Abstract
Background and Objective:
The implementation of quality system and continuous evaluation of all activities of the Blood Transfusion Services (BTS) can help to achieve the maximum quantity and quality of safe blood. Optimizing blood collection and processing would reduce the rate of discard and improve the efficiency of the BTS. The objective of this study is to determine the rate of discard of blood and blood component and identify its reasons at the National Blood Centre (NBC), Kuala Lumpur, during the year of 2007 in order to introduce appropriate intervention.
Study Designs and Methods:
Data on the number of discarded whole blood units and its components, reasons for discard, and the number of blood components processed as well as the number of collected blood units were obtained from the Blood Bank Information System - NBC database. These were analyzed.
Results:
The total number of blood units collected in 2007 was 171169 from which 390636 units of components were prepared. The total number of discarded whole blood units and its components was 8968 (2.3%). Platelet concentrate recorded the highest of discard at 6% (3909) followed by whole blood at 3.7% (647), fresh frozen plasma (FFP) at 2.5% (2839), and cryoprecipitate at 2% (620). The rate of discarded packed red blood cells RBCs, plasma aphaeresis, and PLT aphaeresis was less than 1% at 0.6% (902), 0.6% (37), and 0.29% (14), respectively. RBC contamination of PLT and plasma were the major cause of discard at 40% (3558). Other causes include leakage (26% - 2306), lipemia (25% - 2208), and underweight (4% - 353).
Conclusion:
Good donor selection, training and evaluation of the staff, as well as implementation of automation will help to improve processes and output of BTS. This would reduce discard of blood components and wastage caused by non conformance.
Keywords: Discard blood, National Blood Centre Kuala Lumpur, quality indicators
Introduction
The transfusion of blood and blood components has become an integral part of patient management in modern medicine.[1] Therefore, the Blood Transfusion Services (BTS) plays an important role and is responsible for ensuring sufficiency, quality, and safety of the blood supply. A well-organized and efficient BTS would contribute toward better patients care and contribute toward the development of healthcare in the country.
A major challenge facing the BTS is to supply sufficient amount of safe blood whenever at is required. It needs to double its efforts to collect sufficient amount of safe blood from voluntary, non-remunerated, healthy, and low-risk donors.[1–3] To overcome the lack of blood supply, the performance of BTS can be increased either by increasing the level of resources used in the collection and production of blood components or by utilizing existing resources more efficiently.[4] Another approach is to reduce the amount of blood discarded as a result of inappropriate blood collection and components processing. Reducing the amount of discarded blood can contribute towards decreasing the total cost of blood and its components. In 2006, hospitals in the United States paid $213.94 for a unit of leukocyte-reduced RBCs and $538.72 for apheresis platelet.[5]
The BTS can reach the highest levels of efficiency in terms of quantity and quality of blood and blood components through the implementation of a quality management system in all phases of the collection, processing, and storage of the blood. A functioning quality system would ensure all processes and procedures would adhere to quality standards and this would improve the quality and safety of blood components as well as to reduce the amount of blood discarded to a level that is more acceptable to the set standards.
The efficiency of processing and preparation of the blood components can be monitored by establishing quality indicators that reflect the activities to be evaluated. The rate of discarded blood components is one of those indicators. It is defined as the proportion of a total number of blood components discarded from the total number of blood collections. When the rate of discarded blood is high, the level of efficiency of collection and components preparation process is low.[6] By analyzing the data and the reason for the discards, the BTS can develop plans to improve performance through education and training of staff and introducing new measures in order to minimize the number of discarded blood to a reasonable rate.
Materials and Methods
Study design
This is a retrospective study involving the analyses of discarded blood and blood components data in National Blood Centre (NBC) from January 1 to December 31, 2007, which measured the outcome-based quality of discarded blood and its components. The study included the discarding of whole blood, red cells, platelets, FFP, and cryoprecipitate units due to inappropriate blood collection and processing. These included products that were underweight, overweight, RBCs contamination of plasma and platelets, hemolysis, leakages, clots, lipemic appearances, greenish and yellowish (icterus) discoloration. The discarded units due to expiry date and transfusion-transmitted diseases were not included.
Data collection
All information was retrieved from the Blood Bank Information System (BBIS) in NBC. These included the daily amount of blood collection, the number of units of various components discarded, reason for the discard, and discarded date of each blood components. A total of 390 634 units of blood and blood components were collected and prepared during the year of 2007.
Correlation between the number of daily blood collection and discarded blood units
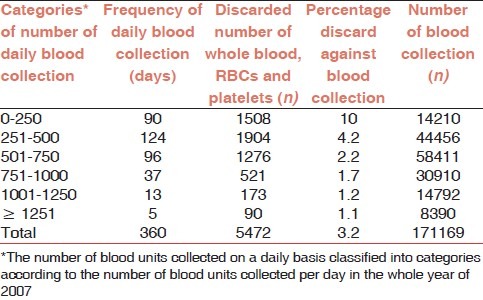
The number of blood units collected on a daily basis was classified into a few categories according to the number of blood units collected per day. Against this category, the amounts of discards were determined as shown in Table 1. This correlation consists of the discarded whole blood, packed RBC, and platelet concentrate as the preparation and discarding components occur in the same day of the blood collection. However, FFP and cryoprecipitate in the frozen components are not mandatory to prepare and discard in the same day of blood collection.
Table 1.
Number of daily blood collection and discarded blood and its components in NBC 2007
Definitions
Quality indicators
Quality indicator is defined as a specific measurement of the performance of functions and processes used to make informed decisions regarding whether a process is in control or identify opportunities for improvement.[7] The American Association of Blood Banks (AABB) defined quality indicators as the specific performance measurements designed to monitor one or more processes during a defined time and are useful for evaluating service demands, production, adequacy of personnel, inventory control, and process stability.[8]
Discarded blood
Blood and blood components are wasted as a result of inappropriate blood collection and components processing as they do not meet the specification that has been specified and defined. These non-conformant products are not issued out to hospitals or patient as they may be ineffective or unstable or show signs of abnormal physical appearance such as underweight, overweight, plasma and platelets with RBCs contamination, hemolysis, leakage, clotted, lipemia, greenish, icterus prior to its expiration date.[9,10]
Rate of discarded blood components
This rate of discard is derived when the number of whole blood, packed RBCs, platelets, FFP, or cryoprecipitate discarded is divided by the number of whole blood, RBCs, platelets, FFP, or cryoprecipitate prepared, respectively multiplied by 100.[11]
Reasons of discarded blood
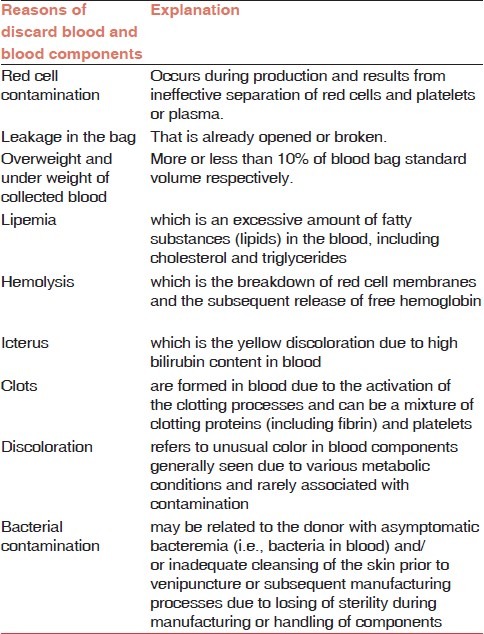
The unsuitable blood components were discarded in NBC according to Guidelines and Principles for safe blood transfusion practice (WHO),[1] Guidelines for the BTS in United Kingdom,[12] and AABB[13] as shown in Table 2.
Table 2.
Explanations of the reasons of discard blood and blood components
The decision to discard a unit of blood was undertaken only after consulting senior medical laboratory technician at the blood center. The date and the cause of discard were recorded and the unit disposed off safely.[1]
Statistic analysis
The correlation between amounts of daily blood collection and discarded whole blood, packed RBCs and platelet concentrates was measured by Spearman's rho. P value <0.01 was considered as statistically significant. The data were analyzed by SPSS version 12.0 (SPSS. Inc., Chicago, USA).
Results
The total number of blood units collected in 2007 were 171169, with 122792 units (71.7%) collected by NBC. However, the three blood collection centers in A, B, and C collected 48377 units (28.3%). All the collected blood units were processed in NBC for screening and preparation of blood components. Five percent of collection was kept as whole blood, while the remaining blood was processed into different blood components. The total amount of the blood components prepared were 390634.[14]
Rates of discarded blood
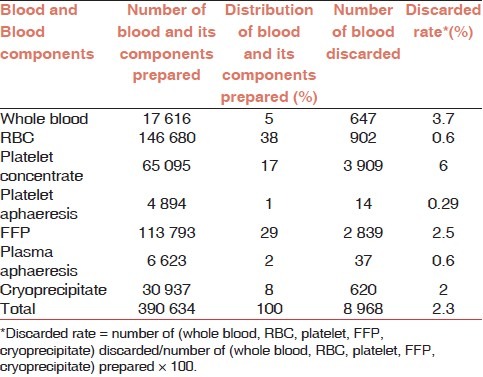
The overall rate of discarded blood and blood components in NBC was 2.3% (8968 of 390634). The rate of discard for platelet concentrate was the highest at 6% (3909 of 65095). The rates of discard for whole blood, FFP, and cryoprecipitate were 3.7% (647 of 17616), 2.5% (2839 of 113793), and 2% (620 of 30937), respectively. The rate of discard for packed RBC was the same as that of plasma aphaeresis at 0.6% (902 of 146680 and 37 of 6623, respectively). The lowest rate of discard was for platelet aphaeresis at 0.29% (14 of 4894) as shown in Table 3.
Table 3.
Distribution numbers prepared and discarded blood and its components at NBC in 2007
Reasons of discarded blood and its components
Table 4 summarizes the reasons for discard of blood and blood components. The major cause of discard in NBC was red cell contamination of the platelet and plasma components which accounted for 3558 (40%). This was followed by leakage at 26% (2306) and thirdly was lipemia at 25% (2208). The other reasons for discard were less than 5%. These included underweight 4.6% (353), greenish discoloration at 3% (246), overweight at 3% (227), clotted units at 0.4% (40), and icterus at 2% (160).
Table 4.
Reasons of discarded blood and blood components in NBC 2007
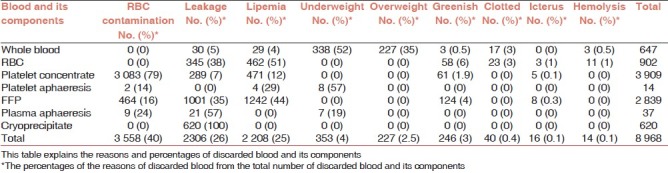
The major reasons for discarding whole blood were underweight, which amounted to 52% (338 of 647) and overweight, 35% (227 of 647). The major reasons of discarding packed RBC components were lipemia at 51% (462 of 902) and leakage of blood bags at 38% (345 of 902).
The majority of platelet concentrates were discarded due to red cell contamination at 79% (3083 of 3906). However, the majority of platelet aphaeresis discarded were due to underweight units at 57% (8 of 17).
The most important reasons for discarded FFP were lipemia at 44% (1242 of 2839) and leakages at 35% (1001 of 2839), whereas the main reasons in discarding aphaeresis plasma were leakage at 57% (21 of 37) and red cells contamination at 24% (9 of 37). The 620 cryoprecipitate bags that were discarded were due to leakage that occurred during thawing before issue. The other reasons for discard are reported in Table 4.
Correlation between number of daily blood collection and discarded blood units
The highest number of collected blood was 58 411 units in the category of 501 to 750 involving collection done in 96 days. The number of discarded whole blood, packed RBC, and platelet concentrates were 1276 units (2.2%) as shown in Table 1.
The maximum number of discarded whole blood, packed RBC, and platelet was 1904 units (4.2%) in the category of 251 to 500. In the same category, the number of collected blood units were 44456 units over a period of 124 days. Meanwhile, in the category of 0 to 250, the highest percentage of discarded units occurred at 10% (1508) against 14210 units of blood collected blood [Table 1].
The study shows that there is no statistical significance between the daily number of discarded whole blood, packed RBCs, and platelets and the daily blood collection (Spearman rho = 0.026, P = 0.311).
Discussion
A total of 390 634 whole blood and blood components units were prepared in 2007 in NBC. Of these, 8 968 (2.3%) units were discarded. There were many reasons for the discard, among them were deviations from established standards during collection such as suboptimal weight at the end of collection, or as a result of components processing which damaged the blood bags or caused red cells contamination of plasma and platelets concentrates.
Platelet concentrate scored the highest at 6% when compared with the other blood components. The discarded rates of whole blood and packed RBCs were 3.7% and 0.6%, respectively. The reasons behind the discard of whole blood can be attributed to procedures carried out during the collection process. A large-scale study conducted in 17 blood centers in 10 European countries from 2000 to 2002 reported that the mean platelet discard rates for the three years were between 6.7% and 25%. However, the annual mean discard rates from 2000 till 2004 remains at 13%. The discarded platelets included all the platelet units which were damaged during processing regardless of the preparation method as well as those that expired.[15] In the same European centers, the mean for packed RBC discard rate was 4.5%, varying annually from 0.2% to 7.7%.[11] The current study showed that the FFP and RBC discard rates were comparable with the Novis study in USA, which reported that the discard rates of FFP ranged from 2% to 2.5% and RBC ranged from 0.1% to 0.7% in 1639 hospitals.[16,17]
Platelet and plasma components contaminated with RBC resulted from ineffective separation of platelet-rich plasma (PRP) or plasma from red cells during centrifugation or processing [Table 2]. This contamination is a known threat to product quality. In this study, 79% (3083) of discarded platelets and 16% (464) plasma were wasted due to this. In this study, all platelets concentrate components were prepared by PRP method. After centrifugation, there were critical steps that can cause red cells contamination of plasma and platelets. The centrifuged blood bags had to be carefully removed from the centrifuge bucket to prevent mixing the red cells with the plasma. Subsequently, these centrifuged blood bags should be carefully reposted onto the plasma extractor (or blood press) and allow gentle pressure to the centrifuged blood bag from the plasma extractor plate. The separation should be done slowly with close monitoring during the transfer of layers of blood components into the satellite bags. The separation is stopped when about 8 mm of plasma remains above the red cells.[18] This technique is labor intensive. Human factor has strong effect on the quality and purity of the blood components preparation. Another source of contamination of the PRP is the tendency of the bags’ content to swirl during rotor deceleration in an effort to keep its angular momentum causing RBCs and WBCs to be mixed with plasma.[19]
In the Components Section of the NBC, there were more than 30 centrifuges. The study could not exactly determine which centrifuge caused significant number of the RBCs contamination with blood components due to lack of records or data on these centrifuges. Although the centrifuge software was able to capture information like the operator's name, date and time, processed details of the centrifuge time, and speed parameters according to the donation identification number (barcode), it was not activated. This needs to be activated so that in the event of any quality problems, full traceability of the centrifugation data is available.[18]
The leakage was the second cause of discarded blood and its components, which represented 26% of discarded blood. The frozen blood components that consist of 43% and 27% of discarded FFP and cryoprecipitate, respectively, were due to the leakage. Mishandling of blood bags during collection, processing, and storage or manufacturing errors may be the major causes of defects and leakages of blood bags.[20] The integrity of plastic bags is essential and precautions should be taken to prevent leakages.[12] The bag may be damaged during the centrifugation. This happens when the bag is forced to a sharp interior bottom/wall junction or corner, resulting in the bag material being stretched too far, causing a tear.[21] The defect and leakage at any part of the plastic blood bags can be detected by visual inspection during the processing, after pressure in a plasma extractor, before freezing, and after thawing.[20]
The FFP should be stored in cardboard or polystyrene protective containers that minimize the risk of breakage of brittle frozen product during storage, handling, and transportation.[1] Another approach to decrease the leakage and contamination immediately before immersion of the frozen blood bags in the water bath is that the whole container should be placed in a sterile plastic bag.[12]
Twenty five percent (2208) of discarded blood were wasted because of gross lipemic blood components. A particular unit of yellowish white milky gross lipemic aphaeresis platelet was traced to a donor who donated after eating a high-fatty meal of hamburger. The triglyceride level of that donor was elevated to 1303 mg/dl (normal range, 35-160 mg/dl) with a normal cholesterol level.[22] The case raised an important question—should we advise donors to refrain from eating fatty foods before donation. This is an important issue for donor's health, selection, and recipient confidence. In the United Kingdom, such donations are not released for issue.[22] Lipaemia itself does not affect the safety of a product but might interfere with the ability to perform viral marker tests.[23,24] The doctors and nurses should make an attempt to identify or suspect donors who are at high risk for hyperlipemia prior to donation by asking the donors during the pre-donation interview if they had eaten a fatty meal prior to coming for blood donation. At the NBC, records of regular blood donors can be traced from the BBIS. If their blood components were discarded because of the lipemia, the donors should be investigated for lipid profile. These may assist in minimizing blood component discard due to hyperlipemia.
In overweight blood bags, the amount of anticoagulant is not enough to prevent the blood from clotting and the clotting process may be initiated. On the other hand, there is an excess of anticoagulant in underweight blood that could denature the blood during storage. Suboptimal weight of blood collected would be unsuitable for transfusion and the ratio between volume of blood collected and volume of anticoagulant in the blood bags should be corrected.[25] In NBC, almost all suboptimal weight of blood units occurred at mobile sessions and not at the center. Small manual spring balances or scales for weighing were still being used in mobile sessions, while blood mixing machines were used in the center. The manual spring balances require the staff nurses to monitor the weight of the blood bags every 30 seconds during the whole blood donation process. Sometimes, the nurses were busy because they have more than the expected donors or the monitoring of the spring balance during blood donation was overlooked. These lead to too much blood going into the collected bags. These problems can be avoided by using the automated blood mixing machines. When sufficient blood has entered the bag, an audiovisual alarm is activated and the blood flow automatically stopped by clamping the tube to prevent further blood flow into the bag.[25] Low volume of collected blood may be due to several reasons including the discontinuation of donation because of donor's reactions and the blood flow from small vein during phlebotomy and the duration of the donation exceeds 15 minutes. Another reason may be due to the spring balance was not calibrated, thus was unable to measure accurately the volume of blood in the bag. Selecting a good donor, training and monitoring the staffs will help to reduce cases of the underweight blood units.
The results of this study showed that there is no correlation between the number of daily blood collection and the number of discarded whole blood, packed RBC, and platelet. This gives rise to the possibility of a correlation between the number of daily blood collection and the numbers of the staff or due to the extended working hours of the experienced staff in Component Section at NBC. In 2007, there were eleven staffs positioned in the Component Section. The section implemented a policy when a large amount of blood was collected on a given day, the Section would request assistance from experienced staffs from other Section in NBC. Magnussen et al. report that there was a difference in the quality control measurements of RBC and platelet, where the change involved replacement of a relatively large inexperienced occasional component manufacturing staffs to an experienced regular manufacturing staff.[26] Some of quality control measurement was out of statistical control. For example, leukocyte count in RBCs and platelet concentration components and volume of the platelets produced by the occasional staff went out of control, but not with the experienced staff. Another study done in 17 Europeans centers noted that there was no association of proportion of discarded platelets with the platelet production.[17]
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
References
- 1.World Health Organization WHO. Quality Systems for Blood Safety: Introductory Module Guidelines and Principles for Safe Blood Transfusion Practice Geneva. 2002:65–75. [Google Scholar]
- 2.Custer B, Johnson E, Sullivan SD, Hazlet TK, Ramsey SD, Murphy EL, et al. Community blood supply model: Development of a new model to assess the safety, sufficiency, and cost of the blood supply. Med Decis Making. 2005;25:571–82. doi: 10.1177/0272989X05280557. [DOI] [PubMed] [Google Scholar]
- 3.Goodnough LT, Shander A, Brecher ME. Transfusion medicine: Looking to the future. Lancet. 2003;361:161–9. doi: 10.1016/S0140-6736(03)12195-2. [DOI] [PubMed] [Google Scholar]
- 4.Pitocco C, Sexton TR. Alleviating blood shortages in a resource-constrained environment. Transfusion. 2005;45:1118–26. doi: 10.1111/j.1537-2995.2005.00176.x. [DOI] [PubMed] [Google Scholar]
- 5.United States Blood Survey. The 2007 national blood collection and utilization survey Report in United State. 2007:41–4. [Google Scholar]
- 6.Veihola M, Aroviita P, Kekomäki R, Linna M, Sintonen H. Discarded cellular components and the technical efficiency of component preparation. Eur J Health Econ. 2007;9:325–31. doi: 10.1007/s10198-007-0079-9. [DOI] [PubMed] [Google Scholar]
- 7. [Last accessed on 2010 Nov 7]. Available from: http://www.mayomedicallaboratories.com/about/quality/framework/glossary.html .
- 8.Robacck JD, Combs MR, Grossman BJ, Hillyer CD. MT (ASCP) SBB. 16th ed. American Association of Blood Banks AABB Technical Manual; 2008. p. 24. [Google Scholar]
- 9.Cobain TJ. Fresh blood product manufacture, issue, and use: A chain of diminishing returns? Transfus Med Rev. 2004;18:279–92. doi: 10.1016/j.tmrv.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Procedure for disposal of unsuitable blood and/or blood components. Document: PDN/CP/Work Instruction-16. 2004:1. [Google Scholar]
- 11.Veihola M. Technical efficiency of blood component preparation in blood centres of 10 European countries, academic dissertation. Finland: Department of Public Health, Faculty of Medicine University of Helsinki; 2008. p. 5. [Google Scholar]
- 12.Guidelines for the Blood Transfusion Services in the United Kingdom. 2005:18–77. [Google Scholar]
- 13.American Association of Blood Banks AABB, American's Blood Centres. Bethesda: 2002. American Red Cross Circular of information for the use of human blood and blood components; pp. 10–40. [Google Scholar]
- 14.Yasmin A, Norris N, Zuraidah Y, Yessy OM, Nor Ashikah MA, Sim Din N, et al. Laporan Tahunan. Pusat Darah Negara, Malaysia. 2007:8. [Google Scholar]
- 15.Veihola M, Aroviita P, Linna M, Sintonen H, Kekomäki R. Variation of platelet production and discard rates in 17 blood centres representing 10 European countries from 2000 to 2002. Transfusion. 2006;46:991–5. doi: 10.1111/j.1537-2995.2006.00832.x. [DOI] [PubMed] [Google Scholar]
- 16.Novis DA, Renner S, Friedberg R, Walsh MK, Saladino AJ. ‘Quality indicators of fresh frozen plasma and platelet utilization three college of American pathologists Q-probes studies of 8 981 796 units of fresh frozen plasma and platelets in 1639 hospitals. Arch Pathol Lab Med. 2002;126:527–31. doi: 10.5858/2002-126-0527-QIOFFP. [DOI] [PubMed] [Google Scholar]
- 17.Novis DA, Renner S, Friedberg R, Walsh MK, Saladino AJ. Quality indicators blood utilization three college of American pathologists Q-probes studies of 12288404 red blood cell units in 1639 hospitals. Arch Pathol Lab Med. 2002;126:150–6. doi: 10.5858/2002-126-0150-QIOBU. [DOI] [PubMed] [Google Scholar]
- 18.Hardwick J. Blood processing: Introduction to blood transfusion technology. ISBT Sci Series. 2008;3:148–76. [Google Scholar]
- 19.Automated system and method for blood components separation and processing 2008. [Last accessed on 2010 Nov 7]. Available from: http://www.freshpatents.com/Automated-system-and-method-for-blood-componentsseparation-and-processing-dt20081009ptan20080248938.php .
- 20.Guide to the preparation, use and quality assurance of blood component. 12th ed. Council of Europe Publishing; 2006. pp. 242–8. [Google Scholar]
- 21.Fullerton CA. Rotor Bucket liner US Beckman Instruments, INC Patent. 1984. [Last accessed on 2010 Nov 7]. Available from: http://www.freepatentsonline.com/4439177.html .
- 22.Mbahi M, Reddy V, Narvios A. Milky platelet concentrate: A second look. Transfusion. 2006;46:877. doi: 10.1111/j.1537-2995.2006.00833.x. [DOI] [PubMed] [Google Scholar]
- 23.Visual Assessment Guide. Canadian Blood Services. 6;2009:4–26. [Google Scholar]
- 24.Visual Inspection Reference Guide. American National Red Cross. 2006:6–26. [Google Scholar]
- 25.Armstrong B. Blood collection. ISBT Sci Series. 2008;3:123–36. [Google Scholar]
- 26.Magnussen K, Quere S, Winke P. Use of statistical process control in the production of blood components. Transfus Med. 2008;18:190–6. doi: 10.1111/j.1365-3148.2008.00864.x. [DOI] [PubMed] [Google Scholar]


