Abstract
The forced swim test is a rodent behavioral test used for evaluation of antidepressant drugs, antidepressant efficacy of new compounds, and experimental manipulations that are aimed at rendering or preventing depressive-like states. Mice are placed in an inescapable transparent tank that is filled with water and their escape related mobility behavior is measured. The forced swim test is straightforward to conduct reliably and it requires minimal specialized equipment. Successful implementation of the forced swim test requires adherence to certain procedural details and minimization of unwarranted stress to the mice. In the protocol description and the accompanying video, we explain how to conduct the mouse version of this test with emphasis on potential pitfalls that may be detrimental to interpretation of results and how to avoid them. Additionally, we explain how the behaviors manifested in the test are assessed.
Keywords: Neuroscience, Issue 59, animal models, behavioral analysis, neuroscience, neurobiology, mood disorder, depression, mood stabilizer, antidepressant, forced swimming test, FST
Protocol
1. Materials and Method
1.1. The water tanks
The cylindrical tanks (30 cm height x 20 cm diameters) required for the mouse forced swim test (FST) in our laboratory are constructed of transparent Plexiglas, as this material is able to withstand the frequent movement of the tanks and accidents better than glass. The water level is 15 cm from the bottom and should be marked on the tank to ensure that the volume of water is consistent across mice. The number of tanks should ideally be at least twice as many as the number of mice being tested at a time, so that the second water tank set can be filled while the first set is in use. The dimensions of the tanks should be selected in a way that the mice will not be able to touch the bottom of the tank, either with their feet or their tails, during the swimming test. The height of the tank should be high enough to prevent the mice from escaping from the tank. Please note that the diameter of tank and the depth of water are important parameters that can be adjusted to change the behavior of mice (for a detailed analysis of these issues see1-3).
1.2. Thermometer
A water resistant infrared thermometer is preferable, since rapid measurement of temperature reduces the amount of time required to conduct the test. However, a glass mercury thermometer will also be sufficient for this task.
1.3. Timer
1.4. Video recording device
We use a video camera supported by a tripod. Since this test usually involves multiple animals being tested at the same time, live scoring will be very difficult and is not advisable. The video camera should record in high enough resolution to render a quality picture that will be used later for behavioral scoring. Always make sure there is sufficient recording memory in the camera before starting the test. We use a video camera that records digitally without the use of mechanical media (i.e. video cassette), allowing for digital transfer of videos. If there are excessive reflections on the tanks, which may occur in laboratory environments with overhead fluorescent illumination, you may want to use a polarizing lens filter with your camera.
1.5. Dividers
In our lab we have two sets of dividers (35 cm height x 22 cm width x 22 cm depth). These are rectangular with three walls and are used as both background and as dividers between tanks to prevent mice from seeing each other during the test and potentially altering their behaviors. One set can be black for albino and light colored animals; the other set can be light colored for dark colored animals in order to render high contrast. The experimenter should make sure that the surfaces of the dividers are not overly reflective so that they alter camera images, or render major differences between illumination levels.
1.6. White noise generator
This is needed in laboratory environments in which sudden loud noises can be heard that would potentially startle the animals. The noise generator will mask such intermittent disturbing sounds. The volume level of the white noise generator should be selected to be above other ambient and unexpected noises. In our experimental room the ambient noise level (without the white noise generator activated) is 60 dB. The total noise level with the white noise generator activated at the location where the tanks are placed is 70-72 dB. However it should be noted that these figures are provided as example only, and each laboratory should select the right noise levels according to their unique environment and circumstances.
1.7. Drying paper and heat lamp
Before returning the animals to their home cages, it is important to dry them gently using paper towels and it is helpful to use a heat lamp (be certain the exposure temperature does not exceed 32°C) to prevent hypothermia.
2. Behavioral Procedures
The overall experimental design should reflect proper counterbalancing between variables specific to your experiment. For example, in our experiments, we try to represent each group equally in every FST session (i.e., if there are four treatment groups, each will be represented in each session). Also, mice are rotated, such that mice from each treatment group are placed in a different tank in each session.
Place the camera and the dividers in position. The camera should be as close as possible in order to obtain the highest possible resolution of the mice.
The tanks should be filled with tap water set at the room temperature (23-25°C) to the determined level, which is marked on the tank walls. If your facility does not have constant hot/cold water, you may want to prepare hot water and/or ice to quickly bring the water to the right temperature. Check the water temperature with the infrared thermometer. Alternatively, if the temperatures of hot and cold water are constant at your facility, you can draw on the tank two marks – one for the level of hot water and a second mark for the addition of cold water – to get close to the correct final water temperature rapidly.
Start the white noise generator, if being used, before the mice are introduced to the testing room. The level of white noise should only be enough to mask external noises. Avoid a high volume and make sure the same level of white noise is being used for all animals.
Bring the animals into the testing room. If the colony room where the animals reside and the testing room are adjacent or very close to each other, the ambient conditions are similar and the disturbance during the moving of the cage is minimal, then no acclimation period will be necessary. Otherwise, place the animals in the testing room for a period of acclimation (generally at least one hour). If an acclimation period is necessary, make sure the acclimated animals will not be affected by the mice being tested at the same time in the same room. Please be aware that olfactory and ultrasonic cues can be sensed by the other animals placed in the same room.
Start video recording before placing the animals into the water tanks.
Hold the animal by its tail, and gently and slowly place in the water. Once the mice is in the water, slowly release the tail. Typically, using this procedure will prevent the animal's head from being submerged under the water.
Place the mice in the tanks in an order in which the obstruction of recording will be minimized. This order, of course, should be decided in conjunction with the counterbalancing of groups and other requirements specific to your experimental design.
Once all mice are in the tanks-start the countdown on the stopwatch. The usual test length for mice is six minutes in the FST.
During the test be certain you are at a reasonable distance from the animals and do not make any movements or noise that may be noticed by the animals. Mice can readily float in water, however, if either a new strain of mice or a new compound being tested without previous knowledge of their effect on swimming behavior, the experimenter should monitor the animals more closely. In contrast to rats, mice do not typically dive during the FST, however in the event of diving the mouse should be removed from the tank. If the experimenter leaves the room the mice should be monitored by video in the event that a mouse cannot maintain swimming and floating behavior and to stop the test if necessary.
At the end of six minute testing period stop the recording. In our lab, we show a note in front of the camera that identifies the animals at the very end of each recording. When using this approach the individual subsequently scoring the recording will not know the identity of the animals since the identity is only shown at the very end of the recording. This prevents any identification and record keeping problems that may occur later related to the recordings. Regardless of the record keeping strategy that is being used, it should clearly identify the animals and also prevent the individual later scoring the test from having knowledge of group assignments.
Remove the animals from the water by their tails in the same order that you put them in and gently dry them with a drying paper and place back into their homecage.
3. Behavior Analysis
The mouse version of FST is typically, from start to finish, six minutes long. However, generally only the last four minutes of the test are analyzed. This is due to the fact that most mice are very active at the beginning of the FST, and the potential effects of the treatment can be obscured during the first two minutes.
In our laboratory we upload the video files directly from the camera to a PC and do the analysis on the PC.
During the behavioral analysis, the time that each mice spends mobile is measured. The total amount of mobility time is then subtracted from the 240 seconds of test time and is then stated as the immobility time. While it is possible to measure the immobility time directly, in our laboratory we have found it easier to detect and measure active movements rather than the lack of such movements.
The most important aspect of behavioral analysis and usually the biggest source of variability between observers in the FST is the correct identification of movements that are counted as bona fide mobility. Our operational definition for mobility in the FST is any movements other than those necessary to balance the body and keep the head above the water4. Mice generally float in water readily, however they still manifest small movements to balance their bodies and keep their heads above the water. These behaviors are not an attempt to escape and should not be scored as mobility. Also, after a single bout of mobility, even though essentially immobile, mice can still drift in the water as a result of momentum. These movements also should not be scored as mobility.
In our laboratory, we use an on-screen stopwatch software (Xnote Stopwatch, dnSoft Research Group) for time measurements. Two separate stopwatches are used on the screen. The first stopwatch counts down from 240 seconds and alerts the observer when the behavioral analysis period ends. The second stopwatch measures the time spent mobile. Some stopwatch software has the ability to assign keys to start and stop functions, so that on-screen stopwatches can be controlled by the keyboard. In our lab, instead of a regular keyboard, we use an input device commonly known as a 'gamepad' to control the stopwatches.
When using a PC to quantify immobility, if there is more than one mouse tested and present on the screen, it is a good idea to cover the other animals (you can use another program window or physically cover the screen with paper), so that their movements will not distract the observer.
If an on-screen stopwatch is used, be certain to cover all but the millisecond decimals of the stopwatch. The reason for this is to prevent bias in the observer, while still allowing the ability to determine if the watch is running or not running. Since the observer, while blind to the group assignments of the animals, will have a general idea of level of mobility in the mice, there might be some bias occurring if she is allowed to see the total amount of mobility time elapsed for that particular mouse before completion of the analysis session. By covering the stopwatch, she will only know whether the stopwatch is on or off at any point but will not know the total time elapsed and therefore cannot be effected by any bias.
An inter-observer reliability test should be conducted for every new observer before beginning to collect data from test animals. In our laboratory, each new observer first watches an experienced observer scoring. After the new observer gains confidence to differentiate mobility from immobility, they then score with the experienced observer watching and pointing out any mistakes. Once this phase is successfully completed, the new observer will analyze a specific set of FST videos that we keep in our laboratory for training purposes. Only after a high level of inter-observer correlation is obtained with the experienced observer does an investigator start analyzing FST videos in actual experiments. We archive the data from these training analyses to constitute an internal standard for the laboratory for future use. We have observed differences between strains in the manner in which they express mobility (and immobility) behaviors, and mean immobility time between sexes. When a new strain, sex, or genetically modified mouse model is tested in the laboratory it is necessary to again undertake this type of reliability analysis.
4. Representative Results
There are marked differences between genetically distinct inbred and outbred mouse strains in terms of their baseline immobility and responding to a specific drug5-11. For example, we identified differential antidepressant-like responses to lithium in a panel of mouse strains (Figure 1)5. Experimental details of this experiment are published in Can et al., 20115.
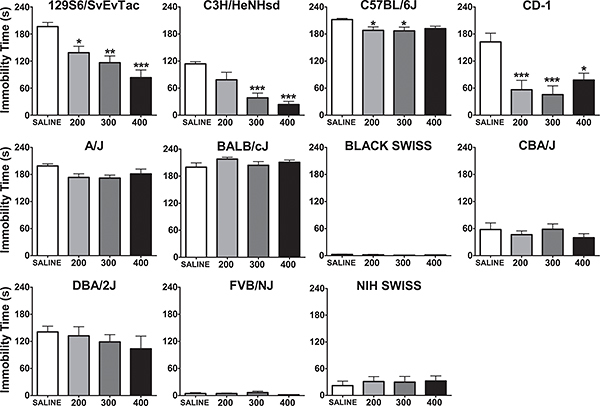 Figure 1. Immobility time (in seconds) in the forced swim test, five hours after a single i.p. injection of saline, 200, 300, or 400 mg/kg in various inbred and outbred mouse strains. *:p<0.05, **:p<0.01, ***:p<0.001 denote a significant difference compared to saline group, Dunnett's post hoc test. Data are expressed as mean ± SEM. Number of animals per group for each strain is 6-8 (Figure reproduced from5).
Figure 1. Immobility time (in seconds) in the forced swim test, five hours after a single i.p. injection of saline, 200, 300, or 400 mg/kg in various inbred and outbred mouse strains. *:p<0.05, **:p<0.01, ***:p<0.001 denote a significant difference compared to saline group, Dunnett's post hoc test. Data are expressed as mean ± SEM. Number of animals per group for each strain is 6-8 (Figure reproduced from5).
Not all mouse strains are suitable for the FST. Some strains, such as Black Swiss, NIH Swiss, and FVB/NJ show little or almost no immobility under control conditions, therefore representing a floor effect (Figure 1)5. The lack of baseline immobility effectively prevents detecting an anti-depressant effect of experimental manipulations. It is also possible, while very rare, that some mouse strains may behave aberrantly and dive into the tank during the test even though they can float. One such strain is DBA/1OlaHsd (unpublished observation in our laboratory). Such strains are not suitable for the FST. Because of this diving risk, however small, when testing a new strain that has not been previously tested in the FST or a mouse harboring a novel genetic manipulation, it is imperative to carefully observe the initial trials to rescue mice if they engage in potentially harmful behaviors.
In the experimental design described here, multiple animals (up to five) are tested at the same time. While the dividers we use prevent mice from seeing each other during the test, and the white noise generator suppresses audible vocalizations, our set-up does not prevent all ultrasonic or olfactory cues from being transmitted. Though unlikely given the nature of the test, these could affect the behaviors of mice. One solution to this problem would be to test the animals individually. However, this approach has its own problems. For example, commonly, the animals tested in each session come from the same homecage. This allows randomization and counterbalancing of the experimental variables. Testing mice individually would mean removing one mouse at a time from the homecage. This will cause repeated stress and disturbance of social hierarchy in the cage among the others left behind. Another issue with testing singly are the time constraints. Testing one mouse at a time will extend the experiment into many hours resulting in a situation in which mice are tested at different times of the circadian cycle. This may create confounding time of day effects. The researchers should keep these issues in mind while designing their experiments.
Discussion
The FST (sometimes called Porsolt swim test) was developed first for rats and then modified for mice by Porsolt and colleagues12,13. In addition to the above-described protocol successful in our laboratory, a number of largely subtle test modifications have been published (see Hascoët and Bourin for a complete review1). It is a common test used for evaluation of the efficacy of anti-depressant drugs and the effects of various behavioral and neurobiological manipulations in basic and preclinical research3,14-16. It has been described as rendering a situation in which "behavioral despair" is induced; that is, the animal loses hope to escape the stressful environment13. The mouse version of the forced swim test is a relatively short and low cost behavioral test that requires no training of the mice and can be conducted with minimal equipment. This is in contrast to the rat version of the test, which generally involves exposure to the water tank one day prior to the test day17 .
Because of its popularity there is a wealth of data regarding the effects of various antidepressants in the FST. This allows researchers to compare and contrast their own results with others (see Hascoët and Bourin for 2009 review1). These characteristics of the FST make it an important tool in academic research and drug discovery in industrial settings where reliability and high throughput screening of novel compounds are essential. An additional feature of the FST is the availability of commercial automated behavior analysis systems that can accelerate the data collection process18-20. However, in our experience, these automated systems require extensive validation by human scoring. Additionally, automated parameters may have to be readjusted when using different strains, especially when the level of background contrast changes, or with mice of different sizes or behavioral responses.
Another area in which the FST is used is neurogenetic research in which the genetic basis of depression-related behaviors is investigated. These types of studies involve comparison of various mouse strains with or without the use of anti-depressant drugs and comparisons of genetically modified or selectively bred mice and their wild type counterparts6,21-23. In this regard, the FST has proven to be useful in basic research related to the neurobiology and genetics of mood disorders. However, the FST is not a full spectrum analog of human depression. Even though there are exceptions, the FST has a considerable level of predictive validity, since it is reasonably sensitive to compounds that are effective in humans as anti-depressants and insensitive to those that are not effective24,25. Since the behavioral outcome of the FST is one-dimensional it can only indicate the antidepressant efficacy of compound or experimental manipulations, but it cannot differentiate mechanistic differences between them. This is in contrast with the rat version of the FST, where rats manifest both swimming and climbing behaviors that can differentiate between serotonin and norepinephrine acting compounds26. Also any manipulations that may affect the overall activity levels may potentially alter immobility in the FST leading to spurious conclusions. Therefore it is important to verify the results of FST with separate behavioral tests that measure overall activity such as the open-field test1,27. It is beneficial to keep in mind that the FST does not represent the human condition, and to extent which underlying neurobiological mechanisms of the behaviors manifested by model animals in the FST and human depression overlap is not entirely clear28. However, these types of limitations should not devalue the usefulness of FST as a drug discovery and validation tool.
Disclosures
Authors declare no conflicts of interest.
Acknowledgments
This study has been supported by the grant NIHM R01 MH091816 and R21 MH084043 to TDG.
References
- Hascoét M, Bourin M. In Mood and Anxiety Related Phenotypes in Mice. 2009;42:85–118. [Google Scholar]
- Sunal R, Gümüçel aB, Kayaalp SO. Effect of changes in swimming area on results of "behavioral despair test". Pharmacology Biochemistry and Behavior. 1994;49:891–896. doi: 10.1016/0091-3057(94)90239-9. [DOI] [PubMed] [Google Scholar]
- Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology. (Berl) 2005;177:245–255. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends in Pharmacological Sciences. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Can A. Antidepressant-like responses to lithium in genetically diverse mouse strains. Genes, Brain and Behavior. 2011;10:434–443. doi: 10.1111/j.1601-183X.2011.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology. (Berl) 2001;155:315–322. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- David DJ, Renard CE, Jolliet P, Hascoet M, Bourin M. Antidepressant-like effects in various mice strains in the forced swimming test. Psychopharmacology (Berl) 2003;166:373–382. doi: 10.1007/s00213-002-1335-4. [DOI] [PubMed] [Google Scholar]
- Bai F, Li X, Clay M, Lindstrom T, Skolnick P. Intra- and interstrain differences in models of "behavioral despair". Pharmacol. Biochem. Behav. 2001;70:187–192. doi: 10.1016/s0091-3057(01)00599-8. [DOI] [PubMed] [Google Scholar]
- Guzzetti S. Strain differences in paroxetine-induced reduction of immobility time in the forced swimming test in mice: Role of serotonin. European Journal of Pharmacology. 2008;594:117–124. doi: 10.1016/j.ejphar.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Cervo L. Genotype-dependent activity of tryptophan hydroxylase-2 determines the response to citalopram in a mouse model of depression. J. Neurosci. 2005;25:8165–8172. doi: 10.1523/JNEUROSCI.1816-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J, Nitzke A, Doukas D, Seiglie M, Dulawa S. Antidepressant response to chronic citalopram treatment in eight inbred mouse strains. Psychopharmacology. 2011;213:509–520. doi: 10.1007/s00213-010-2140-0. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Pichon MLe, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. "Behavioural despair" in rats and mice: strain differences and the effects of imipramine. Eur. J. Pharmacol. 1978;51:291–294. doi: 10.1016/0014-2999(78)90414-4. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav. Brain. Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neuroscience & Biobehavioral Reviews. 2007;31:3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neuroscience & Biobehavioral Reviews. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Jones O'Leary, F O, Lucki I. Automated tests for measuring the effects of antidepressants in mice. Pharmacology Biochemistry and Behavior. 2004;78:269–274. doi: 10.1016/j.pbb.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Kurtuncu M, Luka LJ, Dimitrijevic N, Uz T, Manev H. Reliability assessment of an automated forced swim test device using two mouse strains. Journal of Neuroscience Methods. 2005;149:26–30. doi: 10.1016/j.jneumeth.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Hayashi E, Shimamura M, Kuratani K, Kinoshita M, Hara H. Automated experimental system capturing three behavioral components during murine forced swim test. Life Sciences. 2011;88:411–417. doi: 10.1016/j.lfs.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Cryan J, Page M, Lucki I. Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology. 2005;182:335–344. doi: 10.1007/s00213-005-0093-5. [DOI] [PubMed] [Google Scholar]
- Gould TD. Beta-catenin overexpression in the mouse brain phenocopies lithium-sensitive behaviors. Neuropsychopharmacology. 2007;32:2173–2183. doi: 10.1038/sj.npp.1301338. [DOI] [PubMed] [Google Scholar]
- Can A, Grahame NJ, Gould TD. Affect-related related behaviors in mice selectively bred for high and low voluntary alcohol consumption. Behav. Genet. 2011. [DOI] [PMC free article] [PubMed]
- McKinney WT, Bunney WE. Animal Model of Depression: I Review of Evidence: Implications for Research. Arch. Gen. Psychiatry. 1969;21:240–248. doi: 10.1001/archpsyc.1969.01740200112015. [DOI] [PubMed] [Google Scholar]
- Willner P. The validity of animal models of depression. Psychopharmacology.(Berl) 1984;83:1–16. doi: 10.1007/BF00427414. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Lucki I. Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: the effects of water depth. Behav. Brain Res. 1995;73:43–46. doi: 10.1016/0166-4328(96)00067-8. [DOI] [PubMed] [Google Scholar]
- Gould TD, Dao DT, Kovacsics CE. In: In Mood and Anxiety Related Phenotypes in mice: characterization using behavioral tests. Gould TD, editor. Vol. 42. Humana Press; 2009. [Google Scholar]
- Bourin M, Fiocco AJ, Clenet F. How valuable are animal models in defining antidepressant activity. Human Psychopharmacology: Clinical and Experimental. 2001;16:9–21. doi: 10.1002/hup.178. [DOI] [PubMed] [Google Scholar]


