INTRODUCTION
This Product Profiler introduces health care professionals to BRILINTA (ticagrelor), a P2Y12 platelet inhibitor that reversibly binds with the platelet P2Y12 adenosine diphosphate (ADP) receptor to prevent signal transduction and platelet activation (BRILINTA Prescribing Information 2011). Ticagrelor is not a prodrug and does not require metabolic activation to inhibit the P2Y12 receptor; however, it is converted in the liver into an active metabolite, which is approximately equipotent (James 2009).
BRILINTA has been approved by the FDA to reduce the rate of thrombotic cardiovascular events in patients with acute coronary syndrome (ACS), including unstable angina (UA), non–ST-segment elevation myocardial infarction (NSTEMI), and ST-segment elevation myocardial infarction (STEMI) (FDA 2011, BRILINTA Prescribing Information 2011). BRILINTA has been shown to reduce the rate of a combined end point of cardiovascular (CV) death, myocardial infarction (MI), or stroke compared with clopidogrel. The difference between treatments was driven by CV death and MI with no difference in stroke. In patients treated with PCI, it also reduces the rate of stent thrombosis.
BRILINTA has been studied in ACS in combination with aspirin. Maintenance doses of aspirin above 100 mg decreased the effectiveness of BRILINTA. Avoid maintenance doses of aspirin above 100 mg daily.
In the pivotal phase-3 PLATelet inhibition and patient Outcomes (PLATO) trial, which compared BRILINTA (n = 9,333) with clopidogrel (n = 9,291) in patients with ACS, BRILINTA significantly reduced the rate of first occurrence of the study’s composite end point of cardiovascular death, nonfatal MI (excluding silent MI), or stroke. BRILINTA also reduced the secondary end points of cardiovascular death and MI individually, with no difference in stroke (BRILINTA Prescribing Information 2011).
The following text presents an overview of the evidence supporting the FDA-approved indication for BRILINTA in patients with ACS, and considerations for P&T committee decisions regarding this product.
WARNING: BLEEDING RISK
BRILINTA, like other antiplatelet agents, can cause significant, sometimes fatal, bleeding
Do not use BRILINTA in patients with active pathological bleeding or a history of intracranial hemorrhage
Do not start BRILINTA in patients planned to undergo urgent coronary artery bypass graft (CABG) surgery. When possible, discontinue BRILINTA at least 5 days prior to any surgery
Suspect bleeding in any patient who is hypotensive and has recently undergone coronary angiography, percutaneous coronary intervention (PCI), CABG, or other surgical procedures in the setting of BRILINTA
If possible, manage bleeding without discontinuing BRILINTA. Stopping BRILINTA increases the risk of subsequent cardiovascular events
WARNING: ASPIRIN DOSE AND BRILINTA EFFECTIVENESS
Maintenance doses of aspirin above 100 mg reduce the effectiveness of BRILINTA and should be avoided. After any initial dose, use with aspirin 75 mg to 100 mg per day
Please read additional Important Safety Information on page 2.
Product Information
CONTRAINDICATIONS
History of Intracranial Hemorrhage
BRILINTA™ is contraindicated in patients with a history of intracranial hemorrhage (ICH) because of a high risk of recurrent ICH in this population.
Active Bleeding
BRILINTA is contraindicated in patients with active pathological bleeding, such as peptic ulcer or intracranial hemorrhage.
Severe Hepatic Impairment
BRILINTA is contraindicated in patients with severe hepatic impairment because of a probable increase in exposure, and it has not been studied in these patients. Severe hepatic impairment increases the risk of bleeding because of reduced synthesis of coagulation proteins.
DESCRIPTION
BRILINTA contains ticagrelor, a cyclopentyltriazolopyrimidine, which inhibits platelet activation and aggregation mediated by the P2Y12 ADP receptor. The chemical structure of ticagrelor is shown in Figure 1.
FIGURE 1.
Chemical Structure of Ticagrelor
Source: BRILINTA Prescribing Information 2011.
Ticagrelor is a crystalline powder with an aqueous solubility of approximately 10 mcg/mL at room temperature.
BRILINTA tablets for oral administration contain 90 mg of ticagrelor and the following ingredients: mannitol, dibasic calcium phosphate, sodium starch glycolate, hydroxypropyl cellulose, magnesium stearate, hydroxypropyl methylcellulose, titanium dioxide, talc, polyethylene glycol 400, and ferric oxide yellow.
CLINICAL PHARMACOLOGY
Mechanism of Action
Ticagrelor and its major (active) metabolite (AR-C124910XX) interact with the platelet P2Y12 ADP receptor to prevent signal transduction and platelet activation. Ticagrelor and its active metabolite are approximately equipotent.
Pharmacodynamics
The inhibition of platelet aggregation (IPA) by ticagrelor and clopidogrel was compared in a 6-week study that examined both acute and chronic platelet inhibition effects in response to 20 mcM ADP as the platelet aggregation agonist.
The onset of IPA was evaluated on Day 1 of the study after loading doses of 180 mg ticagrelor or 600 mg clopidogrel. IPA was higher in the ticagrelor group at all time points. The maximum IPA effect of ticagrelor was reached at approximately 2 hours and was maintained for at least 8 hours.
The offset of IPA was examined after 6 weeks on ticagrelor 90 mg twice daily or clopidogrel 75 mg daily, again in response to 20 mcM ADP. The mean maximum IPA after the last dose of ticagrelor and clopidogrel was 88% and 62%, respectively. After 24 hours, IPA in the ticagrelor group (58%) was similar to that in the clopidogrel group (52%), indicating that patients who miss a dose of ticagrelor would still maintain IPA similar to the trough IPA of patients treated with clopidogrel. After 5 days, IPA in the ticagrelor group was similar to IPA in the placebo group. It is not known how either bleeding risk or thrombotic risk correlate with IPA for either ticagrelor or clopidogrel.
Transitioning from clopidogrel to BRILINTA resulted in an absolute IPA increase of 26.4%, and transitioning from BRILINTA to clopidogrel resulted in an absolute IPA decrease of 24.5%. Patients can be transitioned from clopidogrel to BRILINTA without interrupting the drug’s anti-platelet effect.
Pharmacokinetics
Ticagrelor demonstrates dose-proportional pharmacokinetic characteristics, which are similar in patients and healthy volunteers.
Absorption. Absorption of ticagrelor occurs with a median time to maximum plasma concentration (tmax) of 1.5 hours (range, 1.0 to 4.0 hours). The formation of the major (active) circulating metabolite AR-C124910XX from ticagrelor occurs with a median tmax of 2.5 hours (range, 1.5 to 5.0 hours).
The mean absolute bioavailability of ticagrelor is approximately 36% (range, 30% to 42%). Ingestion of a high-fat meal had no effect on the maximum plasma concentration (Cmax) of ticagrelor, but resulted in a 21% increase in the area under the concentration–time curve (AUC). The Cmax of the major metabolite of ticagrelor was decreased by 22%, with no change in AUC. BRILINTA™ can be taken with or without food.
Distribution. The steady-state volume of distribution of ticagrelor is 88 L. Ticagrelor and its active metabolite are extensively bound to human plasma proteins (> 99%).
Metabolism. CYP3A4 is the major enzyme responsible for ticagrelor metabolism and the formation of its major active metabolite. Ticagrelor and its major active metabolite are weak P-glycoprotein substrates and inhibitors. The systemic exposure to the active metabolite is approximately 30% to 40% of the exposure of ticagrelor.
Excretion. The primary route of ticagrelor elimination is hepatic metabolism. When radiolabeled ticagrelor is administered, the mean recovery of radioactivity is approximately 84% (58% in feces, 26% in urine). Recoveries of ticagrelor and the active metabolite in urine were both less than 1% of the dose. The primary route of elimination for the major metabolite of ticagrelor is most likely to be biliary secretion. The mean half-life is approximately 7 hours for ticagrelor and 9 hours for the active metabolite.
Special Populations. The effects of age, gender, ethnicity, renal impairment, and mild hepatic impairment on the pharmacokinetics of ticagrelor are modest and do not require dose adjustment. Ticagrelor has not been evaluated in a pediatric population. No dose adjustment is necessary for ticagrelor based on weight.
Habitual smoking increased the population mean clearance of ticagrelor by approximately 22% when compared with nonsmokers. No dose adjustment is necessary for ticagrelor based on smoking status.
BRILINTA has not been studied in patients with moderate to severe hepatic impairment. For patients with moderate hepatic impairment, consider the risks and benefits of treatment and carefully consider use. BRILINTA is contraindicated in patients with severe hepatic impairment.
BRILINTA was not studied in patients undergoing renal dialysis.
The key pharmacokinetic characteristics of BRILINTA are summarized in Table 1.
TABLE 1.
Key Pharmacokinetic Characteristics of BRILINTA
| Absorption |
|
| Distribution |
|
| Metabolism |
|
| Excretion |
|
CYP = cytochrome P450; t½ = terminal half-life; tmax = time to maximum plasma concentration.
Data from BRILINTA Prescribing Information 2011.
Effects of Other Drugs on BRILINTA
CYP3A4 is the major enzyme responsible for ticagrelor metabolism and the formation of its major active metabolite. Strong CYP3A inhibitors (eg, ketoconazole, itraconazole, and clarithromycin) substantially increase ticagrelor exposure. Moderate CYP3A inhibitors (eg, diltiazem) have lesser effects. CYP3A inducers (eg, rifampin) substantially reduce ticagrelor blood levels.
Effects of BRILINTA on Other Drugs
In vitro metabolism studies demonstrate that ticagrelor and its major active metabolite are weak inhibitors of CYP3A4, potential activators of CYP3A5, and inhibitors of the P-glycoprotein transporter. Ticagrelor and AR-C124910XX were shown to have no inhibitory effect on human CYP1A2, CYP2C9, and CYP2E1 activity.
Clinical Studies
The clinical evidence for the effectiveness of BRILINTA™ was derived from the pivotal PLATelet inhibition and patient Outcomes (PLATO) trial, a randomized double-blind study that compared ticagrelor with clopidogrel, both given in combination with aspirin and other standard therapy, in patients with ACS (James 2009, Wallentin 2009, BRILINTA Prescribing Information 2011). A total of 18,624 patients participated in the study (9,333 in the ticagrelor group and 9,291 in the clopidogrel group). Patients hospitalized with documented evidence of ACS (UA, NSTEMI, or STEMI) within the previous 24 hours were eligible for inclusion. The two treatment groups were well balanced with regard to baseline characteristics (Table 2).
TABLE 2.
Baseline Characteristics of Patients in the PLATO Trial
| Characteristic | Ticagrelor Group (n = 9,333) | Clopidogrel Group (n = 9,291) |
|---|---|---|
| Median age (y) | 62.0 | 62.0 |
| Age ≥ 75 y (%) | 15.0 | 16.0 |
| Sex (%) | ||
| Male | 71.6 | 71.7 |
| Female | 28.4 | 28.3 |
| Median body weight (kg) | 80.0 | 80.0 |
| Median BMIa | 27 | 27 |
| Race (%)b | ||
| White | 91.8 | 91.6 |
| Black | 1.2 | 1.2 |
| Asian | 5.8 | 6.0 |
| Other | 1.2 | 1.2 |
| Cardiovascular risk factors (%) | ||
| Habitual smoker | 36.0 | 35.7 |
| Hypertension | 65.8 | 65.1 |
| Dyslipidemia | 46.6 | 46.7 |
| Diabetes mellitus | 24.9 | 25.1 |
| Other medical history (%) | ||
| Myocardial infarction | 20.4 | 20.7 |
| PCI | 13.6 | 13.1 |
| CABG surgery | 5.7 | 6.2 |
| Congestive heart failure | 5.5 | 5.8 |
| Nonhemorrhagic stroke | 3.8 | 4.0 |
| Peripheral arterial disease | 6.1 | 6.2 |
| Chronic renal disease | 4.1 | 4.4 |
| History of dyspnea | 15.1 | 14.6 |
| COPD | 5.9 | 5.7 |
| Asthma | 2.9 | 2.9 |
| Gout | 2.9 | 2.8 |
| Troponin-I positive at entry (%) | 85.3 | 86.1 |
| Final diagnosis of ACS (%) | ||
| NSTEMI | 42.9 | 42.5 |
| STEMI | 37.5 | 38.0 |
| Unstable angina | 16.6 | 16.8 |
| Other or missing datac | 3.0 | 2.7 |
ACS = acute coronary syndrome; CABG = coronary artery bypass graft; COPD = chronic obstructive pulmonary disease; NSTEMI = non–ST-elevation myocardial infarction; PCI = percutaneous coronary intervention; STEMI = ST-elevation myocardial infarction.
BMI = Body Mass Index (weight in kilograms divided by square of height in meters [kg/m2]).
n = 9,332 for ticagrelor group.
Includes patients with unspecified ACS or no ACS.
Source: Wallentin L, et al. N Engl J Med 2009;36:1045–1057. Copyright 2009 by the Massachusetts Medical Society. Reproduced with permission.
Ticagrelor was administered in a loading dose of 180 mg followed by a dosage of 90 mg twice daily. Patients in the clopidogrel group were given a 300-mg loading dose followed by a dosage of 75 mg daily if they had not received an open-label loading dose and had not used clopidogrel for 5 or more days before randomization. Concomitant aspirin was recommended at a loading dose of 160–500 mg. A daily maintenance dose of aspirin 75–100 mg was recommended, but higher maintenance doses of aspirin were allowed according to local judgment.
Treatment continued for a minimum of 6 months and a maximum of 12 months; the median period of drug exposure was 277 days.
PLATO Trial.
A randomized double-blind study comparing BRILINTA with clopidogrel, both given with aspirin and other standard therapy, in patients with ACS (UA, STEMI, NSTEMI) (N = 18,624)
Patients could be included whether there was intent to manage the ACS medically or invasively
Patients were allowed to receive a loading dose of clopidogrel prior to randomization
Primary end point was the composite of first occurrence of cardiovascular (CV) death, non-fatal MI (excluding silent MI), or nonfatal stroke
BRILINTA as compared with clopidogrel has been shown to decrease the rate of a combined end point of CV death, MI (excluding silent MI), or stroke
The difference in treatments was driven by CV death and MI with no difference in stroke
Maintenance doses of aspirin above 100 mg reduce the effectiveness of BRILINTA and should be avoided. After any initial dose, use with aspirin 75 mg to 100 mg per day.
The primary efficacy end point was the composite of cardiovascular death, nonfatal MI (excluding silent MI), or nonfatal stroke. The components of the primary end point were assessed as secondary end points.
The primary safety end point was the first occurrence of any PLATO-defined major bleeding event (fatal/life-threatening or other). PLATO used the following bleeding severity categorization:
Major bleed – fatal/life-threatening: Any one of the following: fatal; intracranial bleed; intrapericardial bleed with cardiac tamponade; hypovolemic shock or severe hypotension due to bleeding and requiring pressors or surgery; clinically overt or apparent bleeding associated with a decrease in hemoglobin of more than 5 g/dL; or transfusion of 4 or more units of whole blood or PRBCs for bleeding
Major bleed – other: Any one of the following: significantly disabling (eg, intraocular with permanent vision loss); clinically overt or apparent bleeding associated with a decrease in hemoglobin of 3 g/dL; or transfusion of 2 to 3 units of whole blood or PRBCs for bleeding.
Minor bleed: Requires medical intervention to stop or treat bleeding (eg, epistaxis requiring visit to medical facility for packing).
Minimal bleed: All others (eg, bruising, bleeding gums, oozing from injection site) not requiring intervention.
Safety end points also included minor bleeding, dyspnea, bradyarrhythmia, any other clinical adverse event, and results of laboratory safety tests.
EFFICACY RESULTS
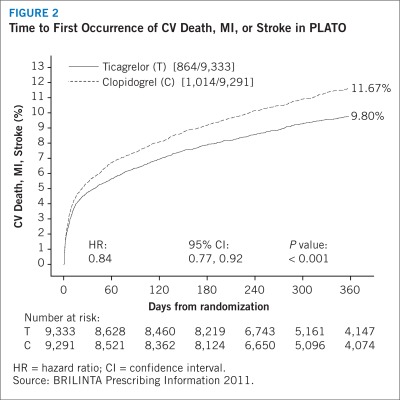
At 12 months, the primary efficacy end point (ie, a composite of cardiovascular death, non-fatal MI [excluding silent MI], or nonfatal stroke) occurred significantly less often in the ticagrelor group than in the clopidogrel group (9.8% vs. 11.7%, respectively; P < 0.001) (Table 3).
TABLE 3.
Patients With Outcome Events in PLATO (KM%)
| Ticagrelor + Aspirin (n = 9,333) | Clopidogrel + Aspirin (n = 9,291) | Hazard ratio (95% CI) | P Value | |
|---|---|---|---|---|
| Composite of CV death, MI, or stroke | 9.8 | 11.7 | 0.84 (0.77, 0.92) | 0.0003 |
| CV death | 2.9 | 4.0 | 0.74 | |
| Nonfatal MI | 5.8 | 6.9 | 0.84 | |
| Nonfatal stroke | 1.4 | 1.1 | 1.24 | |
| Secondary end pointsa | ||||
| CV death | 4.0 | 5.1 | 0.79 (0.69, 0.91) | 0.0013 |
| MIb | 5.8 | 6.9 | 0.84 (0.75, 0.95) | 0.0045 |
| Strokeb | 1.5 | 1.3 | 1.17 (0.91, 1.52) | 0.22 |
| All-cause mortality | 4.5 | 5.9 | 0.78 (0.69, 0.89) | 0.0003 |
First occurrence of specified event at any time.
Includes patients that could have had other nonfatal events or died.
The difference between treatments on the composite resulted from effects on cardiovasacular death and MI; each was statistically significant when considered as a secondary end point, and there was no beneficial effect on strokes. For all-cause mortality, the benefit was also statistically significant (P = 0.0003), with a hazard ratio of 0.78. However, because of the hierarchical test sequence used in PLATO, all-cause mortality was an exploratory analysis, and the P value was considered nominal.
The Kaplan-Meier curve in Figure 2 shows the time to first occurrence of the primary composite end point of cardiovascular death, nonfatal MI, or nonfatal stroke in the overall study. The curves separate by 30 days (RRR 12%) and continue to diverge throughout the 12-month treatment period (RRR 16%).
FIGURE 2.
Time to First Occurrence of CV Death, MI, or Stroke in PLATO
HR = hazard ratio; CI = confidence interval.
REGIONAL DIFFERENCES
Results in the rest of the world compared with effects in North America (U.S. and Canada) show a smaller effect in North America, numerically inferior to the control and driven by the U.S. subset. The statistical test for the U.S./non-U.S. comparison is statistically significant (P = 0.009), and the same trend is present for both cardiovascular death and nonfatal MI. The individual results and nominal P values, like all subset analyses, need cautious interpretation, and they could represent chance findings. The consistency of the differences in both the cardiovascular mortality and nonfatal MI components, however, supports the possibility that the finding is reliable.
A wide variety of baseline and procedural differences between the U.S. and non-U.S. (including intended invasive vs. planned medical management, use of GPIIb/IIIa inhibitors, use of drug eluting vs. bare-metal stents) were examined to see whether they could account for regional differences, but with one exception, aspirin maintenance dose, these differences did not appear to lead to differences in outcome.
ASPIRIN DOSE
The PLATO protocol left the choice of aspirin maintenance dose up to the investigator, and use patterns were very different in the U.S. and elsewhere, with about 8% of non-U.S. investigators using aspirin doses above 100 mg and 2% using aspirin doses above 300 mg, in contrast to U.S. practice, where 57% of patients received doses above 100 mg and 54% received doses above 300 mg. Overall results favored BRILINTA™ when used with low maintenance doses (less than 100 mg) of aspirin, and results analyzed by aspirin dose were similar in the U.S. and elsewhere.
Like any unplanned subset analysis, especially one where the characteristic is not a true baseline characteristic (but may be determined by usual investigator practice), the above analyses must be treated with caution. It is notable, however, that aspirin dose predicts outcome in both regions with a similar pattern, and that the pattern is similar for the two major components of the primary end point, cardiovascular death and nonfatal MI.
Despite the need to treat such results cautiously, there appears to be good reason to restrict aspirin maintenance dosage accompanying ticagrelor to 100 mg. Higher doses do not have an established benefit in the ACS setting, and there is a strong suggestion that use of such doses reduces the effectiveness of BRILINTA.
PHARMACOGENETICS
In a genetic substudy of PLATO (N = 10,285), the effects of BRILINTA compared with clopidogrel on thrombotic events and bleeding were not significantly affected by CYP2C19 genotype.
Regional Differences and Aspirin Dose.
In the North American subgroup, BRILINTA was numerically inferior to clopidogrel. This effect was driven by the US subset
While this could be due to chance, retrospective analyses support the possibility that this finding is reliable and due to aspirin maintenance dose
Because these were unplanned subset analyses, these analyses must be treated with caution
In PLATO, use of > 100 mg of aspirin decreased the effectiveness of BRILINTA
Overall results favored BRILINTA when used with ≤ 100 mg of aspirin
Despite the need to treat such results cautiously, there appears to be good reason to restrict aspirin maintenance dosage accompanying ticagrelor to 100 mg
Higher doses do not have an established benefit in the ACS setting, and there is a strong suggestion that use of such doses reduces the effectiveness of BRILINTA
Safety
WARNING: BLEEDING RISK
BRILINTA™, like other antiplatelet agents, can cause significant, sometimes fatal, bleeding
Do not use BRILINTA in patients with active pathological bleeding or a history of intracranial hemorrhage
Do not start BRILINTA in patients planned to undergo urgent coronary artery bypass graft (CABG) surgery. When possible, discontinue BRILINTA at least 5 days prior to any surgery
Suspect bleeding in any patient who is hypotensive and has recently undergone coronary angiography, percutaneous coronary intervention (PCI), CABG, or other surgical procedures in the setting of BRILINTA
If possible, manage bleeding without discontinuing BRILINTA. Stopping BRILINTA increases the risk of subsequent cardiovascular events
WARNING: ASPIRIN DOSE AND BRILINTA EFFECTIVENESS
Maintenance doses of aspirin above 100 mg reduce the effectiveness of BRILINTA and should be avoided. After any initial dose, use with aspirin 75 mg to 100 mg per day
Please read additional Important Safety Information on page 2.
CONTRAINDICATIONS
BRILINTA is contraindicated in patients with a history of intracranial hemorrhage (ICH) because of a high risk of recurrent ICH in this population.
BRILINTA is contraindicated in patients with active pathological bleeding, such as peptic ulcer or ICH.
BRILINTA is contraindicated in patients with severe hepatic impairment because of a probable increase in exposure, and it has not been studied in these patients. Severe hepatic impairment increases the risk of bleeding because of reduced synthesis of coagulation proteins.
WARNINGS AND PRECAUTIONS
General Risk of Bleeding
Drugs that inhibit platelet function, including BRILINTA, increase the risk of bleeding. BRILINTA increased the overall risk of bleeding (major plus minor) to a somewhat greater extent than did clopidogrel. The increase was seen for non–CABG-related bleeding, but not for CABG-related bleeding. Fatal and life-threatening bleeding rates were not increased.
In general, risk factors for bleeding include older age, a history of bleeding disorders, the performance of percutaneous invasive procedures, and concomitant use of medications that increase the risk of bleeding, such as anticoagulant therapy, higher doses of aspirin, and chronic use of nonsteroidal anti-inflammatory drugs (NSAIDS).
When possible, BRILINTA should be discontinued 5 days prior to surgery. Clinicians should suspect bleeding in any patient who is hypotensive and has recently undergone coronary angiography, PCI, CABG, or other surgical procedures, even if the patient does not have any signs of bleeding.
If possible, clinicians should manage bleeding without discontinuing BRILINTA. Stopping BRILINTA increases the risk of subsequent cardiovascular events.
Concomitant Aspirin Maintenance Dose
In PLATO, the use of BRILINTA with maintenance doses of aspirin above 100 mg decreased the effectiveness of BRILINTA. Therefore, after the initial loading dose of aspirin (usually 325 mg), clinicians should use BRILINTA with a maintenance dose of aspirin of 75 mg to 100 mg.
Moderate Hepatic Impairment
BRILINTA has not been studied in patients with moderate hepatic impairment. Clinicians should consider the risks and benefits of treatment, noting the probable increase in exposure to ticagrelor.
Dyspnea
Dyspnea was reported in 14% of patients treated with BRILINTA and in 8% of patients taking clopidogrel. Dyspnea was usually mild to moderate in intensity and often resolved during continued treatment. If a patient develops new, prolonged, or worsened dyspnea during treatment with BRILINTA, clinicians should exclude underlying diseases that may require treatment. If dyspnea is determined to be related to BRILINTA, no specific treatment is required; BRILINTA may be continued without interruption.
In a substudy, 199 patients from the PLATO trial underwent pulmonary function testing irrespective of whether they reported dyspnea. There was no significant difference between treatment groups for FEV1. There was no indication of an adverse effect on pulmonary function assessed after 1 month or after at least 6 months of chronic treatment.
Discontinuation of BRILINTA
Clinicians should avoid interruption of BRILINTA treatment. If BRILINTA must be temporarily discontinued (eg, to treat bleeding or for elective surgery), clinicians should restart it as soon as possible. Discontinuation of BRILINTA™ will increase the risk of MI, stent thrombosis, and death.
Strong Inhibitors of CYP3A
Ticagrelor is metabolized by CYP3A4/5. Clinicians should avoid the use of BRILINTA with strong CYP3A inhibitors, such as atazanavir, clarithromycin, indinavir, itraconazole, ketoconazole, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin, and voriconazole.
Potent Inducers of CYP3A
Clinicians should avoid the use of BRILINTA with potent CYP3A inducers, such as rifampin, dexamethasone, phenytoin, carbamazepine, and phenobarbital.
ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
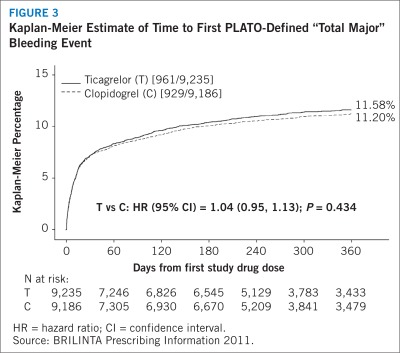
Figure 3 shows major bleeding events over time in PLATO. Many events are early, at a time of coronary angiography, PCI, CABG, and other procedures, but the risk persists during later use of antiplatelet therapy.
FIGURE 3.
Kaplan-Meier Estimate of Time to First PLATO-Defined “Total Major” Bleeding Event
HR = hazard ratio; CI = confidence interval.
Annualized rates of bleeding are summarized in Table 4. About half of the bleeding events occurred during the first 30 days. As shown in Table 4, BRILINTA was associated with a somewhat greater risk of non–CABG-related bleeding than was clopidogrel. No baseline demographic factor altered the relative risk of bleeding with BRILINTA compared with clopidogrel.
TABLE 4.
Non–CABG-Related Bleeds (Kaplan-Meier %)
| Ticagrelor (n = 9,235) | Clopidogrel (n = 9,186) | |
|---|---|---|
| Total (major + minor) | 8.7 | 7.0 |
| Major | 4.5 | 3.8 |
| Fatal/life-threatening | 2.1 | 1.9 |
| Fatal | 0.2 | 0.2 |
| Intracranial (fatal/life-threatening) | 0.3 | 0.2 |
Source: BRILINTA Prescribing Information 2011.
In PLATO, 1,584 patients underwent CABG surgery. The percentages of those patients who bled are shown in Table 5. Rates were very high but similar for BRILINTA and clopidogrel.
TABLE 5.
CABG Bleeds (Kaplan-Meier %)
| Patients With CABG | Ticagrelor (n = 770) | Clopidogrel (n = 814) |
|---|---|---|
| Total major | 85.8 | 86.9 |
| Fata/life-threatening | 48.1 | 47.9 |
| Fatal | 0.9 | 1.1 |
Although the platelet-inhibition effect of BRILINTA has a faster offset than clopidogrel in in vitro tests and BRILINTA is a reversibly binding P2Y12 inhibitor, the PLATO trial did not show an advantage of BRILINTA compared with clopidogrel for CABG-related bleeding. When antiplatelet therapy was stopped 5 days before CABG, major bleeding occurred in 75% of BRILINTA-treated patients and in 79% of patients on clopidogrel.
No data exist with BRILINTA regarding a hemostatic benefit of platelet transfusions.
DRUG DISCONTINUATION
In PLATO, the rates of study drug discontinuation attributed to adverse reactions were 7.4% for BRILINTA and 5.4% for clopidogrel. Bleeding caused permanent discontinuation of study drug in 2.3% of BRILINTA patients and in 1.0% of clopidogrel patients. Dyspnea led to study drug discontinuation in 0.9% of BRILINTA™ patients and in 0.1% of clopidogrel patients.
COMMON ADVERSE EVENTS
A variety of nonhemorrhagic adverse events occurred in the PLATO trial at rates of 3% or more (Table 6). In the absence of a placebo control, whether these events are drug-related cannot be determined in most cases, except where they are more common with BRILINTA or clearly related to the drug’s pharmacologic effect (dyspnea).
TABLE 6.
Percentage of Patients Reporting Nonhemorrhagic Adverse Events (at Least 3% or More in Either Group)
| Ticagrelor (n = 9,235) | Clopidogrel (n = 9,186) | |
|---|---|---|
| Dyspneaa | 13.8 | 7.8 |
| Headache | 6.5 | 5.8 |
| Cough | 4.9 | 4.6 |
| Dizziness | 4.5 | 3.9 |
| Nausea | 4.3 | 3.8 |
| Atrial fibrillation | 4.2 | 4.6 |
| Hypertension | 3.8 | 4.0 |
| Noncardiac chest pain | 3.7 | 3.3 |
| Diarrhea | 3.7 | 3.3 |
| Back pain | 3.6 | 3.3 |
| Hypotension | 3.2 | 3.3 |
| Fatigue | 3.2 | 3.2 |
| Chest pain | 3.1 | 3.5 |
Includes dyspnea, dyspnea exertional, dyspnea at rest, nocturnal dyspnea, and dyspnea paroxysmal nocturnal.
BRADYCARDIA
In clinical studies, BRILINTA has been shown to increase the occurrence of Holter-detected bradyarrhythmias (including ventricular pauses). The PLATO trial excluded patients at increased risk of bradycardic events (e.g., patients who had sick sinus syndrome, second- or third-degree atrioventricular block, or bradycardic-related syncope and not protected with a pacemaker). In PLATO, syncope, pre-syncope, and loss of consciousness were reported by 1.7% and 1.5% of BRILINTA and clopidogrel patients, respectively.
In a Holter substudy of approximately 3,000 patients in the PLATO trial, more patients had ventricular pauses with BRILINTA™ (6.0%) than with clopidogrel (3.5%) in the acute phase; the rates were 2.2% and 1.6%, respectively, after 1 month.
GYNECOMASTIA
In PLATO, gynecomastia was reported by 0.23% of men on BRILINTA and by 0.05% on clopidogrel.
Other sex-hormonal adverse reactions, including sex-organ malignancies, did not differ between the two treatment groups in the PLATO study.
LABORATORY ABNORMALITIES
Serum Uric Acid
Serum uric acid levels increased approximately 0.6 mg/dL from baseline on BRILINTA and approxmately 0.2 mg/dL on clopidogrel in the PLATO trial. The difference disappeared within 30 days of discontinuing treatment. Reports of gout did not differ between treatment groups in PLATO (0.6% in each group).
Serum Creatinine
In the PLATO study, a > 50% increase in serum creatinine levels was observed in 7.4% of patients receiving BRILINTA compared with 5.9% of patients receiving clopidogrel. The increases typically did not progress with ongoing treatment and often decreased with continued therapy. Evidence of reversibility upon discontinuation wasobserved even in those with the greatest on-treatment increases. Treatment groups in PLATO did not differ for renal-related serious adverse events, such as acute renal failure, chronic renal failure, toxic nephropathy, or oliguria.
DRUG INTERACTIONS
Effects of Other Drugs on BRILINTA
Ticagrelor is predominantly metabolized by CYP3A4 and to a lesser extent by CYP3A5.
CYP3A inhibitors. Clinicians should avoid the use of strong inhibitors of CYP3A (eg, ketoconazole, itraconazole, voriconazole, clarithromycin, nefazodone, ritonavir, saquinavir, nelfinavir, indinavir, atazanavir, and telithromycin).
CYP3A inducers. Clinicians should avoid the use of BRILINTA™ with potent inducers of CYP3A (eg, rifampin, dexamethasone, phenytoin, carbamazepine, and phenobarbital).
Aspirin. The use of BRILINTA with aspirin maintenance doses above 100 mg reduced the effectiveness of BRILINTA.
Effect of BRILINTA on Other Drugs
Ticagrelor is an inhibitor of CYP3A4/5 and the P-glycoprotein transporter.
Simvastatin and lovastatin. Treatment with BRILINTA will result in higher serum concentrations of simvastatin and lovastatin because these drugs are metabolized by CYP3A4. Clinicians should avoid simvastatin and lovastatin doses greater than 40 mg.
Digoxin. Because of inhibition of the P-glycoprotein transporter, clinicians should monitor digoxin levels with initiation of or any change in BRILINTA therapy.
Other concomitant therapy. BRILINTA can be administered with unfractionated or low-molecular-weight heparin, glycoprotein IIb/IIIa inhibitors, proton pump inhibitors, beta-blockers, ACE inhibitors, and angiotensin receptor blockers.
63 mg/kg/day (6.8 times the MRHD on a mg/m2basis) had
USE IN SPECIFIC POPULATIONS
Pregnancy
BRILINTA is a Pregnancy Category C drug. There are no adequate and well-controlled studies of the use of BRILINTA™ in pregnant women. In animal studies, ticagrelor caused structural abnormalities at maternal doses approximately 5 to 7 times the maximum recommended human dose (MRHD) based on body surface area. BRILINTA should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
In reproductive toxicology studies, pregnant rats received ticagrelor during organogenesis at dosages from 20 to 300 mg/kg/day. The lowest dose was approximately the same as the MRHD of 90 mg twice daily for a 60-kg human on a mg/m2 basis. Adverse outcomes in offspring occurred at doses of 300 mg/kg/day (16.5 times the MRHD on a mg/m2 basis) and included supernumerary liver lobe and ribs, incomplete ossification of sternebrae, displaced articulation of the pelvis, and misshapen/misaligned sternebrae. When pregnant rabbits received ticagrelor during organogenesis at dosages from 21 to 63 mg/kg/day, fetuses exposed to the highest maternal dosage of delayed gall bladder development, and incomplete ossification of the hyoid, pubis, and sternebrae occurred.
In a prenatal/postnatal study, pregnant rats received ticagrelor at dosages of 10 to 180 mg/kg/day during late gestation and lactation. Pup death and effects on pup growth were observed at a dosage of 180 mg/kg/day (approximately 10 times the MRHD on a mg/m2 basis). Relatively minor effects, such as delays in pinna unfolding and eye opening, occurred at doses of 10 and 60 mg/kg (approximately one half and 3.2 times the MRHD on a mg/m2basis).
Nursing Mothers
It is not known whether ticagrelor or its active metabolites are excreted in human milk. Ticagrelor is excreted in rat milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from BRILINTA™, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
The safety and effectiveness of BRILINTA in pediatric patients have not been established.
Geriatric Use
In the PLATO trial, 43% of patients were 65 years of age and older, and 15% were 75 years of age and older. The relative risk of bleeding was similar in both treatment and age groups.
No overall differences in safety or effectiveness were observed between these patients and younger patients. While this clinical experience has not identified differences in responses between the elderly and younger patients, greater sensitivity of some older individuals cannot be ruled out.
Hepatic Impairment
BRILINTA has not been studied in patients with moderate or severe hepatic impairment. Ticagrelor is metabolized by the liver, and impaired hepatic function can increase the risks for bleeding and other adverse events. Hence, BRILINTA is contraindicated for use in patients with severe hepatic impairment, and its use should be considered carefully in patients with moderate hepatic impairment. No dosage adjustment is needed in patients with mild hepatic impairment.
Renal Impairment
No dosage adjustment is needed in patients with renal impairment. Patients receiving dialysis have not been studied.
Dosage and Administration
DOSAGE AND ADMINISTRATION
BRILINTA™ therapy should be initiated with a 180-mg (two 90-mg tablets) loading dose, and treatment should be continued with 90 mg twice daily.
After the initial loading dose of aspirin (usually 325 mg), BRILINTA should be used with a daily maintenance dose of aspirin of 75 mg to 100 mg.
Patients with ACS who have received a loading dose of clopidogrel may be started on BRILINTA.
BRILINTA can be administered with or without food. A patient who misses a dose of BRILINTA should take one 90-mg tablet (their next dose) at its scheduled time.
HOW SUPPLIED
BRILINTA (ticagrelor) 90 mg is supplied as round, biconvex, yellow, film-coated tablets marked with a “90” above “T” on one side.
The following presentations of BRILINTA are available:
| Bottles of 60 | NDC 0186-0777-60 |
| Bottles of 180 | NDC 0186-0777-18 |
| 100-count Hospital Unit Dose | NDC 0186-0777-39 |
STORAGE AND HANDLING
BRILINTA should be stored at 25°C (77°F); excursions are permitted to 15°–30°C (59°–86°F).
Keep BRILINTA in the container it comes in.
Keep BRILINTA tablets dry.
P&T Committee Considerations
INTRODUCTION
ACS is a leading cause of morbidity and mortality in the United States. According to the Heart Disease and Stroke Statistics 2011 Update from the American Heart Association, 1,172,000 hospital discharges in the U.S. were due to ACS in 2007. Of these, 731,000 (62.4%) were for MI alone; 431,000 (36.8%) were for unstable angina alone; and 10,000 (0.9%) were for both diagnoses (Roger 2011).
BRILINTA CLINICAL EFFECTIVENESS
BRILINTA™ (ticagrelor) is the first reversibly binding oral P2Y12 ADP receptor antagonist (James 2009). Ticagrelor is not a prodrug and does not require metabolic activation to inhibit the P2Y12 receptor; however, it is converted in the liver into an active metabolite, which is approximately equipotent (James 2009).
BRILINTA is indicated to reduce the rate of thrombotic cardiovascular (CV) events in patients with acute coronary syndrome (ACS)(unstable angina, non–ST-elevation myocardial infarction, or ST-elevation myocardial infarction). BRILINTA has been shown to reduce the rate of a combined end point of CV death, myocardial infarction (MI), or stroke compared with clopidogrel. The difference between treatments was driven by CV death and MI with no difference in stroke. In patients treated with PCI, BRILINTA also reduces the rate of stent thrombosis (BRILINTA Prescribing Information 2011).
BRILINTA has been studied in ACS in combination with aspirin. Maintenance doses of aspirin over 100 mg decreased the effectiveness of BRILINTA. Avoid maintenance doses of aspirin over 100 mg daily (BRILINTA Prescribing Information 2011).
The PLATO trial was a phase-3, randomized, double-blind, parallel-group, event-driven study that compared BRILINTA with clopidogrel in more than 18,000 hospitalized patients with ACS (James 2009, Wallentin 2009). In this pivotal study, treatment with BRILINTA, as compared with clopidogrel, significantly reduced the rate of first occurrence of the study’s composite end point of cardiovascular death, nonfatal MI (excluding silent MI), or stroke. BRILINTA also reduced the secondary end points of cardiovascular death and MI individually, with no difference in stroke. BRILINTA and clopidogrel were studied with aspirin and other standard therapies.
BRILINTA was asociated with a somewhat higher rate of non–CABG-related major bleeding compared with clopidogrel (4.5% vs. 3.8%, respectively). In patients who underwent CABG surgery, the rates of total major bleeding were similar in the BRILINTA and clopidogrel groups (85.8% vs. 86.9%, respectively).
RISK EVALUATION AND MITIGATION STRATEGY
The Risk Evaluation and Mitigation Strategy (REMS) for BRILINTA has two goals: (1) to inform health care professionals and patients about the risks associated with BRILINTA, particularly the risk of bleeding, and (2) to inform health care professionals and patients that the maintenance dose of aspirin, coadministered with BRILINTA, should not exceed 100 mg (BRILINTA REMS Document 2011). Components of the BRILINTA REMS initiative include a Medication Guide, a Communication Plan, and the submission of REMS assessments to the FDA.
Medication Guide
A Medication Guide is dispensed with each BRILINTA prescription. The Guide is also available on the BRILINTA REMS Web site (www.brilintarems.com) or by calling 1-800-236-9933. The Medication Guide includes information to support patient counseling regarding the risks and benefits of treatment with BRILINTA.
The Medication Guide is not intended to replace discussions between patients and their health care providers regarding the patient’s condition and its treatment. Rather, the Guide provides information about the use of BRILINTA to lower the risk of a heart attack or stroke. For instance, the Guide informs patients that BRILINTA (and similar drugs) can cause serious bleeding, such as internal bleeding, that can require blood transfusions or surgery, and that this bleeding can result in death. Patients are instructed to contact their physician immediately if they have signs or symptoms of bleeding while taking BRILINTA, such as bleeding that is severe or that cannot be controlled; pink, red, or brown urine; and red or black stools.
The Medication Guide warns patients not to discontinue BRILINTA without first talking to their physician. The Guide points out that if patients with a stent stop taking BRILINTA too soon, they are at increased risk of developing a blood clot in the stent, having a heart attack, or dying. Patients also are told not to stop taking BRILINTA because of bleeding or for other reasons because their risk of a heart attack or stroke may increase.
Patients are advised that BRILINTA is administered with aspirin and that they should not take a dosage of aspirin that is higher than 100 mg daily because it can affect how BRILINTA works.
Other topics covered by the Medication Guide include:
A description of BRILINTA™
Who should not take BRILINTA
What patients should tell their physicians before taking BRILINTA
How BRILINTA should be taken
The possible side effects of BRILINTA
How BRILINTA should be stored
Communication Plan
A Communication Plan tailored for health care professionals who are likely to prescribe and dispense BRILINTA is the second component of the REMS program (BRILINTA REMS Document 2011). The primary objective of the Communication Plan is to inform health care professionals of the serious risks associated with BRILINTA, particularly the increased risk of bleeding, and to inform them that the daily maintenance dose of aspirin, co-administered with BRILINTA, should not exceed 100 mg (BRILINTA REMS Document 2011). The Communication Plan consists of three parts: (1) a “Dear Healthcare Professional” letter; (2) a BRILINTA REMS Web site; and (3) a “Professional Organization” letter.
The “Dear Health Care Professional” letter will be distributed electronically or by postal mail to interventional cardiologists, clinical cardiologists, emergency medicine physicians, internal medicine physicians, primary care physicians, nurse practitioners, physician assistants, pharmacists, critical care nurses, and cardiac nurse specialists. The letter was distributed within 60 days after the initial REMS approval date and will be distributed again at 6, 12, and 24 months after approval. Product labeling and the Medication Guide will be provided in conjunction with the letter.
The BRILINTA REMS Web site was launched within 30 days after REMS approval. The site will provide information for health care professionals and patients for 2 years after product launch. Visitors to the site will learn about the goals of the REMS program and about the risks of treatment with BRILINTA. In addition, they will have access to the product’s package insert, the Medication Guide, and the “Dear Health Care Professional” letter.
A “Professional Organization” letter was distributed within 60 days of the REMS approval date. This letter contains the same information that is found in the “Dear Health Care Professional” letter. AstraZeneca will request that professional organizations disseminate this information to their members. Product labeling and the Medication Guide will be provided in conjunction with the letter.
Recipients of the “Professional Organization” letter will include the American Heart Association, the American College of Cardiologists, the Society for Cardiovascular Angiography and Interventions, the American College of Chest Physicians, the American Academy of Family Physicians, the American Society of Health-System Pharmacists, and other groups.
Submission of REMS Assessments to the FDA
The final component of the REMS program consists of the submission of REMS assessments to the FDA by AstraZeneca at 18 months, 3 years, and 7 years after approval of the REMS plan (BRILINTA REMS Document 2011). To facilitate the inclusion of as much information as possible while allowing reasonable time to prepare the submission, the reporting interval covered by each assessment will conclude no earlier than 60 days before the submission date for that assessment.
Conclusion
In view of the overall health impact of ACS, as well as its significant economic burden, P&T decision makers need to identify optimal treatment approaches to this debilitating and potentially fatal disorder.
BRILINTA™ (ticagrelor) is the first cyclopentyltriazolopyridine in a new class of antiplatelets. BRILINTA interacts with the P2Y12 ADP receptor, which is approved for the reduction of thrombotic cardiovascular events in patients with ACS. Ticagrelor is not a prodrug and does not require metabolic activation to inhibit the P2Y12 receptor; however, hepatic metabolism is needed to produce its active metabolite.
In the pivotal phase-3 PLATO trial, BRILINTA significantly reduced the rate of first occurrence of the study’s composite end point of cardiovascular death, nonfatal MI (excluding silent MI), or stroke versus clopidogrel. BRILINTA also reduced the secondary end points of cardiovascular death and MI individually, with no difference in stroke versus clopidogrel.
BRILINTA has been studied in ACS in combination with aspirin. Maintenance doses of aspirin above 100 mg decreased the effectiveness of BRILINTA. Maintenance doses of aspirin above 100 mg should be avoided.
Like other antiplatelet agents, BRILINTA can cause significant, sometimes fatal, bleeding. BRILINTA should not be used in patients with active pathological bleeding or a history of intracranial hemorrhage. BRILINTA should not be started in patients planned to undergo urgent CABG surgery.
When possible, discontinue BRILINTA at least 5 days prior to any surgery. Suspect bleeding in any patient who is hypotensive and has recently undergone coronary angiography, PCI, CABG, or other surgical procedures in the setting of BRILINTA. If possible, manage bleeding without discontinuing BRILINTA. Stopping BRILINTA increases the risk of subsequent cardiovascular events.
BRILINTA is contraindicated in patients with a history of intracraial hemorrhage and active pathological bleeding, such as peptic ulcer. BRILINTA is also contraindicated in patients with severe hepatic impairment because of a probable increase in exposure. BRILINTA has not been studied in these patients. Severe hepatic impairment increases the risk of bleeding because of reduced synthesis of coagulation proteins.
The clinical use of BRILINTA is supported by a Risk Evaluation and Mitigation Strategy (REMS) initiative, which is designed to inform health care professionals and patients about the risks associated with BRILINTA, particularly the risk of bleeding, and about the need to ensure that the maintenance dose of aspirin, co-administered with BRILINTA, does not exceed 100 mg.
GLOSSARY OF ABBREVIATIONS
- ACS
acute coronary syndrome
- ADP
adenosine diphosphatase
- CABG
coronary artery bypass graft
- CYP
cytochrome P450
- FDA
Food and Drug Administration
- IPA
inhibition of platelet aggregation
- MI
myocardial infarction
- MRHD
maximum recommended human dose
- NSTEMI
non–ST-elevation myocardial infarction
- PCI
percutaneous coronary intervention
- PLATO
PLATelet inhibition and patient Outcomes
- REMS
Risk Evaluation and Mitigation Strategy
- STEMI
ST-elevation myocardial infarction
REFERENCES
- Wilmington, Del.: AstraZeneca LP; Jul, 2011. BRILINTA™ (ticagrelor) Tablets. Medication Guide. Available at: http://www.brilintarems.com. Accessed Sept. 13, 2011. [Google Scholar]
- Wilmington, Del.: AstraZeneca LP; Jul, 2011. BRILINTA™ (ticagrelor) Tablets prescribing information. Available at: http://www1.astrazeneca-us.com/pi/brilinta.pdf. Accessed Sept. 13, 2011. [Google Scholar]
- Wilmington, DE: AstraZeneca LP; Jul, 2011. BRILINTA™ REMS Document. Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM264004.pdf. Accessed Sept. 13, 2011. [Google Scholar]
- FDA (U.S. Food and Drug Administration) FDA approves blood-thinning drug Brilinta to treat acute coronary syndromes [press release. Jul 20, 2011. ]. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm263964.htm. Accessed Sept. 1, 2011.
- James S, Akerblom A, Cannon CP, et al. Comparison of ticagrelor, the first reversible oral P2Y12 receptor antagonist, with clopidogrel in patients with acute coronary syndromes: Rationale, design, and baseline characteristics of the PLATelet inhibition and patient Outcomes (PLATO) trial. Am Heart J. 2009;157(4):599–605. doi: 10.1016/j.ahj.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2011 update: A report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]