Abstract
Observational studies demonstrate an association between physical activity and improved outcomes in breast and colon cancer survivors. To test these observations with a large, randomized clinical trial, an intervention that significantly impacts physical activity in these patients is needed. The Active After Cancer Trial (AACT) was a multicenter pilot study evaluating the feasibility of a telephone-based exercise intervention in a cooperative group setting.
Methods
Sedentary (engaging in < 60 minutes of recreational activity/week) breast and colorectal cancer survivors were randomized to a telephone-based exercise intervention or usual care control group. The intervention was delivered through the University of California at San Diego; participants received 10 phone calls over the course of the 16-week intervention. All participants underwent assessment of physical activity, fitness, physical functioning, fatigue and exercise self-efficacy at baseline and after the 16-week intervention.
Results
One hundred and twenty-one patients were enrolled through 10 Cancer and Leukemia Group B (CALGB) institutions; 100 patients had breast cancer and 21 had colorectal cancer. Participants randomized to the exercise group increased physical activity by more than 100%, vs. 22% in controls (54.5 vs. 14.6 minutes, p=0.13), and experienced significant increases in fitness (increased 6-minute walk test distance by 186.9 vs. 81.9 ft, p=0.006) and physical functioning (7.1 vs. 2.6, p=0.04) as compared to the control group.
Conclusions
Breast and colorectal cancer survivors enrolled in a multicenter, telephone-based physical activity intervention increased physical activity and experienced significant improvements in fitness and physical functioning. Lifestyle intervention research is feasible in a cooperative group setting.
Keywords: Breast cancer, exercise, cooperative group, intervention, physical functioning
INTRODUCTION
Studies suggest that lifestyle factors such as physical activity and functional status are associated with cancer outcomes. The Nurses’ Health Study investigators demonstrated that women with early-stage breast cancer who engaged in more than 9 MET-hours/week of physical activity, equivalent to walking at an average pace for 3 hours/week, had a 50% lower risk of breast cancer recurrence, breast cancer death and all cause mortality than women who were inactive.[1] Subsequent to this report, several additional large prospective cohort studies, encompassing more than 15,000 patients with early stage breast cancer, have demonstrated that women who are physically active after cancer diagnosis have a 30–50% lower risk of disease-specific and overall mortality as compared to sedentary patients.[1–5] Similar findings have also been reported for individuals diagnosed with colon cancer.[6–8] Additionally, poor physical functioning, linked to sedentary physical activity patterns[9], has long been shown to be associated with worse survival in patients with advanced disease[10, 11], and recent work also demonstrates a link between poor physical functioning and decreased overall and disease-specific survival in patients diagnosed with early stage cancers of the breast, head and neck, colon, and lung.[12–15]
These findings have not yet been confirmed in randomized trials. Many small, mostly single-institution, studies have demonstrated that physical activity interventions are safe in breast cancer patients, and that participation in an exercise intervention leads to improvements in physical functioning, fitness, quality of life and other end points.[16, 17] However, there have been no randomized trials looking at the impact of physical activity on disease outcomes, and the single-institution trials performed to date do not provide an adequate foundation for the design of a large-scale trial.
The Active After Cancer Trial (NCT00548236) was designed to evaluate the feasibility of conducting a telephone-based exercise intervention study in a cooperative group setting. The study’s primary endpoint was change in minutes of weekly physical activity. Secondary outcomes included change in physical functioning, fitness, anthropometric measures and quality of life.
METHODS
Study Population
Participants were recruited from medical oncology clinics at 10 Cancer and Leukemia Group B (CALGB) institutions, including both academic institutions and community practices, between November 2007 and November 2009. Eligibility criteria included histological evidence of stage I–III invasive breast, colon or rectal cancer; completion of all surgery, chemotherapy and/or radiation therapy between 2 and 36 months prior to enrollment (adjuvant hormonal therapy and trastuzumab were allowed); BMI ≤ 47 kg/m2; and baseline participation in ≤ 60 minutes of physical activity per week. Baseline exercise was assessed via the Leisure Score Index (LSI) of the Godin Leisure-Time Exercise Questionnaire (modified to include activity duration). Patients were excluded if they had evidence of persistent or recurrent cancer, other malignancy, uncontrolled heart disease or other contraindications to exercise.
Medical clearance was obtained from potential participants’ medical oncologists or primary care providers. The study was approved by the Institutional Review Board at the Dana-Farber Cancer Institute and at each of the participating sites. Informed consent was obtained from all participants prior to enrollment.
Study Design
After enrollment, participants were randomized 1:1 to an exercise intervention group or usual care control group. The intervention group participated in a 16-week telephone-based exercise intervention. The control group received routine care for 16 weeks and was then offered a telephone consultation with an exercise trainer at the end of the control period. Subjects were stratified by type of malignancy (breast vs. colon/rectal) and gender at the time of study entry. Assessment of weekly minutes of physical activity, fitness, anthropometric measures, quality of life, physical functioning, and fatigue was performed at baseline and after the completion of the 16-week study period. Assessment of physical activity was conducted centrally, and all other study measures were collected at the participating sites. Changes in these measures over time were compared between participants randomized to the exercise and control groups.
Exercise Intervention
Social cognitive theory and client-centered counseling techniques[18] were used in a telephone-based intervention to motivate participants to increase physical activity. The intervention consisted of 10–11 semi-structured phone calls over the 16-week intervention period. Calls were delivered by behavioral counselors from a Shared Resource at the Moores UC San Diego Cancer Center. Call duration was 30–45 minutes; calls were more frequent during the early period of the change attempt and became less frequent over time.[19] Initial calls focused on goal setting and performance assessment so as to build self-efficacy for exercise behaviors, while later calls concentrated upon the adequacy of plans for relapse prevention. Each call reviewed performance on the behaviors previously discussed and encouraged the participant to keep using self-regulatory skills to achieve change. The telephone calls were supplemented by a Participant Workbook, which included additional information regarding the importance of exercise in cancer populations, guidelines for exercise safety, and journal pages to track weekly exercise.
The weekly exercise target was performance of at least 180 minutes of moderate-intensity physical activity, based on the results of observational studies demonstrating better survival in patients with early stage breast and colorectal cancer who engaged in 3–5 hours of moderate activity per week.[1–3, 6, 7] Participants were allowed to choose their own form of exercise, as long as it involved moderate to strenuous activity (as defined in Ainsworth’s Compendium of Physical Activities[20]). Participants were provided with a pedometer (New Lifestyle Digi-Walker) and asked to wear this daily. Instructions for using the pedometer were included in the Participant Workbook and were reviewed during the first counseling session. Participants were asked to record the number of minutes of exercise they performed and steps they completed each day in journals, which were reviewed during the telephone counseling calls.
Quality Assurance
The UCSD Cancer Prevention Program counselors complete an intensive 80-h program providing training in conducting physical activity and dietary assessments, the principles and practice of client-centered counseling, and use of computer-based structured counseling protocols. Counselors practice extensive role-playing before conducting their first counseling session. To ensure the fidelity of the intervention, the counselors used a computer-assisted program that provided them with scripted questions that required them to enter respondent answers at each point. These scripted calls were contained within a detailed relational database that provided the call schedule, range checks on keyed responses, and management reports.
Measurements
Demographic data and disease and treatment information were collected at the time of participant enrollment. The study’s primary outcome was change in minutes of weekly physical activity over the course of the 16-week study period. Physical activity was measured with the 7-Day Physical Activity Recall (7-Day PAR) Interview, an instrument that provides information regarding the duration and intensity of physical activity performed. The 7-Day PAR has been widely used to quantify physical activity levels in a variety of epidemiologic and interventional studies[21–23] and has been demonstrated to correlate with changes in VO2 max, body composition[21, 24, 25], and activity patterns generated through direct observation or activity monitors[25, 26]. 7-Day PAR interviews were conducted over the telephone by a blinded member of the study staff at the Dana-Farber Cancer Institute. Weekly minutes of physical activity and weekly metabolic task equivalent-hours (MET-hours) of activity were recorded at baseline and at week 16 for all study participants.
Participants also underwent a series of anthropometric, fitness and quality of life measurements at both time points. Measurements were conducted by study staff at participating institutions. Body weight and height were measured with participants wearing street clothes and no shoes. These data were used to calculate Body Mass Index (BMI) using the formula BMI=weight (kg)/height (m)2. Waist circumference was measured at the bending line, and hip measurement was recorded at the point of maximum girth.
Fitness was assessed through the 6-Minute Walk Test (6MWT), an objective evaluation of functional exercise capacity that has been shown to be highly correlated with the 12 minute walk test[27] (from which it was derived) and with cycle ergometer and treadmill based exercise tests.[28] The 6MWT measures the distance an individual walks on a level, indoor surface in 6 minutes. Given space limitations, each participating site was provided with a stop watch and 100 foot tape measure. Investigators identified a stretch of hallway at least 50 feet in length, and participants walked back and forth along the tape measure for 6 minutes.
Quality of life (QOL) and physical functioning were assessed with the European Organization for Research and Training, Quality of Life Questionnaire - Core 30, Version 3.0 (EORTC QLQ-C30). The EORTC QLQ-C30 is a well-established instrument in cancer clinical trials, and the psychometric properties have been previously reported.[29, 30] This 30-item instrument consists of five functional scales (including physical functioning), a global QOL/health status scale, three multi-item symptom scales, and a number of single-item questions. Items on the multi-item subscales are averaged and then converted to a scale with a range of 0 to 100. Higher scores on the five functional scales and the global QOL/health status scale represent a higher level of functioning. Higher scores on the symptom scales and the single-item questions indicate a higher degree of symptomatology, and thus a poorer QOL.
Fatigue was assessed with the FACIT Fatigue Scale, a validated 13 item scale designed to assess fatigue in terms of its intensity and interference with performing everyday functions.[31, 32] Exercise readiness was assessed with the Physical Activity Self-Efficacy Questionnaire developed by Marcus et al[33], a 5-item scale that rates participants’ confidence regarding their ability to be physically active in various situations.
Statistical Analysis
The study’s primary endpoint was change in minutes of self-reported physical activity, as measured by the 7-Day PAR. With a sample size of 120 patients, we had more than 80% power to detect a difference of 75 minutes of activity per week (change in minutes per week of 165 versus 90) between the arms using a 2-sided 0.05 level Wilcoxon rank-sum test. This was based on the following assumptions: both groups would engage in 60 minutes of moderate-vigorous activity per week at baseline, the control group would increase activity to 90 minutes/week over the study period given a potential increase in activity after the completion of adjuvant therapy, a standard deviation (SD) of 120 minutes per week[34] and a drop out rate of 20%.[35, 36]
Analyses for the changes in minutes of weekly activity, fitness, anthropometric measurements and QOL outcomes included participants for whom both baseline and week 16 measurements were available. Change scores were not imputed for patients who had data missing at either time point and these patients were excluded from the analysis (n=22). The arms were compared using a Wilcoxon rank-sum test or two-sample t-tests, after inspection of histograms to assess distributional assumptions, accounting for unequal variances with Satterthwaite’s method. Pearson correlation coefficients were used to describe the relationship between change in weekly activity and measures of physical function, pain, fatigue, and QOL.
Descriptive statistics were used to summarize minutes of weekly activity and number of daily steps recorded in weekly exercise journals by women randomized to the exercise intervention. For each participant with at least 8 weeks of recorded data, an average number of minutes of weekly physical activity and an average number of steps were calculated. These values were then averaged across all evaluable participants, resulting in an average number of minutes of exercise and an average number of steps performed per week.
Analyses for the changes in minutes of weekly activity, fitness, anthropometric measurements and QOL outcomes were repeated with data from the breast cancer cohort only. As these data were similar to the data from the combined cohort, all analyses reported included all evaluable study participants.
RESULTS
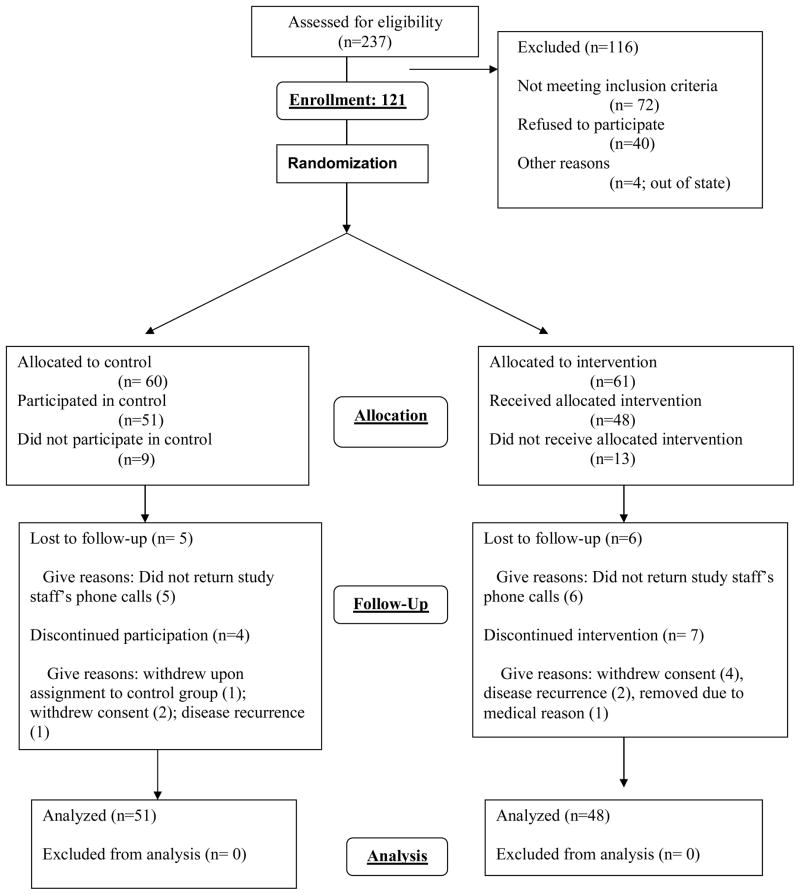
One hundred and twenty-one participants enrolled in the protocol, 100 patients with breast cancer and 21 patients with colorectal cancer (see Consort Diagram in Figure 1). Baseline data are available for 121 participants. Baseline characteristics were distributed similarly in the exercise and control groups (Table 1). The majority of the participants were women, had breast cancer and were treated with chemotherapy, surgery, radiation and hormonal therapy. Mean age was 54 and mean BMI 30.9 kg/m2. Twenty-two patients withdrew consent and/or did not complete the study (Figure 1). There were no significant differences in demographic, disease or treatment variables between patients who completed the protocol and those who dropped out (data not shown).
Figure 1.
Consort Diagram
Table 1.
Baseline & Treatment Characteristics
| Exercise (N=61) | Control (N=60) | |
|---|---|---|
| AGE (± SD) | 53.1 (10.8) | 55.5 (10.6) |
| BMI (kg/m2) | 31.2 (6.2) | 30.6 (5.3) |
| CANCER TYPE | ||
| Breast | 50 (82%) | 50 (83%) |
| Colon | 9 (15%) | 8 (13%) |
| Rectal | 2 (3%) | 2 (3%) |
| SEX | ||
| Female | 56 (92%) | 56 (93%) |
| Male | 5 (8%) | 4 (7%) |
| RACE | ||
| White | 56 (92%) | 55 (92%) |
| Black | 4 (7%) | 5 (8%) |
| Asian | 1 (2%) | 0 (0%) |
| HIGHEST LEVEL OF EDUCATION | ||
| Some/no high school | 1 (2%) | 3 (5%) |
| High school graduate | 11 (18%) | 6 (10%) |
| Technology school/some college | 16 (26%) | 20 (33%) |
| College graduate/advanced degree | 33 (54%) | 31 (52%) |
| EMPLOYMENT STATUS | ||
| Working full time | 22 (36%) | 25 (42%) |
| Working part time | 11 (18%) | 11 (18%) |
| Homemaker | 6 (10%) | 4 (7%) |
| Retired | 7 (11%) | 13 (22%) |
| Disabled | 3 (5%) | 3 (5%) |
| Unemployed | 4 (7%) | 2 (3%) |
| Other | 8 (13%) | 2 (3%) |
| TUMOR STAGE | ||
| Stage I | 20 (33%) | 21 (35%) |
| Stage II | 19 (31%) | 23 (38%) |
| Stage III | 22 (16%) | 16 (27%) |
| SURGERY FOR PRIMARY TUMOR | ||
| Breast (n=100) | ||
| Mastectomy | 25 (50%) | 26 (52%) |
| Lumpectomy | 25 (50%) | 24 (48%) |
| Colon (n=21) | ||
| Partial Colectomy | 4 (36%) | 7 (70%) |
| Low Anterior Resection | 5 (45%) | 0 (0%) |
| Colostomy | 2 (18%) | 2 (20%) |
| CHEMOTHERAPY | 47 (77%) | 43 (72%) |
| RADIATION | 42 (69%) | 33 (55%) |
| HORMONAL THERAPY (Breast Cancer) | 31 (62%) | 36 (72%) |
Exercise Intervention
Sixty-one participants were randomized to the exercise intervention. Although 13 participants ultimately did not complete the intervention, at least partial exercise data was available for all participants. Participants attended a median of 9 calls (range 0–11). For patients who completed the 16 week intervention, the range of calls delivered was 7–11. Forty-one of the 61 participants randomized to the exercise intervention completed at least 8 weekly exercise journals during the 16-week intervention period. Compliance with pedometer use was good, with 30 of the 61 participants randomized to the intervention group reporting daily steps for greater than 90% of days during the 16 week intervention periods, and an additional 9 patients reporting data for more than 50% of days. Participants reported a mean of 153.6 (standard deviation [SD] 74.6) minutes of moderate or strenuous exercise per week and a mean of 7392 (SD 1619) steps per day.
Physical Activity, Physical Functioning, and Fitness
Physical activity behaviors were assessed in all study participants with the 7-Day Physical Activity Recall Interview, physical functioning was assessed with the EORTC QLQ C30, and fitness was assessed with the 6-Minute Walk Test. Baseline and week-16 physical activity and physical functioning data were available for 99 patients; fitness data at both time points were available for 97 patients. At baseline, both groups were relatively inactive (Figure 2); control participants reported a median of 65.7 minutes of moderate or strenuous exercise per week on the 7-Day PAR and intervention participants 44.9 minutes (p=0.12). Over the 16 week study period, the intervention group increased activity by 121% or 54.5 (± 142.0) minutes versus 22% or 14.6 (± 117.0) minutes in control patients (P=0.13). MET-hours/week also increased by a non-significant amount in intervention participants versus controls (3.0 ± 8.2 vs. 1.0 ± 7.6, p=0.23).
Participants randomized to the intervention group significantly increased fitness and physical functioning over the course of the 16-week study period compared to controls (Table 2). Intervention participants increased the distance they walked over 6 minutes by 186.9 (±215.1) feet versus 81.9 (±135.2) feet in control participants (p=0.006). Intervention participants also experienced a significant improvement in self-reported physical functioning as compared to controls (change of 7.1 ± 11.4 points versus 2.6 ± 10.2 points on the EORTC QLQ C30 physical functioning subscale, p=0.04) (Table 2).
Table 2.
Physical Activity Behaviors, Fitness and Physical Functioning at Baseline and Change over 16 Weeksa
| Baseline | Change over 16 weeks | |||||
|---|---|---|---|---|---|---|
| Exercise (n=48) | Control (n=51) | P | Exercise (n=48) | Control (n=51) | P | |
| Physical Activity (min/week)b | 44.9 ± 58.5 | 65.7 ± 84.1 | 0.12 | 54.5 ± 142.0 | 14.6 ± 117.2 | 0.13 |
| MET-hours/weekb | 2.7 ± 3.6 | 4.0 ± 5.0 | 0.10 | 3.0 ± 8.2 | 1.0 ± 7.6 | 0.23 |
| 6-Minute Walk Test (feet) | 1431.9 ± 309.1 | 1495.2 ± 246.3 | 0.22 | 186.9 ± 215.1 | 81.9 ± 135.2 | 0.006 |
| Physical Functioning (EORTC QLQ C-30) | 82.8 ± 17.8 | 85.8 ± 11.9 | 0.29 | 7.1 ± 11.4 | 2.6 ± 10.2 | 0.04 |
All data are presented as means ± SD
As measured by the 7-Day Physical Activity Recall
Quality of Life and Fatigue
Participants completed quality of life, fatigue and exercise self efficacy questionnaires at baseline and 16 weeks (Table 3). At baseline, participants in both groups reported good overall quality of life, and moderate levels of fatigue and exercise self-efficacy. Participants in the intervention group reported trends toward improvement in QOL (4.3 ± 16.0 vs. −1.5 ± 18.8, p=0.10) and exercise self-efficacy (0.1 ± 1.0 vs. −0.3 ± 1.0, p=0.06) as compared with controls. There were no significant differences in change scores for fatigue or other QOL subscales between groups.
Table 3.
Baseline and Change Data for Quality of Life, Fatigue, and Related Outcomesa
| Baseline | Change over 16 weeks | |||||
|---|---|---|---|---|---|---|
| Exercise (n=48) | Control (n=51) | P | Exercise (n=48) | Control (n=51) | P | |
| EORTC QLQ C-30 | ||||||
| Global QOL | 67.1 ± 20.2 | 71.8 ± 18.3 | 0.18 | 4.3 ± 16.0 | −1.5 ± 18.8 | 0.10 |
| Pain | 19.7 ± 24.6 | 21.9 ± 24.1 | 0.61 | −4.9 ± 17.5 | −2.6 ± 27.4 | 0.63 |
| Insomnia | 32.8 ± 29.5 | 35.0 ± 29.7 | 0.68 | −2.1 ± 30.3 | −8.5 ± 29.7 | 0.29 |
| FACIT Fatigue Scale | 36.9 ± 10.9 | 38.6 ± 8.5 | 0.34 | 4.4 ± 8.4 | 2.5 ± 6.8 | 0.23 |
| Exercise Self-Efficacy Scale | 2.8 ± 1.0 | 2.9 ± 1.0 | 0.32 | 0.1 ± 1.2 | −0.3 ± 0.8 | 0.06 |
Data are presented as means (SD)
Physical Measurements
Baseline and week 16 anthropometric data were available for 99 participants (Table 4). At baseline, participants on average weighed about 83 kg and had a BMI slightly less than 31 kg/m2. There were no significant changes in anthropometric measures over the course of the study in either group.
Table 4.
Physical Measurements at Baseline and Change over 16 Weeksa
| Baseline | Change over 16 weeks | |||||
|---|---|---|---|---|---|---|
| Exercise (n=48) | Control (n=51) | P | Exercise (n=48) | Control (n=51) | P | |
| Weight (kg) | 83.5 ± 18.1 | 82.8 ± 16.0 | 0.82 | −0.3 ± 2.9 | −0.4 ± 3.1 | 0.85 |
| Waist circumference (cm) | 96.7 ±20.0 | 94.0 ± 16.1 | 0.41 | 1.4 ± 13.2 | 2.3±9.4 | 0.70 |
| Hip circumference (cm) | 110.1 ± 19.8 | 112.9 ± 18.5 | 0.41 | 2.4 ± 14.6 | 0.8 ± 11.3 | 0.53 |
Data are presented as means (SD)
DISCUSSION
Our study tested the ability of a telephone-based physical activity intervention to increase weekly physical activity and improve physical functioning and fitness in 121 sedentary breast and colorectal survivors recruited from 10 CALGB institutions. The intervention led to statistically significant and clinically meaningful improvements in fitness and functional status. At baseline, both groups walked approximately 1450 feet over the course of 6 minutes, somewhat lower than the average of 1820 feet for women and 1919 feet for men reported in trials of healthy adults.[37] Intervention participants increased their distance on the 6-Minute Walk Test by 186.9 feet (compared to 81.9 feet in controls, p=0.006), a change that has been correlated with significant improvements in functional status in other studies.[38, 39] Self-reported physical functioning also improved by 7.1 points in the intervention group (vs. 2.6 in controls, p=0.04), consistent with a clinically meaningful improvement in functional status.[40, 41] Finally, physical activity increased by 54 minutes/week in the intervention group compared to 14 minutes/week in the control group (p=0.13).
The increase in weekly minutes of physical activity seen in our study is generally consistent with other multicenter, distance-based lifestyle interventions. In RENEW[42], older (age ≥ 65) survivors of breast, prostate and colorectal cancer randomized to a telephone-based diet and exercise intervention increased exercise by an average of 31 minutes/week more than survivors randomized to an education control group (p<0.001). In FRESH START[34], patients with breast or prostate cancer randomized to a mail-based diet and exercise intervention increased weekly physical activity by 59.3 minutes, vs. 39.2 minutes in the education control group (p=0.02). Finally, in ACTION[43] breast cancer survivors provided with pedometers, with or without tailored print materials about exercise, significantly increased self-reported physical activity vs. controls (increase of 30 minutes/week controls, 89 minutes/week pedometers, and 87 minutes pedometer + printed materials, p=0.017 and p=0.022, respectively). However, there were no increases in daily steps in any of the 4 groups.
Despite the modest increase in weekly physical activity seen in our study, intervention participants experienced significant improvements in fitness and physical functioning. Emerging data suggest that physical functioning and physical health may be related to cancer outcomes in patients with early stage disease. A meta-analysis of 30 trials looking at survival and health-related quality of life showed that physical functioning was significantly related to survival in analyses adjusted for disease stage (HR 0.94, 95% CI 0.92–0.96, p<0.001).[13] Gupta et al also demonstrated that women with newly diagnosed breast cancer who had higher physical functioning scores had a mean survival of 35.5 months vs. 17.8 month in patients with lower scores (p=0.0006).[12] These findings could explain, at least in part, the improved survival seen in patients who engage in even modest levels of physical activity after cancer diagnosis. As seen in our study and others[42], even small increases in physical activity can lead to significant improvements in physical functioning and fitness.
Our study also demonstrated the feasibility of conducting lifestyle research in a cooperative group setting. Enrollment of 121 patients was completed over 2 years, and our attrition rate of 18% is similar to other exercise intervention studies targeting inactive cancer survivors, including those involving in-person exercise interventions.[35, 36] Participants received a median of 9 out of a planned 10–11 calls during the intervention period. The data completion rate was >98% for the 99 patients who finished the study, and sites were uniformly successful in collecting study measures, including the 6-Minute Walk test, a novel measure for the majority of the participating sites. This type of distance-based lifestyle intervention could be utilized in a large-scale cooperative group study to test the impact of behavior change upon breast cancer outcomes.
A number of weaknesses of our study should be acknowledged. First, the trial was powered to detect a 75 minute difference in the increase in minutes of weekly activity between the exercise and control groups. Given that the between-group difference was only 40 minutes and that the standard deviations were large, we did not demonstrate that our intervention significantly increased physical activity. Although the improvements in fitness and functional measures suggest that the exercise group did increase activity, a larger sample would have been required to determine the statistical significance of a 40 minute difference in minutes of exercise between the groups. Additionally, our study was initially intended to enroll equal proportions of breast and colorectal survivors, with a plan to conduct separate analyses of our end points in both groups. Given the slower than anticipated enrollment in the colorectal cancer group, the majority of our participants were breast cancer survivors. We were thus not able to conduct a separate analysis in the colorectal cancer subgroup, and it is not clear how applicable the results of this study are for colorectal cancer survivors.
In conclusion, this trial demonstrates the ability of a telephone-based exercise intervention to improve fitness and physical functioning in breast cancer survivors, as well as the feasibility of conducting a lifestyle intervention in a cooperative group setting. Sites without experience in conducting lifestyle research were able to recruit patients and collect study measures, including an objective fitness measure. The lifestyle intervention led to a non-significant increase in weekly minutes of physical activity, but participants significantly improved functional measures linked to survival in observational studies. Further work is needed to determine the most effective lifestyle interventions, and to test the impact of lifestyle change upon outcomes in cancer survivors.
Acknowledgments
This work was supported by a Cancer and Leukemia Group B Pilot Prevention Grant and by the Gloria Spivak Faculty Support Fund at the Dana-Farber Cancer Institute.
Footnotes
Disclosures: The authors report no conflicts of interest
References
- 1.Holmes M, Chen W, Feskanich D, Kroenke C, Colditz G. Physical activity and survival after breast cancer diagnosis. Journal of the American Medical Association. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 2.Holick C, Newcomb P, Trentham-Dietz A, Titus-Ernstoff L, Bersch A, Stampfer M, Baron J, Egan K, Willett W. Physical Activity and Survival after Diagnosis of Invasive Breast Cancer. Cancer Epidemiology, Biomarkers & Prevention. 2008;17:379–386. doi: 10.1158/1055-9965.EPI-07-0771. [DOI] [PubMed] [Google Scholar]
- 3.Pierce J, Stefanick M, Flatt S, Natarajan L, Sternfeld B, Madlensky L, Al-Delaimy W, Thomson C, Kealey S, Hajek R, Parker B, Newman V, Caan B, Rock C. Greater Survival After Breast Cancer in Physically Active Women With High Vegetable-Fruit Intake Regardless of Obesity. Journal of Clinical Oncology. 2007;17:2345–2351. doi: 10.1200/JCO.2006.08.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irwin M, Smith A, McTiernan A, Ballard-Barbash R, Cronin K, Gilliland F, Baumgartner R, Baumgartner K, Bernstein L. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. Journal of Clinical Oncology. 2008;26:3958–3964. doi: 10.1200/JCO.2007.15.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Lu W, Zheng W, Gu K, Matthews CE, Chen Z, Zheng Y, Shu XO. Exercise after diagnosis of breast cancer in association with survival. Cancer Prev Res (Phila) 4:1409–1418. doi: 10.1158/1940-6207.CAPR-10-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyerhardt J, Giovannucci E, Holmes M, Chan A, Chan J, Colditz G, Fuchs C. Physical activity and survival after colorectal cancer diagnosis. Journal of Clinical Oncology. 2006;24:3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 7.Meyerhardt J, Heseltine D, Niedzweicki D, Hollis D, Saltz L, Mayer R, Thomas J, Nelson H, Whittom R, Hantel A, Schilski R, Fuchs C. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: Findings from CALGB 89803. Journal of Clinical Oncology. 2006;22:3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 8.Meyerhardt JA, Giovannucci EL, Ogino S, Kirkner GJ, Chan AT, Willett W, Fuchs CS. Physical activity and male colorectal cancer survival. Arch Intern Med. 2009;169:2102–2108. doi: 10.1001/archinternmed.2009.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faul LA, Jim HS, Minton S, Fishman M, Tanvetyanon T, Jacobsen PB. Relationship of Exercise to Quality of Life in Cancer Patients Beginning Chemotherapy. J Pain Symptom Manage. doi: 10.1016/j.jpainsymman.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee CK, Hudson M, Stockler M, Coates AS, Ackland S, Gebski V, Lord S, Friedlander M, Boyle F, Simes RJ. A nomogram to predict survival time in women starting first-line chemotherapy for advanced breast cancer. Breast Cancer Res Treat. doi: 10.1007/s10549-011-1471-9. [DOI] [PubMed] [Google Scholar]
- 11.Sperduto PW, Chao ST, Sneed PK, Luo X, Suh J, Roberge D, Bhatt A, Jensen AW, Brown PD, Shih H, Kirkpatrick J, Schwer A, Gaspar LE, Fiveash JB, Chiang V, Knisely J, Sperduto CM, Mehta M. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 77:655–661. doi: 10.1016/j.ijrobp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Gupta D, Granick J, Grutsch JF, Lis CG. The prognostic association of health-related quality of life scores with survival in breast cancer. Support Care Cancer. 2007;15:387–393. doi: 10.1007/s00520-006-0165-z. [DOI] [PubMed] [Google Scholar]
- 13.Quinten C, Coens C, Mauer M, Comte S, Sprangers MA, Cleeland C, Osoba D, Bjordal K, Bottomley A. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol. 2009;10:865–871. doi: 10.1016/S1470-2045(09)70200-1. [DOI] [PubMed] [Google Scholar]
- 14.Patterson RE, Saquib N, Natarajan L, Rock CL, Parker BA, Thomson CA, Pierce JP. Improvement in self-reported physical health predicts longer survival among women with a history of breast cancer. Breast Cancer Res Treat. doi: 10.1007/s10549-010-1236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saquib N, Pierce JP, Saquib J, Flatt SW, Natarajan L, Bardwell WA, Patterson RE, Stefanick ML, Thomson CA, Rock CL, Jones LA, Gold EB, Karanja N, Parker BA. Poor physical health predicts time to additional breast cancer events and mortality in breast cancer survivors. Psychooncology. 20:252–259. doi: 10.1002/pon.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galvao D, Newton R. Review of exercise interventions studies in cancer patients. Journal of Clinical Oncology. 2005;23:899–909. doi: 10.1200/JCO.2005.06.085. [DOI] [PubMed] [Google Scholar]
- 17.Markes M, Brockow T, Resch K. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database of Systematic Reviews. 2006:Art. No.: CD005001. doi: 10.1002/14651858.CD005001.pub2. 005010.001002/14651858.CD14005001.pub14651852. [DOI] [PubMed] [Google Scholar]
- 18.Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol. 2003;71:843–861. doi: 10.1037/0022-006X.71.5.843. [DOI] [PubMed] [Google Scholar]
- 19.Zhu S, Pierce J. A new scheduling method for time-limited counseling. Professional Psychology: Research and Practice. 1995;26:624–625. [Google Scholar]
- 20.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, Paffenbarger RS., Jr Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Blair S, Haskell W, Ho P, Paffenbarger R, Vranizan K, Farquhar J, Wood P. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. American Journal of Epidemiology. 1985;122:794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- 22.Nadar P, Baranowski T, Vanderpool N, Dunn K, Dworkin R, Ray L. The Family Health Project: cardiovascular risk reduction education for children and parents. Developmental Behavior in Pediatrics. 1983;4:3–10. [PubMed] [Google Scholar]
- 23.Calfas K, Long B, Sallis J, Wooten W, Pratt M, Patrick K. A controlled trial of physician conseling to promote the adoption of physical activity. Preventive Medicine. 1996;25:225–233. doi: 10.1006/pmed.1996.0050. [DOI] [PubMed] [Google Scholar]
- 24.Dishman R, Steinhardt M. Reliability and concurrent validity for a 7-day recall of physical activity in college students. Medicine & Science in Sports & Exercise. 1988;20:14–25. doi: 10.1249/00005768-198802000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs D, Ainsworth B, Hartman T, Leon A. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Medicine & Science in Sports & Exercise. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Taylor C, Coffey T, Berra K, Iaffaldano R, Casey K, Haskell W. Seven-day activity and self-report compared to a direct measure of physical activity. American Journal of Epidemiology. 1984;120:818–824. doi: 10.1093/oxfordjournals.aje.a113954. [DOI] [PubMed] [Google Scholar]
- 27.Cooper K. A means of assessing maximal oxygen intake. Correlation between field and treadmill testing. Journal of the Amercian Medical Association. 1968;203:201–204. [PubMed] [Google Scholar]
- 28.Butland R, Pang J, Gross E, Woodcock A, Geddes D. Two-, six-, and 12-minute walking tests in respiratory disease. British Journal of Medicine (Clinical Research Edition) 1982;284:1607–1608. doi: 10.1136/bmj.284.6329.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aaronson N, Ahmedzia S, Bergman B, Bullinger M, Cull A, Duez N, Filiberti A, Flechtner H, Fleishman S, de Haes J. The European Organization for Research and Treatment for Cancer QLQ C-30: A quality of life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 30.Osoba D, Zee B, Peter J, Warr D, Kaizer L, Latrelle J. Psychometric properties and responsiveness of the EORTC Quality of Life Questionnaire (QLQ C-30) in patients with breast, ovarian and lung cancer. Quality of Life Research. 1994;3:353–364. doi: 10.1007/BF00451727. [DOI] [PubMed] [Google Scholar]
- 31.Fallowfield L, Cella D, Cuzick J, Francis S. Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) adjuvant breast cancer trial. Journal of Clinical Oncology. 2004;22:4261–4271. doi: 10.1200/JCO.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 32.Cella D, Lai J, Chang C, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94:528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- 33.Marcus BH, Selby VC, Niaura RS, Rossi JS. Self-efficacy and the stages of exercise behavior change. Res Q Exerc Sport. 1992;63:60–66. doi: 10.1080/02701367.1992.10607557. [DOI] [PubMed] [Google Scholar]
- 34.Demark-Wahnefried W, Clipp E, Lipkus I, Lobach D, Snyder D, Sloane R, Peterson B, Macri J, Rock C, McBride C, Kraus W. Main outcomes of the FRESH START Trial: A sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. Journal of Clinical Oncology. 2007;25:2709–2718. doi: 10.1200/JCO.2007.10.7094. [DOI] [PubMed] [Google Scholar]
- 35.Segal R, Evans W, Johnson D, Smith J, Colleta S, Gayton J. Structured exercise improves physical functioning in women with stages I and II breast cancer: Results of a randomized controlled trial. Journal of Clinical Oncology. 2001;19:657–665. doi: 10.1200/JCO.2001.19.3.657. [DOI] [PubMed] [Google Scholar]
- 36.Ligibel J, Campbell N, Partridge A, Chen W, Saldinari T, Chen H, Adloff K, Keshaviah A, Winer E. Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. Journal of Clinical Oncology. 2008;26:907–912. doi: 10.1200/JCO.2007.12.7357. [DOI] [PubMed] [Google Scholar]
- 37.Casanova C, Celli BR, Barria P, Casas A, Cote C, de Torres JP, Jardim J, Lopez MV, Marin JM, Montes de Oca M, Pinto-Plata V. Aguirre-Jaime A The 6-min walk distance in healthy subjects: reference standards from seven countries. Eur Respir J. 37:150–156. doi: 10.1183/09031936.00194909. [DOI] [PubMed] [Google Scholar]
- 38.Puhan MA, Mador MJ, Held U, Goldstein R, Guyatt GH, Schunemann HJ. Interpretation of treatment changes in 6-minute walk distance in patients with COPD. Eur Respir J. 2008;32:637–643. doi: 10.1183/09031936.00140507. [DOI] [PubMed] [Google Scholar]
- 39.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 40.King MT. The interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Qual Life Res. 1996;5:555–567. doi: 10.1007/BF00439229. [DOI] [PubMed] [Google Scholar]
- 41.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 42.Morey MC, Snyder DC, Sloane R, Cohen HJ, Peterson B, Hartman TJ, Miller P, Mitchell DC, Demark-Wahnefried W. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. Jama. 2009;301:1883–1891. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vallance J, Courneya K, Plotnikoff R, Yasui Y, Mackey J. Randomized Controlled Trial of the Effects of Print Materials and Step Pedometers on Physical Activity and Quality of Life in Breast Cancer Survivors. Journal of Clinical Oncology. 2007;25:2352–2359. doi: 10.1200/JCO.2006.07.9988. [DOI] [PubMed] [Google Scholar]