Summary
Background
Breast cancer is the leading cause of cancer deaths in Ghanaian women.
Objective
To describes the characteristics of breast cancer patients attending the Komfo Anokye Teaching Hospital in Kumasi, Ghana.
Method
The study was conducted at the Komfo Anokye Teaching Hospital. Between July 1st 2004 and June 30th 2009 patients presenting with breast lumps were assessed by clinical examination, imaging studies and pathological examination. Relevant clinical and pathological were recorded prospectively data on all patients with microscopically proven breast cancer. The cancers were graded according to the modified Bloom-Richardson system. Tissue immunoperoxidase stains for oestrogen, progesterone receptors and c-erb2 oncogene were performed with commercially prepared antigens and reagents.
Results
Nineteen thousand four hundred and twenty - three (19,423) patients were seen during the study period. There were 330 (1.7%) patients with histologically proven breast cancer. The mean age was 49.1 years. A palpable breast lump was detected in 248 patients (75.2%). Two hundred and eighty -one patients (85.2%) presented with Stages III and IV , 271 (82.1 %) invasive and 230 (85.2%) high grade carcinomas. Oestrogen and progesterone receptors were positive in 32 and 9 cases respectively. Her2 protein was positive in 11 cases.
Conclusion
In Kumasi, as in other parts of Ghana, breast cancer affects mostly young pre-menopausal who present with advanced disease. The cancers have unfavourable prognostic features and are unlikely to respond to hormonal therapy.
Keywords: Breast cancer, late stage presentation, unfavourable characteristics, Kumasi
Introduction
In Ghana data on breast cancer is scant. However the disease is a common cause of hospital admissions and mortality among Ghanaian women.1–2 Reported clinical studies from Ghana and other communities in sub Saharan Africa indicate that breast cancer in indigenous black African populations is often severe with unfavourable prognostic features.3–4 Some of these features include young age at presentation, advanced stage at diagnosis, large tumour size, high grade histologic subtypes and low rate of hormone receptor positivity.4–10 The features are believed to explain why African women are more likely than women from the developed countries to die from the disease.5
There are no universally acceptable explanations for the features that characterize breast cancer in women of indigenous African populations. Data from genetic, clinical and other studies suggest that breast cancer in the indigenous African women has an inherently aggressive biology.11–14 Recently other reported studies support the contrary view that these features only reflect the late stage at diagnosis and the adverse influence of lack of awareness of the disease, non-availability of screening methods and some other epidemiologic risk factors. 15–16 Also breast cancer is considered to be a complex and heterogeneous disease with ethnic and racial variations of histologic subtypes and tumour behaviour. 17
The nature of the disease requires that each community or population must define the characteristics of the disease among its people so as to able to determine the most suitable method to control the disease and limit the mortalities.18 There are some previous reports on breast cancer from Accra. 9,19–20 However there are no reported studies on breast cancer characteristics from Kumasi. It is against this background that this study was designed. This is a report on the clinical and some pathological characteristics of breast cancer as seen in our practice. It provides African-based findings that should contribute to the evidence required for the better understanding of the disease in indigenous black African populations. In addition the data may be useful for policy makers who have to decide on funding for screening for breast cancer in Ghana.
Patients and Methods
Clinical and microscopic diagnosis of breast cancer
The study was conducted at the Komfo Anokye Teaching Hospital the second largest in Ghana. The hospital is located in Kumasi 250 kilometres north of the national capital Accra. It runs an autonomous clinic devoted to breast diseases only on a daily basis. At the Komfo Anokye Teaching hospital - Breast Care Center (KATH-BCC) a detailed proforma - based history is taken from all patients at presentation and a complete clinical examination including the examination of the breast is also performed on these patients. All breast lumps were assessed by a combination of clinical examination, breast imaging (breast ultrasonography and or mammography) and tissue or pathologic diagnosis; the triple assessment. No breast imaging was done on patients with fumigating and ulcerating lesions. Specimens for microscopic diagnoses of cancer included Fine Needle Aspiration Cytology (FNAC), core, excision, and incisional biopsies. In cases that the initial pathological diagnosis was done using FNAC specimens, surgical excision specimens provided tissue for pathologic grading of the cancers.
The estimations of oestrogen and progesterone receptors and Her2/new protein became available from mid 2008. Assistance from the Department of Pathology of the University Hospital of North Norway (UNN) made it possible to have immunohistochemical analysis on a limited number of breast cancer material from our hospital. From July 1st 2004 to June 30th 2009 (five years) details of all patients with microscopically proven breast cancer were recorded in a prospective manner. After each consultation relevant data on the demographic characteristics, stage at presentation and tumour characteristics of all patients with histological confirmation of breast cancer was recorded on a specially designed proforma. Cancer staging was done after relevant investigations using the American Joint Committee on Cancer (AJCC) 2002 system. There were no facilities for radioisotope bone scanning.
Pathologic methods
The tumours that were studied were graded using the modified Bloom-Richardson system. The scoring system used was based on the degree of tubular formation, nuclear pleomorphism and mitotic count as seen on H&E stain. Each category was scored a maximum of 3 points. The tumour grade of each cancer in our series was determined by the sum of the points. Tissue immunoperoxidase stains for oestrogen, progesterone and c-erb2 oncogene (Her2) receptors were performed. Commercially prepared antigens and reagents were used.
The scoring system for ER and PR receptor status were based on the Quick score which scores the percentage of tumour cells with nuclear staining and the intensity of the staining. Cases were classified negative for ER and PR if there is less than 10% of nuclear staining of tumour cells. HER2/neu staining was scored based on membrane staining pattern (intensity and completeness). Scores 0 and 1+ was classified as negative. Score 2+ was inconclusive (weakly positive) and only 4 cases were in this category during this study. They were later classified as negative after Silver In situ hybridization (SISH). Cases that are scored 3+ were regarded as positive.
Results
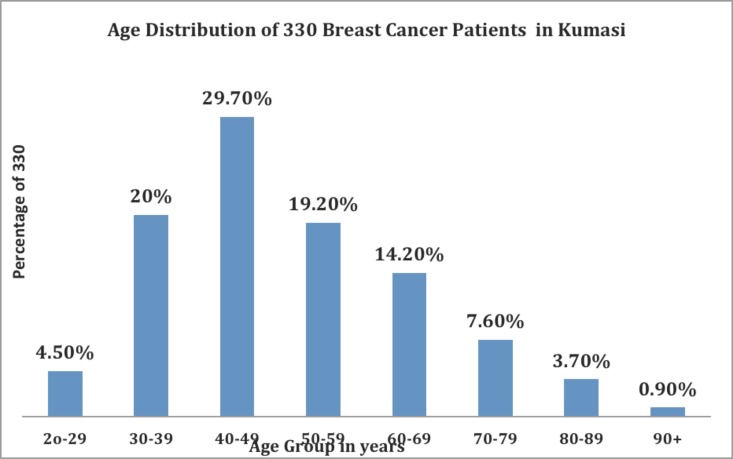
A total of 19,423 patients were seen at the KATH-BCC during the study period. Microscopic diagnosis of breast cancer was established in 330 (1.7%) of the patients: This figure is 22.3% (one -fifth) of the 1481 malignancies diagnosed during the study period. The number of male breast cancers (5 or 1.5%) was considered too small for separate analysis. The demographic characteristics of the 330 patients are shown in Table 1. The frequency distribution of the patients according to age is shown in Figure 1.
Table 1.
The demographic characteristics of breast cancer patients in Kumasi
| Variable | N=330 |
| Age (years) Range Mean+-SD |
20–96 49.1915.3) |
| Sex distribution Male Female |
5(1.5%) 325(98.5%) |
| Age at menarche(years) Range Mean(SD) |
13 to 23 15.4(1.6) |
| Age at first birth(years) Range Mean(SD) |
14 to 34 21.3(4.7) |
| Parity Range Mean(SD) |
0 to 12chidren 4.2(2.7) |
| Duration of breast feeding (months) Range Mean |
4 to 6 13.8(8.7) |
| Menopausal status Pre menopause Post menopause |
n(%) 241(73.0) 89(27.0) |
| Level of education None Primary Post primary |
n(%) 106(32.1) 70(21.2) 154(46.7) |
| Occupation Unemployed Farmers Trader/Artisan Profession |
n(%) 112(33.9) 58(17.6) 122(37.0) 38(11.5) |
| Marital Status Married Not married |
n(%) 155(46.9) 175(53.1) |
| Residence Urban Semi urban Rural |
n(%) 224(67.9) 51(15.4) 55(16.7) |
| Use of hormonal contraceptive Yes No |
n(%) 17(5.1) 313(94.8) |
| Family history of breast cancer Yes No |
n(%) 18(7.9) 209(92.1) |
Figure 1.
Age distribution of 330 breast cancer patients.
Clinical Presentation and Diagnosis
The presenting symptoms were breast lump 248 (75.2%), swelling of the breast 61 (18.5%), breast pain 12 (3.6%) bloody nipple discharge 5 (1.5%), and lump in armpit 4 (1.2%). The duration of symptoms before presentation ranged from one to 82 months, with a mean of 13.8 months with a 95% C.I. (10.9, 16.7).
Most of the tumours (119 or 36.1%) were located in the upper outer quadrant of the breast. The whole breast was involved in 71 patients or 21.5%.
Other locations were central area or the nipple areola complex 55 patients or 16.7%, the lower outer quadrant 28 patients (8.5%), upper inner quadrant 23(7.0%), the lower inner quadrant 22 patients (6.7%) and other less frequent locations 12 patients or 3.6%.
Pathological Diagnosis
FNAC was done in 64 patients or 19.4%, tru-cut biopsies in 30 patients (9.1%), excision biopsies in 96 patients (29.1%) and incisional biopsies in 140 patients (42.4%). Infiltrating ductal carcinoma not otherwise specified was the most frequent pathological type accounting for 82.1% of the breast cancer material. Other less common pathological types were: in situ ductal 2(0.6%), infiltrating lobular 1(0.3%), mucinous 32(9.8%), medullary 13(3.9), adenocystic 5(1.5%) and papillary carcinomas 3(0.9%). There were three cases (0.9%) of adenosarcomas. Of the 270 patients in whom data on the cancer grade was available, 145 cancers (53.7%) were Grade III, 85 (31.5%) Grade II and 40 (14.8%) Grade 1. High-grade tumours (Grades II and III) accounted for 85.2% of all the cancers.
Many patients may have been under staged as no bone scans were done
Biomarkers
Immunoperoxidase nuclear staining of tumour of cells greater than 10% was recorded in 32 ER and 9 PR receptors and these were scored as positive. Of the 54 breast cancer material stained for Her2 receptors 11 showed membrane staining pattern (intensity and completeness) of a score of 3+ and were recorded as positive for Her2 receptor. A total of 54 breast cancer materials were stained for all three receptors and 23 (42.7%) were negative for all three receptors.
Table 3 is a summary of the results of the immunoperoxidase staining for hormone receptors in 68 breast cancer materials.
Table 3.
Hormone receptor status of breast cancer material from Kumasi, Ghana.
| Hormone receptor |
Positive n(%) |
Negative n(%) |
Total n(%) |
| ER | 32(47.06) | 36(52.94) | 68(100) |
| PR | 9(13.24) | 59(86.76) | 68(100) |
| Her2 | 11(20.37) | 43(79.63) | 54(100) |
Legend: ER; oestrogen receptor. PR; progesterone receptor, Her2; Human epidermal growth factor Receptor 2
Discussion
Most of the patients who were diagnosed with breast cancer in this study were young (peak age 40–49 years, Figure 1). Recent clinical and pathological studies from Sub Saharan Africa have reported similar findings.4–6,9 From Uganda, Gakwaya and his colleagues reported that the peak age of the breast cancer patients that they studied was as low as 30–39 years.5 It is unclear why breast cancer patients in this and other studies from Sub Saharan Africa are so young. A possible explanation is the population structure of most countries in the sub region. In Ghana less than 5% of all women are above 50 years21 and in Uganda only 4.2%. of all women are above 50 years.22 The findings of the study may not reflect a true increased incidence of breast cancer in young women as life expectancy in Ghana is low. Over two-thirds (73%) of the patients in the present report were pre menopausal (Table 1). This finding is characteristic of breast cancer in Sub Saharan African women.23 Parkin and his colleagues in a study on African population of Zimbabwe in the 1990s reported some findings that suggested that multi-parity increased breast cancer risk before age 45 years but was protective after that age.24
Recently Palmer and his co-workers reported the dual effect of parity on breast cancer risk in African-American women which is consistent with earlier findings by Parkin on African populations.24–25 In this study parity was high among the women and the average number of children per woman was 4.2 (Table 1). The early age at first birth (Table 1) meant that many women have multiple childbirths in early life. The high parity (multiparty) among these young women may have increased the risk of breast cancer before age 45 years. These observations may explain the high incidence of breast cancer in premenopausal women that was recorded in this series.
Many patients may have been under staged as no bone scans were done. Furthermore the age distribution of breast cancer patients in this study indicates that programmes for breast cancer control in Kumasi (preventive, educational, awareness and screening) should be designed for and targeted to young women.
The unfavourable characteristics of breast cancer patients in Kumasi are evident from the findings of this study. At presentation to hospital 85% of these young women had Stage III or IV disease (Table 2), the most frequent histological type of cancer was the infiltrating ductal carcinoma (82.1%).
Table 2.
Stage (AJCC, 2003) at presentation of 330 breast cancer patients in Kumasi
| Stage | No. of patients |
% |
| Stage 1 | 12 | 3.6 |
| T1N0M0 | 12 | |
| Stage II | 37 | 11.2 |
| Stage IIA | ||
| T0NIM0 | 2 | |
| TINIM0 | 4 | |
| T2N0M0 | 1 | |
| Total | 7 | |
| Stage IIB | ||
| T2NIM0 | 23 | |
| T3N0M0 | 7 | |
| Total | 30 | |
| Stage III | 231 | 70.0 |
| Stage IIIA | ||
| T2N2M0 | 5 | |
| T3N1M0 | 31 | |
| T3N2M0 | 51 | |
| Total | 87 | |
| Stage IIIB | ||
| T4N1M0 | 45 | |
| T4N2M0 | 51 | |
| Total | 96 | |
| Stage IIIC | ||
| Any TN3Mx | 48 | |
| Stage VI | ||
| M1 | 50 | 15.2 |
| Grand Total | 330 | 100 |
Similar findings have been reported from southern Ghana9, Nigeria4,12 and Uganda.5 These findings provide further evidence that breast cancer in indigenous black African populations has poor prognostic factors. The findings may explain why there is a disproportionate mortality from breast cancer in Sub Saharan African women when compared to the incidence and mortality of the disease in Caucasian women.23 Some workers have suggested a genetic basis for the characteristics of breast cancer seen in indigenous African populations.11
There are few studies on hormone receptor status of breast cancer material in African patients due to the lack of the required infrastructure.15–16 In the present study nearly half (47.1 %) of the breast cancer material was oestrogen receptor positive (ER+), (Table 3). The conclusions that may be drawn from these findings have to be assessed in relation to the fact that only 20% of all the specimens were tested for hormone receptor status. Nonetheless the results of the immunohistochemical analysis of breast cancer material from Kumasi as shown in four are comparable with previous reports from Accra where of the 74 tumours analysed 43% were estrogen receptor positive.26 These findings on breast cancer material from Ghana do confirm the generally accepted reports indicating that the rate of estrogen receptor positive cancers in sub-Saharan Africans women is low.4,6,26–28
Over a third (42.7%) of the breast cancer material analysed and reported in this study was negative for expression of oestrogen and progesterone receptors and HER2 protein (triple-negative breast cancer). Triple negative breast cancers have very unfavourable and aggressive clinicopatholigical features including onset at a younger age and high-grade tumours.17,29 The age distribution and grade of tumours of the patients in the present study (figures 1 and 3) are consistent with some of the features of triple negative cancers.28–29 Two recent studies from sub Saharan Africa have reported high rates of hormone receptors in some Nigerian and Sudanese women.15,16
Although researchers Adebamowo and Awaldekarim subsequently recommended that physicians caring for breast cancer patients of indigenous black African origin may use hormone therapy even for patients in whom the receptor status is unknown15,16, the weight of the evidence from world literature including the findings of the present study is in favour of a more selective approach. Breast cancer patients of indigenous black African population as in Kumasi should have their requirements for hormonal treatment determined by the individual biological characteristics of their tumours before the commencement of the therapy.
About a half (53.3 %,) of the patients in the present series have had no education or only up to primary level (Table 1). Also over two-thirds (70.9%) of the women in this study were either unemployed or traders and artisans and hence of low socioeconomic status. As a result of low levels of formal education there is a lack of awareness of early warning signs of breast cancer. Consequently many patients delay for several months before presenting to hospital. The mean duration of symptoms before presentation to hospital in this study was over 12 months. There are other factors that may contribute to the long delay in seeking medical help for any breast complaints.
These include the lack of any form of national breast cancer screening programme in Ghana, a weak health care system, poor access to health facilities and the activities of faith healers and traditional and herbal practitioners who may be more accessible to the population even in the urban and semi –urban communities (Table 1). The combined effect of these adverse factors is the near complete absence of early disease in our series. As shown in Table 3 less than 1% of the tumours were of the early intra-duct or in situ carcinoma histological type and only 3.6% were Stage I at diagnosis (Table 2).
As low levels of education and lower socioeconomic status have been found to be associated with advanced breast cancer at presentation30–31, the findings in this series suggest that any improvement in the levels of education and in the general socioeconomic conditions of the women in Kumasi is likely to lead to a reduction in the number of patients who present to the hospital with advanced breast cancer.
The current study revealed that less than 2% of the patients seen at our center were proven to have cancer of the breast. This finding indicates that in our setting unselected screening for breast cancer may not be cost-effective. Instead breast cancer programmes such as early detection of the disease should aim at health education and (breast cancer) awareness creation among our women.
This is likely to contribute to the effort to reduce late stage presentation of breast cancer in our practice.
Some limitations of the study need to be addressed. There is no population-based Cancer Registry in Kumasi. Consequently the incidence of breast cancer in Kumasi could not be determined. Data on the incidence of breast cancer in Kumasi will be useful information for planning for the control of the disease. Only a limited number of breast cancer cases were subjected to immunohistochemical analysis. The data on hormone receptor status of breast cancer material and triple negative cancers from Kumasi cannot be generalized for the entire country. Many patients will still receive hormone therapy blindly.
Conclusion
In Kumasi most patients with breast cancer present late with advanced disease. The cancers have unfavourable prognostic features and are unlikely to respond to hormonal manipulation.
Acknowledgement
The authors wish to acknowledge the invaluable contribution of all the surgical residents who assisted in the completion of the special proforma. We also acknowledge the co-operation and assistance KATH pathology staff, Pathologists International and the Department of Pathology of the University of North Norway in providing the cytology, histopathology and immunehistochemical data.
References
- 1.Biritwum RB, Amaning AO. Pattern of diseases or conditions leading to hospitalization at the Korle Teaching hospital. Ghana Med J. 2000;34:197–205. [Google Scholar]
- 2.Wiredu EK, Armah HB. Cancer mortality patterns in Ghana: a 10 -year review of autopsies and hospital mortality. BMC Public Health. 2006;6:159–165. doi: 10.1186/1471-2458-6-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amir H, Kitinya JN, Parkin BM. A comparative study of carcinoma of the breast in an African population. East Afr Med J. 1994;71:215–218. [PubMed] [Google Scholar]
- 4.Gukas ID, Jennings BA, Mandong BM, Igun GO, Manasseh AN, Ugwu BT, Leinster SJ. Clinicopatholological features and molecular markers of breast cancer in Jos Nigeria. West Afr J Med. 2005;24:209–213. doi: 10.4314/wajm.v24i3.28220. [DOI] [PubMed] [Google Scholar]
- 5.Gakwaya A, Kigula-Mugambe JB, Kavuma A, Luwaga A, Fualall J, Jombwe J, Galukande M, Kanyike D. Cancer of the breast: 5-year survival in a tertiary hospital in Uganda. Br J Cancer. 2008;99:63–67. doi: 10.1038/sj.bjc.6604435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mbonde MP, Amir H, Mbembati NA, Holland R, Schwartz-Albiez R, Kitinya JN. Characterisation of benign lesions and carcinomas of the female breast in a sub-Saharan population. Pathol Res Pract. 1998;194:623–629. doi: 10.1016/s0344-0338(98)80097-6. [DOI] [PubMed] [Google Scholar]
- 7.Amir H, Azizi MR, Makwaya CK, Jessani S. TNM classification and breast cancer in an African population: a descriptive study. Cent Afr J Med. 1997;43:357–359. [PubMed] [Google Scholar]
- 8.Hassan I, Onukak EE, Mabogunge OA. Breast cancer in Zaria, Nigeria. J R Coll Surg Edinb. 1992;37:159–161. [PubMed] [Google Scholar]
- 9.Clegg-Lamptey JNA, Hodasi WH. A study of breast cancer in Korle Bu teaching hospital: Assessing the impact of health education. Ghana Med J. 2007;41(2):72–77. doi: 10.4314/gmj.v41i2.55305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mbonde MP, Amir H, Schwartz-Albiez R, Akslen LA, Kitinya JN. Expression of estrogen and progesterone receptors in carcinomas of the female breast in Tanzania. Oncol Rep. 2000;7:277–283. doi: 10.3892/or.7.2.277. [DOI] [PubMed] [Google Scholar]
- 11.Gao Q, Adebamowo CA, Fackenthal J, Das S, Sveen li falusi AG, Olopade OI. Protein Truncatimg BRCA1 and BRCA2 mutations in African women with pre -menopausal breast cancer. Hum Genet. 2000;107:192–194. doi: 10.1007/s004390000342. [DOI] [PubMed] [Google Scholar]
- 12.Adesunkanmi ARK, Lawal OO, Adelusola KA, Durosimi MA. The severity, outcome and challenges of breast cancer in Nigeria. The Breast. 2006;15:399–409. doi: 10.1016/j.breast.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Ikpatt OF, Kuopio T, Collan Y. Proliferation in African breast cancer: Biology and prognostication in Nigerian breast cancer material. Mod Pathol. 2002;15:783–789. doi: 10.1097/01.MP.0000021764.03552.BD. [DOI] [PubMed] [Google Scholar]
- 14.Ikpatt OF, Kuopio T, Erekul A, Collan Y. Apoptosis in breast cancer : Nigerian vs. Finish material. Anal Quant Cytol Histol. 2002;24:73–80. [PubMed] [Google Scholar]
- 15.Awadelkarim KD, Arizzi C, Elamin EO, Hamad HM, De Blassio P, Mekki SO, Osman I, Biunno I, Elwali NE, Mariani-Costantini R, Barberis MC. Pathological, clinical and prognostic characteristics of breast cancer in Central Sudan and Northern Italy: implications for breast cancer in Africa. Histopathology. 2008;52:445–456. doi: 10.1111/j.1365-2559.2008.02966.x. [DOI] [PubMed] [Google Scholar]
- 16.Adebamowo CA, Famooto A, Ogundiran TO, Aniagwu T, Nkwodimmah C, Akang EE. Immunohistochemical and molecular subtypes of breast cancer in Nigeria. Breast Cancer Res Treat. 2008;110:183–188. doi: 10.1007/s10549-007-9694-5. [DOI] [PubMed] [Google Scholar]
- 17.Carey LA, Perou CM, Livasy CA, Dressler LG, et al. Race, breast cancer subtypes and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 18.Okobia M, Bunker C, Zmuda J, Kammerer C, Vogel V, Uche E, Anyanwu S, Ezeome E, Ferrel R, Kuller L. Case -controlled study of risk factors for breast cancer in Nigerian women. Int J Cancer. 2006;119:2179–2185. doi: 10.1002/ijc.22102. [DOI] [PubMed] [Google Scholar]
- 19.Anim JT. Breast cancer in Accra. Ghana Med J. 1979;18:161–167. [Google Scholar]
- 20.Quartey-Papafio JB, Anim JT. Cancer of the breast in Accra. Ghana Med J. 1980;19:159–162. [Google Scholar]
- 21.Ghana Statistical Services, author. 2000 Population and Housing Census: Summary of Report of Final Results. Ghana: Ghana Statistical Services; 2000. [Google Scholar]
- 22.Uganda Bureau of Statistics (UBOS), author Uganda Bureau of Statistics. Final Report of the 2002 Population Census of Uganda 2005. www.ubos.org.
- 23.Fregene A, Newman LA. Breast cancer in Sub Saharan Africa: how does it relate to breast cancer in African American women? Cancer. 2005;103(8):1540–1550. doi: 10.1002/cncr.20978. [DOI] [PubMed] [Google Scholar]
- 24.Parkin DM, Vizcain AP, Skinner MEG, Ndhlovu A. Cancer patterns and risk factors in the African population of Southwestern Zimbabwe 1963–1977. Cancer Epidemiology, Biomarkers and Prevention. 1994;3:537–547. [PubMed] [Google Scholar]
- 25.Palmer JR, Wise LA, Horton NJ, et al. Dual effect of parity on breast cancer risk in African - American women. J Natl Cancer Inst. 2003;95:478–483. doi: 10.1093/jnci/95.6.478. [DOI] [PubMed] [Google Scholar]
- 26.Yarney J, Vanderpuye V, Clegg-Lamptey JN. Hormone receptor and Her-2 expression in breast cancers among sub-Saharan African women. Breast J. 2008;14(5):510–1. doi: 10.1111/j.1524-4741.2008.00636.x. [DOI] [PubMed] [Google Scholar]
- 27.Mbonde MP, Amir H, Akslen LA, Kitinya JN. Expression of oestrogen and progesterone receptors, Ki-67, p53 and BCL-2 protiens, cathepsin D, urokinase plasminogen activator and urokinase plasminogen activator-receptors in carcinomas of the female breast in an African population. East Afr Med J. 2001;78:360–5. doi: 10.4314/eamj.v78i7.9009. [DOI] [PubMed] [Google Scholar]
- 28.Nyagol J, Nyong'o A, Byakika B, Muchiri L, Cocco M, de Santi MM, Spina D, Bellan C, Lazzi S, Kostopoulos I, Luzi P, Leoncini L. Routine assessment of hormonal receptor and HER-2/neu status underscores the need for more therapeutic targets in Kenyan women with breast cancer. Anal quant Cytol Histol. 2006;28:97–103. [PubMed] [Google Scholar]
- 29.Dent RTM, Pritchard KI, Hanna WM, et al. Triple -negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 30.Elgaili ME, Abuiris DO, Rahman M, Michalek AM, Mohamed SI. Breast cancer burden in central Sudan. Inter J Women's Health. 2010;2:77–82. doi: 10.2147/ijwh.s8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vorobio DA, Sitas F, Vorobiof G. Breast cancer incidence in South Africa. J clin Oncol. 2001;19(18S):125s–127. [PubMed] [Google Scholar]