Abstract
Should we be concerned about financial conflicts of interest (COI) between doctors and the pharmaceutical industry? Some people will say no as there are clearly doctors who celebrate the relationship. Others say that it does not matter to patients, but the evidence says otherwise. Financial COI is different from other types of conflicts because it is voluntary and can be refused. Finally, it is not just the large gifts that are a problem, the small ones also create a “gift relationship.” Drug companies know about this and spend billions on promotion with good effect from their point of view. Companies also woo doctors who honestly hold pro-industry points of view to speak on behalf of the companies. There are still multiple examples of financial COI, and although there are isolated examples of improvement, this is still an area of deep concern.
Keywords: Doctors, Financial conflict of interest, Gifts, Pharmaceutical industry', Promotion
Introduction
In 2007, I wrote an essay in this journal calling on the medical profession to sever its relationship with the pharmaceutical industry (Lexchin 2007[12]). I ended that piece saying that unless there was bold action the trust that the public has in the medical profession was in danger. Four years on is there any reason for optimism? This question is the subject of this revisit.
The focus here is going to be on the actions of the medical profession and specifically its financial ties with the industry. Despite all of the financial and persuasive powers of the drug companies it takes “two to tango”, and if doctors refused to dance there would not be any problem. However, before leaving the pharmaceutical industry, it is prudent to point out that the industry is not without guilt. In the 5-year period of 2006 – 2010, criminal and civil settlements in the United States between federal and state governments and pharmaceutical companies totaled $14.8 billion (Almashat et al., 2010[1]).
Who is Worried about the Relationship?
Some people will ask what is all the concern about? Tom Stossel, an oncologist in Boston, and some colleagues, argue that the relationship between drug companies and the medical profession should be encouraged and that restrictions stifle research (Kowalczyk, 2009[11]). Stossel and others have gone so far as to form a group - the Association of Clinical Researchers and Educators - to restore some balance to the debate (Kowalczyk, 2009[11]). Stossel claims that there is a silent majority of doctors out there who support him. (Kowalczyk, 2009[11])
Others will ask whether patients and consumers actually care how close doctors are to industry. On this point the evidence is pretty convincing. A recent systematic review looked at research into the attitudes of patients toward physicians’ financial ties to industry (Licurse et al., 2010[14]). Overall, most patients believed that financial ties should be disclosed and, following disclosure, about one-quarter indicated knowledge of these ties would affect their willingness to participate in research.
What about Other Forms of Conflict of Interest?
Next, there is the argument that even if financial conflict of interest (COI) is a problem, other forms of COI are equally bad and need to be dealt with. In December 2011, the Food and Drug Administration (FDA) refused to let Dr. Sidney Wolfe of Public Citizen's Health Research Group take his position as a consumer representative on the FDA's Drug Safety Advisory Committee when it was considering whether to pull the oral contraceptive Yasmin® off the market. Since Wolfe's group had already advocated removing the drug, he was accused of “intellectual conflict of interest” (Goozner, 2011[7]).
However, financial COI is fundamentally different from other types of COI. As Jerome Kassirer, former editor of the New England Journal of Medicine, recognizes:
…there is a substantial difference between financial conflicts and others; namely financial conflicts are optional. When faced with the choice to agree to a financial relationship with a company or not to, one has a choice: either take it or leave it. In contrast…one cannot divest oneself of one's biases or prejudgments because they are so integral; one cannot easily disclose them because they are so internal (Kassirer, 2008[10]).
Are there Really only a Few Villians?
Finally, people will point a finger at psychiatrists like Charles Nemeroff and Alan Schatzberg who took millions in undisclosed payments from pharmaceuticals companies (Harris, 2008[8]) as the villains and vocally maintain that they cannot be bought for a piece of pizza or a trinket with a drug logo on it. In fact, most doctors disdain the notion that they can be influenced by the pharmaceutical industry even as they get their prescribing information directly from the drug companies, attend the continuing medical education (CME) sponsored by the companies, and eat the meals that the companies pay for (Morgan et al., 2006[15]; Rutledge et al., 2003[20]; Steinman et al., 2001[23]). The truth is different and it lies in something known as the gift relationship (Cialdini, 2001[3]). Once you have taken a “gift”, in this case a “free” meal or pen or book, there is the obligation to repay the giver in some way; perhaps by prescribing the company's drug, seeing the sales representative, or just getting a warm and fuzzy feeling about the company because it has been good to you.
Drug Companies and Promotion
Drug companies are run by very smart people, that is one of the reasons why the pharmaceutical industry has consistently been ranked among the most profitable industries for decades (Lexchin, 2011[13]). Smart business executives would not spend $53 billion annually on promoting their products to doctors (Gagnon and Lexchin, 2008[8]) if they were not getting even more back. Not only does drug promotion work but, based on a recent systematic review, with rare exceptions, it never has a positive effect on any aspect of prescribing – appropriateness, cost or frequency (Spurling et al., 2010[22]). This conclusion is a clear indication that not only are we doctors influenced by our interactions with drug companies, but much more importantly, that the results of those interactions are to the detriment of our patients.
How Many Points of View are Heard?
Before I go on let me voice one final thought. Doctors may be incredibly naïve about what happens when we tango with the drug companies, but by and large, with a few exceptions such as Nemeroff and Schatzberg, we are not dishonest. In many cases, when doctors act as spokespeople for drug companies or run CME programmes for them, they are not saying or doing anything that they do not believe in. Rather, they have been selected primarily because their own beliefs coincide with the company's message about the product. The problem here is that the resources available to the pharmaceutical companies dwarfs the resources from any independent source, and so doctors only hear what the companies want them to.
Financial COI is Still a Concern
So back to the question that opened this essay. In 2011 should we still be concerned about the relationship between the medical profession and the pharmaceutical industry? The answer, unfortunately, is yes. If anyone is any doubt, here is just a sample of some of the more recent work in the area: how doctors participate with drug companies in an effort to redefine female sexual dysfunction (Moynihan and Mintzes, 2010[16]); the failure of medical school deans to disclose outside income (Freshwater and Freshwater, 2011[4]); the view from the president of the Australian Medical Association as to the value of seeing drug company representatives (A noble cause, 2011[17]); doctors’ defense of menopausal hormone therapy in the wake of the findings of the Women's Health Initiative study (Fugh-Berman et al., 2011[5]); the prevalence of ghost writing in articles about rofecoxib (Ross et al., 2008[19]) etc.
Progress is Possible
All is not doom and gloom – the American Medical Student Association now has its annual scorecard documenting COI policies at American medical schools (American Medical Student Association, 2011[2]); Emergency Medicine Australasia banned drug advertisements (Jelinek and Brown, 2011[9]); some organizations such as the Oregon Academy of Family Physicians have sworn off drug company money for their educational events (Silverman, 2008[21]); and, beginning in 2013, the Physician Payment Sunshine Act will provide a public record of the value, date and nature of each payment or gift to American doctors from drug and device companies (Pew Prescripiton Project, 2010[18]).
However, these measures by themselves will not suffice. We doctors, collectively and individually, need to say “no” when drug companies ask us to dance.
Concluding Remarks [See also Figure 1: Flowchart of Paper]
Figure 1.
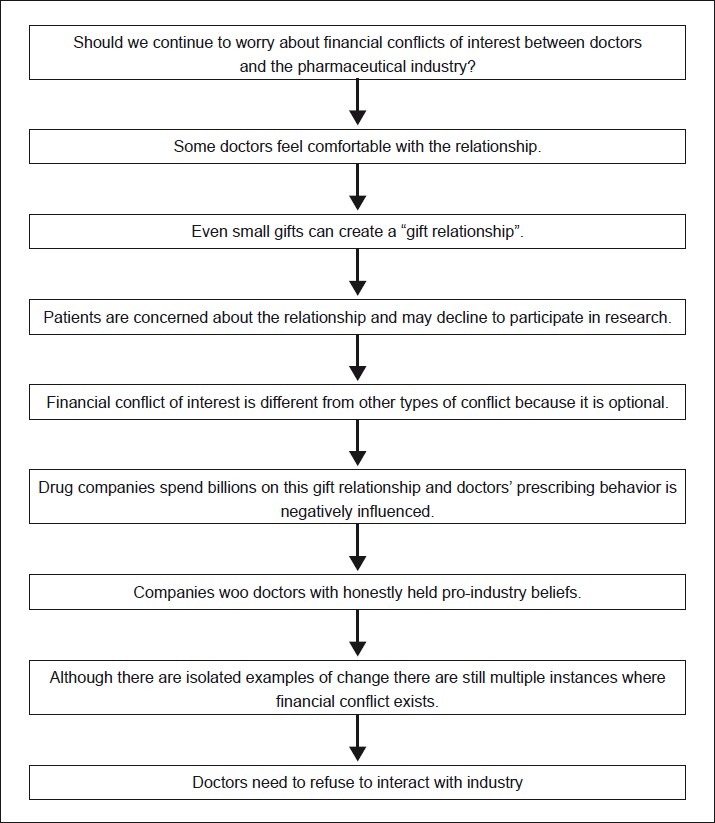
Flowchart of paper
If I write another follow-up to my original article 5 years hence, I hope that I will be able to be more optimistic than I am now about our ability to recapture our independence from the pharmaceutical industry.
Take home message
For the sake of our patients, Doctors need to be on guard and resist entering into financial conflicts of interest with the pharmaceutical industry.
Questions that this Paper Raises
Why do doctors continue to disregard the influence that the pharmaceutical industry has on their practice of medicine?
Is it necessary to avoid all contact with pharmaceutical companies?
Is education about doctor-industry interactions enough to change behavior or is regulation necessary?
What should be done about the financial relations between professional associations and the pharmaceutical industry?
About the Author

Joel Lexchin received his MD from the University of Toronto in 1977. He is currently a Professor in the School of Health Policy and Management at York University and works in the emergency department at the University Health Network. He is the author or co-author of over 115 peer-reviewed articles on a wide variety of topics concerned with Canadian and international pharmaceutical policy
Footnotes
Conflict of interest: Joel Lexchin is part of the Management Group of Healthy Skepticism Inc., is the chair of the Health Action International – Europe Association Board. In 2008, he was an expert witness for the federal government of Canada in its defense of the ban on direct-to-consumer advertising of prescription drugs. In 2010, he was retained as an expert witness for the plaintiff in a case alleging a death from an adverse drug reaction from a product made by Allergan Inc
Declaration
This is an original unpublished manuscript that has not been sent elsewhere for publication.
CITATION: Lexchin J. Of Money and Trust in Medical Care Redux. Mens Sana Monogr 2012; 10: 143-9.
References
- 1.Almashat S, Preston C, Waterman T, Wolfe S. Rapidly increasing criminal and civil monetary penalties against the pharmaceutical industry: 1991 to 2010. Washington D.C.: Public Citizen's Health Research Group; 2010. [Google Scholar]
- 2.American Medical Student Association. 2011. AMSA PharmFree Scorecard 2010. [Accessed on 12 Dec 2011]. Available from: http://www.amsascorecard.org/
- 3.Cialdini RB. The science of persuasion. Sci Am. 2001;284:76–81. [Google Scholar]
- 4.Freshwater DM, Freshwater MF. Failure by deans of academic medical centers to disclose outside income. Arch Intern Med. 2011;171:586–7. doi: 10.1001/archinternmed.2011.71. PMID: 21444851. [DOI] [PubMed] [Google Scholar]
- 5.Fugh-Berman A, McDonald CP, Bell AM, Bethards EC, Scialli AR. Promotional tone in reviews of menopausal hormone therapy after the Women's Health Initiative: An analysis of published articles. PLoS Med 2011. 2011;8:e1000425. doi: 10.1371/journal.pmed.1000425. PMID: 21423581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gagnon MA, Lexchin J. The cost of pushing pills: A new estimate of pharmaceutical promotion expenditures in the United States. PLoS Med. 2008;5:e1. doi: 10.1371/journal.pmed.0050001. PMID: 18177202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goozner M. The latest advisory committee stumbles at FDA. GoozNews.com. 2011. Dec 5, [Accessed on 12 Dec 2011]. Available from: http://gooznews.com/
- 8.Harris G. Top psychiatrist didn’t report drug makers’ pay. New York: New York Times; 2008. [Google Scholar]
- 9.Jelinek GA, Brown AFT. A stand against drug company advertising. Emerg Med Australas. 2011;23:4–6. doi: 10.1111/j.1742-6723.2010.01393.x. PMID: 21284808. [DOI] [PubMed] [Google Scholar]
- 10.Kassirer JP. Medicine's obsession with disclosure of financial conflicts: Fixing the wrong problem. In: Snyder PJ, Mayes LC, Spencer DD, editors. Science and the media: Delgado's brave bulls and the ethics of scientific disclosure. Amsterdam, Boston; London: Academic Press; 2008. pp. 79–89. [Google Scholar]
- 11.Kowalczyk L. Perks policy for doctors challenged: Physician organization wants limits rolled back. Boston: Boston Globe; 2009. [Google Scholar]
- 12.Lexchin J. Of money and trust in medical care. Mens Sana Monographs. 2007;5:7–10. doi: 10.4103/0973-1229.32143. PMID: 22058612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lexchin J. Those who have the gold make the evidence: How the pharmaceutical industry biases the outcome of clinical trials of medications. Sci Eng Ethics. 2011 doi: 10.1007/s11948-011-9265-3. [In Press] PMID: 21327723. [DOI] [PubMed] [Google Scholar]
- 14.Licurse A, Barber E, Joffe S, Gross C. The impact of disclosing financial ties in research and clinical care: A systematic review. Arch Intern Med. 2010;170:675–82. doi: 10.1001/archinternmed.2010.39. PMID: 20421551. [DOI] [PubMed] [Google Scholar]
- 15.Morgan MA, Dana J, Loewenstein G, Zinberg S, Schulkin J. Interactions of doctors with the pharmaceutical industry. J Med Ethics. 2006;32:559–63. doi: 10.1136/jme.2005.014480. PMID: 17012493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moynihan R, Mintzes B. Sex lies and pharmaceuticals: how drug companies plan to profit from female sexual dysfunction. Sydney: Allen & Unwin Pty Ltd; 2010. [Google Scholar]
- 17.A noble cause. ABC Radio National. 2011. Oct 16, [Accessed on 12 Dec 2011]. Available from: http://www.abc.net.au/radionational/programs/backgroundbriefing/a-noble-cause/3583670 .
- 18.Pew Prescription Project. Physician payments sunshine provisions in health care reform. 2011. [Accessed on 12 Dec 2011]. Available from: http://www.prescriptionproject.org/tools/sunshine_docs/files/Sunshinefact-sheet-6.07.10.pdf .
- 19.Ross JP, Hill KP, Egilman DS, Krumholz HM. Guest authorship and ghostwriting in publications related to rofecoxib: A case study of industry documents from rofecoxib litigation. JAMA. 2008;299:1800–12. doi: 10.1001/jama.299.15.1800. PMID: 18413874. [DOI] [PubMed] [Google Scholar]
- 20.Rutledge P, Crookes D, McKinstry B, Maxwell SR. Do doctors rely on pharmaceutical industry funding to attend conferences and do they perceive that this creates a bias in their drug selection. Results from a questionnaire survey? Pharmacoepidemiol Drug Saf. 2003;12:663–7. doi: 10.1002/pds.884. PMID: 14762982. [DOI] [PubMed] [Google Scholar]
- 21.Silverman E. Pharma free: Oregon docs ban CME funding. Pharmalot. 2008. Mar 13, [Accessed on 12 Dec 2011]. Available from: http://www.pharmalot.com/2008/03/pharma-free-oregon-docs-ban-cme-funding/
- 22.Spurling GK, Mansfield PR, Montgomery BD, Lexchin J, Doust J, Othman N, et al. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: A systematic review. PLoS Med. 2010;7:e1000352. doi: 10.1371/journal.pmed.1000352. PMID: 20976098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinman MA, Shlipak MG, McPhee SJ. Of principles and pens: Attitudes and practices of medicine housestaff toward pharmaceutical industry promotions. Am J Med. 2001;110:551–7. doi: 10.1016/s0002-9343(01)00660-x. PMID: 11347622. [DOI] [PubMed] [Google Scholar]


