Abstract
Treatment-resistant symptoms complicate the clinical course of schizophrenia, and a large proportion of patients do not reach functional recovery. In consequence, polypharmacy is frequently used in treatment-refractory cases, addressing psychotic positive, negative and cognitive symptoms, treatment-emergent side effects caused by antipsychotics and comorbid depressive or obsessive-compulsive symptoms. To a large extent, such strategies are not covered by pharmacological guidelines which strongly suggest antipsychotic monotherapy. Add-on strategies comprise combinations of several antipsychotic agents and augmentations with mood stabilizers; moreover, antidepressants and experimental substances are applied. Based on the accumulated evidence of clinical trials and meta-analyses, combinations of clozapine with certain second-generation antipsychotic agents and the augmentation of antipsychotics with antidepressants seem recommendable, while the augmentation with mood stabilizers cannot be considered superior to placebo. Forthcoming investigations will have to focus on innovative pharmacological agents, the clinical spectrum of cognitive deficits and the implementation of cognitive behavioral therapy.
Keywords: Augmentation; Add-on; Combination; Polypharmacy; Schizophrenia, Treatment resistance
Introduction
Antipsychotic medication may alleviate the marked burden of disease caused by schizophrenia, and multimodal treatment approaches are frequently combined. Aside from antipsychotic substances, cognitive behavioral therapy and cognitive remediation as well as psychosocial rehabilitation have proved to be effective. Optimised therapeutic conditions include avoidance of dysfunctional stress, restraint from recreational drug abuse and complete adherence to pharmacological treatment, for which guidelines of several national psychiatric societies strongly suggest monotherapy with second-generation antipsychotics (SGA). After two insufficient monotherapeutic treatment attempts with SGAs, clozapine may be installed which is the agent of choice in treatment refractory schizophrenia (Nielsen et al., 2011[21]). Even under such conditions, however, up to 40% of patients experience only partial remission and even more do not reach a level of full functional recovery. This fact has to stimulate both an improved understanding of treatment refractory schizophrenia and the development of innovative substances. In clinical practice, insufficient response to antipsychotic monotherapy is followed by different modes of polypharmacy comprising the ‘combination’ of equal-class substances (e.g., antipsychotics) and the ‘augmentation’ with substances of different classes (e.g., antipsychotics and mood stabilizers).
Epidemiology
International consensus guidelines strongly recommend monotherapy with antipsychotic agents as first-line treatment for schizophrenia. Yet, a marked increase in both add-on approaches and escalations of cumulative doses have been observed (Centorrino et al., 2010[7]). In consequence, treatment expenses increase, and often, off-label polypharmacy without adequate evidence-based support occurs. More than 40% of schizophrenic outpatients receive two or more antipsychotic agents, and up to 70% are comedicated with other psychotropic drugs (Pickar et al., 2008[22]). Such strategies produce ambiguous results regarding efficacy, efficiency and tolerability with the main concerns to reproach antipsychotic polypharmacy being the risk of cumulative side effects, pharmacokinetic interactions, a potential loss of atypical properties (′atypicity′) and rising treatment costs. Generally, patients with longer courses of illness, lower levels of functioning and high treatment compliance are more likely to receive polypharmacy. In a recent meta-analysis (Weinmann et al., 2009[33]), polypharmacy has been associated with increased mortality rates, whereas two large-scale Scandinavian epidemiological studies failed to replicate these findings (Baandrup et al., 2010[4]; Tiihonen et al., 2009[29]). To date, it remains unclear whether or not polypharmacy is able to enhance the overall treatment outcome in terms of a more profound recovery, psychosocial rehabilitation, and reduced rehospitalisation rates; which is why further randomized controlled trials (RCTs) are necessary to throw light on these open questions differing in their levels of evidence.
Review Method
We conducted a comprehensive search of the online databases “Medline OVID”, “The Cochrane Library”, “PubMed” and “scholar.google.com” for topic-related articles published by November 2011. The terms (schizophrenia OR psychotic disorders OR psychosis) were linked with (combination OR augmentation OR add-on OR addition) as well as (antipsychotic agents OR antidepressive agents OR lithium OR anticonvulsants). In addition, we evaluated currently ongoing subject-related studies on the online database www.clinicaltrials.gov.
Substances Applied in Schizophrenia – An Overview
Multiple classes of substances are used for the treatment of schizophrenia. Combinations of different antipsychotics are employed to address treatment-refractory positive and negative symptoms, whereas antidepressants, mood stabilizers, cognitive enhancers and experimental substances are used to treat chronic aspects such as affective symptoms, cognitive deficits, obsessive-compulsive syndromes and treatment-emergent side effects. Benzodiazepines, finally, are employed mainly in order to manage acute episodes of illness characterized by anxiety, agitation or aggression.
Treatment-resistant Psychotic Positive and Negative Symptoms: Combining SGA Agents
At present, several innovative compounds are in development and address molecular targets outside the dopaminergic and serotonergic neurotransmission. In particular, proglutamatergic agents acting via the metabotropic glutamate receptors or the glycin binding-site of the N-methyl-D-aspartate receptor might prove beneficial in the long run but need further testing and have not yet reached market authorization. To date, clozapine is still the only evidence-based medication for treatment-refractory schizophrenia (Asenjo Lobos et al., 2010[2]; Nielsen et al., 2011[21]). The combination of clozapine with first-generation antipsychotics is common in clinical practice and aims at supplementing the antidopaminergic receptor occupancy. This strategy, however, is not sufficiently substantiated by RCTs and bears the risk of relevant extrapyramidal and other side effects (Barbui et al., 2011[5]). In addition, combinations amongst other SGAs except clozapine await evaluation in controlled clinical trials, and also differences between oral and parenteral modes of application have not yet been sufficiently evaluated in polypharmaceutical approaches.
The SGAs clozapine and olanzapine harbour only low affinity to dopaminergic D2- receptors; however, they have pleiotropic effects on central α1-adrenoceptors, muscarinic M1-cholinoceptors, histaminergic H1-receptors as well as 5-HT2A- and 5-HT2C-receptors. They are the most common and best investigated SGAs used in combination strategies applied for insufficient treatment responses in schizophrenia (Taylor et al., 2011[28]). Their weak antidopaminergic properties can be supplemented by adding amisulpride or sulpiride with the latter one having been established as a favorable option for insufficiently remitted psychotic symptoms (Wang et al., 2010[32]). The benzamide amisulpride exerts analogous properties, but has not yet been evaluated in sufficiently powered trials. Nonetheless, its add-on has been substantiated by several case series, open trials as well as one small RCT (Assion et al., 2008[3]) and has consequently also become very common in clinical practice. The partial D2- and 5-HT1A-receptor agonist aripiprazole exerts differential agonistic and antagonistic effects in both the dopaminergic and the serotonergic systems and has been of beneficial impact on psychotic positive and negative symptoms as well as metabolic parameters when combined with clozapine (Barbui et al., 2011[5]; Fleischhacker et al., 2010[14]). First evaluations of treatment costs during combined clozapine-aripiprazole treatment suggest marked reductions of longitudinal total expenses (Aitchison et al., 2011[1]). The most intensively evaluated SGA added to clozapine is risperidone. Several RCTs ended up with conflicting results with regard to antipsychotic efficiency as an add-on therapeutic (Zink et al., 2010[34]). Treatment adherence and rehospitalization rates, however, could be ameliorated by adding risperidone long-acting injection in clozapine-treated patients (Se et al., 2010[25]). Also ziprasidone has been successfully added to clozapine in numerous case reports and open trials. A head-to-head comparison of ziprasidone or risperidone combined with clozapine revealed similar immediate and long-term efficacy on treatment-resistant schizophrenic symptoms at a diverging range of side effects regarding serum prolactin, extrapyramidal symptoms, and QTc-prolongation (Kuwilsky et al., 2010[16]; Zink et al., 2009[35]).
While clozapine and olanzapine are the most frequent bases for combination strategies, some preliminary data on other SGA combinations (Essock et al., 2011[13]) may be added. In an attempt to overcome insufficient treatment responses, amisulpride has been successfully installed in patients only partially responsive to either risperidone (Lerma-Carrillo et al., 2008[17]) or quetiapine (Englisch et al., 2010a[9]) monotherapy. Similarly, also aripiprazole was used in various combinations (Gomez et al., 2008[15]). As most of the data is derived from observational reports only, the level of evidence for such proceedings, however, remains limited.
Affective and Negative Symptoms
Affective comorbidity in the course of psychotic disorders is common and may emerge as both major depressive episodes and as part of the schizophrenic negative syndrome. As approximately 5% of schizophrenic patients commit suicide in the course of their illness, the necessity of improving affective dysregulation cannot be highlighted enough. Hence, treatment options must be optimized and antidepressant agents as well as mood stabilizers may need to be added.
Augmentation with antidepressants
Antidepressive agents such as citalopram, duloxetine, reboxetine and venlafaxine have been shown to successfully alleviate depressive symptoms in schizophrenia. While tricyclic substances seem to bear a risk of aggravating psychotic symptoms, this risk appears to be low for selective serotonin reuptake inhibitors (SSRIs) combined with SGAs, rendering their utilization generally safe and well-tolerated (Englisch et al., 2009[11]). Noteworthy, pharmacokinetic interactions have to be considered. Furthermore, several SSRIs, trazodone and ritanserine have been found to improve schizophrenic negative symptoms (Singh et al., 2010[26]). This also holds true for mirtazapine which, in addition, showed favorable effects on cognitive deficits usually associated with schizophrenia in several RCTs (Stenberg et al., 2011[27]). The application of mirtazapine in order to ameliorate psychotic positive symptoms, however, yields conflicting results. Owing to the fear of inducing psychotic symptoms, bupropion, a dual dopamine and norepinephrine reuptake inhibitor, had originally been withheld from schizophrenic populations. Meanwhile, however, this substance has been intensively studied as a smoking cessation aid in schizophrenia, and was associated with an amelioration of negative symptoms. Moreover, antidepressive effects have also been successfully observed in a small number of schizophrenic patients (Englisch et al., 2011[12]). Agomelatine, a novel antidepressant exerting its action as an agonist at central melatonergic MT1/MT2 receptors and as a competitive antagonist at serotonergic 5-HT2C-receptors, regulates circadian rhythms and enhances sleep patterns which are frequently disturbed in schizophrenic patients; but further investigations are necessary, before any recommendations in schizophrenic populations can be made.
Augmentation with mood stabilizers
Under the term ‘mood stabilizer’, one subsumes both anticonvulsants (i.e., carbamazepine, lamotrigine, pregabalin, topiramate, valproic acid) as well as lithium. Despite their limited evidence (Citrome, 2009[8]) concerning efficacy, safety and regulatory approval, they are frequently applied in schizophrenia with constantly increasing prescription rates.
The add-on of lithium resulted in an overall improvement of treatment outcome (Leucht et al., 2007[18]), but heterogeneous trials and samples were incorporated into this meta-analysis. Generally, its effects on psychotic core features remain inconclusive, which is why further RCTs are necessary. Quite similarly, carbamazepine was associated with an improvement of global psychopathology compared with placebo in a series of trials (Leucht et al., 2007[19]), whereas – due to methodological issues – its influence on primarily psychotic features still needs to be evaluated. Generally, one has to consider the risk of cumulative side effects in the haematopoietic system which strongly disfavors the add-on of carbamazepine to other myelodepressive substances such as clozapine.
Lamotrigine appears to be beneficial if added in partially remitted clozapine-treated patients (Tiihonen et al., 2009[30]). The GABA-analog pregabalin was successfully used for the management of treatment-resistant anxious syndromes in schizophrenia (Englisch et al., 2010b[10]) and should be further evaluated.
Based on currently available data, the prescription of topiramate in addition to clozapine appears doubtful: while the substance showed only meagre effects regarding clinical symptomatology and reduction of body weight, it harbors the important risk of cognitive impairment (Muscatello et al., 2011[20]) and should be used with caution. Valproic acid shows beneficial impact on aggressive symptoms as well as on tardive dyskinesia. For the management of acute psychotic episodes, however, it was not superior to placebo when added to SGAs (Casey et al., 2009[6]).
Experimental substances
Several experimental substances were tested for beneficial effects on the schizophrenic deficit syndrome. Preliminary positive results have been obtained with the cyclo-ocygenase-2 (COX-2) inhibitor celecoxib, and the phosphodiesterase-5 (PDE-5) inhibitor sildenafil; yet, they lack further verification.
Cognitive Impairment
The importance of assessing and treating cognitive malfunction cannot be overestimated as functional outcome, social and vocational rehabilitation of schizophrenic patients is determined to a great extent by their cognitive skills. Still, as yet, no gold standard has been established for the treatment of schizophrenic thought disturbances. Nonpharmacological interventions such as specifically tailored cognitive remediation and metacognitive training have reproducibly shown moderate results, which are at present not equalled by pharmacological interventions. Several pathophysiological considerations have resulted in trials with predominantly experimental characters and only preliminary evidence.
Cholinergic neurotransmission is involved into memory consolidation and is preponderantly modulated by muscarinic M1-receptors or the α-subunit of nicotinic acetylcholine receptors. Adding choline esterase inhibitors such as donepezil and galantamine to SGA treatment regimens, however, resulted in cognitive improvements no greater than placebo and therefore lacks clinical significance. Preliminary positive findings have been obtained with the α7-partial agonists tropisetrone or with DMXB-A; but they need to be replicated.
The glutamatergic system modulates cognitive processes such as attention, working memory, executive functions and learning via the NMDA receptor. Glycine, D-serine, D-cycloserine and sarcosine, an inhibitor of the glycine-transporter, act as NMDA agonists and were intended to enhance cognition in schizophrenia. More than 800 schizophrenic patients were treated in a total of 26 studies and experienced an improvement of affect regulation, positive and negative symptoms, cognition and general psychopathology under treatment with all of these substances, with the exception of D-cycloserine, as has been revealed by a recent meta-analysis (Tsai & Lin, 2010[31]). Also the tetracyclic antibiotic minocycline modulates the glutamatergic system and was shown to be of favor regarding negative symptoms, global outcome and executive functions, whereas the NMDA-antagonist memantine and modulators of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic (AMPA) receptors such as CX516 brought about no benefit.
Dopaminergic neurotransmission modulates the cognitive domains of working memory and executive functions, and accordingly, the modified dopamine hypothesis of schizophrenia postulates a dysfunctional dopaminergic neurotransmission within the prefrontal cortex. Genetically defined interindividual differences importantly influence the dopaminergic system, and executive dysfunction is strongly associated with schizophrenia. Therefore, dopamine agonists have been applied to counteract such deficits: The D1 agonist dihydrexidine, the combined D1/D2 agonist pergolide and the presynaptic D2/D3 autoreceptor agonist pramipexole have been evaluated with inconsistent results. In parallel, the SSNRI bupropion showed a trend for improving cognitive performance in schizophrenia but needs to be further evaluated.
Aside from the above-mentioned neurotransmitter systems, cognition is also regulated by stimulation of 5-HT1A-, 5HT2A- and 5-HT6-receptors, and modulators of the 5-HT1-4-receptor subtypes have been shown to improve learning abilities. In accordance, favorable effects have been observed with the 5-HT1A partial agonist buspirone, the 5-HT3-antagonist ondansetron and the 5-HT2A/2C-antagonist ritanserine.
Further research on pharmacotherapy of cognitive deficits in schizophrenia might involve cognitive enhancers such as methylphenidate, the norepinephrine reuptake inhibitor atomoxetine or modafinil, sex hormones such as estradiol, allopurinol, gingko biloba, N-acetyl cysteine (NAC), propentofylline, S-adenosyl methinonine (SAM), selegiline and warm-supplementing kidney yang (WSKY). While all of these substances hold conceptual or preliminary justification to some extent, they differ markedly regarding safety and tolerability and cannot be recommended for clinical application at present.
Management of Side Effects Induced by Antipsychotics
Treatment-emergent side effects are common, limit treatment adherence and have to be considered before and during any kind of psychopharmacological treatment. Careful management is necessary to counteract these frequently severe and sometimes stigmatizing symptoms. Side effects often result in polypharmacy, which may be considered outside the scope of this review. However, treatment-emergent side-effects frequently limit antipsychotic dose escalations, may result in switching treatment regimens and consequently result in treatment resistance. While anticholinergic substances such as biperiden, metixen und trihexyphenidyl are well-established remedies for antagonizing acute dystonic movement disorders, their chronic administration, however, must be avoided due to the risk of cognitive side effects. Akathisia may be responsive to the add-on of mirtazapine, but often only disappears after switching to another antipsychotic substance. Weight gain is a highly relevant problem in schizophrenia which may be attributed to some extent to an altered eating behavior one the one hand and disease-inherent deficits in the reward anticipation on the other. Yet, also side effects of antipsychotic drugs must be considered, which is why clinicians struggle to manage obesity, dyslipidemia and other metabolic derangements in schizophrenic patients. Some improvement may be achieved by combining SGAs with less favorable metabolic profiles such as clozapine, olanzapine and quetiapine with SGAs less prone to weight gain such as ziprasidone, amisulpride or aripiprazole (Zink et al., 2010[34]). Hence, relevant dose reductions of the primary SGA may be achieved which can result in significant metabolic improvements. Data regarding metformin as a single adjunct or in combination with sibutramine is still inconsistent, whereas the add-on of the noradrenergic antidepressant reboxetine proved helpful to counteract SGA-associated weight gain.
Sexual dysfunction in schizophrenia is multicausal, with serum prolactin elevations resulting from antipsychotic D2-receptor blockade being of major importance. Consequently, many patients experience menstrual irregularities and galactorrhoea or gynaecomastia and sexual dysfunction, respectively, reducing quality of life and lowering the overall treatment adherence. While dose reductions or switching to another SGA aim at a causative approach, experimental substances such as the PDE-5 inhibitors sildenafile and vardenafile have also been applied. Furthermore, dopaminergic agents, e.g., bromocriptine and cabergoline, as also cyproheptadine, amantadine, shakuyaku-kanzo-to and selegiline, were used symptomatically. Generally, however, it is recommended that physicians focus on prevention, for instance by the choice of antipsychotics with lower risk of endocrine side effects, rather than treating sexual dysfunction.
Obsessive-compulsive symptoms (OCS) in schizophrenia, finally, have been correlated with the administration of antiserotonergic antipsychotics in a time- and dose-dependent manner (Schirmbeck et al., 2011[23]; Schirmbeck & Zink, 2011[24]). Treating this grossly underestimated side effect primarily aims at a dose reduction of the respective underlying antipsychotic agent, which may be achieved by compensating combination and augmentation strategies, for example with neutral (amisulpride) or antiobsessive (aripiprazol and ziprasidone) SGAs.
Concluding Remarks [See also Figure 1: Flowchart of Paper]
Figure 1.
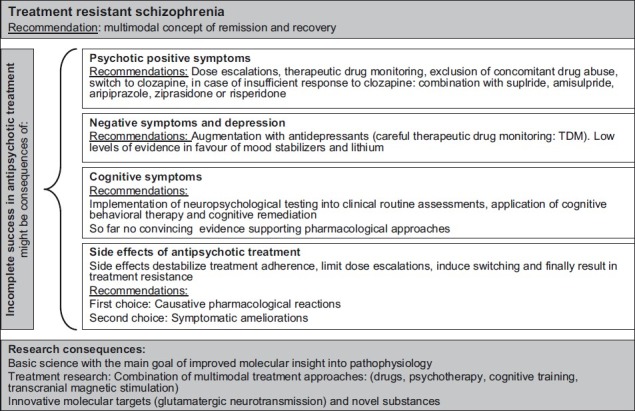
Flowchart of the paper
In light of currently available data, some conclusions seem justified:
Clozapine remains the first-line medication for treatment-refractory psychotic symptoms.
In cases of partial response, the combination with SGAs such as sulpiride, amisulpride, aripiprazole, ziprasidone and risperidone is justified in order to supplement its antidopaminergic properties. Clear differential indications should be elaborated by future head-to-head-trials.
Treatment-refractory negative symptoms and intercurrent major depressive episodes respond to a large extent to antidepressive treatment with SSRIs, SSNRIs or mirtazapine, while mood stabilizers and lithium lack convincing evidence.
Incomplete remission of cognitive dysfunction remains the most important challenge of treatment research in schizophrenia.
Currently available compounds represent only few therapeutic mechanisms which are insufficient to control all aspects of schizophrenia. Thus, there is an urgent need for the scientific exploration of the molecular neurobiology of schizophrenia, the definition of novel treatment targets and the development of innovative substances. Progress along this line might enable clinicians to ease the burden of illness in schizophrenic patients.
Take home message
Treatment resistance is very common and refers not only to psychotic positive and negative symptoms, as well as depression, but most importantly also to manifold cognitive deficits.
Specific pharmacological and psychotherapeutic interventions can be applied based on evidence derived from clinical trials.
Novel molecular targets and innovative substances should be defined in future schizophrenia research.
Questions that the Paper Raises
Which different dimensions of treatment resistance can be addressed by clinical trials?
Which strategies should be chosen if psychotic positive or negative symptoms resist antipsychotic monotherapy?
How prevalent are comorbid depressive or obsessive-compulsive symptoms and which therapeutic consequences are indicated?
Should patients with treatment-resistant cognitive symptoms receive cognitive remediation or pharmacological interventions?
Which methods allow estimating the costs and benefits of antipsychotic polypharmacy regarding subjective as well as socioeconomic burden of disease?
About the Author

Susanne Englisch MD works as a senior resident at the department of psychiatry and psychotherapy of the Central Institute of Mental Health (CIMH). She is also a member of the research group “Molecular schizophrenia research” with her primary focus being on the treatment of affective and cognitive symptoms in schizophrenia.
About the Author

Mathias Zink MD works as a board-certified psychiatrist, senior physician and research group leader at the department of psychiatry and psychotherapy of the Central Institute of Mental Health (CIMH) in Mannheim, Germany. This research institute is associated with the University of Heidelberg and mainly aims at neurobiological research in the pathogenesis and therapy of psychiatric disorders. The research group “Molecular schizophrenia research” is dedicated to animal models of psychosis and antipsychotic treatment, early detection of psychosis, cognitive and metacognitive deficits, neuropsychology and functional imaging in psychosis, treatment resistant symptoms and comorbidity with affective and obsessive-compulsive disorders.
Footnotes
Conflict of interest: S.E. has been provided with travel expenses and consultant fees from AstraZeneca, Bristol-Myers Squibb GmbH & CoKGaA, Eli-Lilly, Janssen Cilag, Lundbeck, Otsuka Pharma, Pfizer Pharma and Servier
M.Z. has received unrestricted scientific grants of German Research Foundation (DFG), ERAB (European Research Advisory Board), Pfizer Pharma GmbH, Bristol-Myers Squibb GmbH & CoKGaA, further speaker and travel support from Pfizer Pharma GmbH, Bristol-Myers Squibb GmbH & CoKGaA, Astra Zeneca, Eli-Lilly and Janssen Cilag
Funding: This work was not supported by any funding.
Declaration
The authors declare that this manuscript is their original unpublished paper and has not been submitted for publication elsewhere.
CITATION: Englisch S., Zink M. Treatment-resistant Schizophrenia: Evidence-based Strategies. Mens Sana Monogr 2012; 10: 20-32.
References
- 1.Aitchison KJ, Mir A, Shivakumar K, McAllister VD, O’Keane V, McCrone P. Costs and outcomes associated with an aripiprazole add-on or switching open-label study in psychosis. J Psychopharmacol. 2011;25:675–84. doi: 10.1177/0269881109358198. PMID: 20176773. [DOI] [PubMed] [Google Scholar]
- 2.Asenjo Lobos C, Komossa K, Rummel-Kluge C, Hunger H, Schwarz SF, Leucht S. Clozapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010;11:CD006633. doi: 10.1002/14651858.CD006633.pub2. PMID: 21069690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assion HJ, Reinbold H, Lemanski S, Bsilowski M, Juckel G. Amisulpride augmentation in patients with schizophrenia partially responsive or unresponsive to clozapine. A randomized, double-blind, placebo-controlled trial, Pharmacopsychiatry. 2008;41:24–8. doi: 10.1055/s-2007-993209. PMID: 18203048. [DOI] [PubMed] [Google Scholar]
- 4.Baandrup L, Gasse C, Jensen VD, Glenthoj BY, Nordentoft M, Lublin H, et al. Antipsychotic polypharmacy and risk of death from natural causes in patients with schizophrenia: a population-based nested case-control study. J Clin Psychiatry. 2010;71:103–8. doi: 10.4088/JCP.08m04818yel. PMID: 19895781. [DOI] [PubMed] [Google Scholar]
- 5.Barbui C, Accordini S, Nose M, Stroup S, Purgato M, Girlanda F, et al. Aripiprazole versus haloperidol in combination with clozapine for treatment-resistant schizophrenia in routine clinical care: a randomized, controlled trial. J Clin Psychopharmacol. 2011;31:266–73. doi: 10.1097/JCP.0b013e318219cba3. PMID: 21508849. [DOI] [PubMed] [Google Scholar]
- 6.Casey DE, Daniel DG, Tamminga C, Kane JM, Tran-Johnson T, Wozniak P, et al. Divalproex ER combined with olanzapine or risperidone for treatment of acute exacerbations of schizophrenia. Neuropsychopharmacol. 2009;34:1330–8. doi: 10.1038/npp.2008.209. PMID: 19052541. [DOI] [PubMed] [Google Scholar]
- 7.Centorrino F, Ventriglio A, Vincenti A, Talamo A, Baldessarini RJ. Changes in medication practices for hospitalized psychiatric patients: 2009 versus 2004. Hum Psychopharmacol. 2010;25:179–86. doi: 10.1002/hup.1095. PMID: 20196186. [DOI] [PubMed] [Google Scholar]
- 8.Citrome L. Adding lithium or anticonvulsants to antipsychotics for the treatment of schizophrenia: Useful strategy or exercise in futility? J Clin Psychiatry. 2009;70:932–3. doi: 10.4088/jcp.09ac05289. PMID: 19573487. [DOI] [PubMed] [Google Scholar]
- 9.Englisch S, Enning F, Grosshans M, Marquardt L, Waltereit R, Zink M. Quetiapine combined with amisulpride in schizophrenic patients with insufficient responses to quetiapine-monotherapy. Clin Neuropharmacol. 2010a;33:227–9. doi: 10.1097/WNF.0b013e3181f0f013. PMID: 20838213. [DOI] [PubMed] [Google Scholar]
- 10.Englisch S, Esser A, Enning F, Hohmann S, Schanz H, Zink M. Augmentation with pregabalin in schizophrenia. J Clin Psychopharmacol. 2010b;30:437–40. doi: 10.1097/JCP.0b013e3181e5c095. PMID: 20531222. [DOI] [PubMed] [Google Scholar]
- 11.Englisch S, Knopf U, Scharnholz B, Kuwilsky A, Deuschle M, Zink M. Duloxetine for major depressive episodes in the course of psychotic disorders: An observational clinical trial. J Psychopharmacol. 2009;23:875–82. doi: 10.1177/0269881108093586. PMID: 18583440. [DOI] [PubMed] [Google Scholar]
- 12.Englisch S, Meyer-Lindenberg A, Zink M. Risks and benefits of Bupropion treatment in Schizophrenia: A meta-analysis. J Psychopharmacol. 2011 doi: 10.1097/WNF.0b013e3182a8ea04. in press. [DOI] [PubMed] [Google Scholar]
- 13.Essock SM, Schooler NR, Stroup TS, McEvoy JP, Rojas I, Jackson C, et al. Effectiveness of switching from antipsychotic polypharmacy to monotherapy. Am J Psychiatry. 2011;168:702–8. doi: 10.1176/appi.ajp.2011.10060908. PMID: 21536693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleischhacker W, Heikkinen ME, Olie JP, Landsberg W, Dewaele P, McQuade RD, et al. Effects of adjunctive treatment with aripiprazole on body weight and clinical efficacy in schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled trial. Int J Neuropsychopharmacol. 2010;13:1115–25. doi: 10.1017/S1461145710000490. PMID: 20459883. [DOI] [PubMed] [Google Scholar]
- 15.Gomez JV, Fuste G, Coronas R, Benito N, Barbero JD, Domenech C, et al. Combination of aripiprazole and other psychopharmacological treatments in resistant and multi-resistant patients. Curr Drug Safety. 2008;3:210–5. doi: 10.2174/157488608785699496. PMID: 18691004. [DOI] [PubMed] [Google Scholar]
- 16.Kuwilsky A, Krumm B, Englisch S, Dressing H, Zink M. Long-term efficacy and tolerability of clozapine combined with ziprasidone or risperidone. Pharmacopsychiatry. 2010;43:216–20. doi: 10.1055/s-0030-1254089. PMID: 20589598. [DOI] [PubMed] [Google Scholar]
- 17.Lerma-Carrillo I, de Pablo BS, del Pozo ML, Pascual-Pinazo F, Molina JD, Baca-Garcia E. Antipsychotic polypharmacy in patients with schizophrenia in a brief hospitalization unit. Clin Neuropharmacol. 2008;31:319–32. doi: 10.1097/WNF.0b013e31815cba78. PMID: 19050409. [DOI] [PubMed] [Google Scholar]
- 18.Leucht S, Kissling W, McGrath J. Lithium for schizophrenia. Cochrane Database Syst Rev. 2007;3:CD003834. doi: 10.1002/14651858.CD003834.pub2. PMID: 17636738. [DOI] [PubMed] [Google Scholar]
- 19.Leucht S, Kissling W, McGrath J, White P. Carbamazepine for schizophrenia. Cochrane Database Syst Rev. 2007:CD001258. doi: 10.1002/14651858.CD001258.pub2. PMID: 17636660. [DOI] [PubMed] [Google Scholar]
- 20.Muscatello MR, Bruno A, Pandolfo G, Mico U, Bellinghieri PM, Scimeca G, et al. Topiramate augmentation of clozapine in schizophrenia: a double-blind, placebo-controlled study. J Psychopharmacol. 2011;25:667–74. doi: 10.1177/0269881110372548. PMID: 20615930. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen J, Damkier P, Lublin H, Taylor D. Optimizing clozapine treatment. Acta Psychiatr Scand. 2011;123:411–22. doi: 10.1111/j.1600-0447.2011.01710.x. PMID: 21534935. [DOI] [PubMed] [Google Scholar]
- 22.Pickar D, Vinik J, Bartko JJ. Pharmacotherapy of schizophrenic patients: Preponderance of off-label drug use. PLoS One. 2008;3:e3150. doi: 10.1371/journal.pone.0003150. PMID: 18781198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schirmbeck F, Esslinger C, Rausch F, Englisch S, Meyer-Lindenberg A, Zink M. Antiserotonergic antipsychotics are associated with obsessive-compulsive symptoms in schizophrenia. Psychol Med. 2011;41:2361–74. doi: 10.1017/S0033291711000419. [DOI] [PubMed] [Google Scholar]
- 24.Schirmbeck F, Zink M. Clozapine-induced obsessive-compulsive symptoms in schizophrenia: A critical review. Curr Neuropharmacol. 2011 doi: 10.2174/157015912799362724. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Se HK, Dong CJ, Yong MA, Yong SK. The combined use of risperidone long-acting injection and clozapine in patients with schizophrenia non-adherent to clozapine: A case series. J Psychopharmacol. 2010;24:981–6. doi: 10.1177/0269881109348174. PMID: 19942641. [DOI] [PubMed] [Google Scholar]
- 26.Singh SP, Singh V, Kar N, Chan K. Efficacy of antidepressants in treating the negative symptoms of chronic schizophrenia: Meta-analysis. Br J Psychiatry. 2010;197:174–9. doi: 10.1192/bjp.bp.109.067710. PMID: 20807960. [DOI] [PubMed] [Google Scholar]
- 27.Stenberg JH, Terevnikov V, Joffe M, Tiihonen J, Tchoukhine E, Burkin M, et al. More evidence on proneurocognitive effects of add-on mirtazapine in schizophrenia. Progr Neuropsychopharmacol Biol Psychiatry. 2011;35:1080–6. doi: 10.1016/j.pnpbp.2011.03.004. PMID: 21402120. [DOI] [PubMed] [Google Scholar]
- 28.Taylor DM, Smith L, Gee SH, Jimmi N. Augmentation of clozapine with a second antipsychotic: a meta-analysis. Acta Psychiatr Scand. 2012;125:15–24. doi: 10.1111/j.1600-0447.2011.01792.x. PMID: 22077319. [DOI] [PubMed] [Google Scholar]
- 29.Tiihonen J, Lonnqvist J, Wahlbeck K, Klaukka T, Niskanen L, Tanskanen A, et al. 11-year follow-up of mortality in patients with schizophrenia: A population-based cohort study (FIN11 study) Lancet. 2009;374:620–7. doi: 10.1016/S0140-6736(09)60742-X. PMID: 19595447. [DOI] [PubMed] [Google Scholar]
- 30.Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: A systematic review and meta-analysis. Schizophr Res. 2009;109:10–4. doi: 10.1016/j.schres.2009.01.002. PMID: 19186030. [DOI] [PubMed] [Google Scholar]
- 31.Tsai GE, Lin PY. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia: A critical review and meta-analysis. Curr Pharmaceut Design. 2010;16:522–37. doi: 10.2174/138161210790361452. PMID: 19909229. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Omori IM, Fenton M, Soares B. Sulpiride augmentation for schizophrenia. Cochrane Database Syst Rev. 2010;1:CD008125. doi: 10.1002/14651858.CD008125.pub2. PMID: 20091661. [DOI] [PubMed] [Google Scholar]
- 33.Weinmann S, Read J, Aderhold V. Influence of antipsychotics on mortality in schizophrenia: Systematic review. Schizophr Res. 2009;113:1–11. doi: 10.1016/j.schres.2009.05.018. PMID: 19524406. [DOI] [PubMed] [Google Scholar]
- 34.Zink M, Englisch S, Meyer-Lindenberg A. Polypharmacy in schizophrenia. Curr Opin Psychiatry. 2010;23:103–11. doi: 10.1097/YCO.0b013e3283366427. PMID: 20051861. [DOI] [PubMed] [Google Scholar]
- 35.Zink M, Kuwilsky A, Krumm B, Dressing H. Efficacy and tolerability of ziprasidone versus risperidone as augmentation in patients partially responsive to clozapine: A randomized controlled clinical trial. J Psychopharmacol. 2009;23:305–14. doi: 10.1177/0269881108089593. PMID: 18562423. [DOI] [PubMed] [Google Scholar]


