Abstract
Human immunodeficiency virus type 1 (HIV-1) coreceptor usage was determined using a phenotypic assay in plasma samples from treatment-naive women infected with subtype C virus who had CD4 cell counts below 200 cells/mm3. Of 148 women 14.9% were infected with dual/mixed (DM) virus; the remainder had R5 virus. A greater proportion of women in the lowest CD4 cell count stratum had DM virus (p=0.026); change in coreceptor use after ART exposure was uncommon. CXCR4-using HIV-1 was less common in subtype C-infected women than reported in subtype B cohorts, but was most prevalent in women with the lowest CD4 cell counts.
Keywords: AIDS, HIV-1 tropism, CCR5, CXCR4, subtype C, antiretroviral therapy
INTRODUCTION
Coreceptor usage by HIV-1 is an important determinant of disease progression and response to CCR5 antagonist therapy. Much of the data relating coreceptor usage to clinical disease is based on studies of subtype B-infected patients. Most HIV-1 transmission involves CCR5-using (R5) viruses, which predominate in the early phases of infection1–4. Over time, CXCR4-using viruses emerge in approximately 50% of patients and are associated with an increased risk of disease progression and death2, 5. Less is known about patterns of coreceptor usage by HIV-1 subtype C (HIV-1C), which accounts for more than half of new infections worldwide. Studies to characterize coreceptor usage of HIV-1C are needed for a better understanding of pathogenesis, treatment and prevention of infection by this highly prevalent subtype.
Data regarding the prevalence of CXCR4-using HIV-1C are conflicting, and come from relatively small, cross-sectional studies. Initial studies suggested that R5 virus predominates in HIV-1C infection throughout the course of disease6–9. Subsequent studies identified CXCR4-using virus in a small number of treatment-naive HIV-1C-infected patients with advanced HIV disease10–13, with some studies showing that 30–50% of patients with AIDS harbor CXCR4-using virus14–16. One study suggested a possible association of ART with higher rates of CXCR4-using virus14, but the relationship between ART and emergence of CXCR4-using virus was inferred by the comparison of prevalence rates between small cohorts of treatment-naïve and – experienced subjects. To obtain a better estimate of the prevalence of CXCR4-using virus in HIV-1C infection and its relationship to antiretroviral therapy we determined co-receptor usage of plasma virus from a large cohort of HIV-1C-infected women from Botswana with advanced HIV-1 disease.
METHODS
Patients
Subjects selected for testing were women participating in the Mashi study, a randomized clinical trial of prevention of MTCT conducted in southeastern Botswana from 2001–2003 (clinicaltrials.gov identifier: NCT00197587)17, 18. All participating women received zidovudine from 34 weeks gestation, and were randomized to single dose nevirapine or placebo intrapartum. Combination ART became available through the Botswana government to participating women with CD4<200 cells/mm3 or AIDS-defining illness beginning in 2002, 19 months into the study. Participants who completed the Mashi study were offered enrollment into a follow-up study, in which clinical status, HIV-1 RNA and CD4 cell count were monitored at 3-month intervals19. For those who initiated ART, virologic failure was defined as confirmed HIV-1 RNA >400 copies/mL 6 or more months after starting ART or failure to achieve at least a 1-log10 drop in HIV-1 RNA by 12 weeks19. Plasma HIV-1 RNA was assessed using the standard COBAS Amplicor HIV-1 Monitor assay (Roche Diagnostics); CD4 cell counts were measured by standard techniques. The use of Mashi samples for this study was approved by the ethics committees and institutional review boards at the Botswana Health Ministry, the Harvard School of Public Health and Partners HealthCare System. All participants had provided written informed consent for use of stored samples for approved HIV-related research.
HIV-1 coreceptor usage assay
Coreceptor usage of plasma virus was determined using a sensitive single-cycle assay validated for subtype C viruses20 at screening or enrollment into Mashi from women with screening CD4 counts below 200 cells/mm3, and for women who initiated ART, at the time of virological failure. Viruses that used only CCR5 were considered R5; those that used only CXCR4 were considered X4; those that used both coreceptors were considered to have dual or mixed (DM) coreceptor usage. The study was powered to estimate the prevalence of CXCR4-using virus among treatment-naïve patients with a precision of ±6.1% (95% confidence interval [CI]), which required a sample size of at least 100 patients.
Statistical methods
Associations of viral tropism with clinical parameters were explored by univariable analyses, using Fisher's exact test for categorical parameters and the Wilcoxon rank-sum test for continuous parameters. The Cochran-Armitage exact test of trend was used to identify significant differences in prevalence across increasing categories of baseline (pre-ART) CD4 cell count and HIV-1 RNA. All reported P values are two-sided. Changes in HIV-1 RNA and CD4 cell count over time were explored using type 3 Tests of Fixed Effects, using SAS version 9.1.
RESULTS
In the Mashi study, 206 women had screening CD4 cell counts below 200 cells/mm3. Of the 165 women with samples available for tropism testing, 12 were excluded because they had initiated ART prior to enrollment, leaving 153 samples for testing. Baseline characteristics of the 153 women with samples tested for co-receptor usage were similar to those of the 53 women who were not tested, except that baseline median viral load was higher (p=0.01) and the time to ART initiation was longer among women with a tested sample (p<0.001;Table 1). The exclusion of the 12 women who initiated ART prior to enrollment likely contributed to the difference in time to ART initiation between the two groups. Among those tested, the median baseline CD4 count was 130 cells/mm3 (interquartile range [IQR], 95–171 cells/mm3) and the plasma HIV-1 RNA level was 5.02 log10copies/ml (IQR, 4.51–5.44 log10copies/ml).
Table 1.
Clinical characteristics and coreceptor usage of women infected with HIV-1C
| Availability for testing of coreceptor usage |
Stratified by coreceptor usage |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline characteristics | total | available | not available | P | total | R5 | DM | P |
| Maternal age (n) | 206 | 153 | 53 | 0.49 | 148 | 126 | 22 | 0.03 |
| median age | 28.3 | 29.4 | 28.1 | 33.1 | ||||
| Baseline CD4 cell count (n) | 206 | 153 | 53 | 0.8 | 148 | 126 | 22 | 0.08 |
| median cells/mm3 (IQR) | 130 (95–171) | 139 (94–167) | 141 (103–172) | 121 (56–157) | ||||
| Baseline HIV-1 RNA (n) | 203 | 151 | 52 | 0.01 | 147 | 125 | 22 | 0.94 |
| median logi10copies/ml (IQR) | 5.0 (4.5–5.4) | 4.6 (3.9–5.2) | 5.0 (4.5–5.4) | 5.2 (4.4–5.4) | ||||
| Time to start of ART (n) | 166 | 119 | 47 | <0.001 | 116 | 99 | 17 | 0.25 |
| months (IQR) | 7.0 (2.1–13.7) | 2.2 (−0.3–5.8) | 7.2 (2.5–14.3) | 3.9 (1.1–10.7) | ||||
| ART initiation (n) | 206 | 153 | 53 | 0.11 | 148 | 126 | 22 | 1 |
| yes (%) | 119 (78) | 47 (89) | 99 (79) | 17 (77) | ||||
| Maternal death (n) | 206 | 153 | 53 | 0.77 | 148 | 126 | 22 | 1 |
| yes (%) | 12 (8) | 5 (9) | 10 (8) | 1 (5) | ||||
| HIV transmission to infant (n) | 206 | 153 | 53 | 0.65 | 148 | 126 | 22 | 1 |
| yes (%) | 22 (14) | 6 (11) | 19 (15) | 3 (14) | ||||
Prevalence of CXCR4-using viruses and the characteristics of the women by coreceptor usage
The coreceptor usage assay generated results for 148 of 153 (96.7%) women. Among those with an assay result, 22 (14.9%) had DM virus and 126 (85.1%) had R5 virus; no samples were X4 (Table 1). A maximum likelihood tree built from sequences of the C2–V4 region of a subset of env amplicons (N=61) confirmed that each sequence was unique and clustered with reference subtype C sequences (bootstrap value 98%).
Median baseline viral load, time to initiation of ART, death and MTCT did not differ significantly between women with DM versus R5 virus, but women with DM virus were significantly older than women with R5 virus (median age 33.1 years versus 28.1 years, respectively; p=0.025). Comparison of the median baseline CD4 cell count in women harboring DM virus to those with exclusively R5 virus showed differences that approached statistical significance (p=0.08) (Table 1). The distribution of women infected with DM virus varied significantly across the range of CD4 cell counts between 0 and 200 cells/mm3, with the highest proportion having baseline CD4 cell counts below 50 cells/mm3 (Cochran-Armitage exact test of trend, p=0.026) (Figure 1).
Figure 1.
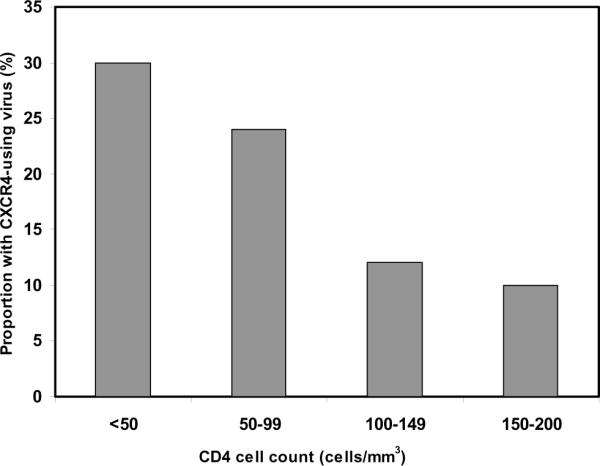
Proportion of patients with CXCR4-using virus by CD4 cell count category (p=0.026, Cochran-Armitage exact test of trend).
CXCR4-using virus as predictor of clinical progression
The association of baseline coreceptor usage with change in CD4 cell count and virus load from study entry to ART initiation was examined, but the number of women not on ART decreased substantially over time; the majority started ART shortly after enrollment when ART became available in 2002 through the Botswana government treatment program. By 12 months after enrollment 82% of women infected with R5 or DM virus had initiated ART; among those who remained treatment-naïve the mean CD4 cell count was 130 cells/mm3 for DM virus (n=4) compared to 170 cells/mm3 for R5 virus (n=23). The mean CD4 cell counts at sequential 3-month intervals were always lower among women with DM virus compared those with R5 virus, but these differences did not achieve statistical significance (p=0.149).
Change in coreceptor usage over time
Paired samples from 24 women collected prior to ART and at virological failure were tested for HIV coreceptor usage to determine the association of ART exposure with emergence of CXCR4-using virus. The mean interval between entry and virologic failure was 28 months (range, 8 to 64 months). The majority of women (n=17, 71%) had R5 virus at both time points. In four women (17%) with R5 virus at entry, DM virus emerged after ART exposure. Seven women without a sample at study entry had R5 virus at virological failure.
DISCUSSION
This study determined the prevalence and clinical correlates of CXCR4-using virus in a cohort of ART-naïve HIV-1 C-infected women in Botswana. Because previous studies suggested a much lower frequency of CXCR4-using virus in HIV-1C infection, we selected women with low CD4 cell counts for testing in order to increase the likelihood of identifying DM or X4 virus. Because some studies suggest that ART may select for DM or X4 virus, the prevalence of CXCR4-using virus was examined in ART-naïve women.
Nearly 15% of women with CD4 cell counts below 200 cells/mm3 harbored DM virus. By contrast, studies in subtype B-infected patients with similarly advanced HIV-1 disease show a prevalence of CXCR4-using virus of 50% or more2, 3, 5, 21, 22. Previous studies among treatment-naïve patients infected with HIV-1C virus show a prevalence of DM virus of 0 to 30%. These studies were generally based on small numbers of patients in Africa, with the largest having 40 patients6–9, 11, 14, 15, 23–25. One study of 174 HIV-1C-infected patients in India showed a prevalence of 3.5% for CXCR4-using virus in treatment-naïve patients who had a wide range of CD4 cell counts26. Because our study focused on patients with lower CD4 cell counts, the prevalence of DM and X4 virus among HIV-infected patients in Botswana overall most likely is considerably lower than 15%. The relatively low frequency of CXCR4-using virus even among persons with advanced disease suggests that CCR5 antagonists such as maraviroc may be particularly useful in the treatment of HIV-1C infection. These data also support evaluating the role of CCR5 antagonists as agents for the prevention of HIV-1 transmission in areas of the world where HIV-1 C predominates.
The association of HIV-1 coreceptor usage and disease is well-established in HIV-1 subtype B infection but less so for other subtypes4, 27–29. Because our study only examined HIV-1 coreceptor usage among patients within a narrow range of CD4 cell counts, it was not possible to assess fully the association between CD4 cell count and presence of CXCR4-using virus. Nevertheless, even among patients with CD4 counts below 200 cells/mm3 there was a significant trend towards higher prevalence of CXCR4-using HIV-1 among patients with the lowest CD4 counts (p=0.02).
In cross-sectional studies of subtype B-infected patients, CXCR4-using virus is detected in approximately 20% of treatment-naive patients but in up to 50% of treatment-experienced patients27, 29–32. This difference suggests to some that ART selects for CXCR4-using viruses, independent of CD4 cell count. However, after adjusting for nadir CD4 cell count the association of ART and CXCR4 is no longer statistically significant33, 34. In a cross-sectional study of 28 HIV-1C-infected patients, CXCR4-using virus was not detected in any ART-naïve patients but was found in 50% of ART-experienced patients. This finding led the authors to propose that ART exerts a greater effect on coreceptor usage in HIV-1C infection as compared to subtype B14. In the current study, the majority of women with R5 virus at the start of ART remained R5 at the time of virologic failure; DM virus emerged in only 4 (19%) women. This rate is consistent with observations from natural history studies, in which DM or X4 virus emerged in 23% of patients over time22.
This study has several limitations. As noted above, the inclusion only of women with low CD4 cell counts may have limited the ability to detect significant associations between coreceptor usage and other clinical factors. In addition, data were obtained from pregnant women only; gender and pregnancy, however, are not known to affect the emergence of CXCR4-using virus. The phenotypic assay used in the current study can detect minority CXCR4-using variants present at 1% to 5% of the population, depending on the plasma virus load. It is possible that a somewhat higher frequency of CXCR4-using virus would have been detected using the enhanced-sensitivity Trofile assay (Monogram BioSciences, South San Francisco, CA), which has a threshold of detection of 0.3% for CXCR4-using variants35
In summary, DM virus was readily detected in HIV-1C-infected women with advanced disease, but its prevalence was considerably lower than that reported in subtype B- or D-infected patients with similarly advanced disease36, 37. Exposure to ART did not appear to be a major factor in emergence of CXCR4-using virus in the cohort studied here. Such knowledge is essential for an improved understanding of the biological and clinical differences between HIV-1 subtypes, and for the design of therapeutic agents and preventive vaccines targeted against the most prevalent HIV-1 subtype.
ACKNOWLEDGMENTS
We would like to express our gratitude to all the participants and the study staff of the Mashi study for making this study possible. We thank Erik van Widenfelt and Jean Leidner for assistance in sample identification and data management, Keikantse Matlhagela for sharing the treatment failure samples, Roger Shapiro and the Harvard Initiative for Global Health (HIGH) for providing support for overseas work, Daniel Negusse for assistance in developing the tropism assay, Zixin Hu, Noriaki Hosoya, Athe Tsibris and Manish Sagar for valuable scientific insights.
Funding support: This work was supported in part by Public Health Services grants from the National Institutes of Health (NIH) including K24 RR016482 and U01 AI068636 (an ACTG Virology Support Laboratory contract) (to D.R.K.); R01 HD037793 (to M.E.); R01 HD044391 (to S.L.); a Scholar Award from the Harvard Center for AIDS Research (CFAR) (P30 AI060354), a Harvard Institute for Global Health International Fellowship, a Bristol-Myer's Squibb Virology Fellowship, and a Burroughs Wellcome Fund/American Society of Tropical Medicine and Hygiene post-doctoral fellowship (to N.H.L.); the NIH Fogarty grant R24TW007988 (to R.M.M) and the CFAR Biostatistical core grant award 2P30AI060354-06 (to L.M.S.). Preliminary data were presented at the 2010 Conference of Retroviruses and Opportunistic Infections in San Francisco, CA (February 8–11, 2010).
REFERENCES
- 1.Connor RI, Sheridan KE, Ceradini D, et al. Change in coreceptor use coreceptor use correlates with disease progression in HIV-1--infected individuals. J Exp Med. 1997 Feb 17;185(4):621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koot M, Keet IP, Vos AH, et al. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann Intern Med. 1993 May 1;118(9):681–688. doi: 10.7326/0003-4819-118-9-199305010-00004. [DOI] [PubMed] [Google Scholar]
- 3.Schuitemaker H, Koot M, Kootstra NA, et al. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J Virol. 1992 Mar;66(3):1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bozzette SA, McCutchan JA, Spector SA, et al. A cross-sectional comparison of persons with syncytium- and non-syncytium-inducing human immunodeficiency virus. J Infect Dis. 1993 Dec;168(6):1374–1379. doi: 10.1093/infdis/168.6.1374. [DOI] [PubMed] [Google Scholar]
- 5.Richman DD, Bozzette SA. The impact of the syncytium-inducing phenotype of human immunodeficiency virus on disease progression. J Infect Dis. 1994 May;169(5):968–974. doi: 10.1093/infdis/169.5.968. [DOI] [PubMed] [Google Scholar]
- 6.Abebe A, Demissie D, Goudsmit J, et al. HIV-1 subtype C syncytium- and non-syncytium-inducing phenotypes and coreceptor usage among Ethiopian patients with AIDS. Aids. 1999 Jul 30;13(11):1305–1311. doi: 10.1097/00002030-199907300-00006. [DOI] [PubMed] [Google Scholar]
- 7.Cecilia D, Kulkarni SS, Tripathy SP, et al. Absence of coreceptor switch with disease progression in human immunodeficiency virus infections in India. Virology. 2000 Jun 5;271(2):253–258. doi: 10.1006/viro.2000.0297. [DOI] [PubMed] [Google Scholar]
- 8.Bjorndal A, Sonnerborg A, Tscherning C, et al. Phenotypic characteristics of human immunodeficiency virus type 1 subtype C isolates of Ethiopian AIDS patients. AIDS Res Hum Retroviruses. 1999 May 1;15(7):647–653. doi: 10.1089/088922299310944. [DOI] [PubMed] [Google Scholar]
- 9.Ping LH, Nelson JA, Hoffman IF, et al. Characterization of V3 sequence heterogeneity in subtype C human immunodeficiency virus type 1 isolates from Malawi: underrepresentation of X4 variants. J Virol. 1999 Aug;73(8):6271–6281. doi: 10.1128/jvi.73.8.6271-6281.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Rensburg EJ, Smith TL, Zeier M, et al. Change in co-receptor usage of current South African HIV-1 subtype C primary isolates. Aids. 2002 Dec 6;16(18):2479–2480. doi: 10.1097/00002030-200212060-00015. [DOI] [PubMed] [Google Scholar]
- 11.Cilliers T, Nhlapo J, Coetzer M, et al. The CCR5 and CXCR4 coreceptors are both used by human immunodeficiency virus type 1 primary isolates from subtype C. J Virol. 2003 Apr;77(7):4449–4456. doi: 10.1128/JVI.77.7.4449-4456.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papathanasopoulos MA, Cilliers T, Morris L, et al. Full-length genome analysis of HIV-1 subtype C utilizing CXCR4 and intersubtype recombinants isolated in South Africa. AIDS Res Hum Retroviruses. 2002 Aug 10;18(12):879–886. doi: 10.1089/08892220260190362. [DOI] [PubMed] [Google Scholar]
- 13.Tien PC, Chiu T, Latif A, et al. Primary subtype C HIV-1 infection in Harare, Zimbabwe. J Acquir Immune Defic Syndr Hum Retrovirol. 1999 Feb 1;20(2):147–153. doi: 10.1097/00042560-199902010-00006. [DOI] [PubMed] [Google Scholar]
- 14.Johnston ER, Zijenah LS, Mutetwa S, et al. High frequency of syncytium-inducing and CXCR4-tropic viruses among human immunodeficiency virus type 1 subtype C-infected patients receiving antiretroviral treatment. J Virol. 2003 Jul;77(13):7682–7688. doi: 10.1128/JVI.77.13.7682-7688.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connell BJ, Michler K, Capovilla A, et al. Emergence of X4 usage among HIV-1 subtype C: evidence for an evolving epidemic in South Africa. Aids. 2008 Apr 23;22(7):896–899. doi: 10.1097/QAD.0b013e3282f57f7a. [DOI] [PubMed] [Google Scholar]
- 16.Michler K, Connell BJ, Venter WD, et al. Genotypic characterization and comparison of full-length envelope glycoproteins from South African HIV type 1 subtype C primary isolates that utilize CCR5 and/or CXCR4. AIDS Res Hum Retroviruses. 2008 May;24(5):743–751. doi: 10.1089/aid.2007.0304. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro RL, Thior I, Gilbert PB, et al. Maternal single-dose nevirapine versus placebo as part of an antiretroviral strategy to prevent mother-to-child HIV transmission in Botswana. Aids. 2006 Jun 12;20(9):1281–1288. doi: 10.1097/01.aids.0000232236.26630.35. [DOI] [PubMed] [Google Scholar]
- 18.Thior I, Lockman S, Smeaton LM, et al. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana: a randomized trial: the Mashi Study. Jama. 2006 Aug 16;296(7):794–805. doi: 10.1001/jama.296.7.794. [DOI] [PubMed] [Google Scholar]
- 19.Lockman S, Shapiro RL, Smeaton LM, et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med. 2007 Jan 11;356(2):135–147. doi: 10.1056/NEJMoa062876. [DOI] [PubMed] [Google Scholar]
- 20.Lin NH, Negusse DM, Beroukhim R, et al. The design and validation of a novel phenotypic assay to determine HIV-1 coreceptor usage of clinical isolates. J Virol Methods. 2010 Oct;169(1):39–46. doi: 10.1016/j.jviromet.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tersmette M, de Goede RE, Al BJ, et al. Differential syncytium-inducing capacity of human immunodeficiency virus isolates: frequent detection of syncytium-inducing isolates in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. J Virol. 1988 Jun;62(6):2026–2032. doi: 10.1128/jvi.62.6.2026-2032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shepherd JC, Jacobson LP, Qiao W, et al. Emergence and persistence of CXCR4-tropic HIV-1 in a population of men from the multicenter AIDS cohort study. J Infect Dis. 2008 Oct 15;198(8):1104–1112. doi: 10.1086/591623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choge I, Cilliers T, Walker P, et al. Genotypic and phenotypic characterization of viral isolates from HIV-1 subtype C-infected children with slow and rapid disease progression. AIDS Res Hum Retroviruses. 2006 May;22(5):458–465. doi: 10.1089/aid.2006.22.458. [DOI] [PubMed] [Google Scholar]
- 24.Coetzer M, Cilliers T, Ping LH, et al. Genetic characteristics of the V3 region associated with CXCR4 usage in HIV-1 subtype C isolates. Virology. 2006 Dec 5–20;356(1–2):95–105. doi: 10.1016/j.virol.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 25.Ndung'u T, Sepako E, McLane MF, et al. HIV-1 subtype C in vitro growth and coreceptor utilization. Virology. 2006 Apr 10;347(2):247–260. doi: 10.1016/j.virol.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 26.Tressler R, Greenacre A, Rajicic N, et al. Distribution of HIV-1 coreceptor tropism among individuals in India infected with clade C HIV-1. 5th IAS Conference on HIV Pathogenesis Treatment and Prevention; Cape Town, South Africa. 2009. [Google Scholar]
- 27.Brumme ZL, Goodrich J, Mayer HB, et al. Molecular and clinical epidemiology of CXCR4-using HIV-1 in a large population of antiretroviral-naive individuals. J Infect Dis. 2005 Aug 1;192(3):466–474. doi: 10.1086/431519. [DOI] [PubMed] [Google Scholar]
- 28.Furrer H, Wendland T, Minder C, et al. Association of syncytium-inducing phenotype of HIV-1 with CD4 cell count, viral load and sociodemographic characteristics. Aids. 1998 Jul 30;12(11):1341–1346. doi: 10.1097/00002030-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Moyle GJ, Wildfire A, Mandalia S, et al. Epidemiology and predictive factors for chemokine receptor use in HIV-1 infection. J Infect Dis. 2005 Mar 15;191(6):866–872. doi: 10.1086/428096. [DOI] [PubMed] [Google Scholar]
- 30.Melby T, Despirito M, Demasi R, et al. HIV-1 coreceptor use in triple-class treatment-experienced patients: baseline prevalence, correlates, and relationship to enfuvirtide response. J Infect Dis. 2006 Jul 15;194(2):238–246. doi: 10.1086/504693. [DOI] [PubMed] [Google Scholar]
- 31.Wilkin TJ, Su Z, Kuritzkes DR, et al. HIV type 1 chemokine coreceptor use among antiretroviral-experienced patients screened for a clinical trial of a CCR5 inhibitor: AIDS Clinical Trial Group A5211. Clin Infect Dis. 2007 Feb 15;44(4):591–595. doi: 10.1086/511035. [DOI] [PubMed] [Google Scholar]
- 32.Fatkenheuer G, Nelson M, Lazzarin A, et al. Subgroup analyses of maraviroc in previously treated R5 HIV-1 infection. N Engl J Med. 2008 Oct 2;359(14):1442–1455. doi: 10.1056/NEJMoa0803154. [DOI] [PubMed] [Google Scholar]
- 33.Delobel P, Sandres-Saune K, Cazabat M, et al. R5 to X4 switch of the predominant HIV-1 population in cellular reservoirs during effective highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2005 Apr 1;38(4):382–392. doi: 10.1097/01.qai.0000152835.17747.47. [DOI] [PubMed] [Google Scholar]
- 34.Hunt PW, Harrigan PR, Huang W, et al. Prevalence of CXCR4 tropism among antiretroviral-treated HIV-1-infected patients with detectable viremia. J Infect Dis. 2006 Oct 1;194(7):926–930. doi: 10.1086/507312. [DOI] [PubMed] [Google Scholar]
- 35.Su Z, Gulick RM, Krambrink A, et al. Response to vicriviroc in treatment-experienced subjects, as determined by an enhanced-sensitivity coreceptor tropism assay: reanalysis of AIDS clinical trials group A5211. J Infect Dis. 2009 Dec 1;200(11):1724–1728. doi: 10.1086/648090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herbeck JT, Lyagoba F, Moore SW, et al. Prevalence and genetic diversity of HIV type 1 subtypes A and D in women attending antenatal clinics in Uganda. AIDS Res Hum Retroviruses. 2007 May;23(5):755–760. doi: 10.1089/aid.2006.0237.A. [DOI] [PubMed] [Google Scholar]
- 37.Kaleebu P, Nankya IL, Yirrell DL, et al. Relation between chemokine receptor use, disease stage, and HIV-1 subtypes A and D: results from a rural Ugandan cohort. J Acquir Immune Defic Syndr. 2007 May 1;45(1):28–33. doi: 10.1097/QAI.0b013e3180385aa0. [DOI] [PubMed] [Google Scholar]


