Abstract
Serotonin is an ancient molecular signal and a recognized neurotransmitter brainwide distributed with particular presence in hippocampus. Almost all serotonin receptor subtypes are expressed in hippocampus, which implicates an intricate modulating system, considering that they can be localized as autosynaptic, presynaptic, and postsynaptic receptors, even colocalized within the same cell and being target of homo- and heterodimerization. Neurons and glia, including immune cells, integrate a functional network that uses several serotonin receptors to regulate their roles in this particular part of the limbic system.
1. Serotonin
Serotonin (5-hydroxytryptamine; 5-HT), named by Rapport et al. (1948) [1], is one of the ubiquitous molecules acting as messengers, well known as a neurotransmitter and neuromodulator. Serotonin (Figure 1) is mostly found outside the central nervous system [2]; it was first identified in enterochromaffin cells and named as “enteramine” by Vialli and Erspamer in 1937 and confirmed to be the same entity with the “clotted blood” vasoconstriction effects in 1952 [3].
Figure 1.
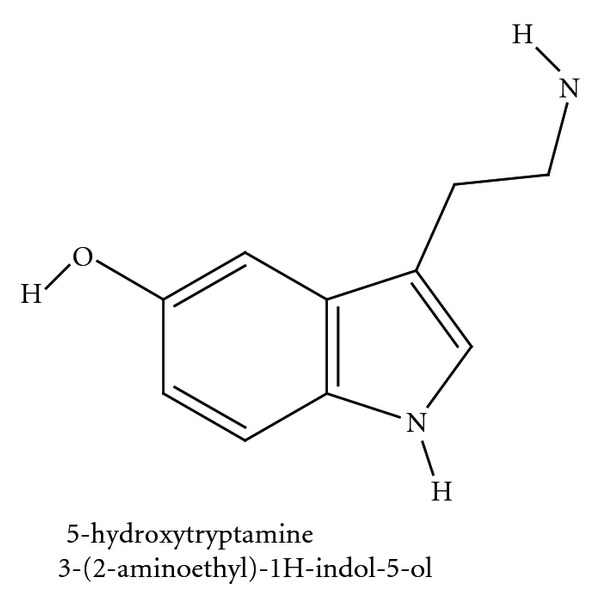
Serotonin (5-HT). Modified image from NCBI PubChem Substance Database CID 5202.
2. Serotonin as an Ancient Molecular Signal
The serotonergic system is an ancient sensor of diverse stimuli and molecular signaling in single-celled eukaryotes, plants, and animals [4–6].
The regulated expression of genetic material in every cell is very important and a “regulatory lesson” learned over the years is that small metabolites are often regulatory signals to control gene expression. For “expensive” biosynthesis, as the required for the serotonin precursor tryptophan, common pathways are found in organisms that take advantage of the aromatic structures; tryptophan serves as the precursor not only of serotonin (Figure 2), but also of very important compounds as niacin in eukaryotes, indoleacetic acid in plants, and indole in bacteria. Regulatory strategies could be compatible with other metabolic goals as organisms evolved capable of obtaining tryptophan by feeding, with specific plasma membrane transporters [7, 8].
Figure 2.
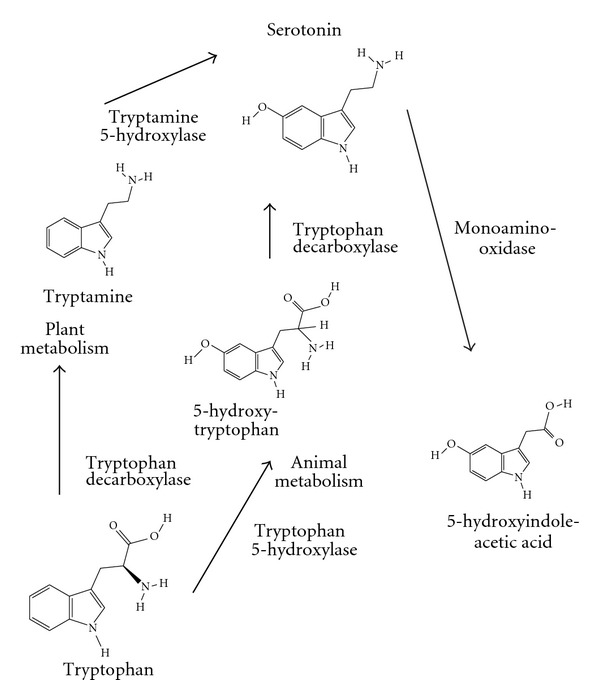
Serotonin metabolism. Tryptophan is the precursor for serotonin synthesis, with different enzymatic reactions in plant and animals [6]; hydroxylation is the rate limiting step (enzyme mediated by tryptophan hydroxylase in animals or tryptamine hydroxylase in plants), while decarboxylation is a rapid conversion by the aromatic amino acid decarboxylase (tryptophan decarboxylase). The catabolic metabolite of serotonin is 5-hydroxyindoleacetic acid, via 5-hydroxyindole acetaldehyde enzymatically converted by the membrane-bound mitochondrial flavoprotein monoamino oxidase. Modified images from NCBI PubChem Substance Database.
Beyond the heterotrophic theory of the very first living organisms [9], serotonin could be used as specific signal, after direct relation with tryptophan synthesis was controlled, and specific monoamine transporters that do not need the missing carboxyl group of the aminoacids [7, 10] were present; later, it acquired functions of “hormone” and growth factor, and serotonin activity as neurotransmitter was achieved at last [4]. In prenervous stages, serotonin regulates basic developmental processes from cleavage divisions after fertilization (proliferator) to morphogenetic cell movements during gastrulation (morphogen) in sea urchin [11]. Presence of serotonin and its metabolite 5-hydroxyindoleacetic acid in unicellular ciliate Tetrahymena pyriformis [12] and increasing RNA production in the 5-HT stimulated protozoa [13] suggested an active biogenic amine system with relevant functions; interaction with GTPases might represent some of the earlier functions of serotonin (and biogenic amines) before it could be vesiculated and its exocytosis could be regulated for metazoan serotonergic systems [14, 15].
3. Serotonin as a Regulatory Molecule in Animals
This happy hormone, as recalled by Dr. Barnes [16], plays a modulatory role in almost every physiological function and is involved in many biological processes [2, 17]; furthermore, the three related metabolites, 5HT, tryptophan, and melatonin, are important regulators of feed intake, reproduction, immunity, neurological function, and antistress responses [18].
Serotonin is involved in natural reward-related physiology and behaviour, from feeding to sexual activity [19] with many actions correlated to the involved location (cellular-tissue-organ concentration) and the different signaling can also be associated with its more than fourteen receptor subtypes, regulating physiological processes through different, even opposing mechanisms; these indoleamine effects include also serotonylation and interaction with GTPases [2, 14, 15]. Serotonin influences body temperature, breathing rhythms (respiratory system), heart rate (cardiovascular function in general), eating and bowel motility (gastrointestinal system), ejaculatory latency and bladder control, muscle contraction/relaxation and locomotion, sleep, arousal, pain and sensory perception, emotions, and cognition [2, 5, 20] with a well-known signaling role in immune cells [21].
4. Serotonin in Central Nervous System
Serotonergic neurons, first discovered in the brainstem by Dahlström and Fuxe in 1964 [22], release 5-HT throughout the CNS [23, 24] as expected after the brain serotonin discovery [25]. 5-HT cell bodies are mainly localized in the raphe nuclei with their axons innervating almost every brain region [17]. The hippocampus is a principal target of serotonergic afferents along with all the limbic system [26].
The serotonin projections to hippocampus stem in a topographic order from the midbrain dorsal and median raphe nuclei [27–29]. The rat ventral hippocampus receive moderately dense projections from the caudal dorsal raphe and essentially none from the rostral dorsal raphe, with fine serotonergic axons and small varicosities widely distributed throughout the hippocampus. Furthermore, beaded serotonergic axons with large, spherical varicosities are also found in hippocampus; median raphe nucleus predominantly innervate the stratum lacunosum moleculare of the CA1 and CA3 regions and the dentate hilus [26, 28, 30, 31]. The density of serotonergic axons is highest in CA3, lower in dentate gyrus and lowest in CA1 [26, 30]. Almost all subtypes of serotonin receptors are expressed in hippocampus during ontogeny, so the regulation of the serotonergic system is more than complex [32, 33].
5. Serotonin Receptors
Heterogeneity in serotonin receptors was established by the late 1950s, with Gaddum and Picarelli [34] proposing two tryptamine receptors in the guinea-pig ileum: M and D, blocked with morphine and dibenzyline, respectively; binding to serotonin receptors was also studied with [3H] 5-HT and [3H] LSD [35, 36] and more than twenty years later a new classification was proposed by Peroutka and Snyder (1979): 5-HT1 and 5-HT2 receptors based on radioligand binding techniques ([3H] 5-HT, [3H] LSD and [3H] spiroperidol) [37].
With the use of specific radiolabelled ligands, there was a new classification [38] proposing 5-HT3 receptors although 5-HT1-like receptors were still considered a heterogeneous entity. Others tried to adjust the new information and finally, with the advances in molecular biology, the serotonin receptors were cloned, finding more than three subtypes. The Serotonin Club Receptor Nomenclature Committee (SCRNC), reporting directly to the IUPHAR Committee for Receptor Nomenclature, described a new classification of 5-HT receptors [39]. This classification was based in different operational (selective agonists, antagonists, and ligand-binding affinities), structural (molecular structure), and transductional (intracellular transduction mechanisms) criteria.
Serotonergic receptors (Figure 3) were grouped in seven classes 5-HT1–7, all of them belonging to the G-protein-coupled receptor (GPCR) superfamily [40], except 5-HT3 which is a ligand-gated ion channel that belongs to the nicotinic acetylcholine receptor superfamily: cystein-loop transmitter gated superfamily which constitutes heteropentamers [5, 41, 42]. Particularly, subindex for the different receptors were arranged and the former 5-HT1C was renamed as 5-HT2C, for its transductional properties and molecular structure [39]. In the paper, subscript will be used for 5-HT subtype receptors after SCRNC, and normal line of type for previous findings in subtype receptor will be written.
Figure 3.
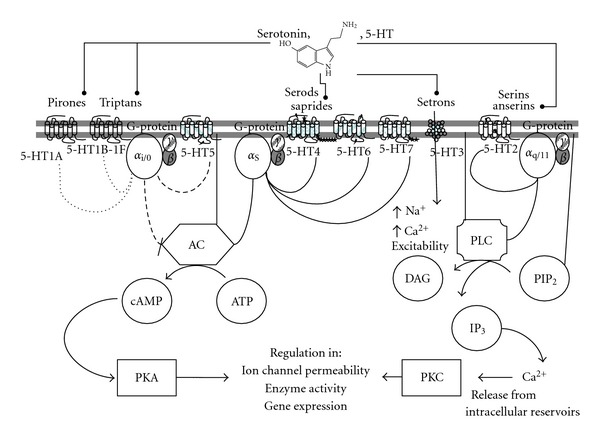
Serotonin main signaling pathways. 5-HT or agonists/antagonists for each receptor (•) interact in the extracellular side and the conformational changes of 5-HTRs modify the activity of specific intracellular enzymes, which in time modify other targets state to provoke different cellular responses [43]. G-protein βγ pathways are not represented in the figure. All of the serotonin receptor subtypes are represented for a hippocampal pyramidal cell, as reported, but subpopulations of these neurons might differentially express 5-HT receptors. AC, adenylate cyclase; PLC, phospholipase C. The 7TMD images of each subtype receptor are represented with the defined number of exons that code for the mature protein [44]; putative intron location in correspondent pre-mRNA is marked by a lightning symbol (↯), and alternative splicing sites are marked with stars (⋆⋆⋆).
6. Ion Channel Serotonin Receptor
The 5-HT3 receptor is a cation-selective ion channel which activation evokes neuronal excitation and neurotransmitter release. There are two well-recognized genes encoding A and B subunits, but additional C, D, and E genes expand the diversity to heterooligomer formation of the pentameric channel [45]. The different composition might reflect distinct pharmacology and relevance to their function representing each one a different subtype of receptor. These subunits can interact with other members of the Cys-loop superfamily, regarding the previous “M”-type serotonin of Gaddum and Picarrelli classification [46].
7. Metabotropic Serotonin Receptors
The seven transmembrane domain (7TMD) serotonin receptors belong to the “type A” family of GPCR, rhodopsin-like receptors, grouped by Fredricksson et al. (2003) in the amine receptor cluster [47]. They display a heterogeneous phylogenetic pattern with 5-HT2 forming one group and 5-HT1B-1F forming another group; the rest of 5-HT receptor subtypes can be related with other biogenic amine receptors clusters. In other classification [48], 7TMD 5-HT receptors can be grouped in type 1 family that contains GPCRs for small ligands binding in a cavity formed by TM-III to TM VI [49].
The 7TMD serotonin receptors are coupled to different G proteins. The 5-HT1 receptors couple to Gα i/Gα o proteins; the 5-HT2 receptors couple to Gα q proteins; the 5-HT4, 5-HT6 and 5-HT7 receptors couple to Gα s proteins, and the 5-HT5 receptors are related to Gα i/Gα o proteins [44].
Activation of Gα s coupled receptors (Figure 4) leads to the stimulation of adenylyl cyclases elevating cyclic AMP (cAMP), which as a second messenger interacts with other proteins including ion channels and activating the protein kinase A (PKA). This phosphorylating enzyme also activates cAMP-responsive transcription factors like CREB modifying gene expression. The interaction with other exchange proteins directly activated by cAMP leads to alternative signaling cascades besides the classical PKA. The interaction with Gα i leads to inhibition of adenylyl cyclases, decreasing production of cAMP [5].
Figure 4.
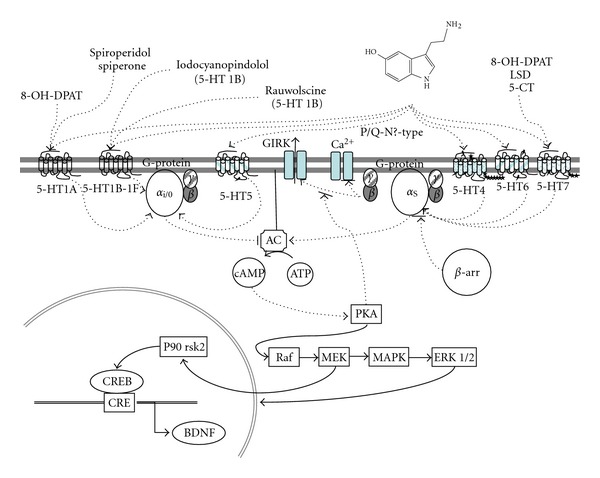
cAMP signaling pathways. Serotonin receptors 5-HT1 and 5-HT5 interact with α i/0 G-protein inhibiting the formation of cyclic adenylate monophosphate (cAMP) by adenylate cyclase (AC), while 5-HT4, 5-HT6, and 5-HT7 activate AC by means of α S G-protein. βγ subunits of G-protein may interact in other signaling pathways, for example, modulating GIRK or calcium voltage gated channels. Representation of β-arrestin (β-arr) is made to indicate other signaling pathways. Traditional ligands to study different subtype receptors are written in the extracellular zone; note that 5-HT7 may bind the traditional ligand for 5-HT1A as well as LSD (5-HT2 ligand).
The activation of Gα q/11 coupled receptors (Figure 5) lead to the hydrolysis of membrane phosphoinositides resulting in the formation of diacyl glycerol (DAG) and inositol phosphates (IP3). IP3 can interact with the calcium reservoirs, elevating intracellular levels and activating protein kinase C [5, 50]. Serotonin receptors may also be coupled to Gα 12/13, mediating structural changes within the cell through activation of the Rho signaling pathway [41].
Figure 5.
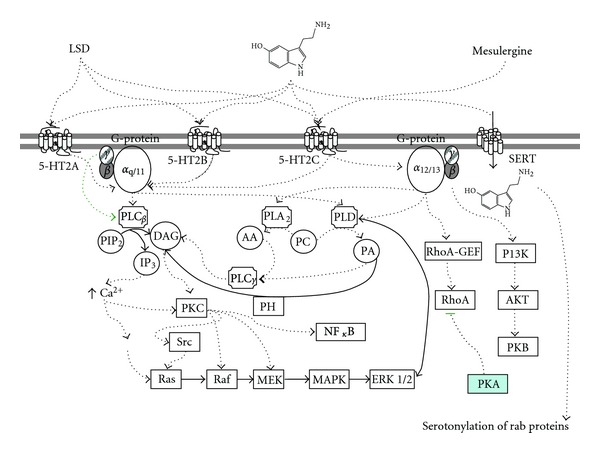
5-HT2 receptors signaling. Main pathways of intracellular signaling for these serotonine receptors subtype involve rupture of membrane phospholipids, particularly with phospholipase C (PLC) producing diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3) from phosphatidylinositol 4,5-bisphosphate (PIP2). These second messengers activate protein kinase C (PKC) which in time may activate the extracellular signal-regulated kinases 1 and 2 (ERK1/2) [50]. Phospholipase A2 is eventually activated producing arachidonic acid (AA) from phosphatidylcholine (PC), or phosphatidic acid (PA) by means of phospholipase D (PLD) [44, 50]. SERT is included in the diagram, coexisting in astrocytes for example, to emphasize the intracellular participation of serotonin itself [14]. Other pathways including (Rho-GEF) and (PI3K) are shown [51]. MEK, mitogen-activated protein kinase; PH, phosphohydrolase enzyme; PKA, protein kinase A-relation to cAMP pathways; SERT, serotonin transporter.
The Gβγ dimeric subunit can interact with a variety of enzymatic effectors within the cell, like their action on gated ion channels, regulation of particular isoforms of adenylyl cyclase and phospholipase C, and phosphoinositide-3-kinase isoforms (and ERK signaling) [51].
If so many receptor subtypes of serotonin make it complex to understand, plethora of activities can be found with the coupling to multiple G-proteins. There are different parameters in the activation pathway of the GPCR receptors, considering multiple states instead of the traditional two-state model of activation and forming dimers that may have distinct pharmacology with respect to activation, signaling, and internalization and the organization in microdomains at the membrane level that may affect coupling and trafficking of G-proteins [52].
Promiscuous coupling of GPCRs to G-proteins is not a surprise, and they can also signal without coupling to them; they can activate a variety of cascades by arrestin-ergic signalling, beside the original function of these proteins in terminating coupling and endocytosis [53, 54].
In brief, there are thirteen genes coding for GPCR serotonin receptors that may couple almost every G-protein in the cell membrane and probably act without coupling to them, and two recognized genes coding for the subunits of cation-selective 5-HT3 ligand-gated ion channel pentameric receptor.
This diversity is further complexed by the posttranslational and co/posttransductional modifications of the protein to be produced, without talking about oligomerization of the serotonin receptors and single-nucleotide polymorphisms. There are examples of this modifications in the different receptor families with alternative splicing, RNA editing, palmitoylation, glycosylation, phosphorylation, and proteolysis, to mention a few [55].
8. Serotonin Receptors Expression in Hippocampus
All the serotonin receptor families are remarkably expressed in hippocampus, which is part of the limbic system, a whole structure related with memory processing, emotional association with memory, judgment, affect, and motivation or the organization of planned actions [26]. The innervation of serotonergic pathways in hippocampus and the diverse expression of serotonin receptors in this brain area reflect the overall functions related to 5-HT, in particular with cognition, mood and food intake. After recognition of hippocampal serotonergic afferents by histochemical methods (fluorescence, potassium dichromate), uptake of tritiated serotonin was achieved corroborating the wide spread of 5-HT pathways [56]. Molecular biology of the specific receptors for serotonin confirmed this knowledge.
8.1. 5-HT1 Receptors
The hippocampus contains a high density of 5-HT1 sites, most of which belong to the 5-HT1A subtype [39]. Before classification of serotonin receptors on the basis of their molecular biology, distinction between the receptors in this group was based on the affinities for 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) distinguishing 5-HT1A, lysergic acid diethylamide (LSD) and mesulergine detecting 5-HT1C, later renamed as 5-HT2C, and rauwolscine for 5-HT1D receptors, for example, but findings of new receptors with affinity for these ligands may clarify error in quantitation of the former groups.
8.2. 5-HT1A
Fargin et al. characterized the genomic clone G-21 that corresponded to 5-HT1A sequence [57]. Gozlan et al. (1983) [58] had previously reported the existence of 5-HT1—like receptors in hippocampus on the basis of the binding experiments of [3H] 8-OH-DPAT. In 1986, Hoyer et al. [59] and Vergé et al. [60] confirmed these results and compared binding of 5-HT1A and 5-HT1B; later characterization was performed by chromatographic analyses of the serotonin 5-HT1A receptor solubilized from the rat hippocampus [61]. Activation of somatodendritic autoreceptors diminished 5-HT synaptic transmission [62] suggesting that 5-HT1A might represent presynaptic receptors as well as postsynaptic neurotransmission in hippocampus. At cellular levels, 5-HT1A receptors are located postsynaptically in pyramidal and granular neurons of the hippocampus as well as extrasynaptic structures, by studies using highly selective 5-HT1A antibodies that allowed confirmation and refinement of autoradiographic results [41]. They function as somatodendritic inhibitory receptors in raphe nuclei and presynaptically in hippocampus [63]. 5-HT1A has also been detected in some astrocytes, radial glia, and ependymal and endothelial cells [64].
8.3. 5-HT1B
Molecular cloning of rat 5-HT1B receptor was performed by Voigt et al. in 1991 [65]. Previously, 5-HT1B was defined as the nonspiperone sensitive [3H]5-HT binding in brain [41]; localization of 5-HT1B was described with low densities in hippocampus (gyrus dentatus > CA1 ≥ CA3) by affinity differences with [3H] 8-OH-DPAT [60] and binding studies with [125I]iodocyanopindolol [66]. Immunohistochemistry analysis had also shown coexpression of 5-HT1B in hippocampal cells with other serotonin receptors [67]. 5-HT1B receptors are responsible for the presynaptic inhibition of neurotransmission at the local synapses between axon collaterals of CA1 pyramidal cells and other CA1 pyramidal neurons and interneurons [68]. Projection neurons from hippocampus reach the bed nucleus of the stria terminalis, where presynaptic 5-HT1B receptors are involved in the inhibition of glutamate transmission [69]. Furthermore, 5-HT1B hippocampal GABAergic axon terminal heteroreceptors inhibit neurotransmitter release [70].
8.4. 5-HT1D
Hamblin and Metcalf in 1991 [71] described sequence of human 5-HT1D serotonin receptor and two genes known as 5-HT1Da and 5-HT1Db were reported [72]. It was clear later that 5-HT1Db was the homologue receptor of rat 5-HT1B, so called 5-HT1B. Operational profiles between the former 5-HT1Da and 5-HT1Db receptors were almost indistinguishable, and similarities are still very present [41]. 5-HT1Da remained as the homologue of rat 5-HT1D, and so-called 5-HT1D. 5-HT1D binding sites resemble those of 5-HT1B receptors in hippocampus with very low presence [41, 73]. 5-HT1B/1D receptors are found at pre- and postsynaptic sites but presynaptic receptors are predominantly located on 5-HT hippocampal nerve terminals [63].
8.5. 5-HT1E
There is not a clear characterization of 5-HT1E due to the lack of specific ligands that might differentiate this receptor subtype; furthermore, expression of 5-HT1E has not been found in rodents, because there is a stop codon in the correspondent mRNA [41]. Cloning of this receptor was achieved using cDNA synthesized from monkey cortex and human hippocampal cDNA library [74] though confirming its presence in hippocampus, previously reported by the existence of a 5-HT1E subtype in human brain with findings in radioligand studies [75].
8.6. 5-HT1F
When 5-HT1F was found [76], it was designated as 5-HT1Eb due to its related pharmacological profile; 5-HT1F-labeling was moderate in granule cells of the dentate gyrus and hippocampal pyramidal cells in CA1–CA3, confirming its expression in hippocampus [77].
8.7. 5-HT2 Receptors
Receptors from this group were originally recognized by ligands like ketanserin, mesulergine, LSD, and spiperone, which were reported to have high affinities for 5-HT2 receptors compared to 5-HT1 group [78]. These receptors are coupled to phosphatidylinositol hydrolysis although some effects may involve intracellular calcium release via an independent mechanism [79]. Hoyer et al. [80] used Ketanserin binding though localizing 5-HT2 receptors recognition sites in hippocampus.
8.8. 5-HT2A
On the basis of the similarity in exerting the cellular effects which reflected the structural relationship with the former 5-HT1C receptor, Pritchett et al. (1988) used oligonucleotides encoding this serotonin receptor and found 5-HT2A sequence [81]. Julius et al. (1990) also found an encoding sequence for 5-HT2 which was expressed in hippocampus in a 10-fold lower level than in rat cortex [82]. The 5-HT2A receptor refers to the classical D receptor described by Gaddum and Picarelli in 1957 and defined later as 5-HT2 by Peroutka and Snyder in 1979 [37]. 5-HT2A expression in human hippocampus was confirmed with RT-PCR technique [83]. Immunoreactivity for 5-HT2A receptor in hippocampus was found primarily in the pyramidal cell layer of CA1–CA3 and in the granular layer of dentate gyrus [84]. Agonist studies with 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) indicate postsynaptic receptors for 5-HT2A [63]; in prelimbic prefrontal cortex, most 5-HT2A receptors were postsynaptically located, but presynaptic axons and varicosities locations were found [85]. Cellular localization of 5-HT2A receptors in astrocytes has been found in hippocampus [86].
8.9. 5-HT2B
The “last” 5-HT2-like receptor subtype to be cloned was 5-HT2B [87] from rat stomach fundus. The origin and comparable sequence to 5-HT1C/2 led them to designate it as 5-HT2F (for fundus) and renamed as 5-HT2B after consensus of SCRNC in 1994. Cloned human 5-HT2B receptors had a high degree of homology with mouse and rat receptors although with higher affinity for ketanserin and a lower affinity for yohimbine; it was found at very low presence in the whole brain [88]. Expression of 5-HT2B receptors in cultured astrocytes from hippocampus with Ca2+ increases after stimulation with alpha-methyl 5-HT has been reported [89]. The presence of this receptor in astrocytes was verified with immunohistochemistry and westernblot analysis. Furthermore, microglial cell cultures expresses 5-HT2B receptors, and they are involved in the regulation of inflammatory cytokine production from blood cells [90].
8.10. 5-HT2C
Lübbert and colleagues cloned in 1987 [91] the mouse 5-HT1C-mRNA (actually 5-HT2C) extracted from choroid plexus tumors; Julius et al. (1988) characterized a cDNA encoding this protein and confirmed the receptor expression in neurons of many regions of central nervous system by in situ hybridization and RNA blot analysis [92]. It was first identified in porcine choroid plexus on the basis of its pharmacological properties [93] and localized by autoradiographic mapping in rat [94] and human brain, particularly in hippocampus [80].
The overall distribution of 5-HT2C receptor was reported by several studies with mRNA in situ hybridization [95–98]. The specificity of radioligand binding ([3H] mesulergine) was compared with in situ hybridization by Mengod et al. (1990), finding high signal in the pyramidal layer of the CA3 field of rostral rodent hippocampal formation, while intense hybridization was found in the strata oriens and radiatum of the caudal CA1 area and in the ventral subiculum [97]. Furthermore, Abramowski et al. (1995) compared [3H] mesulergine binding with specific antibody-binding in rat and human brain [99]; Clemett et al. (2000) also studied the presence of 5-HT2C protein with immunohistochemistry and western blotting with abundant expression in rat hippocampus [100].
8.11. 5-HT3 Receptors
5-HT3 receptor belongs to the ligand-gated ion channel superfamily and corresponds to the M receptor of Gaddum and Picarelli [41, 101]; five subunits have been cloned although only 5-HT3A and 5-HT3B are recognized for rodents [102–106]. The various subtypes of 5-HT3 may well-correspond to the pentameric heterodimer assembled between all subunits and their splice variants, and also with other members of the cys-loop superfamily, like a4-nAChR nicotinic receptor [46, 107] although this association has not been detected in porcine native 5-HT3 brain receptors [108]. On the contrary, association and coimmunoprecipitation of 5-HT3 and P2X2 ATP-gated channels has been reported [109].
All subunits have been found mainly in human intestine [110]. 5-HT3 mRNA was found in rat hippocampus primarily on interneurons, mediating indirect inhibitory effects on pyramidal neuron populations [111]. On the contrary, 5-HT3 was found in human hippocampus with predominant immunoreactivity associated with pyramidal neurons in CA2 and CA3; transcripts were also identified so hippocampal cells can produce 5-HT3A and 5-HT3B functionally isoforms of this ion channel [112].
8.12. 5-HT4 Receptors
The 5-HT4 receptor was first described in the central nervous system [113] stimulating adenylate cyclase; with some useful radioligands, it was showed to be distributed in hippocampus. It was cloned [114] and mRNA was localized in hippocampus by in situ hybridization [115].
The 5-HT4 receptor gene is very complex and has several possible splice variants; there are at least nine receptor splice variants reported with a number of carboxy-terminal variants but no difference in affinity for agonists or antagonists [41]. There is evidence that suggests that 5-HT4 receptor activity enhances cognition and provides neuroprotection, particularly on hippocampal effects [116]; 5-HT4 receptors on hippocampal cholinergic axon terminals are neurotransmitter release facilitating [70].
8.13. 5-HT5 Receptors
The 5-HT5 receptor group consists of two members: 5-HT5A and 5-HT5B; human 5-HT5B has been described, but it fails to encode a functional protein due to the presence of stop codons in the sequence [117–119]. They still lack physiological correlation, in part for the lack of selective agonists; the transductions pathways have not been well established although negatively coupling to adenylate cyclase has been reported [41, 43, 120].
8.14. 5-HT5A
Cloning and distribution of 5-HT5A receptor has been reported, finding high concentration in hippocampus [119, 121, 122]. Although this receptor is a well-recognized GPCR protein, the negatively coupling to adenylated cyclase is not well established [120, 123–125], and furthermore, its coupling to multiple signal transduction pathways has been reported [126]. The 5-HT5A receptor is expressed predominantly by astrocytes with very weak neuronal immunoreactivity [120].
8.15. 5-HT5B
Cloning and distribution of 5-HT5B receptor has been reported as well, finding this receptor in hippocampus [119, 127]. The levels of expression of 5-HT5B mRNA in hippocampus were high, with predominant expression in CA1 pyramidal cells [128]. It is a pseudogene in man [129], and it has been proposed that the upregulation found (particularly in hippocampus) for mice 5-HT5B receptor, in response of social isolation stress, might be undertaken in humans by another receptor like 5-HT5A [130].
8.16. 5-HT6 Receptors
Ruat et al. (1993) cloned 5-HT6 receptor [131], starting from the sequence of rat histamine H2 receptor with two transcripts evidenced. mRNA was detected in hippocampus and in transfected COS-7 cells 5-HT6 receptor was positively coupled to adenylate cyclase. Hybridization signal of 5-HT6 mRNA was detected in CA1, CA2, and CA3 fields of hippocampus as well as in dentate gyrus [128].
8.17. 5-HT7 Receptors
Ruat et al. (1993) also cloned the putative 5-HT7 receptor and localized it at hippocampus [132]. It is differentially expressed in CA1 cells preferentially localized on the cell body but absent in interneurons [133]. The expression in the limbic areas suggests that these receptors mediate serotoninergic controls in functions like mood, learning, or neuroendocrine and vegetative behaviors. The emerging functions of hippocampus involve several neurotransmitter networks, where 5-HT7 receptors can be functioning. AMPA receptor-mediated transmission between CA3 and CA1 pyramidal neurons is enhanced postsynaptically by 5-HT7, while 5-HT1A receptors inhibit this transmission both pre- and postsynaptically [134].
9. Serotonergic Modulation in Hippocampus
Among the various major neurotransmitter signaling, like monoaminergic, glutamatergic, and nitrergic neurotransmitter systems that might be involved in some plastic modifications of hippocampus particularly after stress exposure [135], serotonergic system is very interesting for its complexity and regulation.
Almost all pre- and postsynaptic serotonin receptors have been identified in hippocampus; furthermore, the 5-HT transporter (SERT, 5-HTT) plays a key role in serotonergic neurotransmission, and it is condition-regulated in hippocampus [136, 137]. In addition, tryptophan hydroxylase (TPH), the rate-limiting enzyme for producing serotonin, plays another key role in the regulation of this system; TPH1 and TPH2 have been found in hippocampus [138]. The other key enzyme in serotonergic system is monoamine oxidase A, responsible for 5-HT degradation [139], expressed in hippocampus as well.
Regulation of serotonin system is very important and disturbances in this matter are related to anatomical, functional and behavioural anomalies, including neurologic and psychiatric disorders as obsessive-compulsive disorder, bulimia, chronic impulsivity, obesity and drug addiction, aggression, -major- depression, suicide, anxiety, schizophrenia, mania, autism, Alzheimer's disease and also sudden infant death syndrome [43, 139–142].
The function of serotonin as neurotransmitter seems to be developed at last in evolution, and ionotropic channels are related to rapid neuronal activation, particularly in enteric nervous system [4]. Serotonin, as metabotropic effector, has been recognized as a trophic factor, particularly during development including morphogenetic activities as cell proliferation, migration and differentiation [137, 143]; during adulthood, depletion in serotonin decreases neurogenesis in the dentate gyrus [144] though 5-HT plays a critical role in the neuronal organization of the hippocampus [145].
Several metabotropic effects of serotonin have been related to brain-derived neurotrophic factor (BDNF) expression [144] and BDNF itself promotes the development and function of serotonergic neurons [140]. This kind of interaction between neurotrophic factors and neurotransmitters has been reported also with steroids; the regulation of HPA axis by serotonin and vice versa is well documented [146, 147]; sexual steroids have this intricate correlation as well [148]. The key for understanding these relationships is the existence of multiple receptors and ligand interaction for molecular signaling.
On the other hand, hippocampus-dependent memory formation uses long-term potentiation (LTP) as a pivotal role. Cross-talk between the cAMP signal transduction system and LTP has been reported, with a critical linkage between Ca2+ and cAMP signaling [149]. At this level, all of the serotonin receptors seem to be directly involved in the normal function of hippocampus in mood regulation and memory formation; neurogenesis is thought to be one of the involved processes for long lasting changes related to hippocampal function, particularly because dentate gyrus is one of the prominent areas of adult brain neurogenesis [150].
The 5-HT1A is the most likely involved receptor in regulation of neurogenesis in the dentate gyrus [150]; it is expressed on raphe serotonin neurons as an autoreceptor [151], acting as a negative regulator of neuronal activity in presynaptic locations in hippocampus, with very important function in the balance of serotonin reservoirs. 5-HT1A also inhibits neuronal firing, activating G-protein-gated inwardly rectifying potassium (GIRK) currents and inhibiting Ca2+ channels [44]; it is involved in the inhibition of long-term potentiation (LTP) by the inhibition of NMDA function [152].
As one of the most “important” members of serotonin receptors, 5-HT1A receptor is the best characterized and its ligands are used extensively. The mutant (knockout) mice lacking this receptor exhibits enhanced anxiety-related behaviour [153, 154]. The “specific” 5-HT1A ligand 8-OH-DPAT has been used to establish the roles of this receptor as trophic factor and in neurotransmission as well, but 5-HTT (SERT) recognizes this ligand and likewise modulates anxiety-related behaviour [136, 155].
The therapeutic effects of serotonin-selective reuptake inhibitors (SSRI), “specifically” acting on SERT function, are well documented, and several theories are proposed to explain the retarded actions in successfully treated patients [156–158]. SSRIs are the most widely prescribed class of antidepressants, which increases synaptic levels of 5-HT in hours or days, but exerts the therapeutic response several weeks later [159]. The increasing levels of 5-HT cause a desensitization of 5-HT1A autoreceptors with a lesser inhibition caused by this receptor in raphe neurons, leading to a facilitation of 5-HT signaling [160]. There is a differential response of SSRI's desensitizing 5-HT1A presynaptic or postsynaptic receptors; the specific serotonin receptor antagonist WAY 100635 also promotes differential changes in autoreceptors compared to postsynaptic 5-HT1A receptors [160, 161].
SERT and 5-HT1A are the most studied therapeutic targets although several serotonin receptors are involved in hippocampus activities, particularly 5-HT4, 5-HT6, and 5-HT7 that activate cAMP signaling increasing CREB, which may increase the expression of BDNF [150]. Furthermore, 5-HT4 activation may cause a faster direct activation of 5-HT neurons, increasing their firing and causing desensitization of 5-HT1A [159]. 5-HT2 receptors involve an alternative signaling pathway to cAMP, where increasing Ca2+ levels is of particular importance, relying on the crosstalk between cAMP signaling and Ca2+-regulated adenylyl cyclases. Knockout phenotype for 5-HT2A shows decreased, anxiety while the one for 5-HT2C shows increased appetite, overweight, and cognitive impairment. Serotonin receptor 5-HT2C is probably the most important receptor related to food intake and energy balance (satiety and obesity), with viable targeting for weight control [20].
The most representative neurotransmitter receptor for serotonin in rapid actions is the ionotropic 5-HT3, which is also involved in LTP modulation in hippocampus [162]. The knockout phenotype for 5-HT3A has reduced pain perception and variants of the 5-HT3A receptor have been associated with bipolar disorder and schizophrenia [43].
Serotonergic neuronal-glial interactions (Figure 6) have been proposed to play a significant role in the development of several CNS pathologies [163]. Some serotonin receptors are mainly expressed in glia. 5-HT5A correlates with astrocyte maturity and activity, increasing its levels after induced gliosis [120] although its expression in pyramidal cells of hippocampus has been reported [117]. Addition of cAMP analogues to astrocyte cultures decreases 5-HT1A expression and increases 5-HT5A, therefore suggesting a direct neuronal regulation of astrocyte homeostasis, as cAMP intracellular increases might activate and sensitize astrocytes to respond at serotonin signaling from neurons that can supress gliosis in vivo [120].
Figure 6.
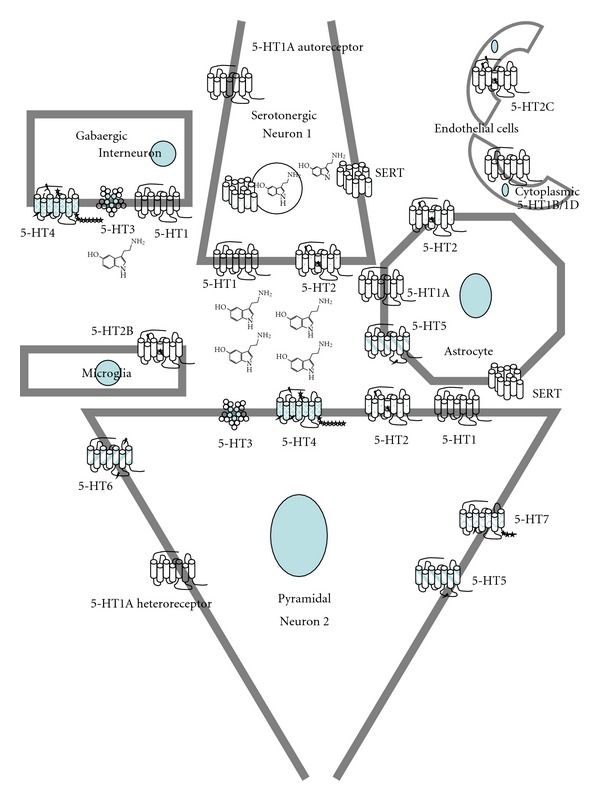
Serotonin receptors in hippocampus. The functional glia-neuron-vascular cells network uses several serotonin receptors (5-HTRs). The 7TMD images of each subtype receptor are represented with the defined number of exons that code for the mature protein (Bockaert et al., 2006) [44]; putative intron location in correspondent pre-mRNA is marked by a lightning symbol (↯); alternative splicing sites are marked with stars (⋆⋆⋆). Neuron metabotropic 5-HTRs are mainly somatodendritic volume receptors although there is an association with synaptic specializations for some of them. 5-HT3 with the five 4TMD subunits of a ligand activated ion channel is shown as synaptic receptor although this fact remains to be determined in hippocampus. Microglia is also included in the network for its relevance in pathophysiological responses, with 5-HT2B receptor expression (Capone et al., 2007) [90]. The 12TMD image of the serotonin transporter (SERT; 5-HTT) and vesicular monoamine transporter (VMAT) are represented in the serotonergic neuron and only SERT in the astrocyte.
Each cell type can modify its serotonin receptor expression depending on the differentiation time and relationship in a particular network. Mouillet-Richard et al. (2000) have shown the differentiating changes than inducted serotonergic 1C11∗5HT cells can exhibit [164], sequentially expressing three different serotonin receptor subtypes (5-HT1B/1D, 5-HT2B, and 5-HT2A). Although cell cultures do not represent reliable conditions of in vivo differentiation, they help us understand how cells can adapt to changing media. The 5-HT2 receptors are referred to as programmable receptors that may not influence development although this process affect their number, affinity, or function; the coupling efficiency of the receptor may change in time, in correlation to a developmental change of phosphatidylinositol hydrolysis-second messenger system [165].
In conclusion, the specific changes that modulate serotonin signaling can be performed by serotonin itself; the levels of serotonin that can be reached in the synapses, or as a volume transmission, is of outstanding importance to understand the rate of change in the 5-HT signaling itself, time of action might conduce to one response or the contrary, considering that all the cell types in hippocampus are involved in this modulation and function. Serotonin can act directly into neuron and glia after SERT incorporation, an ancient function for this biogenic amine and probably with more importance during development.
Conflict of Interests
The authors do not have conflict of interests. Support for publication is received from Universidad Autónoma de Querétaro.
Acknowledgments
The authors appreciate the suggestions of Jesica Escobar in the preparation of this paper. They would also like to acknowledge Salvador and Diego Lecona for editing the English content of this paper.
References
- 1.Rapport MM, Green AA, Page IH. Serum vasoconstrictor (serotonin). IV. Isolation and characterization. The Journal of Biological Chemistry. 1948;176:1243–1251. [PubMed] [Google Scholar]
- 2.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annual Review of Medicine. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erspamer V, Asero B. Identification of enteramine, the specific hormone of the enterochromaffin cell system, as 5-hydroxytryptamine. Nature. 1952;169(4306):800–801. doi: 10.1038/169800b0. [DOI] [PubMed] [Google Scholar]
- 4.Turlejski K. Evolutionary ancient roles of serotonin: long-lasting regulation of activity and development. Acta Neurobiologiae Experimentalis. 1996;56(2):619–636. doi: 10.55782/ane-1996-1167. [DOI] [PubMed] [Google Scholar]
- 5.Nichols DE, Nichols CD. Serotonin receptors. Chemical Reviews. 2008;108(5):1614–1641. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- 6.Park S, Kang K, Lee SW, Ahn MJ, Bae JM, Back K. Production of serotonin by dual expression of tryptophan decarboxylase and tryptamine 5-hydroxylase in Escherichia coli. Applied Microbiology and Biotechnology. 2011;89(5):1387–1394. doi: 10.1007/s00253-010-2994-4. [DOI] [PubMed] [Google Scholar]
- 7.Rudnick G. What is an antidepressant binding site doing in a bacterial transporter. ACS Chemical Biology. 2007;2(9):606–609. doi: 10.1021/cb7001818. [DOI] [PubMed] [Google Scholar]
- 8.Yanofsky C. RNA-based regulation of genes of tryptophan synthesis and degradation, in bacteria. RNA. 2007;13(8):1141–1154. doi: 10.1261/rna.620507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazcano A. Historical development of origins research. Cold Spring Harbor Perspectives in Biology. 2010;2(11) doi: 10.1101/cshperspect.a002089. Article ID a002089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy DL, Fox MA, Timpano KR, et al. How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology. 2008;55(6):932–960. doi: 10.1016/j.neuropharm.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buznikov GA, Lambert HW, Lauder JM. Serotonin and serotonin-like substances as regulators of early embryogenesis and morphogenesis. Cell and Tissue Research. 2001;305(2):177–186. doi: 10.1007/s004410100408. [DOI] [PubMed] [Google Scholar]
- 12.Essman EJ. The serotonergic system in Tetrahymena pyriformis. La Ricerca in Clinica e in Laboratorio. 1987;17(1):77–82. [PubMed] [Google Scholar]
- 13.Csaba G. Presence in and effects of pineal indoleamines at very low level of phylogeny. Experientia. 1993;49(8):627–634. doi: 10.1007/BF01923943. [DOI] [PubMed] [Google Scholar]
- 14.Paulmann N, Grohmann M, Voigt JP, et al. Intracellular serotonin modulates insulin secretion from pancreatic β-cells by protein serotonylation. PLoS Biology. 2009;7(10) doi: 10.1371/journal.pbio.1000229. Article ID e1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mercado CP, Ziu E, Kilic F. Communication between 5-HT and small GTPases. Current Opinion in Pharmacology. 2011;11(1):23–28. doi: 10.1016/j.coph.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnes NM. 5-HT: the promiscuous and happy hormone! editorial overview. Current Opinion in Pharmacology. 2011;11(1):1–2. doi: 10.1016/j.coph.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Charnay Y, Léger L. Brain serotonergic circuitries. Dialogues in Clinical Neuroscience. 2010;12(4):471–487. doi: 10.31887/DCNS.2010.12.4/ycharnay. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao K, Fang J, Yin YL, Feng ZM, Tang ZR, Wu G. Tryptophan metabolism in animals: important roles in nutrition and health. Frontiers in Bioscience. 2011;3:286–297. doi: 10.2741/s152. [DOI] [PubMed] [Google Scholar]
- 19.Hayes DJ, Greenshaw AJ. 5-HT receptors and reward-related behaviour: a review. Neuroscience and Biobehavioral Reviews. 2011;35(6):1419–1449. doi: 10.1016/j.neubiorev.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Feijó FM, Bertoluci MC, Reis C. Serotonin and hypothalamic control of hunger: a review. Revista da Associação Médica Brasileira. 2011;57(1):74–77. [PubMed] [Google Scholar]
- 21.Ahern GP. 5-HT and the immune system. Current Opinion in Pharmacology. 2011;11(1):29–33. doi: 10.1016/j.coph.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dahlström A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964;20(7):398–399. doi: 10.1007/BF02147990. [DOI] [PubMed] [Google Scholar]
- 23.Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6(4):557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- 24.Wisden W. Cre-ating ways to serotonin. Frontiers in Neuroscience. 2010;4:p. 167. doi: 10.3389/fnins.2010.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Twarog BM, Page IH. Serotonin content of some mammalian tissues and urine and a method for its determination. The American Journal of Physiology. 1953;175(1):157–161. doi: 10.1152/ajplegacy.1953.175.1.157. [DOI] [PubMed] [Google Scholar]
- 26.Hensler JG. Serotonergic modulation of the limbic system. Neuroscience and Biobehavioral Reviews. 2006;30(2):203–214. doi: 10.1016/j.neubiorev.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Molliver ME. Serotonergic neuronal systems: what their anatomic organization tells us about function. Journal of Clinical Psychopharmacology. 1987;7(supplement 6):3S–23S. [PubMed] [Google Scholar]
- 28.Freund TF, Gulyás AI, Acsády L, Görcs T, Tóth K. Serotonergic control of the hippocampus via local inhibitory interneurons. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(21):8501–8505. doi: 10.1073/pnas.87.21.8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bijak M. Monoamine modulation of the synaptic inhibition in the hippocampus. Acta Neurobiologiae Experimentalis. 1996;56(1):385–395. doi: 10.55782/ane-1996-1142. [DOI] [PubMed] [Google Scholar]
- 30.Mamounas LA, Mullen CA, O’Hearn E, Molliver ME. Dual serotoninergic projections to forebrain in the rat: morphologically distinct 5-HT axon terminals exhibit differential vulnerability to neurotoxic amphetamine derivatives. Journal of Comparative Neurology. 1991;314(3):558–586. doi: 10.1002/cne.903140312. [DOI] [PubMed] [Google Scholar]
- 31.Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. Journal of Comparative Neurology. 1991;313(4):643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- 32.García-Alcocer G, Sarabia-Altamirano G, Martínez-Torres A, Miledi R. Developmental expression of 5-HT5A receptor mRNA in the rat brain. Neuroscience Letters. 2005;379(2):101–105. doi: 10.1016/j.neulet.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 33.García-Alcocer G, Segura LCB, Peña MG, Martínez-Torres A, Miledi R. Ontogenetic distribution of 5-HT2C, 5-HT5A, and 5-HT7 receptors in the rat hippocampus. Gene Expression. 2005;13(1):53–57. doi: 10.3727/000000006783991935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaddum JH, Picarelli ZP. Two kinds of tryptamine receptor. British Journal of Pharmacology and Chemotherapy. 1957;12(3):323–328. doi: 10.1111/j.1476-5381.1957.tb00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bennett JL, Aghajanian GK. D LSD binding to brain homogenates: possible relationship to serotonin receptors. Life Sciences. 1974;15(11):1935–1944. doi: 10.1016/0024-3205(74)90044-7. [DOI] [PubMed] [Google Scholar]
- 36.Fillion G, Fillion MP, Jacob J, Rousselle JC. 5 HT and LSD high affinity binding sites to brain synaptosomal membranes. British Journal of Pharmacology. 1976;58(3):425P–426P. [PMC free article] [PubMed] [Google Scholar]
- 37.Peroutka SJ, Snyder SH. Multiple serotonin receptors: differential binding of [3H]5-hydroxytryptamine, [3H]lysergic acid diethylamide and [3H]spiroperidol. Molecular Pharmacology. 1979;16(3):687–699. [PubMed] [Google Scholar]
- 38.Bradley PB, Engel G, Feniuk W. Proposals for the classification and nomenclature of functional receptors for 5-hydroxytryptamine. Neuropharmacology. 1986;25(6):563–576. doi: 10.1016/0028-3908(86)90207-8. [DOI] [PubMed] [Google Scholar]
- 39.Hoyer D, Clarke DE, Fozard JR, et al. International union of pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacological Reviews. 1994;46(2):157–203. [PubMed] [Google Scholar]
- 40.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological adn functional diversity of 5-HT receptors. Pharmacology Biochemistry and Behavior. 2002;71(4):533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 41.Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behavioural Brain Research. 2008;195(1):198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 42.Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology. 2009;56(1):2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Filip M, Bader M. Overview on 5-HT receptors and their role in physiology and pathology of the central nervous system. Pharmacological Reports. 2009;61(5):761–777. doi: 10.1016/s1734-1140(09)70132-x. [DOI] [PubMed] [Google Scholar]
- 44.Bockaert J, Claeysen S, Bécamel C, Dumuis A, Marin P. Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell and Tissue Research. 2006;326(2):553–572. doi: 10.1007/s00441-006-0286-1. [DOI] [PubMed] [Google Scholar]
- 45.Barnes NM, Hales TG, Lummis SCR, Peters JA. The 5-HT3 receptor-the relationship between structure and function. Neuropharmacology. 2009;56(1):273–284. doi: 10.1016/j.neuropharm.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Hooft JA, Spier AD, Yakel JL, Lummis SCR, Vijverberg HPM. Promiscuous coassembly of serotonin 5-HT3 and nicotinic α4 receptor subunits into Ca2+-permeable ion channels. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(19):11456–11461. doi: 10.1073/pnas.95.19.11456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fredriksson R, Lagerström MC, Lundin LG, Schiöth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Molecular Pharmacology. 2003;63(6):1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 48.Kumari T, Pant B, Pardasani KR. A model for the evaluation of domain based classification of GPCR. Bioinformation. 2009;4(4):138–142. [PMC free article] [PubMed] [Google Scholar]
- 49.Bockaert J, Pin JP. Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO Journal. 1999;18(7):1723–1729. doi: 10.1093/emboj/18.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Werry TD, Gregory KJ, Sexton PM, Christopoulos A. Characterization of serotonin 5-HT2C receptor signaling to extracellular signal-regulated kinases 1 and 2. Journal of Neurochemistry. 2005;93(6):1603–1615. doi: 10.1111/j.1471-4159.2005.03161.x. [DOI] [PubMed] [Google Scholar]
- 51.Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiological Reviews. 2005;85(4):1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- 52.DeFea K. β-arrestins and heterotrimeric G-proteins: collaborators and competitors in signal transduction. British Journal of Pharmacology. 2008;153(supplement 1):S298–S309. doi: 10.1038/sj.bjp.0707508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeFea KA. β-arrestins as regulators of signal termination and transduction: how do they determine what to scaffold? Cellular Signalling. 2010;23(4):621–629. doi: 10.1016/j.cellsig.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 54.Roth BL. Irving Page lecture 5-HT(2A) serotonin receptor biology: interacting proteins, kinases and paradoxical regulation. Neuropharmacology. 2011;61(3):348–354. doi: 10.1016/j.neuropharm.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davies MA, Chang CY, Roth BL. Polymorphic and posttranscriptional modifications of 5-HT receptor structure. functional and pathological implications. In: Roth BL, editor. The Serotonin Receptors. New Jersy, NJ, USA: Humana Press; 2006. pp. 59–90. [Google Scholar]
- 56.Azmitia EC, Marovitz WF. In vitro hippocampal uptake of tritiated serotonin (3H-5HT): a morphological, biochemical, and pharmacological approach to specificity. Journal of Histochemistry and Cytochemistry. 1980;28(7):636–644. doi: 10.1177/28.7.6967079. [DOI] [PubMed] [Google Scholar]
- 57.Fargin A, Raymond JR, Lohse MJ, Kobilka BK, Caron MG, Lefkowitz RJ. The genomic clone G-21 which resembles a β-adrenergic receptor sequence encodes the 5-HT1A receptor. Nature. 1988;335(6188):358–360. doi: 10.1038/335358a0. [DOI] [PubMed] [Google Scholar]
- 58.Gozlan H, El Mestikawy S, Pichat L. Identification of presynaptic serotonin autoreceptors using a new ligand: 3H-PAT. Nature. 1983;305(5930):140–142. doi: 10.1038/305140a0. [DOI] [PubMed] [Google Scholar]
- 59.Hoyer D, Pazos A, Probst A, Palacios JM. Serotonin receptors in the human brain. I. Characterization and autoradiographic localization of 5-HT(1A) recognition sites. Apparent absence of 5-HT(1B) recognition sites. Brain Research. 1986;376(1):85–96. doi: 10.1016/0006-8993(86)90902-9. [DOI] [PubMed] [Google Scholar]
- 60.Vergé D, Daval G, Marcinkiewicz M, et al. Quantitative autoradiography of multiple 5-HT1 receptor subtypes in the brain of control or 5,7-dihidroxytryptamine-treated rats. Journal of Neuroscience. 1986;6(12):3474–3478. doi: 10.1523/JNEUROSCI.06-12-03474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El Mestikawy S, Taussig D, Gozlan H, Emerit MB, Ponchant M, Hamon M. Chromatographic analyses of the serotonin 5-HTA receptor solubilized from the rat hippocampus. Journal of Neurochemistry. 1989;53(5):1555–1566. doi: 10.1111/j.1471-4159.1989.tb08552.x. [DOI] [PubMed] [Google Scholar]
- 62.Chaput Y, Blier P, de Montigny C. In vivo electrophysiological evidence for the regulatory role of autoreceptors on serotonergic terminals. Journal of Neuroscience. 1986;6(10):2796–2801. doi: 10.1523/JNEUROSCI.06-10-02796.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muchimapura S, Mason R, Marsden CA. Effect of isolation rearing on pre- and post-synaptic serotonergic function in the rat dorsal hippocampus. Synapse. 2003;47(3):209–217. doi: 10.1002/syn.10167. [DOI] [PubMed] [Google Scholar]
- 64.Azmitia EC, Gannon PJ, Kheck NM, Whitaker-Azmitia PM. Cellular localization of the 5-HT(1A) receptor in primate brain neurons and glial cells. Neuropsychopharmacology. 1996;14(1):35–46. doi: 10.1016/S0893-133X(96)80057-1. [DOI] [PubMed] [Google Scholar]
- 65.Voigt MM, Laurie DJ, Seeburg PH, Bach A. Molecular cloning and characterization of a rat brain cDNA encoding a 5-hydroxytryptamine 1B receptor. EMBO Journal. 1991;10(13):4017–4023. doi: 10.1002/j.1460-2075.1991.tb04977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoyer D, Engel G, Kalkman HO. Characterization of the 5 − HT1B recognition site in rat brain: binding studies with [125I]iodocyanopindolol. European Journal of Pharmacology. 1985;118(1-2):1–12. doi: 10.1016/0014-2999(85)90657-0. [DOI] [PubMed] [Google Scholar]
- 67.Egeland M, Warner-Schmidt J, Greengard P, Svenningsson P. Co-expression of serotonin 5-HT(1B) and 5-HT(4) receptors in p11 contaning cells in cerebral cortex, hippocampus, caudate-putamen and cerebellum. Neuropharmacology. 2011;61(3):442–450. doi: 10.1016/j.neuropharm.2011.01.046. [DOI] [PubMed] [Google Scholar]
- 68.Mlinar B, Falsini C, Corradetti R. Pharmacological characterization of 5-HT1B receptor-mediated inhibition of local excitatory synaptic transmission in the CA1 region of rat hippocampus. British Journal of Pharmacology. 2003;138(1):71–80. doi: 10.1038/sj.bjp.0705026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guo JD, Rainnie DG. Presynaptic 5-HT1B receptor-mediated serotonergic inhibition of glutamate transmission in the bed nucleus of the stria terminalis. Neuroscience. 2010;165(4):1390–1401. doi: 10.1016/j.neuroscience.2009.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fink KB, Göthert M. 5-HT receptor regulation of neurotransmitter release. Pharmacological Reviews. 2007;59(4):360–417. doi: 10.1124/pr.107.07103. [DOI] [PubMed] [Google Scholar]
- 71.Hamblin MW, Metcalf MA. Primary structure and functional characterization of a human 5-HT(1D)-type serotonin receptor. Molecular Pharmacology. 1991;40(2):143–148. [PubMed] [Google Scholar]
- 72.Weinshank RL, Zgombick JM, Macchi MJ, Branchek TA, Harting PR. Human serotonin 1D receptor is encoded by a subfamily of two distinct genes: 5-HT1D α and 5-HT1D β . Proceedings of the National Academy of Sciences of the United States of America. 1992;89(8):8630–8634. doi: 10.1073/pnas.89.8.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bruinvels AT, Palacios JM, Hoyer D. Autoradiographic characterisation and localisation of 5-HT1D compared to 5-HT1B binding sites in rat brain. Naunyn-Schmiedeberg’s Archives of Pharmacology. 1993;347(6):569–582. doi: 10.1007/BF00166939. [DOI] [PubMed] [Google Scholar]
- 74.McAllister G, Charlesworth A, Snodin C, et al. Molecular cloning of a serotonin receptor from human brain (5HT1E): a fifth 5HT1-like subtype. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(12):5517–5521. doi: 10.1073/pnas.89.12.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leonhardt S, Herrick-Davis K, Titeler M. Detection of a novel serotonin receptor subtype (5-HT1E) in human brain: interaction with a GTP-binding protein. Journal of Neurochemistry. 1989;53(2):465–471. doi: 10.1111/j.1471-4159.1989.tb07357.x. [DOI] [PubMed] [Google Scholar]
- 76.Amlaiky N, Ramboz S, Boschert U, Plassat JL, Hen R. Isolation of a mouse “5HT1E-like” serotonin receptor expressed predominantly in hippocampus. Journal of Biological Chemistry. 1992;267(28):19761–19764. [PubMed] [Google Scholar]
- 77.Adham N, Kao HT, Schechter LE, et al. Cloning of another human serotonin receptor (5-HT1F): a fifth 5-HT1 receptor subtype coupled to the inhibition of adenylate cyclase. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(2):408–412. doi: 10.1073/pnas.90.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pazos A, Cortés R, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain Research. 1985;346(2):231–249. doi: 10.1016/0006-8993(85)90857-1. [DOI] [PubMed] [Google Scholar]
- 79.Ullmer C, Boddeke HG, Schmuck K, Lübbert H. 5-HT2B receptor-mediated calcium release from ryanodine-sensitive intracellular stores in human pulmonary artery endothelial cells. British Journal of Pharmacology. 1996;117(6):1081–1088. doi: 10.1111/j.1476-5381.1996.tb16700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoyer D, Pazos A, Probst A, Palacios JM. Serotonin receptors in the human brain. II. Characterization and autoradiographic localization of 5-HT1C and 5-HT2 recognition sites. Brain Research. 1986;376(1):97–107. doi: 10.1016/0006-8993(86)90903-0. [DOI] [PubMed] [Google Scholar]
- 81.Pritchett DB, Bach AW, Wozny M, et al. Structure and functional expression of cloned rat serotonin 5HT-2 receptor. EMBO Journal. 1988;7(13):4135–4140. doi: 10.1002/j.1460-2075.1988.tb03308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Julius D, Huang KN, Livelli TJ, Axel R, Jessell TM. The 5HT2 receptor defines a family of structurally distinct but functionally conserved serotonin receptors. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(3):928–932. doi: 10.1073/pnas.87.3.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burnet PW, Eastwood SL, Harrison PJ. Detection and quantitation of 5-HT1A and 5HT(2A) receptor mRNAs in human hippocampus using a revel-se transcriptase-polymerase chain reaction (RT-PCR) technique and their correlation with binding site densities and age. Neuroscience Letters. 1994;178(1):85–89. doi: 10.1016/0304-3940(94)90296-8. [DOI] [PubMed] [Google Scholar]
- 84.Li QH, Nakadate K, Tanaka-Nakadate S, Nakatsuka D, Cui Y, Watanabe Y. Unique expression patterns of 5-HT2A and 5-HT2C receptors in the rat brain during postnatal development: western blot and immunohistochemical analyses. Journal of Comparative Neurology. 2004;469(1):128–140. doi: 10.1002/cne.11004. [DOI] [PubMed] [Google Scholar]
- 85.Miner LA, Backstrom JR, Sanders-Bush E, Sesack SR. Ultrastructural localization of serotonin2A receptors in the middle layers of the rat prelimbic prefrontal cortex. Neuroscience. 2003;116(1):107–117. doi: 10.1016/s0306-4522(02)00580-8. [DOI] [PubMed] [Google Scholar]
- 86.Xu T, Pandey SC. Cellular localization of serotonin(2A) (5HT(2A)) receptors in the rat brain. Brain Research Bulletin. 2000;51(6):499–505. doi: 10.1016/s0361-9230(99)00278-6. [DOI] [PubMed] [Google Scholar]
- 87.Kursar JD, Nelson DL, Wainscott DB, Cohen ML, Baez M. Molecular cloning, functional expression, and pharmacological characterization of a novel serotonin receptor (5-hydroxytryptamine2F) from rat stomach fundus. Molecular Pharmacology. 1992;42(4):549–557. [PubMed] [Google Scholar]
- 88.Bonhaus DW, Bach C, DeSouza A, et al. The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT(2A) and 5-HT(2C) receptors. British Journal of Pharmacology. 1995;115(4):622–628. doi: 10.1111/j.1476-5381.1995.tb14977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sandén N, Thorlin T, Blomstrand F, Persson PA, Hansson E. 5-Hydroxytryptamine2B receptors stimulate Ca2+ increases in cultured astrocytes from three different brain regions. Neurochemistry International. 2000;36(4-5):427–434. doi: 10.1016/s0197-0186(99)00134-5. [DOI] [PubMed] [Google Scholar]
- 90.Capone C, Fabrizi C, Piovesan P, et al. 2-Aminotetraline derivative protects from ischemia/reperfusion brain injury with a broad therapeutic window. Neuropsychopharmacology. 2007;32(6):1302–1311. doi: 10.1038/sj.npp.1301255. [DOI] [PubMed] [Google Scholar]
- 91.Lübbert H, Hoffman BJ, Snutch TP, et al. cDNA cloning of a serotonin 5-HT1C receptor by electrophysiological assays of mRNA-injected Xenopus oocytes. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(12):4332–4336. doi: 10.1073/pnas.84.12.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Julius D, MacDermott AB, Axel R, Jessell TM. Molecular characterization of a functional cDNA encoding the serotonin 1c receptor. Science. 1988;241(4865):558–564. doi: 10.1126/science.3399891. [DOI] [PubMed] [Google Scholar]
- 93.Pazos A, Hoyer D, Palacios JM. The binding of serotonergic ligands to the porcine choroid plexus: characterization of a new type of serotonin recognition site. European Journal of Pharmacology. 1984;106(3):539–546. doi: 10.1016/0014-2999(84)90057-8. [DOI] [PubMed] [Google Scholar]
- 94.Pazos A, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Research. 1985;346(2):205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- 95.Hoffman BJ, Mezey E. Distribution of serotonin 5 − HT1C receptor mRNA in adult rat brain. FEBS Letters. 1989;247(2):453–462. doi: 10.1016/0014-5793(89)81390-0. [DOI] [PubMed] [Google Scholar]
- 96.Molineaux SM, Jessell TM, Axel R, Julius D. 5 − HT1C receptor is a prominent serotonin receptor subtype in the central nervous system. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(17):6793–6797. doi: 10.1073/pnas.86.17.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mengod G, Nguyen H, Le H, Waeber C, Lübbert H, Palacios JM. The distribution and cellular localization of the serotonin 1C receptor mRNA in the rodent brain examined by in situ hybridization histochemistry. Comparison with receptor binding distribution. Neuroscience. 1990;35(3):577–591. doi: 10.1016/0306-4522(90)90330-7. [DOI] [PubMed] [Google Scholar]
- 98.Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Molecular Brain Research. 1994;23(1-2):163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 99.Abramowski D, Rigo M, Duc D, Hoyer D, Staufenbiel M. Localization of the 5-hydroxytryptamine2C receptor protein in human and rat brain using specific antisera. Neuropharmacology. 1995;34(12):1635–1645. doi: 10.1016/0028-3908(95)00138-7. [DOI] [PubMed] [Google Scholar]
- 100.Clemett DA, Punhani T, Duxon MS, Blackburn TP, Fone KC. Immunohistochemical localisation of the 5-HT2C receptor protein in the rat CNS. Neuropharmacology. 2000;39(1):123–132. doi: 10.1016/s0028-3908(99)00086-6. [DOI] [PubMed] [Google Scholar]
- 101.Richardson BP, Engel G, Donatsch P, Stadler PA. Identification of serotonin M-receptor subtypes and their specific blockade by a new class of drugs. Nature. 1985;316(6024):126–131. doi: 10.1038/316126a0. [DOI] [PubMed] [Google Scholar]
- 102.Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science. 1991;254(5030):432–437. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- 103.Davies PA, Pistis M, Hanna MC, et al. The S-HT3B subunit is a major determinant of serotonin-receptor function. Nature. 1999;397(6717):359–363. doi: 10.1038/16941. [DOI] [PubMed] [Google Scholar]
- 104.Karnovsky AM, Gotow LF, McKinley DD, et al. A cluster of novel serotonin receptor 3-like genes on human chromosome 3. Gene. 2003;319(1-2):137–148. doi: 10.1016/s0378-1119(03)00803-5. [DOI] [PubMed] [Google Scholar]
- 105.Niesler B, Frank B, Kapeller J, Rappold GA. Cloning, physical mapping and expression analysis of the human 5-HT3 serotonin receptor-like genes HTR3C, HTR3D and HTR3E. Gene. 2003;310(1-2):101–111. doi: 10.1016/s0378-1119(03)00503-1. [DOI] [PubMed] [Google Scholar]
- 106.Holbrook JD, Gill CH, Zebda N, et al. Characterization of 5-HT3C, 5-HT3D and 5-HT3E receptor subunits: evolution, distribution and function. Journal of Neurochemistry. 2009;108(2):384–396. doi: 10.1111/j.1471-4159.2008.05775.x. [DOI] [PubMed] [Google Scholar]
- 107.Sudweeks SN, Van Hooft JA, Yakel JL. Serotonin 5-HT3 receptor in rat CA1 hippocampal interneurons: functional and molecular characterization. Journal of Physiology. 2002;544(3):715–726. doi: 10.1113/jphysiol.2002.029736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fletcher S, Lindstrom JM, McKernan RM, Barnes NM. Evidence that porcine native 5-HT3 receptors do not contain nicotinic acetylcholine receptor subunits. Neuropharmacology. 1998;37(3):397–399. doi: 10.1016/s0028-3908(98)00052-5. [DOI] [PubMed] [Google Scholar]
- 109.Boué-Grabot É, Barajas-López C, Chakfe Y, et al. Intracellular cross talk and physical interaction between two classes of neurotransmitter-gated channels. Journal of Neuroscience. 2003;23(4):1246–1253. doi: 10.1523/JNEUROSCI.23-04-01246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kapeller J, Möller D, Lasitschka F, et al. Serotonin receptor diversity in the human colon: expression of serotonin type 3 receptor subunits 5-HT3C, 5-HT3D, and 5-HT3E. Journal of Comparative Neurology. 2011;519(3):420–432. doi: 10.1002/cne.22525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tecott LH, Maricq AV, Julius D. Nervous system distribution of the serotonin 5-HT3 receptor mRNA. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(4):1430–1434. doi: 10.1073/pnas.90.4.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brady CA, Dover TJ, Massoura AN, Princivalle AP, Hope AG, Barnes NM. Identification of 5-HT3A and 5-HT3B receptor subunits in human hippocampus. Neuropharmacology. 2007;52(5):1284–1290. doi: 10.1016/j.neuropharm.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 113.Dumuis A, Bouhelal R, Sebben M, Bockaert J. A 5-HT receptor in the central nervous system, positively coupled with adenylate cyclase, is antagonized by ICS 205 930. European Journal of Pharmacology. 1988;146(1):187–188. doi: 10.1016/0014-2999(88)90503-1. [DOI] [PubMed] [Google Scholar]
- 114.Gerald C, Adham N, Kao HT, et al. The 5-HT4 receptor: molecular cloning and pharmacological characterization of two splice variants. EMBO Journal. 1995;14(12):2806–2815. doi: 10.1002/j.1460-2075.1995.tb07280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vilaró MT, Cortés R, Gerald C, Branchek TA, Palacios JM, Mengod G. Localization of 5-HT4 receptor mRNA in rat brain by in situ hybridization histochemistry. Molecular Brain Research. 1996;43(1-2):356–360. doi: 10.1016/s0169-328x(96)00248-3. [DOI] [PubMed] [Google Scholar]
- 116.Mohler EG, Shacham S, Noiman S, et al. VRX-03011, a novel 5-HT4 agonist, enhances memory and hippocampal acetylcholine efflux. Neuropharmacology. 2007;53(4):563–573. doi: 10.1016/j.neuropharm.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 117.Plassat JL, Boschert U, Amlaiky N, Hen R. The mouse 5HT5 receptor reveals a remarkable heterogeneity within the 5HT1D receptor family. EMBO Journal. 1992;11(13):4779–4786. doi: 10.1002/j.1460-2075.1992.tb05583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Erlander MG, Lovenberg TW, Baron BM, et al. Two members of a distinct subfamily of 5-hydroxytryptamine receptors differentially expressed in rat brain. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(8):3452–3456. doi: 10.1073/pnas.90.8.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Matthes H, Boschert U, Amlaiky N, et al. Mouse 5-hydroxytryptamine5A and 5-hydroxytryptamine5B receptors define a new family of serotonin receptors: cloning, functional expression, and chromosomal localization. Molecular Pharmacology. 1993;43(3):313–319. [PubMed] [Google Scholar]
- 120.Carson MJ, Thomas EA, Danielson PE, Sutcliffe JG. The 5-HT5A serotonin receptor is expressed predominantly by astrocytes in which it inhibits cAMP accumulation: a mechanism for neuronal suppression of reactive astrocytes. GLIA. 1996;17(4):317–326. doi: 10.1002/(SICI)1098-1136(199608)17:4<317::AID-GLIA6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 121.Pasqualetti M, Ori M, Nardi I, Castagna M, Cassano GB, Marazziti D. Distribution of the 5-HT5A serotonin receptor mRNA in the human brain. Molecular Brain Research. 1998;56(1-2):1–8. doi: 10.1016/s0169-328x(98)00003-5. [DOI] [PubMed] [Google Scholar]
- 122.Oliver KR, Kinsey AM, Wainwright A, Sirinathsinghji DJS. Localization of 5 − ht5A receptor-like immunoreactivity in the rat brain. Brain Research. 2000;867(1-2):131–142. doi: 10.1016/s0006-8993(00)02273-3. [DOI] [PubMed] [Google Scholar]
- 123.Francken BJ, Jurzak M, Vanhauwe JF, Luyten WH, Leysen JE. The human 5 − ht5A receptor couples to G(i)/G(o) proteins and inhibits adenylate cyclase in HEK 293 cells. European Journal of Pharmacology. 1998;361(2-3):299–309. doi: 10.1016/s0014-2999(98)00744-4. [DOI] [PubMed] [Google Scholar]
- 124.Francken BJB, Josson K, Lijnen P, Jurzak M, Luyten WHML, Leysen JE. Human 5-hydroxytryptamine(5A) receptors activate coexpressed G(i) and G(o) proteins in Spodoptera frugiperda 9 cells. Molecular Pharmacology. 2000;57(5):1034–1044. [PubMed] [Google Scholar]
- 125.Francken BJB, Vanhauwe JF, Josson K, Jurzak M, Luyten WH, Leysen JE. Reconstitution of human 5-hydroxytryptamine5A receptor-G protein coupling in E. coli and Sf9 cell membranes with membranes from Sf9 cells expressing mammalian G proteins. Receptors and Channels. 2001;7(4):303–318. [PubMed] [Google Scholar]
- 126.Noda M, Yasuda S, Okada N, et al. Recombinant human serotonin5A receptors stably expressed in C6 glioma cells couple to multiple signal transduction pathways. Journal of Neurochemistry. 2003;84(2):222–232. doi: 10.1046/j.1471-4159.2003.01518.x. [DOI] [PubMed] [Google Scholar]
- 127.Wisden W, Parker EM, Mahle CD, et al. Cloning and characterizatin of the rat 5 − HT5B receptor. Evidencie that the 5 − HT5B receptor couples to a G protein in mammalian cell membranes. FEBS Letters. 1993;333(1-2):25–31. doi: 10.1016/0014-5793(93)80368-5. [DOI] [PubMed] [Google Scholar]
- 128.Kinsey AM, Wainwright A, Heavens R, Sirinathsinghji DJ, Oliver KR. Distribution of 5-ht(5A), 5-ht(5B), 5-ht(6) and 5-HT(7) receptor mRNAs in the rat brain. Molecular Brain Research. 2001;88(1-2):194–198. doi: 10.1016/s0169-328x(01)00034-1. [DOI] [PubMed] [Google Scholar]
- 129.Grailhe R, Grabtree GW, Hen R. Human 5-HT5 receptors: the 5 − HT5A receptor is functional but the 5 − HT5B receptor was lost during mammalian evolution. European Journal of Pharmacology. 2001;418(3):157–167. doi: 10.1016/s0014-2999(01)00933-5. [DOI] [PubMed] [Google Scholar]
- 130.Maekawa T, Kim S, Nakai D, et al. Social isolation stress induces ATF-7 phosphorylation and impairs silencing of the 5-HT 5B receptor gene. The EMBO Journal. 2010;29(1):196–208. doi: 10.1038/emboj.2009.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ruat M, Traiffort E, Arrang JM, et al. A novel rat serotonin (5-HT6) receptor: molecular cloning, localization and stimulation of cAMP accumulation. Biochemical and Biophysical Research Communications. 1993;193(1):268–276. doi: 10.1006/bbrc.1993.1619. [DOI] [PubMed] [Google Scholar]
- 132.Ruat M, Traiffort E, Leurs R, et al. Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(18):8547–8551. doi: 10.1073/pnas.90.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bickmeyer U, Heine M, Manzke T, Richter DW. Differential modulation of I(h) by 5-HT receptors in mouse CA1 hippocampal neurons. European Journal of Neuroscience. 2002;16(2):209–218. doi: 10.1046/j.1460-9568.2002.02072.x. [DOI] [PubMed] [Google Scholar]
- 134.Costa L, Trovato C, Musumeci SA, Catania MV, Ciranna L. 5-HT(1A) and 5-HT(7) receptors differently modulate AMPA receptor-mediated hippocampal synaptic trasnmission. doi: 10.1002/hipo.20940. Hippocampus. In press. [DOI] [PubMed] [Google Scholar]
- 135.Joca SR, Ferreira FR, Guimaraes FS. Modulation of stress consequences by hippocampal monoaminergic, glutamatergic and nitrergic neurotransmitter systems. Stress. 2007;10(3):227–249. doi: 10.1080/10253890701223130. [DOI] [PubMed] [Google Scholar]
- 136.Schoemaker H, Langer SZ. [3H]8-OH-DPAT labels the serotonin transporter in the rat striatum. European Journal of Pharmacology. 1986;124(3):371–373. doi: 10.1016/0014-2999(86)90243-8. [DOI] [PubMed] [Google Scholar]
- 137.Lesch KP, Mössner R. Genetically driven variation in serotonin uptake: is there a link to affective spectrum, neurodevelopmental, and neurodegenerative disorders? Biological Psychiatry. 1998;44(3):179–192. doi: 10.1016/s0006-3223(98)00121-8. [DOI] [PubMed] [Google Scholar]
- 138.Sugden K, Tichopad A, Khan N, Craig IW, D’Souza UM. Genes within the serotonergic system are differentially expressed in human brain. BMC Neuroscience. 2009;15:10–50. doi: 10.1186/1471-2202-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nordquist N, Oreland L. Serotonin, genetic variability, behaviour, and psychiatric disorders—a review. Upsala Journal of Medical Sciences. 2010;115(1):2–10. doi: 10.3109/03009730903573246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Martinowich K, Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology. 2008;33(1):73–83. doi: 10.1038/sj.npp.1301571. [DOI] [PubMed] [Google Scholar]
- 141.Siever LJ. Neurobiology of aggression and violence. American Journal of Psychiatry. 2008;165(4):429–442. doi: 10.1176/appi.ajp.2008.07111774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Waters K. Serotonin in the sudden infant death syndrome. Drug News and Perspectives. 2010;23(9):537–548. doi: 10.1358/dnp.2010.23.9.1453626. [DOI] [PubMed] [Google Scholar]
- 143.Moiseiwitsch JR, Lauder JM. Serotonin regulates mouse cranial neural crest migration. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(16):7182–7186. doi: 10.1073/pnas.92.16.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience. 1999;89(4):999–1002. doi: 10.1016/s0306-4522(98)00693-9. [DOI] [PubMed] [Google Scholar]
- 145.Brezun JM, Daszuta A. Serotonergic reinnervation reverses lesion-induced decreases in PSA-NCAM labeling and proliferation of hippocampal cells in adult rats. Hippocampus. 2000;10(1):37–46. doi: 10.1002/(SICI)1098-1063(2000)10:1<37::AID-HIPO4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 146.Lanfumey L, Mongeau R, Cohen-Salmon C, Hamon M. Corticosteroid-serotonin interactions in the neurobiological mechanisms of stress-related disorders. Neuroscience and Biobehavioral Reviews. 2008;32(6):1174–1184. doi: 10.1016/j.neubiorev.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 147.Haj-Dahmane S, Shen RY. Modulation of the serotonin system by endocannabinoid signaling. Neuropharmacology. 2011;61(3):414–420. doi: 10.1016/j.neuropharm.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Banasr M, Hery M, Brezun JM, Daszuta A. Serotonin mediates oestrogen stimulation of cell proliferation in the adult dentate gyrus. European Journal of Neuroscience. 2001;14(9):1417–1424. doi: 10.1046/j.0953-816x.2001.01763.x. [DOI] [PubMed] [Google Scholar]
- 149.Wang H, Storm DR. Calmodulin-regulated adenylyl cyclases: cross-talk and plasticity in the central nervous system. Molecular Pharmacology. 2003;63(3):463–468. doi: 10.1124/mol.63.3.463. [DOI] [PubMed] [Google Scholar]
- 150.Djavadian RL. Serotonin and neurogenesis in the hippocampal dentate gyrus of adult mammals. Acta Neurobiologiae Experimentalis. 2004;64(2):189–200. doi: 10.55782/ane-2004-1505. [DOI] [PubMed] [Google Scholar]
- 151.Albert PR, le Francois B, Millar AM. Transcriptional dysregulation of 5-HT1A autoreceptors in mental illness. Molecular Brain. 2011;4(1):p. 21. doi: 10.1186/1756-6606-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Staubli U, Otaky N. Serotonin controls the magnitude of LTP induced by theta bursts via an action on NMDA-receptor-mediated responses. Brain Research. 1994;643(1-2):10–16. doi: 10.1016/0006-8993(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 153.Gardier AM. Mutant mouse models and antidepressant drug research: focus on serotonin and brain-derived neurotrophic factor. Behavioural Pharmacology. 2009;20(1):18–32. doi: 10.1097/FBP.0b013e3283243fcd. [DOI] [PubMed] [Google Scholar]
- 154.Saxena R, Chattopadhyay A. Membrane organization and dynamics of the serotonin1A receptor in live cells. Journal of Neurochemistry. 2011;116(5):726–733. doi: 10.1111/j.1471-4159.2010.07037.x. [DOI] [PubMed] [Google Scholar]
- 155.Schmitt A, Benninghoff J, Moessner R, et al. Adult neurogenesis in serotonin transporter deficient mice. Journal of Neural Transmission. 2007;114(9):1107–1119. doi: 10.1007/s00702-007-0724-6. [DOI] [PubMed] [Google Scholar]
- 156.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Archives of General Psychiatry. 1997;54(7):597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 157.Hindmarch I. Beyond the monoamine hypothesis: mechanisms, molecules and methods. European Psychiatry. 2002;17(supplement 3):294–299. doi: 10.1016/s0924-9338(02)00653-3. [DOI] [PubMed] [Google Scholar]
- 158.Sharp T, Cowen PJ. 5-HT and depression: is the glass half-full? Current Opinion in Pharmacology. 2011;11(1):45–51. doi: 10.1016/j.coph.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 159.Duman RS. A silver bullet for the treatment of depression? Neuron. 2007;55(5):679–681. doi: 10.1016/j.neuron.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 160.Corradetti R, Laaris N, Hanoun N, et al. Antagonist properties of (-)-pindolol and WAY 100635 at somatodendritic and postsynaptic 5-HT1A receptors in the rat brain. British Journal of Pharmacology. 1998;123(3):449–462. doi: 10.1038/sj.bjp.0701632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.le Poul E, Boni C, Hanoun N, et al. Differential adaptation of brain 5-HT1A and 5-HT1B receptors and 5-HT transporter in rats treated chronically with fluoxetine. Neuropharmacology. 2000;39(1):110–122. doi: 10.1016/s0028-3908(99)00088-x. [DOI] [PubMed] [Google Scholar]
- 162.Passani MB, Pugliese AM, Azzurrini M, Corradetti R. Effects of DAU 6215, a novel 5-hydroxytryptamine3 (5-HT3) antagonist on electrophysiological properties of the rat hippocampus. British Journal of Pharmacology. 1994;112(2):695–703. doi: 10.1111/j.1476-5381.1994.tb13132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hertz L. Neuronal-astrocytic interactions in brain development, brain function and brain disease. Advances in Experimental Medicine and Biology. 1991;296:143–159. doi: 10.1007/978-1-4684-8047-4_15. [DOI] [PubMed] [Google Scholar]
- 164.Mouillet-Richard S, Mutel V, Loric S, Tournois C, Launay JM, Kellermann O. Regulation by neurotransmitter receptors of serotonergic or catecholaminergic neuronal cell differentiation. Journal of Biological Chemistry. 2000;275(13):9186–9192. doi: 10.1074/jbc.275.13.9186. [DOI] [PubMed] [Google Scholar]
- 165.Whitaker-Azmitia PM. The role of serotonin and serotonin receptors in development of the mammalian nervous system. In: Zagon ISN, McLaughlin PJ, editors. Receptors in the Developing Nervous System. Vol. 2. London, UK: Chapman and Hall; 1993. pp. 43–50. [Google Scholar]


