Abstract
Poly(ethylene glycol) (PEG) conjugation (i.e. PEGylation) is a commonly used strategy to increase the circulatory half-life of therapeutic proteins and colloids, however, few viable alternatives exist to replicate its functions. Herein, we report a method for the rapid site-selective glycosylation of proteins with various sized carbohydrates, up to a molecular weight (MW) of 10,000 Da, thus, serving as a potential alternative for PEGylation. More importantly, the method developed has two unique features. First, traditional protecting group strategies that typically accompany the modification of the carbohydrate fragments are circumvented, allowing for the facile site-selective glycosylation of a desired protein with various sized glycans. Second, the methodology employed is not limited by oligosaccharide size; consequently, glycans of a similar MW to that of PEG, used in the PEGylation of therapeutic proteins, can be employed. To demonstrate the usefulness of this technology, hemoglobin (Hb) was site-selectively glycosylated with a series of carbohydrates of increasing MW (504 to ~10,000 Da). Hb was selected based on the vast wealth of biochemical and biophysical knowledge present in the literature and because of its use as a precursor in the synthesis/formulation of artificial red blood cell substitutes. Following the successful site-selective glycosylation of Hb, the impact of increasing the glycan MW on Hb’s biophysical properties was investigated in vitro.
Keywords: Glycosylation, glycoprotein synthesis, hemoglobin cysteine 93, PEGylation, carbohydrate, saccharide
INTRODUCTION
The covalent coupling of poly(ethylene glycol) (PEG) to therapeutic proteins and colloids, PEGylation, has long provided an effective means to increase the pharmacological effectiveness of such biopharmaceuticals1–4. PEGylation serves to increase the hydrodynamic radius of the protein/colloid, effectively shielding its surface and preventing recognition by the reticuloendothelial system. Benefits of PEGylation include increased circulatory half-life, decreased degradation by metabolic enzymes and reduced protein immunogenicity. However, despite the numerous benefits of PEGylation, questions regarding its universal application have arisen in the literature5,6. In certain cases, PEGylation has been observed to promote decreased biological activity and can accumulate over long periods of treatment, giving rise to macromolecular syndrome3.
Currently, various groups are exploring potential alternatives to PEGylation for improving the pharmaceutical effectiveness of therapeutic proteins7. In certain cases, inspiration has been taken from nature, in which the glycosylation of proteins, with even small oligosaccharides, functions in much the same way as PEGylation8,9. Similar to PEGylation, glycosylation provides a large hydration shell capable of protecting proteins from proteolysis10. Interestingly though, glycosylation has the added benefit of being able to stabilize the protein by forming hydrogen bonds with a protein’s amino acid residues. Furthermore, glycosylation has been shown to increase thermal stability while decreasing precipitation and aggregation11,12. Consequently, due to the importance of glycoproteins in nature, the glycosylation of proteins in the laboratory (glycoprotein synthesis) has long been an area of interest among researchers, and many elegant methods exist in the literature13,14.
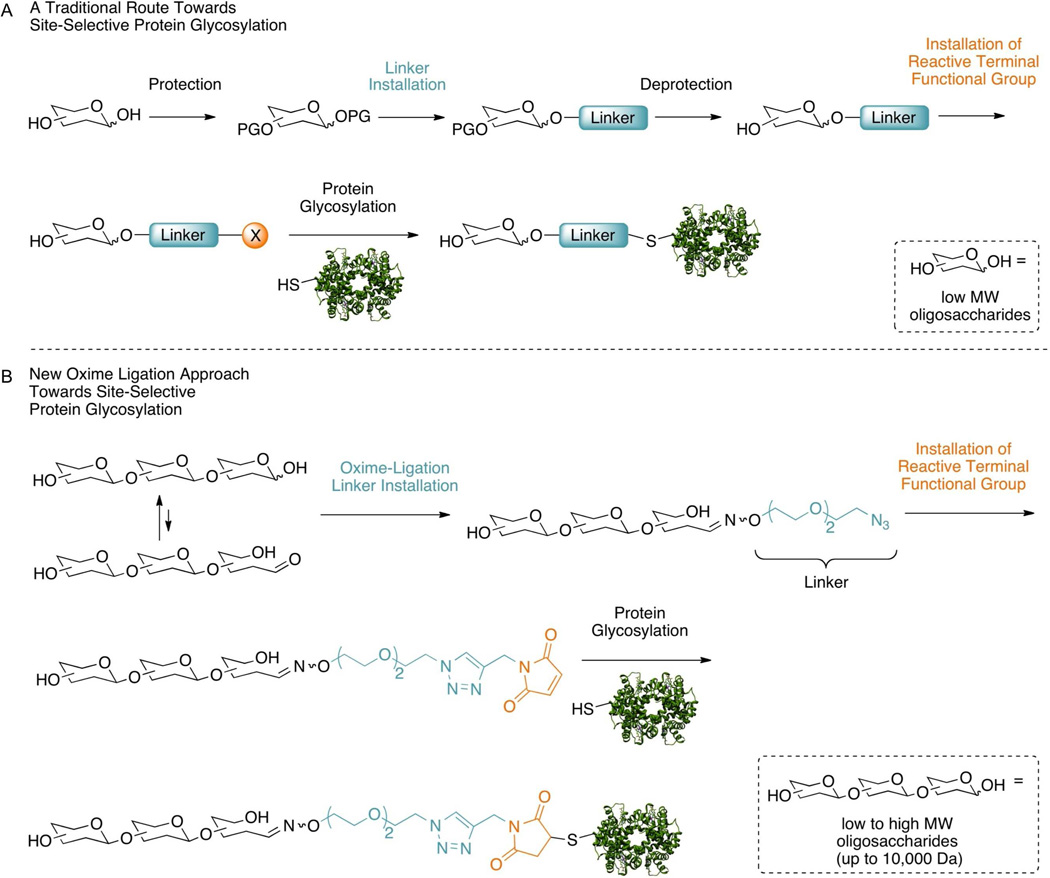
Unfortunately, two limiting factors are frequently associated with the ability to chemically glycosylate a protein in the laboratory. The first is the rate at which the carbohydrate derivatives, or carbohydrate linkers, used in protein glycosylation can be synthesized—a bottleneck mainly due the tedious protecting group strategies that accommodate carbohydrate modification, as shown in Figure 1A13,14. The second limiting factor is that current methods for protein glycosylation are conceivably confined to small oligosaccharides due, in part, to the low solubility of large oligosaccharides and polysaccharides in many organic solvents. This low solubility, or lack thereof, significantly reduces the routes available for glycan modification necessary to employ them in glycoprotein synthesis. Consequently, based on these limitations, we sought to develop a method that would circumvent carbohydrate protecting group chemistry altogether and be aqueous-friendly, thus allowing a desired protein to be glycosylated with a wide variety of carbohydrates, regardless of size.
Figure 1.
(A) Traditional route towards glycoprotein synthesis involving protecting group manipulation. (B) New oxime-ligation approach avoiding the need for protecting group manipulation.
Herein, we report a versatile method for the site-selective glycosylation of proteins that requires no protecting group chemistry on the carbohydrate fragment and, importantly, allows for the use of large molecular weight (MW) oligosaccharides. By taking advantage of the aldehyde available in the open-chain conformation on the reducing end carbohydrate, we were able to employ oxime-ligation chemistry for the installation of a linker fragment (Figure 1B). It is important to note that this method enabled us to employ glycans of a comparable MW to that of PEG, commonly used in the PEGylation of therapeutic proteins, to be conjugated site-selectively to proteins. Thus, this method of site-selectively glycosylating proteins with high MW oligosaccharides may provide a new viable alternative to site-selective PEGylation, as carbohydrates are biodegradable, whereas PEG is not, and carry with them further advantageous properties. In addition, the ease by which this method can be employed may allow for the facile fine-tuning of the pharmacokinetic and pharmacodynamic properties of therapeutic proteins, as is done with the PEGylation of similar therapeutics. To the best of our knowledge, this is the first reported method for the site-selective chemical glycosylation of proteins with oligosaccharides of such high MWs.
To demonstrate the usefulness of this methodology, hemoglobin (Hb) was site-selectively glycosylated with three different maltose-type sugars (MWs ranging from 504–1,153 Da) and a dextran sugar (MW ~10,000 Da). After determining the glycosylation reactions were successful and indeed site-selective, the biophysical properties of the oligosaccharide conjugated Hbs were subsequently studied in vitro. Hb was chosen as the test protein for the conjugation chemistry based on several factors including: (1) the significant amount of information present in the literature regarding Hb’s biochemistry, (2) the feature of bovine Hb (bHb) containing one cysteine residue on the β chain at position 93 and none on the α chain, making site-selective modification possible and (3) the potential of Hb to serve as an oxygen therapeutic for use in transfusion medicine to treat moderate to severe blood loss15–17. Thus, using thiol-targeting chemistry we were able to successfully conjugate various MW glycans to the two cysteine residues on the Hb tetramer (α2β2) and, subsequently, investigate the biophysical effects of glycosylation. Not only did this methodology allow us to explore the potential of glycosylated Hb to serve as an oxygen therapeutic, but the methodology holds the promising ability to be applied to a wide variety of other therapeutic proteins bearing naturally occurring and accessible cysteine residues or to therapeutic proteins that have undergone site-directed mutagenesis to incorporate cysteine residues.
RESULTS AND DISCUSSION
Design of the Carbohydrate Linkers
Typically, site-selective protein glycosylation occurs in a fashion similar to that depicted in Figure 1A in which the chosen glycan is chemically modified and then allowed to react with the desired protein13,14,18. Chemical modification of the glycan typically involves initial protection of the hydroxyl groups followed by activation at the anomeric position, thus allowing for the subsequent installation of a substituent (referred to as the linker herein) responsible for linking the glycan moiety to the desired protein. Lastly, the carbohydrate must be deprotected and the linker modified to accommodate a functional group capable of reacting site-selectively with the protein. Consequently, when new glycans are chosen for protein glycosylation, an exponentially greater number of synthetic steps are required, slowing the rate at which a protein can be glycosylated with various glycans and subsequent investigation of the biophysical effects of glycosylation.
Thus, in an attempt to abstain from the use of this lengthy process, we searched for a method that would circumvent the requirement of protecting the desired glycan and be aqueous-friendly, allowing for the use of high MW oligosaccharides not soluble in most organic solvents. As a result, we chose to employ an oxime-ligation for the installation of a linker and “click” chemistry for its subsequent modification to allow for site-selective glycosylation (Figure 1B). Employing an oxime-ligation allowed us to take advantage of the ability of an aminooxy group to react with a ketone or aldehyde—in our case, the aldehyde present in the open-chain conformation on the reducing end carbohydrate of the oligosaccharide—forming an oxime bond. Notably, oxime-ligation conjugates have been shown to be stable under physiological conditions and a wide pH range19–21. This type of oxime-ligation chemistry has previously been applied to the development of carbohydrate microarrays22,23, glyconanoparticles24,25 and solution-phase tagging of glycans26–28. Furthermore, at the time of conception, it was our belief that the addition of aniline to the oxime-ligation would catalyze the reaction, functioning as a nucleophilic catalyst under slightly acidic conditions, as noted by Dawson and coworkers, but which had yet to be applied to carbohydrate chemistry29. Confirming our suspicions, while we were carrying out the synthetic portion of this project, Jensen and coworkers published a paper diligently detailing the effects of nucleophiic catalysis on carbohydrate oxime formation by aniline30. Accordingly, the linker was designed to incorporate an aminooxy functional group on one end and an azido group on the opposite end, with the two being separated by a short oligoethyleneglycol spacer. The aminooxy group was selected to partake in the aforementioned oxime-ligation, while the azido group was selected to remain inert during the oxime-ligation to subsequently allow for the installation of a maleimide group—capable of site-selectively reacting with the cysteine residues of a protein—via a CuAAC (copper(I) catalyzed azide-alkyne cycloaddition) reaction. It is important to note that the overall design of the synthesis was constructed to allow for easy scale-up with a minimal number of synthetic steps.
Four different sized carbohydrates, spanning a MW range of 504–10,000 Da, were chosen for the glycosylation of bHb. Of the four chosen carbohydrates, three were maltose sugars, maltotriose, maltopentaose and maltoheptaose, with the other being a dextran sugar with an average MW of 10,000 Da. Both the maltose and dextran sugars are oligomers of glucose with differing linkages, the maltoses being composed of α(1→4) linkages and the dextran being composed of α(1→6) linkages. Although the maltose carbohydrates have a lower MW range (504–1,153 Da), conjugation of smaller oligosaccharides could still be advantageous based on reports that PEGylation with low MW PEG (300–2,500 Da) effectively increases the pharmacokinetics and immunogenicity profiles of therapeutic proteins31. The dextran glycan, in addition to being of a much higher MW—comparable to that of PEG commonly used in therapeutic protein conjugation—belongs to a family of carbohydrates (dextrans) that are utilized as plasma expanders, coatings for certain biomedical devices and components of biomedical hydrogels32–35.
Synthesis of the Carbohydrate Linkers
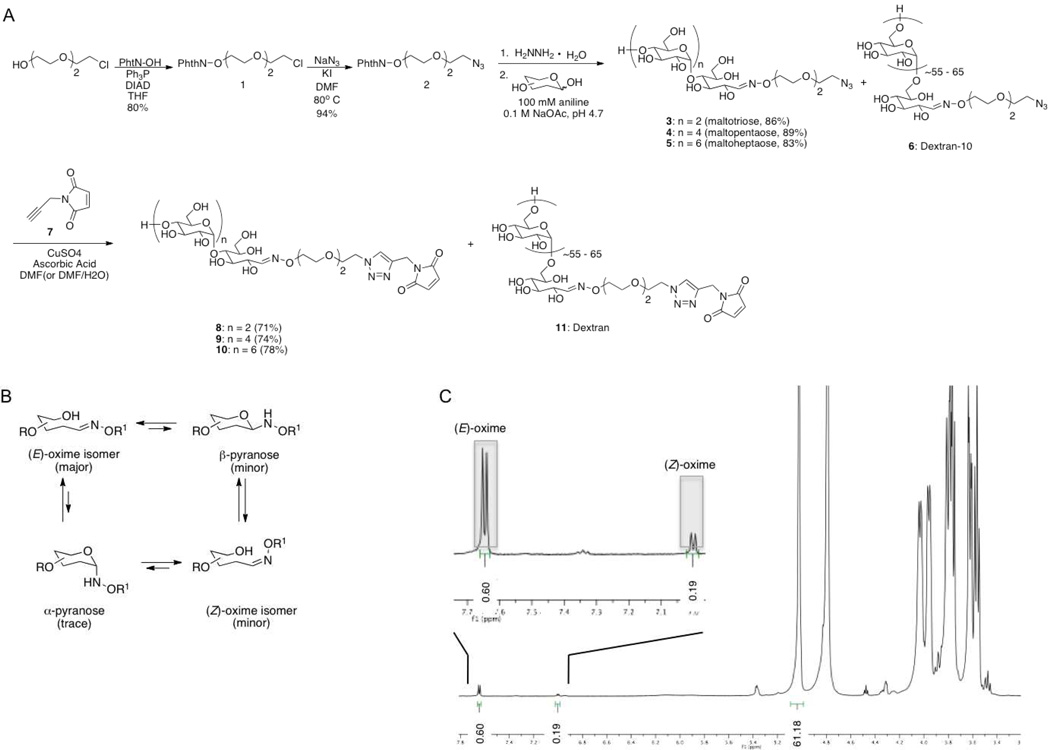
Synthesis of the carbohydrate linkers is depicted in Figure 2A and began with 2-(2-[2-chloroethoxy]ethoxy) ethanol to which a protected amiooxy functional group was installed via a Mitsunobu reaction with N-hydroxyphtalimide giving substrate 1. Subsequent azide displacement of the chloro group gave linker 2, which was capable of being synthesized on a multi-gram scale.
Figure 2.
(A) Synthesis of the aminooxy-bearing linker and subsequent synthesis of maltotriose-, maltopentaose-, maltoheptaose- and dextran-linkers. (B) Ring-chain tautonomers of the oximine-ligation products including (E)-oxime, (Z)-oxime, β-pyranose and α-pyranose24,36. (C) 1H-NMR of dextran-linker 6 showing the presence of the (E)-oxime (0.6H) and (Z)-oxime (0.19H) H-1 with the integration of the C1-H of the repeating glucose residues being (61.18H)37.
Following the removal of the phthalimide protecting group using hydrazine monohydrate, oxime ligations between linker 2 and the desired glycans were carried out under slightly acidic conditions (pH 4.7) in the presence of 100 mM aniline, giving the carbohydrate-azide linkers 3–6. All reactions proceeded with relatively high yields, were purified by size exclusion chromatography and subsequently characterized by LC-MS, 1H NMR and 13C NMR. The oxime-ligation products 3–6 can exist as several ring-chain tautonomeric forms (Figure 2B), of which, the (E)-oxime and (Z)-oxime predominate over the α- and β-pyranoses with each giving rise to distinctive 1H NMR and 13C NMR peaks for H-1 and C-1 of the conjugated glycan24,30. Importantly, the 1H NMR of the dextran linker 6 (Figure 2C) confirmed that the aminooxy linker 2, once deprotected, was capable of reacting with the high MW dextran oligosaccharide as shown by the presence of the (E)-oxime and (Z)-oxime H-1 1H NMR peaks. With the assembly of the four carbohydrate azide linkers complete, a maleimide functional group was installed via a CuAAC reaction between linkers 3–6 and compound 7, which itself was synthesized by a short one step procedure (see Supporting Information), giving the desired carbohydrate maleimide linkers 8–11.
Preparation and Glycosylation of bHb
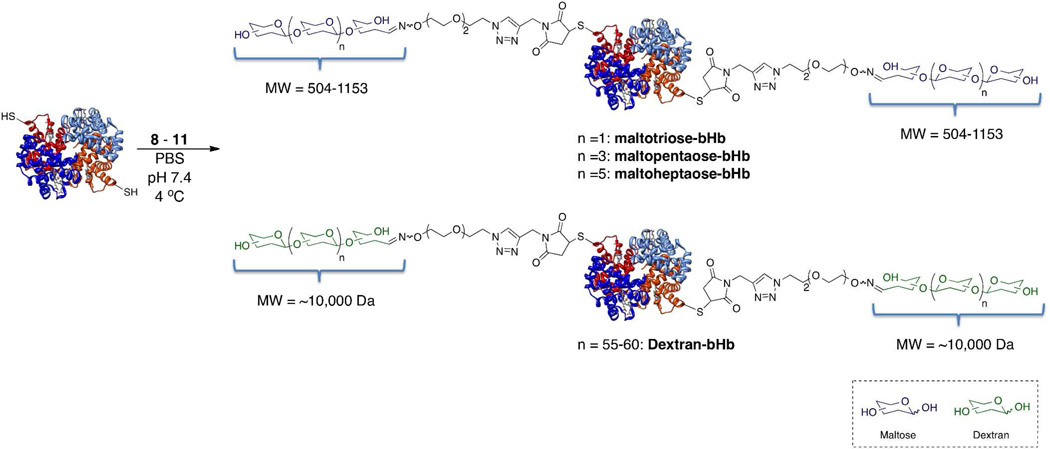
bHb is a readily available type of Hb capable of being purified on a multi-kilogram scale, making it an attractive precursor for the synthesis/formulation of oxygen therapeutics38,39. Like other forms of Hb, bHb is a tetramer consisting of two identical α chains and two identical β chains. More importantly for our purposes, the two β chains both display one cysteine residue (Cys-93), which have been previously shown to be capable of modification with maleimide-modified compounds18,40.
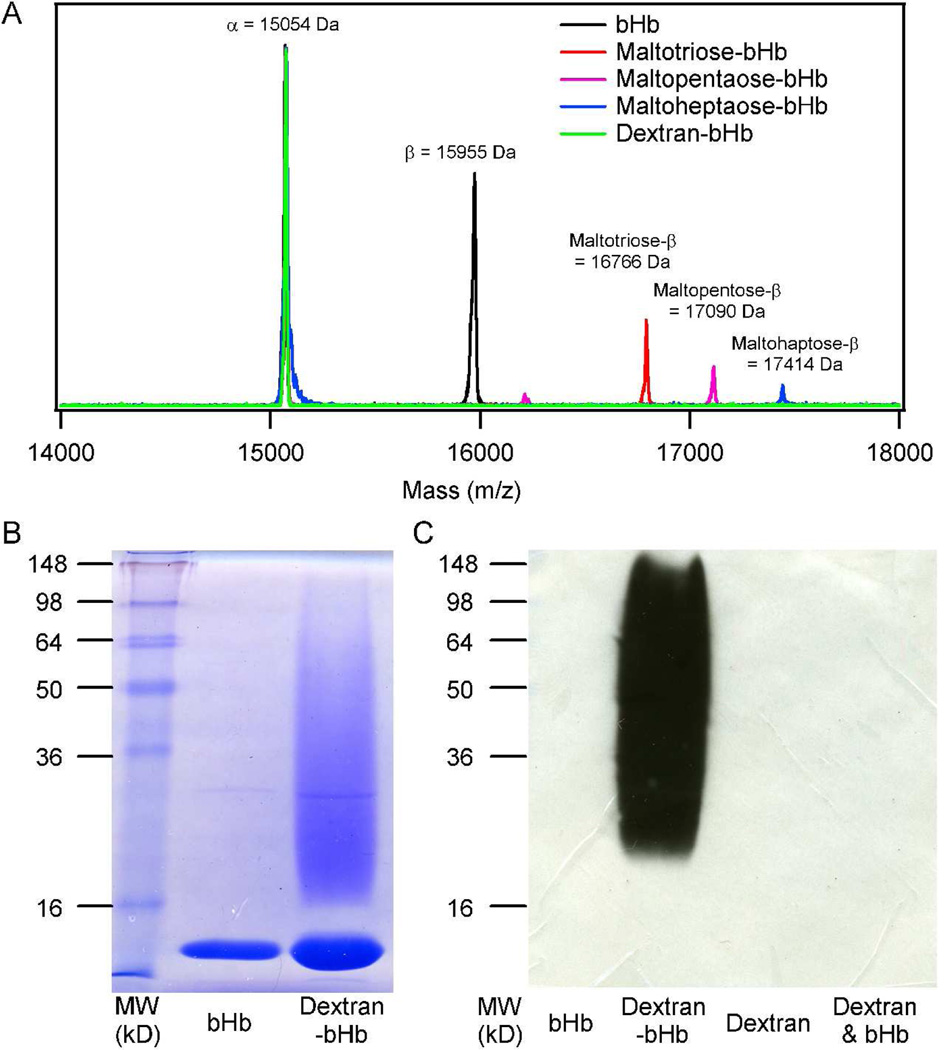
Using a minimal excess of the synthesized glycan-linkers 8–11, bHb was successfully site-selectively glycosylated, via a maleimide conjugation reaction—introducing a novel chiral center on the resultant succinimide thioether—giving the desired maltotriose-bHb, maltopentaose-bHb, maltoheptaose-bHb and dextran-bHb glycoconjugates (Figure 3). After purification, the glycosylated bHb samples were submitted for analysis by MALDI, LC-MS and tryptic digest followed by LC-MS/MS. Initial analysis by MALDI for the linker-treated bHb showed successful glycosylation of the β subunit (Figure 4A). Glycosylation of the β-subunit was evident through the complete disappearance of the MW peak corresponding to the native β-subunit (15,955 Da) and the emergence of a new peak corresponding to that of the carbohydrate modified β-subunit. For instance, when bHb was treated with the maltotriose linker (MW 811 Da) the detected mass shifted from the original mass of 15,955 Da to 16,766 Da (β-subunit + linker). For bHb treated with the dextran-linker, the disappearance of the native β-subunit MW was observed, however, the presence of the dextran modified β-subunit could not be detected by MALDI due to the interference of ionization imposed by the large oligosaccharide dextran, a trait commonly seen with proteins bearing large oligosaccharides41. Thus, to further analyze the dextran-modified Hb and confirm glycosylation, SDS-PAGE and Western blot analysis, with an anti-dextran antibody, were performed (Figure 4B and 4C, respectively)42, 43. Both the SDS-PAGE and Western blot analysis showed a large shift in the MW of the β-subunit, with the Western blot staining positive for dextran in the region corresponding to the modified β-subunit. Further analysis of the glycosylated bHb by LC-MS demonstrated that the glycosylation reaction went to completion leaving no unmodified β-subunit and showed an increase in MW corresponding to the linker that the bHb was treated with, as was observed by MALDI. Furthermore, completion of the glycosylation reaction was evident in the RP-HPLC chromatograms of the LC-MS analysis, in which only two peaks were present, corresponding to the α and modified β-subunits. Deconvolution of the ESI mass spectrums for the peaks corresponding to the modified β-subunits yielded molecular ions with masses of 15,955, 16,767, 17,091 and 17,415 Da, correlating to native bHb (Figure S1), maltotriose-bHb (Figure S2), maltopentaose-bHb (Figure S3) and maltoheptaose-bHb (Figure S4), respectively. Additionally, trypsin digestion, followed by LC-MS/MS analysis was used to confirm that Cys 93 of the β-subunit was indeed the site of glycosylation (Table S1, S2 and S3), with the results indicating that the maleimide moiety was present in the open maleimic acid form due to the partial hydrolysis of the succinimidyl thioether that occurred during the procedure—a side reaction that has been reported in the literature.44
Figure 3.
Synthesis of maltotriose-bHb, maltopentaose-bHb, maltoheptaose-bHb and dextran-bHb.
Figure 4.
A) MALDI-ESI of bHb, maltotriose-bHb, maltopentaose-bHb, maltoheptaose-bHb and dextran-bHb. B) SDS-PAGE of bHb and dextran-bHb. C) Western blot of bHb, dextran-bHb, dextran, and dextran-bHb.
Evaluation of the Biophysical Properties of bHb
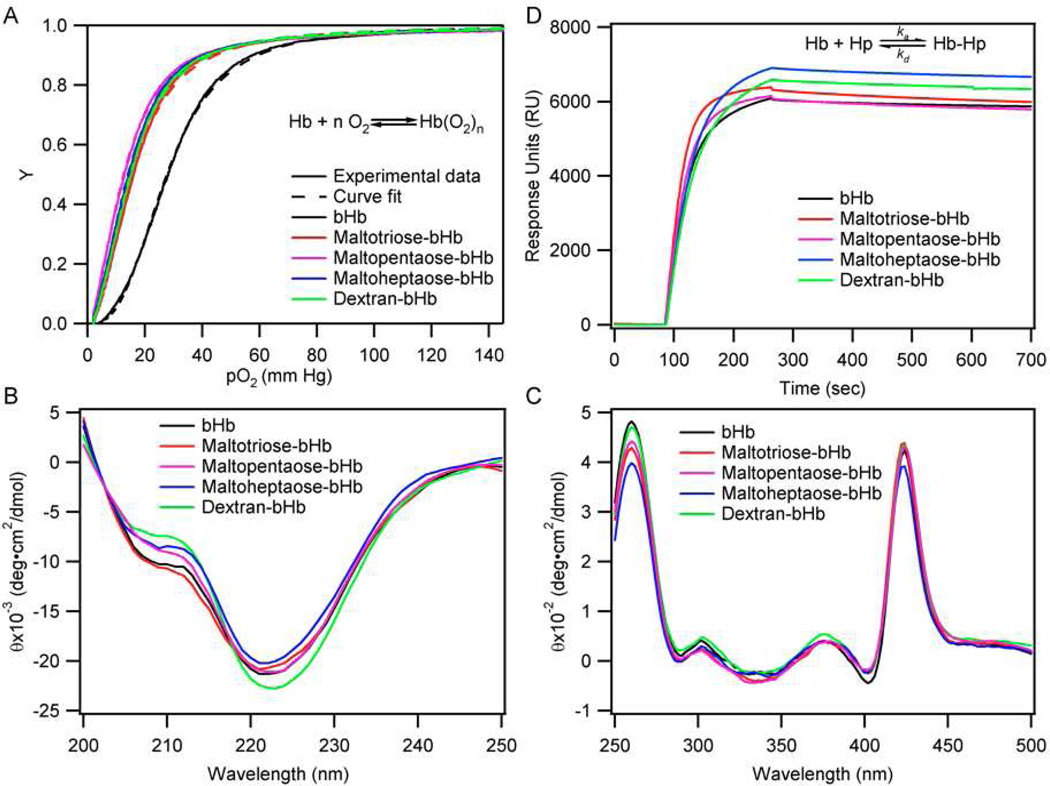
After confirming that bHb was indeed glycosylated at the Cys-β93 position, we proceeded to study the effect of glycosylation on the biophysical properties of bHb. In particular, we were interested in examining the effect of glycosylation on the equilibria between oxygen and bHb, the secondary structure and heme environment of bHb, the kinetics of haptoglobin (Hp) binding to bHb and the 3-dimensional structure of bHb.
Oxygen-bHb/Glycosylated bHb Equilibria
Hb is primarily responsible for gaseous ligand storage and transport in the circulatory system and thus has received significant attention as a precursor for the synthesis/formulation of oxygen therapeutics, i.e. Hb-based oxygen carriers (HBOCs)15–17. Consequently, in this study, the equilibria between oxygen and bHb/glycosylated bHb was measured in order to investigate the effects of glycosylation on the oxygen affinity (P50) and cooperativity coefficients (n) of bHb. The P50 is an indicator of oxygen affinity and refers to the partial pressure oxygen (pO2) at which half the HBOC is saturated with oxygen. The cooperativity coefficient (n) of Hb is associated with the allosteric transition from the tense (T) to the relaxed (R) quaternary state upon oxygenation45. For native bHb, the P50 was determined to be 27.61 mm Hg, while the glycosylated Hbs had values ranging from 12–16 mm Hg (Table 1). It is apparent from the oxygen equilibrium curves (Figure 5A), decreased cooperativity coefficients, and decreased P50 values (Table 1) that the glycosylated Hbs possess a higher affinity for oxygen compared to native Hb. The observed reduction in cooperativity upon glycosylation of Cys β93 indicates a reduction in the available motion of the α1β2 interface in which the Cys β93 is located. Similar modifications of Cys β93 by other chemical agents have been shown to have an analogous effect by influencing the heme pocket of the β chain46–48.
Table 1.
Oxygen affinity (P50) and cooperativity coeffient of (n) of native bHb and glycosylated bHbs.
| bHb | Maltotriose- bHb |
Maltopentaose- bHb |
Maltohaptaose- bHb |
Dextran-bHb | |
|---|---|---|---|---|---|
| P50 (mm Hg) | 27.61±0.02 | 15.75±0.01 | 12.57±0.01 | 14.09±0.01 | 14.91±0.01 |
| n | 2.862±0.010 | 2.106±0.004 | 1.835±0.004 | 2.002±0.004 | 2.065±0.004 |
Figure 5.
(A) Oxygen equilibrium curves of bHb, maltotriose-bHb, maltopentaose-bHb, maltoheptaose-bHb and dextran-bHb. Circular dichroism (CD) spectra of native and glycosylated Hbs in the: (B) far-ultraviolet region and (C) near-ultraviolet regions. (D) Surface plasmon resonance (SPR) analysis of native and glycosylated bHbs.
Interestingly, the low P50 of glycosylated Hbs may be beneficial in any future development of glycosylated Hbs as potential HBOCs. This lies in the fact that one of the few HBOCs to reach phase III clinical trials, Hemospan® (PEGylated human Hb), has a low P50 value of 5–6 mm Hg, whereas other HBOCs that have failed in Phase II clinical trials typically lie in the range of 26–38 mm Hg49,50. It has been postulated that lower P50 values may be beneficial by limiting arteriole oxygen transport, thus, targeting oxygen transport to tissues and organs with low partial pressure and avoiding induced high blood pressure caused by an autoregulatory response to high arteriole O2 levels, resulting in vasoconstriction51.
Circular Dichroism (CD) Analysis
To investigate the effects of glycosylation on the structural characteristics of Hb and its glycosylated variants, the CD spectra were obtained, in the far-ultraviolet (200–250 nm) region (Figure 4B) and near-ultraviolet (250–500 nm) region (Figure 5C). An initial concern in performing CD spectroscopy was the potential of the conjugated carbohydrates themselves altering the obtained spectra of the modified Hb, however, it is known that the absorbance of unsubstituted polysaccharides does not lie above wavelengths greater than 200 nm, and thus, the spectra obtained were expected to yield accurate information about the secondary structure and heme environment of glycosylated Hbs52. Comparison of the glycosylated and native Hb spectra in the far-ultraviolet region reveals that glycosylation does not adversely affect the structure of bHb as indicated by the analogous spectra. In the near-ultraviolet spectra, the L band (in the vicinity of 260 nm) provides information indicative of the heme ligand, and examination of this region shows no significant change in intensity upon glycosylation. Likewise, investigation of the Soret region (around 420 nm), which provides information regarding the interactions of the heme prosthetic group with surrounding aromatic residues, shows little deviation for the glycosylated Hb compared to native Hb. Overall, glycosylation does not appear to alter the secondary structure and heme environment of bHb, as indicated by examining the CD spectra.
Surface Plasmon Resonance (SPR) Analysis
Haptoglobin-hemoglobin (Hp-Hb) complexation is an important biological process in which Hp binds to free Hb in the blood to effectively remove it from systemic circulation53. Thus, SPR analysis was used to investigate the effect of glycosylation at Cys-β93 on the formation of Hp-Hb complexes. The binding-dissociation curves (Figure 5D) indicate that glycosylation at Cys-β93 has only a slight influence on the interaction between glycosylated bHb and Hp. Compared to bHb with an association rate constant (ka) of 1.30 × 105 M−1·S−1, maltotriose and maltopentaose modification resulted in a slightly higher association rate constant (1.83 × 105 M−1·S−1 and 1.49 × 105 M−1·S−1, respectively), while maltoheptaose and dextran modification resulted in a lower association rate constant, 1.12 × 105 M−1·S−1 and 1.30 × 105 M−1·S−1, respectively (Table 2). Furthermore, glycosylation increased the dissociation rate constant (kd) from 5.23 × 10−5s−1 for native bHb to a value greater than 6.45 × 10−5s−1 for all four forms of glycosylated bHb (Table 2). Collectively, glycosylation increased the equilibrium dissociation constant (KD) from 4.02 × 10−10 M to a value above 5.46 × 10−10 M for the lower MW glycosylated forms of bHb with the dextran-bHb reaching as high as 8.23 × 10−10 M (Table 2). Consequently, it can be inferred that glycosylation appears to slightly interfere with the formation of Hp-Hb complexes, which may be expected considering the glycans rest on the surface of Hb and likely interfere with Hp’s recognition of Hb.
Table 2.
Association/dissociation rate constants and equilibrium dissociation constant of native and glycosylated bHbs.
| Sample | bHb | Maltotriose- bHb |
Maltopentaose- bHb |
Maltohaptaose- bHb |
Dextran-bHb |
|---|---|---|---|---|---|
| ka (M−1·s−1) | (1.30±0.01)×105 | (1.83±0.13)×105 | (1.49±0.07)×105 | (1.12±0.05)×105 | (1.00±0.04)×105 |
| kd (s−1) | (5.23±0.04)×10−5 | (10.00±0.05)×10−5 | (8.85±0.06)×10−5 | (6.45±0.04)×10−5 | (8.23±0.07)×10−5 |
| KD (M) | (4.02±0.03)×10−10 | (5.46±0.10)×10−10 | (5.94±0.07)×10−10 | (5.76±0.05)×10−10 | (8.23±0.06)×10−10 |
X-Ray Crystallography
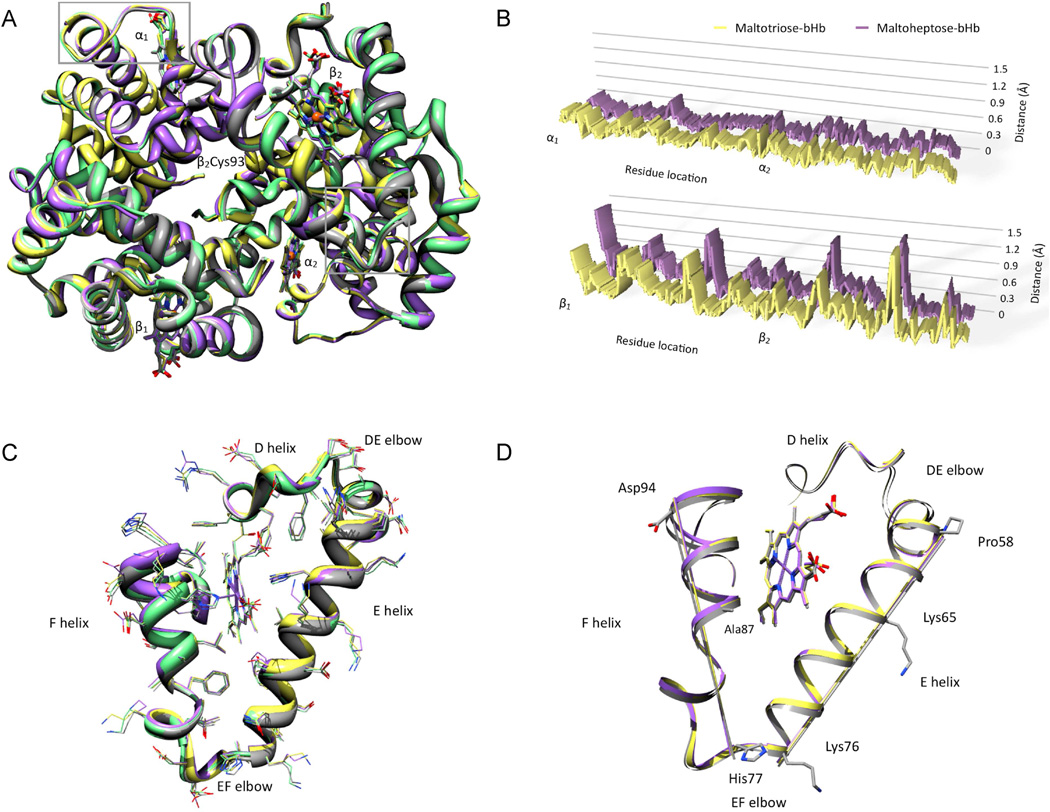
Hb is a α2β2 tetramer having one true 2-fold rotational symmetry axis and two 2-fold pseudosymmetry axes. Functional properties of Hb, such as its equilibrium oxygen binding characteristics, are regulated at the structural level by the relative orientation and interaction of its constituent subunits54,55. For example, interactions at the α1β1 (and the corresponding α2β2) intersubunit interface as well as the interactions at the α2β1 (and the corresponding α1β2) interface launch Hb into quaternary conformations that have distinct oxygen affinities56–59. Furthermore, in the absence of certain salt bridges Hb’s ‘central cavity’ assumes a more relaxed, high oxygen affinity state referred to as the R-state55,60. Consequently, the quaternary structure of Hb directly reflects its functional behavior making it paramount to investigate any significant changes upon glycosylation. Accordingly, the crystal structures of maltotriose-bHb, maltopentose-bHb and maltoheptose-bHb were obtained and reveal that the tetrameric state was preserved upon glycosylation. More importantly, a superposition of only the α1β1 subunits of tetrameric bHb and glycosylated bHbs shows that the quaternary state of the glycosylated bHbs corresponds exactly to the quaternary state of native bHb (data not shown). A complete structural alignment of the 572 Cα atoms of the α2β2 subunits of bHb and the glycosylated bHbs shows that their tertiary and quaternary features correspond well (Figure 6A). The average root mean square deviations from bHb are 0.337 Å for maltotriose-bHb and 0.371 Å for maltoheptose-bHb. These analyses strongly suggest that the overall tertiary and quaternary features of bHb are preserved upon glycosylation.
Figure 6.
(A) Superposition of bHb (grey), maltotriose-bHb (yellow), maltopentaose-bHb (green) and maltoheptaose-bHb (purple). The overall tertiary and quaternary structures of all three glycosylated bHbs are quite similar to native bHb. Grey rectangles enclose two regions exhibiting conformational change: the CE elbow on the α-subunit (top left rectangle) and the EF elbow on the β-subunit (lower bottom rectangle). (B) Variable deviation from native bHb in the α and β subunits of maltotriose (yellow) and maltoheptaose bHb (purple). Five residues at the N- and C- termini of each of the four subunits were deleted and the Cα atoms of all of the remaining residues were used to generate an optimal superposition. The peaks represent the separation of a Cα atom of each of the glycosylated Hbs from the corresponding Cα atom of native bHb. (C) Overall preservation of the side chain conformations in the ‘EF’ region of the β1 subunit. The side chain of bHb (grey), maltotriose-bHb (yellow), maltopentaose-bHb (green) and maltoheptaose-bHb (purple) are depicted as thin wires. (D) Axial displacement of E and F helices of the β1 subunit upon glycosylation. Axes were computed through the geometric centroid for Cα atoms of 3 residues on the E helix and Cα atoms of residues selected within the EF elbow, middle of the F helix and the carboxy end of the F helix. The axes passing through the maltotriose-bHb (yellow) and maltoheptaose-bHb (purple) are in a very similar position especially on the F helix. This position is distinct from the axes passing through the corresponding position of native bHb (grey).
The comparison between the structures of glycosylated bHbs and the structure of native bHb reveals that the deviations from the structure of native bHb are not uniformly distributed throughout the bHb molecule, but instead localized (Figure 5B). These deviations were found to be present mostly in the β1 and β2 subunits. However, this was expected to a certain degree, since it is the β Cys-93 residue located near the carboxy edge of the F helix, which is glycosylated. Although electron density for the glycan linker is not defined, the position of structural deviations suggests that the glycan linker interacts closely with the β subunit. The α2β1 interface is defined by a close interaction between the C helix and the CE elbow on the α1 subunit and the carboxy edge of the F helix and the FG elbow on the β2 subunit (Figure 6A). Given the minor structural deviations observed in C-helices on α subunits, it is possible that the interaction influences the conformation of the α2β1 interface. Inspection of the β subunit region of glycosylated bHbs for structural deviation from the corresponding region in native bHb reveals the region is comprised mainly of the E helix, the EF elbow, the F helix and the FG elbow. Interestingly, the side chain conformations in this region closely resemble each other (Figure 6C). This raises the intriguing possibility of a global movement of the helices in the region that accommodates glycosylation, which likely serves as a buffer to restrict transmission of these changes to other regions of the quaternary structure of the glycosylated bHbs.
The Cα atoms of 3 residues contained in the E helix of the β1 subunit namely Pro-57, Lys-66 and Lys-76 were selected for calculating an axis passing through the geometric centroid of the atoms. This procedure was repeated for bHb, maltotriose-bHb and maltoheptaose-bHb. In a similar fashion, axes were computed through the geometric centroid of Cα atoms of 3 residues on the β1 subunit, namely His-77 that is located at the EF elbow, Ala-87 that is located in the middle of the F helix and Asp-94 that is located at the carboxy end of the F helix. These two sets of axes revealed some interesting features (Figure 6D) that may have profound implications. Firstly, the axes drawn through the β1 subunits of maltotriose-bHb and maltoheptaose-bHb are in almost identical positions. Secondly, the axes computed for the β1 subunit of native bHb are in a position that is close to, yet distinctly apart from the corresponding axes in maltotriose-bHb and maltoheptaose-bHb. Thirdly, these analyses reveal the structure contained within the EF helices undergoes a ‘global’ movement farther away from the α2β1 interface. Finally, the β1 superposition in the region of the FG elbow and amino edge of the G helix as well as the β1 superposition in the region of the D helix and the DE elbow indicates that these conformational changes are not transmitted further. Conformational changes in the F helix of β subunits, especially near its carboxy edge are deemed important in determining the oxygen affinity of Hb61. Thus upon oxygen binding, this region is displaced farther from the α2β1 interface, which in turn, ‘relaxes’ Hb and increases its oxygen affinity. The fact that glycosylation at Cys-β93 also results in a small displacement farther from the α2β1 interface rationalizes the observation that glycosylated Hbs have a higher oxygen affinity than native Hb. A measurement of surface area and volume of the heme pocket of bHb, maltotriose-bHb and maltopentose-bHb further reveals these values are quite similar across all the three structures (Table 3), suggesting the conformational changes upon glycosylation are accommodated and compensated for in such a manner that allows the quaternary structure to be preserved.
Table 3.
Dimensions of the heme pocket for selected Hbs.
| bHb | maltotriose-bHb | maltoheptaose-bHb | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α1 | α2 | β1 | β2 | α1 | α2 | β1 | β2 | α1 | α2 | β1 | β2 | |
| Heme pocket surface area (Å2) | 800 | 680 | 600 | 600 | 730 | 640 | 580 | 530 | 730 | 820 | 530 | 520 |
| Heme pocket volume (Å3) | 960 | 950 | 760 | 740 | 920 | 900 | 790 | 740 | 880 | 1010 | 700 | 710 |
CONCLUSIONS
In this work, we have presented a novel method allowing for the facile site-selective glycosylation of proteins with carbohydrates of variable MWs. To the best of our knowledge this is the first reported methodology for the site-selective glycosylation of proteins with oligosaccharides of such a high MW. To demonstrate the feasibility of the reported methodology, Hb was site-selectively glycosylated with four glycans of increasing MW, up to 10,000 Da. Although the present study was limited to oligosaccharides with a maximum MW of 10,000, it is envisioned—due to the aqueous nature of the reactions employed in both the synthesis of the carbohydrate-linkers and the bioconjugation reaction—that larger saccharides could be utilized. Furthermore, upon examining the biophysical properties, of the glycosylated Hb, it was discovered that glycosylation of Hb lowered the P50 to levels similar to that of the commercially developed oxygen therapeutic Hemospan®, now in phase III clinical trials. It has been postulated that a low P50 value may be critical to the development of HBOCs because it facilitates oxygen transport to tissues with low levels of oxygen and decreases arteriole oxygen transport, potentially eliminating undesired cardiovascular effects such as induced high blood pressure51. Consequently, the presented methodology may be of significant importance for the future development of glycosylated-Hbs as potential HBOCs. Specifically, the dextran-conjugated bHb has a significant potential to serve as a “Red Blood Cell (RBC) substitute,” for through site-specific glycosylation it combines Hb, an oxygen carrier, and dextran, a plasma expander.
Moreover, the method developed has the ability to be applied towards the site-specific glycosylation of other therapeutic proteins with high MW oligosaccharides/polysaccharides, thus serving as a viable alternative to site-specific PEGylation. Glycans carry with them a vast array of unique biological properties spread out over a wide range of MWs; consequently, the method reported herein, which easily allows for high MW glycan conjugation to a desired therapeutic protein, could be of significant usefulness. Furthermore, glycans are quite biodegradable in comparison to PEG, making their use advantageous when administration must occur over long periods of time or in large doses, thus preventing long-term accumulation. Future potential applications range form the conjugation of high MW polysacchardies to carrier proteins used in vaccine conjugation—a task that has been daunting in the past—to the conjugation of large oligosaccharides/polysaccharides to therapeutic proteins as a means to extend circulation half-life and thus their pharmacological effectiveness.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants R01HL078840 and R01DK070862 to AFP.
Footnotes
Supporting Information Available. Experimental procedures, compound characterization, biophysical characterization methods along with data and crystallographic information files. This material is available free of charge via the Internet at http://pubs.acs.org
REFERENCES
- 1.Fishburn CS. J. Pharm. Sci. 2008;97:4167. doi: 10.1002/jps.21278. [DOI] [PubMed] [Google Scholar]
- 2.Harris JM, Chess RB. Nat. Rev. Drug Discovery. 2003;2:214. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 3.Veronese FM, Pasut G. Drug Discovery Today. 2005;10:1451. doi: 10.1016/S1359-6446(05)03575-0. [DOI] [PubMed] [Google Scholar]
- 4.Otsuka H, Nagasaki Y, Kataoka K. Advanced Drug Delivery Reviews. 2003;55:403. doi: 10.1016/s0169-409x(02)00226-0. [DOI] [PubMed] [Google Scholar]
- 5.Gaberc-Porekar V, Zore I, Podobnik B, Menart V. Curr Opin Drug Discov Devel. 2008;11:242. [PubMed] [Google Scholar]
- 6.Veronese FM. Biomaterials. 2001;22:405. doi: 10.1016/s0142-9612(00)00193-9. [DOI] [PubMed] [Google Scholar]
- 7.Pisal DS, Kosloski MP, Balu-Iyer SV. Journal of Pharmaceutical Sciences. 2010;99:2557. doi: 10.1002/jps.22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell D, Oldham NJ, Davis BG. Carbohydr. Res. 2009;344:1508. doi: 10.1016/j.carres.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 9.Sato M, Sadamoto R, Niikura K, Monde K, Kondo H, Nishimura SI. Angewandte Chemie International Edition. 2004;43:1516. doi: 10.1002/anie.200353058. [DOI] [PubMed] [Google Scholar]
- 10.Dwek RA. Chem Rev. 1996;96:683. doi: 10.1021/cr940283b. [DOI] [PubMed] [Google Scholar]
- 11.Sola RJ, Griebenow K. BioDrugs. 2010;24:9. doi: 10.2165/11530550-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sola RJ, Rodriguez-Martinez JA, Griebenow K. Cell Mol Life Sci. 2007;64:2133. doi: 10.1007/s00018-007-6551-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis BG. Chem. Rev. (Washington, D. C.) 2002;102:579. doi: 10.1021/cr0004310. [DOI] [PubMed] [Google Scholar]
- 14.Gamblin DP, Scanlan EM, Davis BG. Chem. Rev. (Washington, DC, U. S.) 2009;109:131. doi: 10.1021/cr078291i. [DOI] [PubMed] [Google Scholar]
- 15.Riess JG. Chem Rev. 2001;101:2797. doi: 10.1021/cr970143c. [DOI] [PubMed] [Google Scholar]
- 16.Buehler PW, D'Agnillo F, Schaer DJ. Trends Mol. Med. 2010;16:447. doi: 10.1016/j.molmed.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Awasthi V. Curr. Drug Delivery. 2005;2:133. doi: 10.2174/1567201053586029. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Bhatt VS, Sun G, Wang PG, Palmer AF. Bioconjug Chem. 2008;19:2221. doi: 10.1021/bc8003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Feizi T, Carnpanero-Rhodes MA, Childs RA, Zhang YN, Muiioy B, Evans PG, Osborn HMI, Otto D, Crocker PR, Chai WC. Chem Biol. 2007;14:847. doi: 10.1016/j.chembiol.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Forget D, Renaudet O, Boturyn D, Defrancq E, Dumy P. Tetrahedron Lett. 2001;42:9171. [Google Scholar]
- 21.Forget D, Boturyn D, Defrancq E, Lhomme J, Dumy P. Chem-Eur J. 2001;7:3976. doi: 10.1002/1521-3765(20010917)7:18<3976::aid-chem3976>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Feizi T, Campanero-Rhodes MA, Childs RA, Zhang Y, Mulloy B, Evans PG, Osborn HM, Otto D, Crocker PR, Chai W. Chem Biol. 2007;14:847. doi: 10.1016/j.chembiol.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Lee MR, Shin I. Org Lett. 2005;7:4269. doi: 10.1021/ol051753z. [DOI] [PubMed] [Google Scholar]
- 24.Thygesen MB, Sauer J, Jensen KJ. Chemistry – A European Journal. 2009;15:1649. doi: 10.1002/chem.200801521. [DOI] [PubMed] [Google Scholar]
- 25.Thygesen MB, Sorensen KK, Clo E, Jensen KJ. Chem Commun (Camb) 2009:6367. doi: 10.1039/b911676a. [DOI] [PubMed] [Google Scholar]
- 26.Pauly M, York WS, Guillen R, Albersheim P, Darvill AG. Carbohydr Res. 1996;282:1. doi: 10.1016/0008-6215(95)00362-2. [DOI] [PubMed] [Google Scholar]
- 27.Ramsay SL, Freeman C, Grace PB, Redmond JW, MacLeod JK. Carbohydr Res. 2001;333:59. doi: 10.1016/s0008-6215(01)00115-x. [DOI] [PubMed] [Google Scholar]
- 28.Namavari M, Cheng Z, Zhang R, De A, Levi J, Hoerner JK, Yaghoubi SS, Syud FA, Gambhir SS. Bioconjug Chem. 2009;20:432. doi: 10.1021/bc800422b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dirksen A, Hackeng TM, Dawson PE. Angewandte Chemie International Edition. 2006;45:7581. doi: 10.1002/anie.200602877. [DOI] [PubMed] [Google Scholar]
- 30.Thygesen MB, Munch H, Sauer Jr, CloÃÅ E, J√Πrgensen MR, Hindsgaul O, Jensen KJ. J Org Chem. 2010;75:1752. doi: 10.1021/jo902425v. [DOI] [PubMed] [Google Scholar]
- 31.Novikov BN, Grimsley JK, Kern RJ, Wild JR, Wales ME. Journal of Controlled Release. 2010;146:318. doi: 10.1016/j.jconrel.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Clay JG, Zierold D, Grayson K, Battistella FD. J. Surg. Res. 2009;155:89. doi: 10.1016/j.jss.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 33.Moriyama K, Yui N. Journal of Controlled Release. 1996;42:237. doi: 10.1016/s0168-3659(98)00183-7. [DOI] [PubMed] [Google Scholar]
- 34.Ferreira L, Rafael A, Lamghari M, Barbosa MA, Gil MH, Cabrita AMS, Dordick JS. Journal of Biomedical Materials Research Part A. 2004;68A:584. doi: 10.1002/jbm.a.20102. [DOI] [PubMed] [Google Scholar]
- 35.Sun G, Shen Y-I, Kusuma S, Fox-Talbot K, Steenbergen CJ, Gerecht S. Biomaterials. 2010;32:95. doi: 10.1016/j.biomaterials.2010.08.091. [DOI] [PubMed] [Google Scholar]
- 36.Finch P, Merchant Z. J Chem Soc Perk T 1. 1975:1682. [Google Scholar]
- 37.Integration for the (E)-oxime and (Z)-oxime were based on previous reports which showed a ration of approximately 0.6:0.20 following 2D NMR experiments. See references 21 and 33.
- 38.Palmer AF, Sun G, Harris DR. Biotechnol Prog. 2009;25:189. doi: 10.1002/btpr.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun G, Palmer AF. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;867:1. doi: 10.1016/j.jchromb.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manjula BN, Tsai A, Upadhya R, Perumalsamy K, Smith PK, Malavalli A, Vandegriff K, Winslow RM, Intaglietta M, Prabhakaran M, Friedman JM, Acharya AS. Bioconjug Chem. 2003;14:464. doi: 10.1021/bc0200733. [DOI] [PubMed] [Google Scholar]
- 41.Harvey DJ. Journal of Chromatography A. 1996;720:429. doi: 10.1016/0021-9673(95)00307-x. [DOI] [PubMed] [Google Scholar]
- 42.The broad nature of the band in Figure 4(c) arose primarily due to a combination of: (1) the ‘smearing’ effect seen when polysaccharides are analyzed via SDS-polyacrylamide gel electrophoresis; and (2) the heterogeneity of the dextran used. See Reference 43 for a description of the ‘smearing’ effect seen when utilizing SDS-polyacrylamide gel electrophoresis for the analysis of protein glycosylation.
- 43.Jacobs PP, Geysens S, Vervecken W, Contreras R, Callewaert N. Nat Protoc. 2009;4:58. doi: 10.1038/nprot.2008.213. [DOI] [PubMed] [Google Scholar]
- 44.Kalia J, Raines RT. Bioorg Med Chem Lett. 2007;17:6286. doi: 10.1016/j.bmcl.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vasquez GB, Karavitis M, Ji X, Pechik I, Brinigar WS, Gilliland GL, Fronticelli C. Biophys J. 1999;76:88. doi: 10.1016/S0006-3495(99)77180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manjula BN, Tsai AG, Intaglietta M, Tsai CH, Ho C, Smith PK, Perumalsamy K, Kanika ND, Friedman JM, Acharya SA. Protein J. 2005;24:133. doi: 10.1007/s10930-005-7837-2. [DOI] [PubMed] [Google Scholar]
- 47.Khan I, Dantsker D, Samuni U, Friedman AJ, Bonaventura C, Manjula B, Acharya SA, Friedman JM. Biochemistry. 2001;40:7581. doi: 10.1021/bi010051o. [DOI] [PubMed] [Google Scholar]
- 48.Juszczak LJ, Manjula B, Bonaventura C, Acharya SA, Friedman JM. Biochemistry. 2002;41:376. doi: 10.1021/bi011212r. [DOI] [PubMed] [Google Scholar]
- 49.Vandegriff KD, Malavalli A, Wooldridge J, Lohman J, Winslow RM. Transfusion. 2003;43:509. doi: 10.1046/j.1537-2995.2003.00341.x. [DOI] [PubMed] [Google Scholar]
- 50.Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Jama. 2008;299:2304. doi: 10.1001/jama.299.19.jrv80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rohlfs RJ, Bruner E, Chiu A, Gonzales A, Gonzales ML, Magde D, Magde MD, Jr, Vandegriff KD, Winslow RM. J Biol Chem. 1998;273:12128. doi: 10.1074/jbc.273.20.12128. [DOI] [PubMed] [Google Scholar]
- 52.Parra A, Stevens ES. Carbohydrate Polymers. 2000;41:111. [Google Scholar]
- 53.Wintrobe MM, Lee GR. Wintrobe's clinical hematology. 10th ed. Baltimore: Williams & Wilkins; 1999. [Google Scholar]
- 54.Perutz MF. Annu Rev Biochem. 1979;48:327. doi: 10.1146/annurev.bi.48.070179.001551. [DOI] [PubMed] [Google Scholar]
- 55.Perutz MF, Fermi G, Poyart C, Pagnier J, Kister J. J Mol Biol. 1993;233:536. doi: 10.1006/jmbi.1993.1530. [DOI] [PubMed] [Google Scholar]
- 56.Tsai CH, Ho C. Biophys Chem. 2002;98:15. doi: 10.1016/s0301-4622(02)00081-9. [DOI] [PubMed] [Google Scholar]
- 57.Baldwin J, Chothia C. J Mol Biol. 1979;129:175. doi: 10.1016/0022-2836(79)90277-8. [DOI] [PubMed] [Google Scholar]
- 58.Zhang J, Hua ZQ, Tame JRH, Lu GY, Zhang RJ, Gu XC. J Mol Biol. 1996;255:484. doi: 10.1006/jmbi.1996.0040. [DOI] [PubMed] [Google Scholar]
- 59.Mueser TC, Rogers PH, Arnone A. Biochemistry. 2000;39:15353. doi: 10.1021/bi0012944. [DOI] [PubMed] [Google Scholar]
- 60.Shaanan B. J Mol Biol. 1983;171:31. doi: 10.1016/s0022-2836(83)80313-1. [DOI] [PubMed] [Google Scholar]
- 61.Baldwin J, Chothia C. J Mol Biol. 1979;129:175. doi: 10.1016/0022-2836(79)90277-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.