of 1, to which expression in miR-21-null mice is compared. (N=4 mice per group). In appropriate panels, error bars reflect SEM; (*) denotes p≤ 0.05 (N≥3), NS signifies p>0.05 (N≥3).
Discussion
Here, a unique network biology approach coupled with experimental data in cultured cells, animal models, and diseased human tissue identifies miR-21 as a critical regulatory factor that controls the downstream development of PH. This is the first report describing a reliable network-based bioinformatic approach to identify miRNA that control PH. As a result, we present a molecular model of the pulmonary vasculature whereby hypoxia, inflammation, and BMP-dependent signaling up-regulate miR-21 (Figure 7). In response, miR-21 represses Rho kinase activation, and perhaps other pathways, as a homeostatic brake to protect against PH in vivo.
Figure 7.
MiR-21 integrates multiple pathogenic signals to regulate pulmonary hypertension. A molecular model is presented whereby hypoxia, inflammation, and BMP-dependent signaling up-regulate miR-21 in the pulmonary vasculature. In response, miR-21 represses Rho kinase activation and, perhaps, other pathways to modulate the development of PH in vivo.
The direct regulation of Rho/Rho kinase by miR-21 augments our evolving understanding of this pathway in the diseased pulmonary vasculature. Up-regulation of RhoA is known to induce Rho kinase activity leading to pulmonary vascular pathology 28. Less is known about the functions of RhoB in the vasculature; although in cell culture, it suppresses angiogenesis 41 and can induce a vasoconstrictive phenotype by activating endothelin-1 42 and repressing NOS3 31. Thus, our findings emphasize the underappreciated importance of RhoB in control of the dysregulated pulmonary vessel.
Furthermore, integration of various PH-relevant stimuli by miR-21 correlates with the known pleiotropic activity of this miRNA in various pathological contexts [as reviewed in 39]. While modulation of RhoB reflects an important function of miR-21, other direct targets of miR-21 may affect downstream PH development. Among these, BMPRII may represent a key target, given its role in PH development [as reviewed in 1] as well as our finding that miR-21 regulates its expression (Figure 2E). However, unlike RhoB, BMPRII has several predicted targeting miRNA (Figure 1D), making miR-21’s precise control of the BMP pathway more complex. Additional gene targets of miR-21 could influence PH, particularly tumor suppressor genes such as PDCD4 39,19, 20. However, PDCD4 is not substantially altered in miR-21-null mice with exaggerated PH (Supplemental Figure 7), consistent with a prior study of cardiac-specific PDCD4 in miR-21-null mice 43. Thus, control of each direct target by miR-21 clearly differs depending on biological context. Accordingly, future comparison of the roles of miR-21 in different cellular and disease contexts may further highlight these differences 1.
Beyond these specific insights regarding miR-21, additional PH-relevant miRNA that were identified by the network-based approach await validation. Of 4 other top-ranked miRNA (miR-20a, miR-27a, miR-130a, and miR-375, Table 1) that are expressed in the lung, directionally consistent changes in expression are observed for miR-20a, miR-27a, and miR-375 in two animal models of PH (accompanied by alteration of miR-130a in the hypoxia + SU5416 mouse model) (Supplemental Figure 8A–B). This supports the value of these predictions and, given the significant overlap of their targets (Figure 1D), emphasizes the need to consider coordinate control of PH progression by networks of miRNA and their integrated targets.
Although some of the same miRNA predictions are certainly possible through other methods besides network bioinformatics, such a network-based approach appears well-suited to better predict the influence of miRNA on other complex diseases. Based on the weak predictive power of a poorly connected gene module (Supplemental Figure 2B), simple assembly of gene sets without regard for the interconnectivity of the gene sets appears suboptimal in identifying miRNA that target multiple related genes. In part, this may stem the wide variability in how data are curated from scientific reports, leading to literature-curated gene sets that are incomplete, biased and perhaps dramatically different, even if derived for the same disease. Thus, such gene sets may not always segregate into easily discernible functionally connected groups. For instance, we have curated a set of 46 genes from the scientific literature and expression arrays associated with the complex pregnancy-related disease, preeclampsia. Unlike the PH-network, these genes carried no discernible interconnectivity (39 genes were recognized in the consolidated interactome, LCC=1 without any edges), despite all genes being associated with preeclampsia. Thus, there exists little confidence in using this gene set for an analysis to capture miRNA that synergistically regulate discernible functional pathways (Cottrill and Chan, MD, PhD, unpublished data, 2012). Such examples highlight the importance of first confirming a defined network of genes in order to then identify miRNA that regulate multiple targets in concert for a unified functional effect.
To date, the discovery of bona fide disease-modifying miRNA has depended upon observations of altered levels of miRNA during disease manifestation. However, such an identification process can be limited by paucity of diseased human tissue samples, as in PH. In addition, unlike the in silico method presented here, it provides little insight into functional relationships among miRNA and cannot identify changes in miRNA function in the absence of direct alterations in miRNA expression. The predictive utility of this method should continue to improve with future variations in the design of this approach. Therefore, along with more traditional direct miRNA screening methods, we expect that network-based predictions will result in a more rapid and comprehensive identification of disease-modifying miRNA.
Previously, only one other miRNA, miR-204, has been rigorously studied in its direct control of PH, effectively protecting against disease by targeting SHP2 7. Significant changes in miR-21 were not appreciated in that study, potentially resulting from a relatively focused screen of a single cell type garnered from a narrow set of etiologic categories of PH. Conversely, our use of network biology predicts the importance of both miR-21 and miR-204 in PH as well as emphasizes their unique and shared targets in the PH-network (Supplemental Table 2). Thus, these findings emphasize the utility of such bioinformatics in expanding predictive power and suggest that miR-21 and miR-204 carry synergistic actions to coordinate protection against PH.
Finally, in contrast to a previous report describing down-regulation of miR-21 in lung homogenate in PH 44, our data unambiguously describe a pervasive, time-dependent up-regulation of miR-21 in remodeled pulmonary vessels after injury in multiple rodent and human subjects suffering from end-stage PH (Figure 4–5). When considering the well-established observations of enhanced hypoxic/HIF-dependent signaling and inflammatory IL-6 expression in rodent models of PH 45, 46 and human disease 47, 48, such an up-regulation of miR-21 in PH is mechanistically supported by the transcriptional induction of miR-21 by IL-6 49 as well as hypoxia/HIF (Figure 2 and Supplemental Figure 3). Differences in RNA extraction from historical formalin-fixed lung samples or differences in the clinical context of disease may partially explain the contrasting observations of Caruso and colleagues. Nonetheless, our findings, consistent in both RT-PCR and in situ staining across several animal and human disease types, clearly demonstrate that miR-21 expression is increased most prominently during the latter stages of PH. Taken together with our findings of a protective role for miR-21 in PH, these observations also suggest that an inappropriate down-regulation of miR-21 may predispose to PH. Consistent with this view, recent observations indicate that naturally occurring human mutations in the BMP pathway lead to decreased miR-21 expression 25. These findings are consistent with reduced expression of miR-21 in BMPR2-haploinsufficient mice (Figure 2D), which are more susceptible to PH induced by hypoxia 24 and inflammation 50. Thus, these data support a possible mechanism by which to explain how genetic deficiencies in BMP signaling predispose to PAH -- via decreased miR-21 expression in response to injury and loss of protection against disease progression.
In summary, a combination of in silico predictions, cell culture data, and animal modeling demonstrates that miR-21 integrates diverse pathogenic signaling to control the development of PH. Future attempts to combine network-based bioinformatics and biological validation may offer a more efficient and comprehensive approach to identifying additional miRNA important in PH and other complex diseases. Furthermore, the finding of such a central role for miR-21 provides much needed insight into the fundamental development of PH with broad implications for future therapeutic approaches.
Supplementary Material
Acknowledgments
We thank S.K. Chan (critical reading of manuscript) and S. Tribuna (administrative assistance).
Funding Sources: This work was supported by the Sarnoff Cardiovascular Research Foundation (V.N.P.); American Heart Association grant 0825906D, NIH-KO8 grant, the Lerner, Harris, and Watkins Funds, Gilead Research Scholars Fund, and the Pulmonary Hypertension Association (S.Y.C.); NIH grants R37HL061795, U54HL070819, PO1HL48743, P50HL107192, and U01HL108630 (J.L.); and the NIH-Center of Excellence in Genomic Sciences (S.R., N.G., A.-L.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None
References
- 1.Chan SY, Loscalzo J. Pathogenic mechanisms of pulmonary arterial hypertension. J Mol Cell Cardiol. 2008;44:14–30. doi: 10.1016/j.yjmcc.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation. 2010;121:2045–2066. doi: 10.1161/CIRCULATIONAHA.108.847707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu X, Chang M, Mitsialis S, Kourembanas S. Hypoxia regulates bone morphogenetic protein signaling through C-terminal-binding protein 1. Circ Res. 2006;99:240–247. doi: 10.1161/01.RES.0000237021.65103.24. [DOI] [PubMed] [Google Scholar]
- 4.Hagen M, Fagan K, Steudel W, Carr M, Lane K, Rodman DM, West J. Interaction of interleukin-6 and the BMP pathway in pulmonary smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1473–L1479. doi: 10.1152/ajplung.00197.2006. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.Courboulin A, Paulin R, Giguere NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher S, Cote J, Simard MJ, Bonnet S. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med. 2011;208:535–548. doi: 10.1084/jem.20101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu M, Zhang Q, Deng M, Miao J, Guo Y, Gao W, Cui Q. An analysis of human microRNA and disease associations. PloS One. 2008;3:e3420. doi: 10.1371/journal.pone.0003420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D, Wang J, Lu M, Song F, Cui Q. Inferring the human microRNA functional similarity and functional network based on microRNA-associated diseases. Bioinformatics. 2010;26:1644–1650. doi: 10.1093/bioinformatics/btq241. [DOI] [PubMed] [Google Scholar]
- 10.Shirdel EA, Xie W, Mak TW, Jurisica I. NAViGaTing the micronome--using multiple microRNA prediction databases to identify signalling pathway-associated microRNAs. PloS One. 2011;6:e17429. doi: 10.1371/journal.pone.0017429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ewens WJ, Gregory RG. Statistical Methods in Bioinformatics: An Introduction. New York: Springer; 2005. Probability Theory (i): One Random Variable; pp. 10–13. [Google Scholar]
- 13.Kajstura J, Rota M, Hall SR, Hosoda T, D'Amario D, Sanada F, Zheng H, Ogorek B, Rondon-Clavo C, Ferreira-Martins J, Matsuda A, Arranto C, Goichberg P, Giordano G, Haley KJ, Bardelli S, Rayatzadeh H, Liu X, Quaini F, Liao R, Leri A, Perrella MA, Loscalzo J, Anversa P. Evidence for human lung stem cells. N Engl J Med. 2011;364:1795–1806. doi: 10.1056/NEJMoa1101324. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Fredenburgh LE, Liang OD, Macias AA, Polte TR, Liu X, Riascos DF, Chung SW, Schissel SL, Ingber DE, Mitsialis SA, Kourembanas S, Perrella MA. Absence of cyclooxygenase-2 exacerbates hypoxia-induced pulmonary hypertension and enhances contractility of vascular smooth muscle cells. Circulation. 2008;117:2114–2122. doi: 10.1161/CIRCULATIONAHA.107.716241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johns C, Gavras I, Handy DE, Salomao A, Gavras H. Models of experimental hypertension in mice. Hypertension. 1996;28:1064–1069. doi: 10.1161/01.hyp.28.6.1064. [DOI] [PubMed] [Google Scholar]
- 16.Song Y, Coleman L, Shi J, Beppu H, Sato K, Walsh K, Loscalzo J, Zhang YY. Inflammation, endothelial injury, and persistent pulmonary hypertension in heterozygous BMPR2-mutant mice. Am J Physiol Heart Circ Physiol. 2008;295:H677–H690. doi: 10.1152/ajpheart.91519.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones J, Walker J, Song Y, Weiss N, Cardoso W, Tuder R, Loscalzo J, Zhang Y. Effect of 5-lipoxygenase on the development of pulmonary hypertension in rats. Am J Physiol Heart Circ Physiol. 2004;286:H1775–H1784. doi: 10.1152/ajpheart.00281.2003. [DOI] [PubMed] [Google Scholar]
- 18.Brock M, Trenkmann M, Gay RE, Michel BA, Gay S, Fischler M, Ulrich S, Speich R, Huber LC. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ Res. 2009;104:1184–1191. doi: 10.1161/CIRCRESAHA.109.197491. [DOI] [PubMed] [Google Scholar]
- 19.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarkar J, Gou D, Turaka P, Viktorova E, Ramchandran R, Raj JU. MicroRNA-21 plays a role in hypoxia-mediated pulmonary artery smooth muscle cell proliferation and migration. Am J Physiol Lung Cell Mol Physiol. 2010;299:L861–L871. doi: 10.1152/ajplung.00201.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulshreshtha R, Ferracin M, Wojcik S, Garzon R, Alder H, Agosto-Perez F, Davuluri R, Liu C, Croce C, Negrini M, Calin G, Ivan M. A microRNA signature of hypoxia. Mol Cell Biol. 2007;27:1859–1867. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorospe M, Tominaga K, Wu X, Fahling M, Ivan M. Post-Transcriptional Control of the Hypoxic Response by RNA-Binding Proteins and MicroRNAs. Front Mol Neurosci. 2011;4:7. doi: 10.3389/fnmol.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polytarchou C, Iliopoulos D, Hatziapostolou M, Kottakis F, Maroulakou I, Struhl K, Tsichlis PN. Akt2 regulates all Akt isoforms and promotes resistance to hypoxia through induction of miR-21 upon oxygen deprivation. Cancer Res. 2011;71:4720–4731. doi: 10.1158/0008-5472.CAN-11-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beppu H, Ichinose F, Kawai N, Jones R, Yu P, Zapol W, Miyazono K, Li E, Bloch K. BMPR-II heterozygous mice have mild pulmonary hypertension and an impaired pulmonary vascular remodeling response to prolonged hypoxia. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1241–L1247. doi: 10.1152/ajplung.00239.2004. [DOI] [PubMed] [Google Scholar]
- 25.Drake KM, Zygmunt D, Mavrakis L, Harbor P, Wang L, Comhair SA, Erzurum SC, Aldred MA. Altered microRNA Processing in Heritable Pulmonary Arterial Hypertension: an Important Role for Smad-8. Am J Respir Crit Care Med. 2011;184:1400–1408. doi: 10.1164/rccm.201106-1130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin W, Zhao B, Shi Y, Yao C, Jin L, Jin Y. BMPRII is a direct target of miR-21. Acta Biochim Biophys Sin. 2009;41:618–623. doi: 10.1093/abbs/gmp049. [DOI] [PubMed] [Google Scholar]
- 27.Connolly EC, Van Doorslaer K, Rogler LE, Rogler CE. Overexpression of miR-21 promotes an in vitro metastatic phenotype by targeting the tumor suppressor RHOB. Mol Cancer Res. 2010;8:691–700. doi: 10.1158/1541-7786.MCR-09-0465. [DOI] [PubMed] [Google Scholar]
- 28.Connolly MJ, Aaronson PI. Key role of the RhoA/Rho kinase system in pulmonary hypertension. Pulm Pharmacol Ther. 2011;24:1–14. doi: 10.1016/j.pupt.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 2009;10:273–284. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 31.Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998;273:24266–24271. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- 32.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 33.Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res. 2009;104:236–244. doi: 10.1161/CIRCRESAHA.108.182014. 228p following 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minamishima YA, Moslehi J, Bardeesy N, Cullen D, Bronson RT, Kaelin WG., Jr Somatic inactivation of the PHD2 prolyl hydroxylase causes polycythemia and congestive heart failure. Blood. 2008;111:3236–3244. doi: 10.1182/blood-2007-10-117812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hickey MM, Richardson T, Wang T, Mosqueira M, Arguiri E, Yu H, Yu QC, Solomides CC, Morrisey EE, Khurana TS, Christofidou-Solomidou M, Simon MC. The von Hippel-Lindau Chuvash mutation promotes pulmonary hypertension and fibrosis in mice. J Clin Invest. 2010;120:827–839. doi: 10.1172/JCI36362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J. 2001;15:427–438. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- 37.Ciuclan L, Bonneau O, Hussey M, Duggan N, Holmes AM, Good R, Stringer R, Jones P, Morrell NW, Jarai G, Walker C, Westwick J, Thomas M. A Novel Murine Model of Severe Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2011;184:1171–1182. doi: 10.1164/rccm.201103-0412OC. [DOI] [PubMed] [Google Scholar]
- 38.Ghodsi F, Will JA. Changes in pulmonary structure and function induced by monocrotaline intoxication. Am J Physiol. 1981;240:H149–H155. doi: 10.1152/ajpheart.1981.240.2.H149. [DOI] [PubMed] [Google Scholar]
- 39.Krichevsky AM, Gabriely G. miR-21: a small multi-faceted RNA. J Cell Mol Med. 2009;13:39–53. doi: 10.1111/j.1582-4934.2008.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu TX, Hartner J, Lim EJ, Fabry V, Mingler MK, Cole ET, Orkin SH, Aronow BJ, Rothenberg ME. MicroRNA-21 Limits In Vivo Immune Response-Mediated Activation of the IL-12/IFN-{gamma} Pathway, Th1 Polarization, and the Severity of Delayed-Type Hypersensitivity. J Immunol. 2011;187:3362–3373. doi: 10.4049/jimmunol.1101235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sabatel C, Malvaux L, Bovy N, Deroanne C, Lambert V, Gonzalez ML, Colige A, Rakic JM, Noel A, Martial JA, Struman I. MicroRNA-21 exhibits antiangiogenic function by targeting RhoB expression in endothelial cells. PloS One. 2011;6:e16979. doi: 10.1371/journal.pone.0016979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hernandez-Perera O, Perez-Sala D, Soria E, Lamas S. Involvement of Rho GTPases in the transcriptional inhibition of preproendothelin-1 gene expression by simvastatin in vascular endothelial cells. Circ Res. 2000;87:616–622. doi: 10.1161/01.res.87.7.616. [DOI] [PubMed] [Google Scholar]
- 43.Patrick DM, Montgomery RL, Qi X, Obad S, Kauppinen S, Hill JA, van Rooij E, Olson EN. Stress-dependent cardiac remodeling occurs in the absence of microRNA-21 in mice. J Clin Invest. 2010;120:3912–3916. doi: 10.1172/JCI43604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caruso P, MacLean MR, Khanin R, McClure J, Soon E, Southgate M, MacDonald RA, Greig JA, Robertson KE, Masson R, Denby L, Dempsie Y, Long L, Morrell NW, Baker AH. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol. 2010;30:716–723. doi: 10.1161/ATVBAHA.109.202028. [DOI] [PubMed] [Google Scholar]
- 45.Bhargava A, Kumar A, Yuan N, Gewitz MH, Mathew R. Monocrotaline induces interleukin-6 mRNA expression in rat lungs. Heart Dis. 1999;1:126–132. [PubMed] [Google Scholar]
- 46.Lai YL, Law TC. Chronic hypoxia- and monocrotaline-induced elevation of hypoxia-inducible factor-1 alpha levels and pulmonary hypertension. J Biomed Sci. 2004;11:315–321. doi: 10.1007/BF02254435. [DOI] [PubMed] [Google Scholar]
- 47.Humbert M, Monti G, Brenot F, Sitbon O, Portier A, Grangeot-Keros L, Duroux P, Galanaud P, Simonneau G, Emilie D. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med. 1995;151:1628–1631. doi: 10.1164/ajrccm.151.5.7735624. [DOI] [PubMed] [Google Scholar]
- 48.Hanze J, Weissmann N, Grimminger F, Seeger W, Rose F. Cellular and molecular mechanisms of hypoxia-inducible factor driven vascular remodeling. Thromb Haemost. 2007;97:774–787. [PubMed] [Google Scholar]
- 49.Loffler D, Brocke-Heidrich K, Pfeifer G, Stocsits C, Hackermuller J, Kretzschmar AK, Burger R, Gramatzki M, Blumert C, Bauer K, Cvijic H, Ullmann AK, Stadler PF, Horn F. Interleukin-6 dependent survival of multiple myeloma cells involves the Stat3-mediated induction of microRNA-21 through a highly conserved enhancer. Blood. 2007;110:1330–1333. doi: 10.1182/blood-2007-03-081133. [DOI] [PubMed] [Google Scholar]
- 50.Song Y, Jones J, Beppu H, Keaney JJ, Loscalzo J, Zhang Y. Increased susceptibility to pulmonary hypertension in heterozygous BMPR2-mutant mice. Circulation. 2005;112:553–562. doi: 10.1161/CIRCULATIONAHA.104.492488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.