Abstract
Adenosine is an endogenously released purine nucleoside that signals via four widely expressed G-protein coupled receptors: A1, A2A, A2B, and A3. In the setting of inflammation, the generation and release of adenosine is greatly enhanced. Neutrophils play an important role in host defense against invading pathogens and are the cellular hallmark of acute inflammation. Neutrophils both release adenosine and can respond to it via expression of all four adenosine receptor subtypes. At low concentrations, adenosine can act via the A1 and A3 adenosine receptor subtypes to promote neutrophil chemotaxis and phagocytosis. At higher concentrations, adenosine acts at the lower-affinity A2A and A2B receptors to inhibit neutrophil trafficking and effector functions such as oxidative burst, inflammatory mediator production, and granule release. Modulation of neutrophil function by adenosine is relevant in a broad array of disease models, including ischemia reperfusion injury, sepsis, and non-infectious acute lung injury. This review will summarize relevant research in order to provide a framework for understanding how adenosine directly regulates various elements of neutrophil function.
Keywords: adenosine, neutrophil, chemotaxis, adhesion, host defense
Introduction
Neutrophils are professional phagocytes that play a critical role in host defense against infection and are important to pathogenesis of many inflammatory diseases. Neutrophils are the most abundant leukocyte in human blood, and are among the first cells recruited in response to invading pathogens. They are short-lived cells that do not replicate once differentiated in the bone marrow, and ultimately undergo one of several cell death programs, which can contribute to their anti-microbial functionality in tissue targets. Neutrophil antimicrobial effector mechanisms include phagocytosis and intracellular killing, release of extracellular enzymes and anti-microbial granule contents and neutrophil extracellular trap (NET) formation. Two critical mechanisms to neutrophil effector functions are generation of oxidative burst and resultant elaboration of reactive oxygen species, and the generation of an extracellular chromatin fibrillary matrix, known as NETs, which serve to co-localize microbes and antimicrobial molecules. In addition to their well-recognized role as effector cells in antimicrobial immunity and inflammatory tissue damage, neutrophils are increasingly recognized for a multi-faceted array of immunoregulatory functions, including release of soluble mediators and cross-talk with other leukocytes.
Adenosine is an endogenous purine nucleoside with a very short tissue half-life and potent signaling functions. Under baseline conditions, adenosine is released constitutively from multiple cell types, with extracellular concentrations in the nanomolar range.1-4 In settings of inflammation and tissue injury, adenosine release and generation is greatly augmented and tissue levels can rise 100-fold.5 Adenosine is a breakdown product of ATP, a process mediated intracellularly by the soluble intracellular 5’-nucleotidase CD73. CD73 also exists as an ectoenzyme, and functions with the apyrase CD39 to produce adenosine from ATP in the extracellular space. Once generated, adenosine is rapidly degraded by adenosine deaminase into inosine, or phosphorylated by adenosine kinase into AMP, resulting in a biological half-life of under 10 seconds.6 Adenosine signals via 4 G protein-coupled receptors: A1, A2A, A2B, and A3, encoded by Adora1, Adora2a, Adora2b, and Adora3, respectively. The A1 receptor is Gi/o-coupled and inhibits formation of cyclic AMP (cAMP), while the A2A and A2B receptors are Gs-coupled and promote formation of cAMP. The A3 receptor is both Gi/o and Gq/11-coupled, inhibiting cAMP production and enhancing inositol P3 production. The A1 and A3 receptors have high-affinity for adenosine, with EC50 values between 0.2-0.5 μM, while the A2A receptor has a somewhat lower affinity (EC50 0.6-0.9 μM). The A2B receptor exhibits much lower affinity for adenosine than all other receptor subtypes, with an EC50 between 16 and 64 μM.7 The effects of adenosine in a given tissue are therefore complex, being determined by the interplay of the dynamics of production and clearance of adenosine and the receptor repertoire of the cells present.
Neutrophils both produce adenosine and can respond to it via all four adenosine receptors.7-12 Neutrophils can be a major source of tissue adenosine, and adenosine can stimulate or inhibit neutrophil function depending on its concentration in the microenvironment and the receptor profile of the neutrophil.2,5,7
Neutrophils as a source of adenosine
Activated neutrophils secrete adenosine and its precursors, which are converted to adenosine and can subsequently act in an autocrine fashion to regulate neutrophil function. Early work demonstrated that adenosine is continuously generated in unstimulated neutrophils independent of ectoenzymes and then secreted,13 and that this intracellular production was mediated by a cytosolic 5’ nucleotidase.14 Endogenous adenosine production by neutrophils is also inducible after stimulation by a variety of stimulants, including phorbol 12-myristate 13-acetate (PMA), fMLP, calcium ionophore and complement C5a.15-20
In addition to adenosine, neutrophils release ATP after stimulation, thereby providing a substrate for extracellular generation of adenosine. ATP is released from activated neutrophils via connexin 43 hemichannels and is rapidly converted to 5’ AMP and adenosine by the ectoenzymes CD39 and CD73, respectively, expressed on the neutrophil surface.21,22 ATP can act independently of adenosine to regulate neutrophil function; recent reviews have characterized these effects in detail.2,7,20,23 Neutrophil-derived 5’ AMP promotes chloride secretion from epithelial cells and enhances endothelial barrier function; however, both functions are mediated after extracellular conversion to adenosine by CD73.24-26
Several studies have provided insight into how adenosine levels are maintained in the extracellular space during inflammation. Stimulation of neutrophils enhances extracellular adenosine accumulation in part by inactivation of extracellular adenosine deaminase, an enzyme that converts adenosine into inosine.20 Adenosine is cleared from the extracellular space in part by passive uptake by equilibrative nucleoside transporters. Their expression is inhibited during hypoxic conditions, thus promoting extracellular adenosine accumulation.27,28
Modulation of neutrophil movement by adenosine
Recruitment
Adenosine produced by neutrophils can act in an autocrine and paracrine fashion to promote or inhibit neutrophil chemotaxis. The early literature was dominated by inhibitory effects of adenosine on neutrophils.17,29-31 One of the first reports of adenosine promoting neutrophil function attributed enhanced neutrophil chemotaxis toward complement C5 fragments and fMLP to the A2 (later A2A) receptor;32 but a follow-up study determined that adenosine promotes chemotaxis by acting at the A1 receptor by using N6-cyclopentyladenosine (CPA), a selective A1 receptor agonist.9 The A1 receptor was also noted to have a higher affinity for adenosine than the A2 receptor, and therefore, at early stages of inflammation, lower local concentrations of adenosine promoted neutrophil recruitment, whereas later high concentrations of adenosine limit neutrophil recruitment by action at A2 receptors.9
The role of adenosine A3 receptors in neutrophil chemotaxis was addressed more recently.33 Human neutrophils subjected to a chemotactic gradient of fMLP were shown to release ATP from their leading edge. The extracellular ATP was then converted to adenosine by CD73 on the neutrophil cell surface, generating an adenosine gradient that subsequently acts on neutrophil A3 receptors, also concentrated at the leading edge, to augment chemotaxis.33 The relevance of A3-directed neutrophil chemotaxis was demonstrated in a mouse model of intra-abdominal infection in A3 receptor knockout mice (Adora3-/-). Using the cecal ligation and puncture model of sepsis, Adora3-/- mice exhibited decreased recruitment of neutrophils to the lung and peritoneum and increased numbers of circulating neutrophils compared to wild-type treated mice.34
In addition to CD73, the ecto-nucleoside triphosphate diphosphohydrolase 1 (E-NTPDase 1, CD39) is capable of converting extracellular ATP to adenosine, and has also been found to associate with the leading edge of neutrophils migrating along a chemotactic gradient. Neutrophils from CD39-deficient mice or neutrophils in which CD39 was inhibited pharmacologically exhibited impaired chemotaxis but not polarization.35 It has therefore been postulated that release of ATP and conversion to adenosine by migrating neutrophils, along with polarized expression of A3 receptors, CD73 and CD39 at the leading edge, represents an autocrine positive feedback mechanism that amplifies chemotactic signals and promotes forward movement of neutrophils.7,36 In contrast, due to its uniform distribution across the surface of neutrophils, the A2A receptor may act as a “global inhibition” signal that promotes chemotaxis by inhibiting backward movement.7,37 Neutrophil-derived adenosine can also inhibit release of the potent neutrophil chemoattractant, CXCL8, from endothelial cells, potentially allowing extravasated neutrophils to migrate away from endothelial cells, further into the damaged tissue region.38
Recently, the neuronal guidance molecule netrin-1 has been implicated in the regulation of neutrophil chemotaxis in an adenosine A2B receptor-dependent fashion. Exogenous netrin-1 dampens infiltration of neutrophils in models of LPS-induced acute lung injury, hypoxia-induced lung inflammation, and acute experimental colitis, effects dependent on the expression of the A2B receptor. 39-41 In addition, netrin-1 engagement of the A2B receptor in vitro limits neutrophil transepithelial migration.41 However, it has been proposed that netrin-1 does not bind A2B directly, but instead activation of the A2B receptor closely regulates expression of the netrin-1 receptor UNC5B, producing the observed anti-inflammatory effects.42
Taken together, these data suggest that sub-micromolar concentrations of adenosine can augment neutrophil chemotaxis toward inflammatory stimuli by autocrine action at the high-affinity A1 and A3 adenosine receptors. In addition, neutrophil chemotaxis is inhibited by the interaction of netrin-1 and the A2B receptor.
Adhesion and transmigration
Neutrophil adhesion and transmigration across the endothelial cell barrier is a multi-step process. Neutrophils are captured from the circulation and loosely tethered to the vascular endothelium near sites of injury by selectins, which facilitate “rolling” of neutrophils along the endothelial surface, allowing local inflammatory stimuli to interact with and further activate the neutrophils. These stimuli, including ELR-containing CXC chemokine ligands such as CXCL8, activate integrins on the surface of neutrophils. Neutrophil integrins, such as β2 (CD11a/CD18 and CD11b/CD18) and very late antigen-4 (VLA-4) tightly bind cell adhesion molecules on the vascular endothelium (ICAMs and VCAMs). This is followed by diapedesis into the interstitium and further migration toward the site of injury. Adenosine acts on both neutrophils and endothelial cells to inhibit neutrophil adhesion and transmigration.43
Several studies have demonstrated that adenosine attenuates adhesion and neutrophil-induced damage to the endothelium. The adenosine analog 2-choloradenosine inhibits adherence of fMLP-stimulated neutrophils to endothelial monolayers and inhibits neutrophil-mediated endothelial cell damage.29 A similar effect was shown with the pan-adenosine receptor agonist N-ethylcarboxamidoadenosine (NECA).44 NECA and the A2A-receptor agonist CGS-21680 decreased adherence of PMA-stimulated neutrophils to porcine aortic endothelium45 and adenosine and CGS-21680 inhibited neutrophil adhesion and damage to endothelial cells in isolated canine coronary arteries.46 These effects were attributed to inhibition of selectin-based adhesion.47
Additional studies revealed that adenosine inhibits both the shedding of L-selectin and expression of β2 integrins (mainly CD11b/CD18) on the surface of neutrophils, thus limiting adhesion.12 These effects are potentiated by the addition of dipyridamole, a nucleoside uptake inhibitor that enhances extracellular concentrations of adenosine, and attenuated by addition of adenosine deaminase.12 Neutrophil adhesion to fibrinogen, a ligand for CD11b/CD18, can also be inhibited by NECA.48 NECA was also shown to attenuate CD11b/CD18 expression on fMLP-stimulated neutrophils in a dose-dependent manner.12,49 The inhibitory effects of adenosine on neutrophil adhesion appear to be at least partially mediated via the A2A receptor, since ATL146e, a selective A2A receptor agonist, inhibits human neutrophil CD49d expression, a component of the VLA-4 integrin complex, and adhesion to a VCAM-1 coated surface, whereas the A2A receptor antagonist, ZM-241385, blocked this inhibition.50 Similarly, pre-incubation of endothelial cells with the anti-inflammatory drug sulfasalazine resulted in a dose-dependent inhibition of fMLP-stimulated neutrophil adhesion that was lost with the addition of adenosine deaminase or an A2A receptor antagonist.51
Several in vivo models demonstrate that A2A-mediated inhibition of neutrophil adhesion can have anti-inflammatory effects. Infusion of ATL146e in a model of canine myocardial infarction reduced P-selectin expression and neutrophil infiltration, and was correlated with reduction in infarct size after reperfusion.52 Similarly, ATL146e infusion in a murine model of carotid ligation and repair resulted in reduced neutrophil recruitment, VCAM-1, ICAM-1, and P-selectin expression, an effect that correlated with sustained reduction in neointimal tissue formation and subsequent vessel constriction.53
The adenosine A3 receptor has also been implicated in the regulation of neutrophil adhesion. Activation of the A3 receptor with the selective agonist 2-Chloro-N6-(3-iodobenzyl)adenosine-5’-N-methyluronamide (Cl-IB-MECA) reduced platelet activating factor (PAF)-stimulated neutrophil adherence to coronary endothelium. This effect was reversed by MRS-1220, an A3-selective antagonist.54 Furthermore, in a model of reperfusion injury in isolated rabbit hearts Cl-IB-MECA attenuated the neutrophil-mediated reduction in cardiac contractile recovery. Thus agonism of A3 promoted recovery after reperfusion in part by inhibiting neutrophil adhesion.54
Adenosine also attenuates neutrophil accumulation though actions on the endothelium: adenosine inhibits release of the neutrophil chemoattractant CXCL8 from endothelial cells, and reduces expression of adhesion molecules endothelial-selectin (E-selectin) and VCAM-1 on the endothelial cell surface.38 Endogenous adenosine and 5’ AMP induce surface expression of CD73 on endothelial cells, generating additional adenosine in a positive feedback loop while promoting endothelial barrier function.55 This enhanced barrier function is due in part to adenosine A2B receptor activation on endothelial cells,26 which is upregulated on post-hypoxic endothelial cells along with CD39 and CD73. Furthermore, activated neutrophils can promote barrier function in these post-hypoxic cells, an effect inhibitable by A2B antagonism.56 Taken together, these studies suggest paracrine regulation of neutrophil accumulation by adenosine at the endothelial cell barrier; production of adenosine by activated neutrophils enhances adenosine production by endothelial cells, and acts on endothelial A2B receptors to enhance barrier function and limit neutrophil transmigration.
In contrast, the A1 receptor has been shown to enhance neutrophil adhesion to the endothelium. The A1 receptor-specific agonist CPA enhances neutrophil adhesion to gelatin-coated plates, via a CD11b/CD18-independent mechanism.48 A different A1 receptor agonist, 2-chloro-N6-cyclopentyladenosine (COPA), promoted PMA-stimulated adhesion of neutrophils to cultured porcine aortic endothelial cells.45
Thus, A1 receptors may promote adhesion via an integrin-independent mechanism, while A2A receptors attenuate adhesion via inhibition of integrins. Alternatively, it is possible that A1-mediated adhesion functions similarly to A1 and A3-mediated chemotaxis; sub-micromolar concentrations of adenosine promote neutrophil recruitment during early stages of inflammation. Once the inflammatory reaction is under way, elevated concentrations of adenosine can activate the lower-affinity A2A and A2B receptors, inhibiting neutrophil adhesion and transmigration, respectively.
Modulation of neutrophil effector mechanisms by adenosine
Inflammatory mediators
Activated neutrophils release numerous cytokines, chemokines, and arachadonic acid-derived lipid mediators, with diverse effects on the ongoing inflammatory reaction. Adenosine receptor activation inhibits pro-inflammatory mediator release from activated neutrophils, while promoting release of anti-inflammatory mediators. The adenosine A1 agonist COPA, and the combined A2A/A2B agonist 5’-(N-cyclopropyl)-carboxamido-adenosine (CPCA), both inhibit the release of TNF from LPS-stimulated human neutrophils, with A2 agonism 1000-times more effective than A1 agonism.57 Similarly, activation of the A2A receptor with CGS-21680 inhibited release of TNF and inflammatory chemokines from LPS-stimulated neutrophils.58
Induction of COX-2 activity converts arachadonic acid into prostaglandin E2 (PGE2) and thromboxane A2 (TXA2). PGE2 is a potent anti-inflammatory agent capable of inhibiting neutrophil chemotaxis, aggregation, and superoxide production, while TXA2 is a pro-inflammatory mediator that activates platelet aggregation and clotting. A series of studies reported that leukocyte COX-2 induction is attenuated in A2A receptor-deficient mice (Adora2a-/-). Furthermore, CGS-21680 activation of A2A receptors on fMLP-stimulated human neutrophils potentiates the induction of COX-2 and enhances PGE2 generation without affecting production of TXA2.59,60
Arachadonic acid can also be converted into leukotrienes via the 5-lipoxygenase (5-LO) pathway. Leukotriene B4 (LTB4) is a potent neutrophil chemoattractant and can also stimulate oxidative burst and degranulation. Early studies found that adenosine analogs inhibit fMLP-induced synthesis of LTB4 in whole blood.61 A subsequent study examined ligand-stimulated neutrophils in isolation and found that removal of endogenous adenosine or blockade of A2A receptors enhanced LTB4 synthesis.62 Similarly, activated neutrophils are unable to transform arachadonic acid into 5-LO products without removal of adenosine or addition of an A2A antagonist.63 Finally, endogenous adenosine inhibits the ability of neutrophils to produce LTA4, a precursor of LTB4, LTC4, and lipoxins, which modulate functional responses of phagocytes. 64,65
Taken together, these studies provide evidence that activation of A2A receptors on neutrophils can influence the broader inflammatory response by modulating production and release of pro- and anti-inflammatory mediators, such as chemokines, leukotrienes, and prostaglandins.
Phagocytosis
Neutrophil phagocytosis is facilitated by opsonization of microbes by complement, antibodies and other opsonins. As such, the neutrophil Fc receptors (FcR) and complement receptors are critical to their phagocytic activity. After fusion of the phagosome with lysosomes, pathogens in the resulting phagolysosome are killed by superoxide radical production as well as non-oxidative microbicidal granule components.
The regulation of neutrophil phagocytosis by adenosine is concentration- and receptor-dependent. A1 receptor agonism enhances FcRγ-mediated phagocytosis in human neutrophils at pico- to nanomolar concentrations of adenosine.66,67 In contrast, micromolar concentrations of adenosine or NECA inhibit FcR-mediated phagocytosis.67 Few subsequent studies have specifically examined effects of adenosine receptors on neutrophil phagocytosis, but it can be postulated that, similar to regulation of neutrophil chemotaxis, low concentrations of adenosine promote phagocytosis via A1 receptor binding, while elevated concentrations of adenosine inhibit phagocytosis via activation of A2A receptors.
Degranulation
Neutrophils contain primary, secondary, and tertiary granules that contain antimicrobial molecules and enzymes that degrade extracellular matrix components. Primary or azurophilic granules are defined by containing myeloperoxidase and CD63; they also contain a number of antimicrobial molecules including neutrophil elastase, acid hydrolase, defensins and bacterial permeability increasing (BPI) protein. Secondary (or specific) granules contain lactoferrin whereas tertiary (or gelatinase) granules no not; secondary and tertiary granules otherwise contain similar compounds including gelatinase, lysozyme, lipocalin, collagenase, as well as components of the NADPH oxidase complex. In general, engagement of adenosine receptors on neutrophil inhibits granule release, limiting neutrophil-mediated injury. Early work demonstrated that micromolar concentrations of adenosine inhibit degranulation of human neutrophils, as measured by lactoferrin secretion in response to fMLP68 and release of BPI, neutrophil elastase and defensins in response to LPS and TNFα.11 Subsequent studies showed that agonism of the A2A and A3 receptor, but not the A1 receptor, inhibit elastase release from neutrophils in response to fMLP69 that was associated with cAMP-dependent sequestration of cytosolic calcium.69, 70 Consistent with this, in vivo administration of the A2A agonist WRC-0470 inhibited degranulation of secondary neutrophil granules in a rat model of meningitis, as measured by extracellular lysozyme concentration.71 These data suggest that adenosine appears to inhibit neutrophil granule release, which is at least in part mediated via binding to the A2A receptor.
Oxidative burst
Neutrophil oxidative burst requires assembly of the NADPH oxidase subunits and culminates in transfer of electrons to molecular oxygen to produce superoxide, O2-, a reactive free radical that both spontaneously and enzymatically dismutes to generate hydrogen peroxide, the substrate for the generation of additional reactive oxygen intermediaries including hypochlorous acid, singlet oxygen, ozone, hypohalous acids, chloramine, and hydroxyl radical.72 These reactive oxygen species mediate oxidative damage against invading pathogens as well as host tissues and also have a subtler immunomodulatory role.
Regulation of oxidative burst is among the earliest and best characterized effects of adenosine on neutrophils, first described by Cronstein and colleagues who found that oxidative burst activity in fMLP-stimulated neutrophils is inhibited by micromolar concentrations of adenosine by nearly 50 percent,18 an effect that could be replicated by the adenosine analogue NECA and antagonized by theophylline.73 A similar effect was seen when adenosine was administered in vivo in a porcine model of endotoxemia.74 Subsequent studies have linked inhibition of oxidative burst to adenosine action at the neutrophil A2A receptor: the A2A agonists WRC-0470, CGS-21680, ATL193 and ATL146e inhibit neutrophil oxidative burst in response to a variety of neutrophil stimuli including fMLP, TNFα, PAF and IgG;70,75-78 after exposure to complement C3b-coated zymosan particles79 and during rat bacterial meningitis.71 The mechanism of adenosine-mediated inhibition of neutrophil oxidative burst was examined in fMLP-stimulated human neutrophils and was found to correlate with a reduction of flavocytochrome b (the heterodimer of gp91phox and p22phox) content in neutrophil plasma membranes and primary granules.80 Activation of A2A receptors increases cyclic AMP and intracellular calcium, but inhibition of PKA does not restore superoxide anion synthesis,81 suggesting additional pathways, such as EPAC signaling, may also be involved in these effects. In this context, phospholipase D (PLD) may be an important mediator of adenosine regulation of neutrophil function, since adenosine signaling via A2A inhibits PLD activation by blocking membrane recruitment of small GTPases.82
Regulation of neutrophil oxidative burst by other adenosine receptors has been less thoroughly characterized. The A2B receptor agonist BAY 60-6583 inhibited superoxide production in fMLP-stimulated murine neutrophils with a peak effect of approximately 50%, an effect that was absent in neutrophils from A2B-deficient mice (Adora2b-/-).83 Interestingly, the inhibitory effect of this agonist on neutrophils that were first primed with TNF before stimulation with fMLP or neutrophils harvested from LPS-treated mice was much more modest.83 In contrast, the A1 receptor agonist CPA enhanced superoxide generation during FcRγ-mediated stimulation of human neutrophils, an effect that could be blocked by the adenosine antagonist 8-(p-Sulfophenyl)theophylline and by pertussis toxin.66 The activation of neutrophil A3 receptors with Cl-IB-MECA had no effect on superoxide production by canine neutrophils stimulated with platelet-activating factor.54 A careful study of A3 agonists and antagonists could not rule out the contribution of A3 receptors to inhibition of oxidative burst but concluded inhibition is predominantly mediated through A2A activation.10
Neutrophil death
Cell death is essential to both homeostatic turnover of neutrophils in the resting state and during tissue inflammation. Neutrophil death occurs via several distinct apoptosis subroutines, by necrosis, and as the end-result of NET production (‘NETosis’). Several studies have demonstrated that adenosine analogs delay apoptosis of resting human neutrophils in culture.84,85 Adenosine agonists with higher affinity for the A2A receptor had more potency in inhibiting neutrophil apoptosis.84 Similarly, theophylline and its analogs promote apoptosis of resting neutrophils in culture.85 The role of adenosine signaling in neutrophil death in the context of inflamed tissues has not been investigated to our knowledge.
Applications in pre-clinical models of human disease
Ischemia-reperfusion injury (IRI) is an important pathogenic mechanism relevant to many diseases, including myocardial infarction, sickle cell crises and early graft dysfunction in solid organ transplantation. Neutrophil infiltration is a prominent feature of IRI and mediates tissue injury in part due to oxidative damage to the endothelium. Inhibition of neutrophil adherence and superoxide production by adenosine has been shown to attenuate IRI.86,87 In this context, administeration of the A2A agonists CGS-21680 or ATL146e reduce infarct size in a canine model of myocardial IRI using coronary ligation.88-91 Adenosine A2A receptor agonists have also been shown to improve hepatic IRI and sickle cell disease-induced IRI by inhibiting iNKT cell activation,89,92 thought to be upstream of neutrophil-mediated tissue damage.
Adenosine receptor modulation has also been investigated in the context of several other inflammatory diseases. In a model of LPS-induced acute lung injury, chimeric mice lacking the A2A receptor on bone marrow-derived cells exhibited more neutrophil recruitment into the alveolar space. In addition, pretreatment of wild-type mice with the A2A agonist ATL202 reduced neutrophil recruitment and cytokine release, an effect that required the expression of A2A receptors on myeloid cells.90 In the context of anti-microbial host defense, agonism of the A2A receptor with ATL146e resulted in improved survival after intra-peritoneal administration of LPS;91 conversely, genetic deletion of the A2A receptor or its pharmacological antagonism enhanced bacterial clearance and survival in a models of intra-abdominal infection.91,93 Thus the modulation of A2A receptor activity to inhibit neutrophil function may be beneficial in non-infectious inflammatory diseases but may impair defense against infections.
Conclusions
Neutrophils are involved in generation of tissue adenosine, and adenosine can activate or inhibit various neutrophil functions. Adenosine regulation of neutrophils is highly dependent on the inflammatory microenvironment and, in part, regulated by expression of adenosine receptors on neutrophils and the affinity of these receptors for adenosine. Nanomolar concentrations of adenosine act via A1 and A3 receptors to promote neutrophil chemotaxis toward inflammatory stimuli and phagocytosis, whereas micromolar concentrations result in activation of the low-affinity A2A and A2B receptors which inhibits neutrophil phagocytosis, granule release, and oxidative burst, limits excessive tissue damage, and promotes endothelial barrier function and repair.
There are numerous avenues for additional research in the field of neutrophil-adenosine biology. Recent pharmacologic developments have resulted in potent, selective A2B and A3 receptor agonists and antagonists, which should facilitate study of these receptors. The impact of A2B and A3 receptor signaling on neutrophil effector functions, including phagocytosis and oxidative burst, granule release, and inflammatory mediator production, requires further characterization. The downstream signaling pathways that couple adenosine receptor activation with inhibition or activation of neutrophil function should be further elucidated. Adenosine receptor modulation of neutrophil cell death pathways, including the recently characterized process of NETosis, would also benefit from additional study. Insight gained into the basic biology of adenosine-neutrophil interactions should be applied to the study of relevant disease models; adenosine receptor biology has numerous therapeutic applications, which can be advanced through translational research.
Finally, there are numerous factors involved in the regulation of neutrophil activation and function, for example ATP and the neuronal guidance molecule netrin-1. Study of the interplay between adenosine and these molecules is important to achieve a more complete understanding of neutrophil biology.
Figure 1.
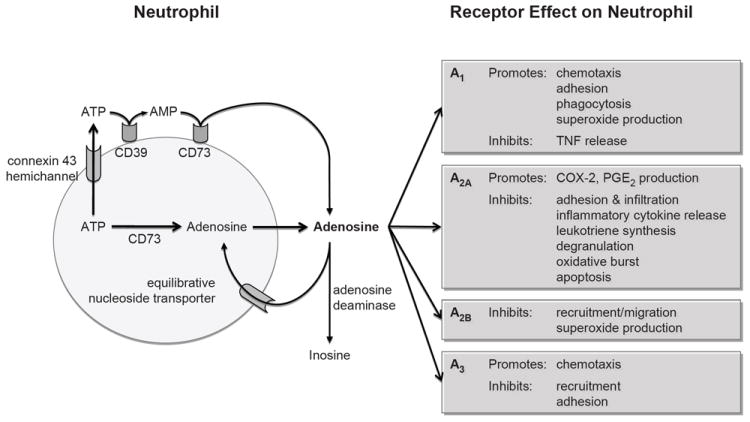
Diagrammatic overview of adenosine regulation of neutrophils.
Table 1.
Pharmacologic ligands of adenosine receptors.
| Receptor Target | Ligand | Effect |
|---|---|---|
| global | 2-choloradenosine | agonist |
| global | N-ethylcarboxamidoadenosine (NECA) | agonist |
| global | 8-(p-Sulfophenyl)theophylline | antagonist |
| global | theophylline | antagonist |
| A1 | N6-cyclopentyladenosine (CPA) | agonist |
| A1 | 2-chloro-N6-cyclopentyladenosine (COPA) | agonist |
| A2A | CGS-21680* | agonist |
| A2A | WRC-0470* | agonist |
| A2A | ATL146e | agonist |
| A2A | ATL193 | agonist |
| A2A | ATL202 | agonist |
| A2A | ZM-241385* | antagonist |
| A2A/A2B | 5’-(N-cyclopropyl)-carboxamido-adenosine (CPCA) | agonist |
| A2B | BAY 60-6583* | agonist |
| A3 | 2-Chloro-N6-(3-iodobenzyl)adenosine-5’-N-methyluronamide (Cl-IB-MECA) | agonist |
| A3 | MRS-1220* | antagonist |
Abbreviations:
CGS-21680, 3-[4-[2-[[6-amino-9-[(2R,3R,4S,5S)-5-(ethylcarbamoyl)-3,4-dihydroxy-oxolan-2-yl]purin-2-yl]amino]ethyl]phenyl]propanoic acid;
WRC-0470, 2-cyclohexylmethylidenehydrazinoadenosine;
ZM-241385, 4-(2-[7-Amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol;
BAY 60-6583, 2-[6-amino-3,5-dicyano-4-[4-(cyclopropylmethoxy)phenyl]pyridin-2-ylsulfanyl]acetamide;
MRS-1220, N-[9-Chloro-2-(2-furanyl)[1,2,4]-triazolo[1,5-c]quinazolin-5-yl]benzene acetamide.
Table 2.
Brief summary of neutrophil-adenosine receptor literature.
| Receptor | System | Stimulus | Manipulation | Effect on Neutrophil | Ref |
|---|---|---|---|---|---|
| unspecified | human neutrophil | fMLP | 2-chloroadenosine | inhibits adherence to endothelium | 29 |
| unspecified | human neutrophil | fMLP | NECA | inhibits neutrophil-mediated endothelial damage | 29,44 |
| unspecified | human neutrophil | fMLP | adenosine kinase inhibition | inhibits selectin-based adhesion | 47 |
| unspecified | human neutrophil | fMLP | NECA | inhibits oxidative burst | 18,73 |
| A1 | human neutrophil | fMLP | CPA | promotes chemotaxis | 9 |
| A1 | human neutrophil | fMLP | CPA | promotes adhesion to gelatin-coated plates | 48 |
| A1 | porcine neutrophil | PMA | COPA | promotes adhesion | 45 |
| A1 | human neutrophil | antibody-coated erythrocytes | CPA | promotes FcRγ-mediated phagocytosis | 66,67 |
| A1 | human neutrophil | FcRγ stimulation | CPA | enhances superoxide production | 66 |
| A1, A2A/A2B | human neutrophil | LPS | COPA, CPCA | inhibits TNF release | 57 |
| A2 | human neutrophil | complement C5, fMLP | NECA | promotes chemotaxis | 32 |
| A2 | human neutrophil | fMLP | dipyrimidole, NECA | inhibits selectin shedding, expression of β2 integrins | 12,49 |
| A2 | human neutrophil | fMLP | NECA | inhibits adhesion to fibrinogen-coated plates | 48 |
| A2 | human neutrophil | antibody-coated yeast | NECA | inhibits FcRγ-mediated phagocytosis | 66,67 |
| A2A | porcine neutrophil | PMA | NECA, CGS-21680 | inhibits adhesion | 45 |
| A2A | canine neutrophil | PAF | CGS-21680 | inhibits adhesion | 46 |
| A2A | human neutrophil | fMLP, TNF | ATL146e | inhibits adhesion to VCAM-1 coated surface | 50 |
| A2A | canine neutrophil | myocardial infarction | ATL146e | inhibits p-selectin expression, infiltration | 52 |
| A2A | murine neutrophil | carotid ligation | ATL146e | inhibits recruitment, integrin and selectin expression | 53 |
| A2A | human neutrophil | LPS | CGS-21680 | inhibits inflammatory chemokine release | 58 |
| A2A | Adora2a-/- mice | air pouch inflammation | - | inhibits COX-2 induction | 59 |
| A2A | human neutrophil | fMLP | CGS-21680 | promotes COX-2, PGE2 generation | 59,60 |
| A2A | whole blood, human neutrophil | fMLP | NECA, CGS-21680 | inhibits LTB4 synthesis | 61,62 |
| A2A | human neutrophil | fMLP | CGS-21680 | inhibits leukotriene synthesis | 63 |
| A2A | human neutrophil | various | CGS-21680 | inhibits LTA4 production | 64,65 |
| A2A | rat neutrophil | meningitis | WRC-0470 | inhibits degranulation | 71 |
| A2A | human, murine, rat neutrophil | fMLP, TNF, PAF, IgG, C3b-zymosan, bacterial meningitis | WRC-0470, CGS-21680, ATL193, ATL146e | inhibits oxidative burst | 70,75-79 |
| A2A | human neutrophil | - | CGS-21680 | inhibits neutrophil apoptosis | 84,85 |
| A2A, A3 | human neutrophil | fMLP, TNF, LPS | - | inhibits degranulation | 68-70 |
| A2B | murine, human neutrophil | LPS, hypoxia, colitis | netrin-1 | inhibits recruitment, transepithelial migration | 39-41 |
| A2B | murine neutrophil | fMLP | BAY 60-6583 | inhibits superoxide production | 83 |
| A3 | human neutrophil | fMLP | - | promotes chemotaxis | 33 |
| A3 | Adora3-/- mice | sepsis | - | inhibits recruitment | 34 |
| A3 | canine, rabbit neutrophil | PAF | Cl-IB-MECA | inhibits adhesion to endothelium | 54 |
Abbreviations: N-formyl-methionine-leucine-phenylalanine (fMLP), Phorbol myristate acetate (PMA), lipopolysaccharide (LPS), platelet activating factor (PAF), prostaglandin (PG), arachadonic acid (AA), leukotriene (LT), lipoxygenase (LO). See Table 1 for ligand information.
Acknowledgments
Source of funding: Supported by NIH grants HL073848 and HL098329
References
- 1.Harkness RA, Simmonds RJ, Coade SB. Purine transport and metabolism in man: the effect of exercise on concentrations of purine bases, nucleosides and nucleotides in plasma, urine, leucocytes and erythrocytes. Clin Sci. 1983;64:333–340. doi: 10.1042/cs0640333. [DOI] [PubMed] [Google Scholar]
- 2.Bours MJL, Swennen ELR, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5’-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Ontyd J, Schrader J. Measurement of adenosine, inosine, and hypoxanthine in human plasma. J Chromatogr. 1984;307:404–409. doi: 10.1016/s0378-4347(00)84113-4. [DOI] [PubMed] [Google Scholar]
- 4.Yoneyama Y, Suzuki S, Sawa R, Araki T. Plasma adenosine concentrations increase in women with hyperemesis gravidarum. Clin Chim Acta. 2004;342:99–103. doi: 10.1016/j.cccn.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Fredholm BB. Purines and neutrophil leukocytes. Gen Pharmacol. 1997;28:345–350. doi: 10.1016/s0306-3623(96)00169-3. [DOI] [PubMed] [Google Scholar]
- 6.Möser GH, Schrader J, Deussen A. Turnover of adenosine in plasma of human and dog blood. Am J Physiol. 1989;256:C799–C806. doi: 10.1152/ajpcell.1989.256.4.C799. [DOI] [PubMed] [Google Scholar]
- 7.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11:201–212. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fredholm BB, Zhang Y, van der Ploeg I. Adenosine A2A receptors mediate the inhibitory effect of adenosine on formyl-Met-Leu-Phe-stimulated respiratory burst in neutrophil leucocytes. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:262–267. doi: 10.1007/BF00171056. [DOI] [PubMed] [Google Scholar]
- 9.Cronstein BN, Daguma L, Nichols D, Hutchison AJ, Williams M. The adenosine/neutrophil paradox resolved: human neutrophils possess both A1 and A2 receptors that promote chemotaxis and inhibit O2 generation, respectively. J Clin Invest. 1990;85:1150–1157. doi: 10.1172/JCI114547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gessi S, Varani K, Merighi S, Cattabriga E, Iannotta V, Leung E, Baraldi PG, Borea PA. A(3) adenosine receptors in human neutrophils and promyelocytic HL60 cells: a pharmacological and biochemical study. Mol Pharmacol. 2002;61:415–424. doi: 10.1124/mol.61.2.415. [DOI] [PubMed] [Google Scholar]
- 11.Bouma MG, Jeunhomme TM, Boyle DL, Dentener MA, Voitenok NN, van den Wildenberg FA, Buurman WA. Adenosine inhibits neutrophil degranulation in activated human whole blood: involvement of adenosine A2 and A3 receptors. J Immunol. 1997;158:5400–5408. [PubMed] [Google Scholar]
- 12.Thiel M, Chambers JD, Chouker A, Fischer S, Zourelidis C, Bardenheuer HJ, Arfors KE, Peter K. Effect of adenosine on the expression of beta(2) integrins and L-selectin of human polymorphonuclear leukocytes in vitro. J Leukoc Biol. 1996;59:671–682. doi: 10.1002/jlb.59.5.671. [DOI] [PubMed] [Google Scholar]
- 13.Newby AC, Holmquist CA. Adenosine production inside rat polymorphonuclear leucocytes. Biochem J. 1981;200:399–403. doi: 10.1042/bj2000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Worku Y, Newby AC. The mechanism of adenosine production in rat polymorphonuclear leucocytes. Biochem J. 1983;214:325–330. doi: 10.1042/bj2140325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bengtsson T, Zalavary S, Stendahl O, Grenegård M. Release of oxygen metabolites from chemoattractant-stimulated neutrophils is inhibited by resting platelets: role of extracellular adenosine and actin polymerization. Blood. 1996;87:4411–4423. [PubMed] [Google Scholar]
- 16.Mann JS, Renwick AG, Holgate ST. Release of adenosine and its metabolites from activated human leucocytes. Clin Sci. 1986;70:461–468. doi: 10.1042/cs0700461. [DOI] [PubMed] [Google Scholar]
- 17.Cronstein BN, Kramer SB, Weissmann G, Hirschhorn R. A new physiological function for adenosine: regulation of superoxide anion production. Trans Assoc Am Physicians. 1983;96:384–391. [PubMed] [Google Scholar]
- 18.Cronstein BN, Kramer SB, Weissmann G, Hirschhorn R. Adenosine: a physiological modulator of superoxide anion generation by human neutrophils. J Exp Med. 1983;158:1160–1177. doi: 10.1084/jem.158.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitakaze M, Hori M, Morioka T, Takashima S, Minamino T, Sato H, Inoue M, Kamada T. Attenuation of ecto-5’-nucleotidase activity and adenosine release in activated human polymorphonuclear leukocytes. Circ Res. 1993;73:524–533. doi: 10.1161/01.res.73.3.524. [DOI] [PubMed] [Google Scholar]
- 20.van Waeg G, Van den Berghe G. Purine catabolism in polymorphonuclear neutrophils. Phorbol myristate acetate-induced accumulation of adenosine owing to inactivation of extracellularly released adenosine deaminase. J Clin Invest. 1991;87:305–312. doi: 10.1172/JCI114987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eltzschig HK, Eckle T, Mager A, Küper N, Karcher C, Weissmüller T, Boengler K, Schulz R, Robson SC, Colgan SP. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- 22.Eltzschig HK, Macmanus CF, Colgan SP. Neutrophils as sources of extracellular nucleotides: functional consequences at the vascular interface. Trends Cardiovasc Med. 2008;18:103–107. doi: 10.1016/j.tcm.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grassi F. Purinergic control of neutrophil activation. J Mol Cell Biol. 2010;2:176–177. doi: 10.1093/jmcb/mjq014. [DOI] [PubMed] [Google Scholar]
- 24.Madara JL, Parkos C, Colgan S, MacLeod RJ, Nash S, Matthews J, Delp C, Lencer W. Cl- secretion in a model intestinal epithelium induced by a neutrophil-derived secretagogue. J Clin Invest. 1992;89:1938–1944. doi: 10.1172/JCI115800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madara JL, Patapoff TW, Gillece-Castro B, Colgan SP, Parkos CA, Delp C, Mrsny RJ. 5’-adenosine monophosphate is the neutrophil-derived paracrine factor that elicits chloride secretion from T84 intestinal epithelial cell monolayers. J Clin Invest. 1993;91:2320–2325. doi: 10.1172/JCI116462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lennon PF, Taylor CT, Stahl GL, Colgan SP. Neutrophil-derived 5’-adenosine monophosphate promotes endothelial barrier function via CD73-mediated conversion to adenosine and endothelial A2B receptor activation. J Exp Med. 1998;188:1433–1443. doi: 10.1084/jem.188.8.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baldwin SA, Beal PR, Yao SYM, King AE, Cass CE, Young JD. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. 2004;447:735–743. doi: 10.1007/s00424-003-1103-2. [DOI] [PubMed] [Google Scholar]
- 28.Eltzschig HK, Abdulla P, Hoffman E, Hamilton KE, Daniels D, Schönfeld C, Löffler M, Reyes G, Duszenko M, Karhausen J, Robinson A, Westerman KA, Coe IR, Colgan SP. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med. 2005;202:1493–1505. doi: 10.1084/jem.20050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cronstein BN, Levin RI, Belanoff J, Weissmann G, Hirschhorn R. Adenosine: an endogenous inhibitor of neutrophil-mediated injury to endothelial cells. J Clin Invest. 1986;78:760–770. doi: 10.1172/JCI112638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cronstein BN, Kubersky SM, Weissmann G, Hirschhorn R. Engagement of adenosine receptors inhibits hydrogen peroxide (H2O2) release by activated human neutrophils. Clin Immunol Immunopathol. 1987;42:76–85. doi: 10.1016/0090-1229(87)90174-7. [DOI] [PubMed] [Google Scholar]
- 31.Schrier DJ, Imre KM. The effects of adenosine agonists on human neutrophil function. J Immunol. 1986;137:3284–3289. [PubMed] [Google Scholar]
- 32.Rose FR, Hirschhorn R, Weissmann G, Cronstein BN. Adenosine promotes neutrophil chemotaxis. J Exp Med. 1988;167:1186–1194. doi: 10.1084/jem.167.3.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 34.Inoue Y, Chen Y, Hirsh MI, Yip L, Junger WG. A3 and P2Y2 receptors control the recruitment of neutrophils to the lungs in a mouse model of sepsis. Shock. 2008;30:173–177. doi: 10.1097/shk.0b013e318160dad4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corriden R, Chen Y, Inoue Y, Beldi G, Robson SC, Insel PA, Junger WG. Ecto-nucleoside triphosphate diphosphohydrolase 1 (E-NTPDase1/CD39) regulates neutrophil chemotaxis by hydrolyzing released ATP to adenosine. J Biol Chem. 2008;283:28480–28486. doi: 10.1074/jbc.M800039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Junger WG. Purinergic regulation of neutrophil chemotaxis. Cell Mol Life Sci. 2008;65:2528–2540. doi: 10.1007/s00018-008-8095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Linden J. Cell biology. Purinergic chemotaxis. Science. 2006;314:1689–1690. doi: 10.1126/science.1137190. [DOI] [PubMed] [Google Scholar]
- 38.Bouma MG, van den Wildenberg FA, Buurman WA. Adenosine inhibits cytokine release and expression of adhesion molecules by activated human endothelial cells. Am J Physiol. 1996;270:C522–C529. doi: 10.1152/ajpcell.1996.270.2.C522. [DOI] [PubMed] [Google Scholar]
- 39.Mirakaj V, Thix CA, Laucher S, Mielke C, Morote-Garcia JC, Schmit MA, Henes J, Unertl KE, Köhler D, Rosenberger P. Netrin-1 dampens pulmonary inflammation during acute lung injury. Am J Respir Crit Care Med. 2010;181:815–824. doi: 10.1164/rccm.200905-0717OC. [DOI] [PubMed] [Google Scholar]
- 40.Aherne CM, Collins CB, Masterson JC, Tizzano M, Boyle TA, Westrich JA, Parnes JA, Furuta GT, Rivera-Nieves J, Eltzschig HK. Neuronal guidance molecule netrin-1 attenuates inflammatory cell trafficking during acute experimental colitis. Gut. 2011 doi: 10.1136/gutjnl-2011-300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC, Unertl K, Eltzschig HK. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009;10:195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- 42.Linden J. Regulation of Leukocyte Function by Adenosine Receptors. 1. Elsevier Inc.; 2011. pp. 95–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Säve S, Mohlin C, Vumma R, Persson K. Activation of adenosine A2A receptors inhibits neutrophil transuroepithelial migration. Infect Immun. 2011;79:3431–3437. doi: 10.1128/IAI.05005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gunther GR, Herring MB. Inhibition of neutrophil superoxide production by adenosine released from vascular endothelial cells. Ann Vasc Surg. 1991;5:325–330. doi: 10.1007/BF02015292. [DOI] [PubMed] [Google Scholar]
- 45.Felsch A, Stöcker K, Borchard U. Phorbol ester-stimulated adherence of neutrophils to endothelial cells is reduced by adenosine A2 receptor agonists. J Immunol. 1995;155:333–338. [PubMed] [Google Scholar]
- 46.Zhao ZQ, Sato H, Williams MW, Fernandez AZ, Vinten-Johansen J. Adenosine A2-receptor activation inhibits neutrophil-mediated injury to coronary endothelium. Am J Physiol. 1996;271:H1456–H1464. doi: 10.1152/ajpheart.1996.271.4.H1456. [DOI] [PubMed] [Google Scholar]
- 47.Firestein GS, Bullough DA, Erion MD, Jimenez R, Ramirez-Weinhouse M, Barankiewicz J, Smith CW, Gruber HE, Mullane KM. Inhibition of neutrophil adhesion by adenosine and an adenosine kinase inhibitor. The role of selectins. J Immunol. 1995;154:326–334. [PubMed] [Google Scholar]
- 48.Cronstein BN, Levin RI, Philips M, Hirschhorn R, Abramson SB, Weissmann G. Neutrophil adherence to endothelium is enhanced via adenosine A1 receptors and inhibited via adenosine A2 receptors. J Immunol. 1992;148:2201–2206. [PubMed] [Google Scholar]
- 49.Wollner A, Wollner S, Smith JB. Acting via A2 receptors, adenosine inhibits the upregulation of Mac-1 (Cd11b/CD18) expression on FMLP-stimulated neutrophils. Am J Respir Cell Mol Biol. 1993;9:179–185. doi: 10.1165/ajrcmb/9.2.179. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan GW, Lee DD, Ross WG, DiVietro JA, Lappas CM, Lawrence MB, Linden J. Activation of A2A adenosine receptors inhibits expression of alpha 4/beta 1 integrin (very late antigen-4) on stimulated human neutrophils. J Leukoc Biol. 2004;75:127–134. doi: 10.1189/jlb.0603300. [DOI] [PubMed] [Google Scholar]
- 51.Gadangi P, Longaker M, Naime D, Levin RI, Recht PA, Montesinos MC, Buckley MT, Carlin G, Cronstein BN. The anti-inflammatory mechanism of sulfasalazine is related to adenosine release at inflamed sites. J Immunol. 1996;156:1937–1941. [PubMed] [Google Scholar]
- 52.Glover DK, Riou LM, Ruiz M, Sullivan GW, Linden J, Rieger JM, Macdonald TL, Watson DD, Beller GA. Reduction of infarct size and postischemic inflammation from ATL-146e, a highly selective adenosine A2A receptor agonist, in reperfused canine myocardium. Am J Physiol Heart Circ Physiol. 2005;288:H1851–H1858. doi: 10.1152/ajpheart.00362.2004. [DOI] [PubMed] [Google Scholar]
- 53.McPherson JA, Barringhaus KG, Bishop GG, Sanders JM, Rieger JM, Hesselbacher SE, Gimple LW, Powers ER, Macdonald T, Sullivan G, Linden J, Sarembock IJ. Adenosine A(2A) receptor stimulation reduces inflammation and neointimal growth in a murine carotid ligation model. Arterioscler Thromb Vasc Biol. 2001;21:791–796. doi: 10.1161/01.atv.21.5.791. [DOI] [PubMed] [Google Scholar]
- 54.Jordan JE, Thourani VH, Auchampach JA, Robinson JA, Wang NP, Vinten-Johansen J. A(3) adenosine receptor activation attenuates neutrophil function and neutrophil-mediated reperfusion injury. Am J Physiol. 1999;277:H1895–H1905. doi: 10.1152/ajpheart.1999.277.5.H1895. [DOI] [PubMed] [Google Scholar]
- 55.Narravula S, Lennon PF, Mueller BU, Colgan SP. Regulation of endothelial CD73 by adenosine: paracrine pathway for enhanced endothelial barrier function. J Immunol. 2000;165:5262–5268. doi: 10.4049/jimmunol.165.9.5262. [DOI] [PubMed] [Google Scholar]
- 56.Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thiel M, Chouker A. Acting via A2 receptors, adenosine inhibits the production of tumor necrosis factor-alpha of endotoxin-stimulated human polymorphonuclear leukocytes. J Lab Clin Med. 1995;126:275–282. [PubMed] [Google Scholar]
- 58.McColl SR, St-Onge M, Dussault A-A, Laflamme C, Bouchard L, Boulanger J, Pouliot M. Immunomodulatory impact of the A2A adenosine receptor on the profile of chemokines produced by neutrophils. FASEB J. 2006;20:187–189. doi: 10.1096/fj.05-4804fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cadieux J-S, Leclerc P, St-Onge M, Dussault A-A, Laflamme C, Picard S, Ledent C, Borgeat P, Pouliot M. Potentiation of neutrophil cyclooxygenase-2 by adenosine: an early anti-inflammatory signal. J Cell Sci. 2005;118:1437–1447. doi: 10.1242/jcs.01737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pouliot M, Fiset M-E, Massé M, Naccache PH, Borgeat P. Adenosine up-regulates cyclooxygenase-2 in human granulocytes: impact on the balance of eicosanoid generation. J Immunol. 2002;169:5279–5286. doi: 10.4049/jimmunol.169.9.5279. [DOI] [PubMed] [Google Scholar]
- 61.Krump E, Lemay G, Borgeat P. Adenosine A2 receptor-induced inhibition of leukotriene B4 synthesis in whole blood ex vivo. Br J Pharmacol. 1996;117:1639–1644. doi: 10.1111/j.1476-5381.1996.tb15334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krump E, Picard S, Mancini J, Borgeat P. Suppression of leukotriene B4 biosynthesis by endogenous adenosine in ligand-activated human neutrophils. J Exp Med. 1997;186:1401–1406. doi: 10.1084/jem.186.8.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Surette ME, Krump E, Picard S, Borgeat P. Activation of leukotriene synthesis in human neutrophils by exogenous arachidonic acid: inhibition by adenosine A(2a) receptor agonists and crucial role of autocrine activation by leukotriene B(4) Mol Pharmacol. 1999;56:1055–1062. doi: 10.1124/mol.56.5.1055. [DOI] [PubMed] [Google Scholar]
- 64.Flamand N, Surette ME, Picard S, Bourgoin S, Borgeat P. Cyclic AMP-mediated inhibition of 5-lipoxygenase translocation and leukotriene biosynthesis in human neutrophils. Mol Pharmacol. 2002;62:250–256. doi: 10.1124/mol.62.2.250. [DOI] [PubMed] [Google Scholar]
- 65.Flamand N, Boudreault S, Picard S, Austin M, Surette ME, Plante H, Krump E, Vallée MJ, Gilbert C, Naccache P, Laviolette M, Borgeat P. Adenosine, a potent natural suppressor of arachidonic acid release and leukotriene biosynthesis in human neutrophils. Am J Respir Crit Care Med. 2000;161:S88–S94. doi: 10.1164/ajrccm.161.supplement_1.ltta-18. [DOI] [PubMed] [Google Scholar]
- 66.Salmon JE, Cronstein BN. Fc gamma receptor-mediated functions in neutrophils are modulated by adenosine receptor occupancy. A1 receptors are stimulatory and A2 receptors are inhibitory. J Immunol. 1990;145:2235–2240. [PubMed] [Google Scholar]
- 67.Zalavary S, Stendahl O, Bengtsson T. The role of cyclic AMP, calcium and filamentous actin in adenosine modulation of Fc receptor-mediated phagocytosis in human neutrophils. Biochim Biophys Acta. 1994;1222:249–256. doi: 10.1016/0167-4889(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 68.Richter J. Effect of adenosine analogues and cAMP-raising agents on TNF-, GM-CSF-, and chemotactic peptide-induced degranulation in single adherent neutrophils. J Leukoc Biol. 1992;51:270–275. doi: 10.1002/jlb.51.3.270. [DOI] [PubMed] [Google Scholar]
- 69.Visser SS, Theron AJ, Ramafi G, Ker JA, Anderson R. Apparent involvement of the A(2A) subtype adenosine receptor in the anti-inflammatory interactions of CGS 21680, cyclopentyladenosine, and IB-MECA with human neutrophils. Biochem Pharmacol. 2000;60:993–999. doi: 10.1016/s0006-2952(00)00414-7. [DOI] [PubMed] [Google Scholar]
- 70.Anderson R, Visser SS, Ramafi G, Theron AJ. Accelerated resequestration of cytosolic calcium and suppression of the pro-inflammatory activities of human neutrophils by CGS 21680 in vitro. Br J Pharmacol. 2000;130:717–724. doi: 10.1038/sj.bjp.0703344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sullivan GW, Linden J, Buster BL, Scheld WM. Neutrophil A2A adenosine receptor inhibits inflammation in a rat model of meningitis: synergy with the type IV phosphodiesterase inhibitor, rolipram. J Infect Dis. 1999;180:1550–1560. doi: 10.1086/315084. [DOI] [PubMed] [Google Scholar]
- 72.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 73.Cronstein BN, Rosenstein ED, Kramer SB, Weissmann G, Hirschhorn R. Adenosine; a physiologic modulator of superoxide anion generation by human neutrophils. Adenosine acts via an A2 receptor on human neutrophils. J Immunol. 1985;135:1366–1371. [PubMed] [Google Scholar]
- 74.Thiel M, Holzer K, Kreimeier U, Moritz S, Peter K, Messmer K. Effects of adenosine on the functions of circulating polymorphonuclear leukocytes during hyperdynamic endotoxemia. Infect Immun. 1997;65:2136–2144. doi: 10.1128/iai.65.6.2136-2144.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sullivan GW, Rieger JM, Scheld WM, Macdonald TL, Linden J. Cyclic AMP-dependent inhibition of human neutrophil oxidative activity by substituted 2-propynylcyclohexyl adenosine A(2A) receptor agonists. Br J Pharmacol. 2001;132:1017–1026. doi: 10.1038/sj.bjp.0703893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stewart AG, Harris T. Adenosine inhibits platelet-activating factor, but not tumour necrosis factor-alpha-induced priming of human neutrophils. Immunology. 1993;78:152–158. [PMC free article] [PubMed] [Google Scholar]
- 77.Zalavary S, Bengtsson T. Adenosine inhibits actin dynamics in human neutrophils: evidence for the involvement of cAMP. Eur J Cell Biol. 1998;75:128–139. doi: 10.1016/S0171-9335(98)80055-1. [DOI] [PubMed] [Google Scholar]
- 78.Sullivan GW, Carper HT, Mandell GL. The specific type IV phosphodiesterase inhibitor rolipram combined with adenosine reduces tumor necrosis factor-alpha-primed neutrophil oxidative activity. Int J Immunopharmacol. 1995;17:793–803. doi: 10.1016/0192-0561(95)00073-b. [DOI] [PubMed] [Google Scholar]
- 79.Kubersky SM, Hirschhorn R, Broekman MJ, Cronstein BN. Occupancy of adenosine receptors on human neutrophils inhibits respiratory burst stimulated by ingestion of complement-coated particles and occupancy of chemoattractant but not Fc receptors. Inflammation. 1989;13:591–599. doi: 10.1007/BF00916765. [DOI] [PubMed] [Google Scholar]
- 80.Swain SD, Siemsen DW, Nelson LK, Sipes KM, Hanson AJ, Quinn MT. Inhibition of the neutrophil NADPH oxidase by adenosine is associated with increased movement of flavocytochrome b between subcellular fractions. Inflammation. 2003;27:45–58. doi: 10.1023/a:1022639228723. [DOI] [PubMed] [Google Scholar]
- 81.Cronstein BN, Haines KA, Kolasinski S, Reibman J. Occupancy of G alpha s-linked receptors uncouples chemoattractant receptors from their stimulus-transduction mechanisms in the neutrophil. Blood. 1992;80:1052–1057. [PubMed] [Google Scholar]
- 82.Thibault N, Harbour D, Borgeat P, Naccache PH, Bourgoin SG. Adenosine receptor occupancy suppresses chemoattractant-induced phospholipase D activity by diminishing membrane recruitment of small GTPases. Blood. 2000;95:519–527. [PubMed] [Google Scholar]
- 83.van der Hoeven D, Wan TC, Gizewski ET, Kreckler LM, Maas JE, Van Orman J, Ravid K, Auchampach JA. A Role for the Low-Affinity A2B Adenosine Receptor in Regulating Superoxide Generation by Murine Neutrophils. J Pharmacol Exp Ther. 2011;338:1004–1012. doi: 10.1124/jpet.111.181792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Walker BA, Rocchini C, Boone RH, Ip S, Jacobson MA. Adenosine A2a receptor activation delays apoptosis in human neutrophils. J Immunol. 1997;158:2926–2931. [PubMed] [Google Scholar]
- 85.Yasui K, Agematsu K, Shinozaki K, Hokibara S, Nagumo H, Nakazawa T, Komiyama A. Theophylline induces neutrophil apoptosis through adenosine A2A receptor antagonism. J Leukoc Biol. 2000;67:529–535. doi: 10.1002/jlb.67.4.529. [DOI] [PubMed] [Google Scholar]
- 86.Thiagarajan RR, Winn RK, Harlan JM. The role of leukocyte and endothelial adhesion molecules in ischemia-reperfusion injury. Thromb Haemost. 1997;78:310–314. [PubMed] [Google Scholar]
- 87.Polanowska-Grabowska R, Wallace K, Field JJ, Chen L, Marshall MA, Figler R, Gear ARL, Linden J. P-selectin-mediated platelet-neutrophil aggregate formation activates neutrophils in mouse and human sickle cell disease. Arterioscler Thromb Vasc Biol. 2010;30:2392–2399. doi: 10.1161/ATVBAHA.110.211615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jordan JE, Zhao ZQ, Sato H, Taft S, Vinten-Johansen J. Adenosine A2 receptor activation attenuates reperfusion injury by inhibiting neutrophil accumulation, superoxide generation and coronary endothelial adherence. J Pharmacol Exp Ther. 1997;280:301–309. [PubMed] [Google Scholar]
- 89.Wallace KL, Linden J. Adenosine A2A receptors induced on iNKT and NK cells reduce pulmonary inflammation and injury in mice with sickle cell disease. Blood. 2010;116:5010–5020. doi: 10.1182/blood-2010-06-290643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reutershan J, Cagnina RE, Chang D, Linden J, Ley K. Therapeutic anti-inflammatory effects of myeloid cell adenosine receptor A2a stimulation in lipopolysaccharide-induced lung injury. J Immunol. 2007;179:1254–1263. doi: 10.4049/jimmunol.179.2.1254. [DOI] [PubMed] [Google Scholar]
- 91.Sullivan GW, Fang G, Linden J, Scheld WM. A2A adenosine receptor activation improves survival in mouse models of endotoxemia and sepsis. J Infect Dis. 2004;189:1897–1904. doi: 10.1086/386311. [DOI] [PubMed] [Google Scholar]
- 92.Lappas CM, Day Y-J, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Németh ZH, Csóka B, Wilmanski J, Xu D, Lu Q, Ledent C, Deitch EA, Pacher P, Spolarics Z, Haskó G. Adenosine A2A receptor inactivation increases survival in polymicrobial sepsis. J Immunol. 2006;176:5616–5626. doi: 10.4049/jimmunol.176.9.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]


