Abstract
Background/Aim:
The immunopathogenesis of occult Hepatitis C virus (HCV) infection is a matter of great controversy and has been suggested to involve a complex balance between cytokines with pro- and anti-inflammatory activity. This work aimed at studying the serum Th1 and Th2 cytokine production in patients with occult HCV infection.
Materials and Methods:
Serum levels of cytokines of Th1 (interleukin [IL]-2, INF-γ) and Th2 (IL-4) were measured in 27 patients with occult HCV infection and 28 patients with chronic hepatitis C infection.
Results:
The levels of IL-2 and interferon-γ were highly significantly increased in patients with chronic HCV infection (P<0.001). IL-4 was highly significantly increased in occult HCV infection (P<0.001). Significant increases were noted in chronic HCV infection regarding bilirubin (P<0.001), ALT (P = 0.009), AST (P = 0.013), AFP (P<0.001), while serum albumin was significantly higher in occult HCV infection (P<0.001). Necroinflammation (P<0.001), fibrosis (P<0.001), and cirrhosis (P = 0.03) were significantly increased in chronic HCV infection.
Conclusion:
Our data revealed a high prevalence of occult HCV infection (25%) in patients with unexplained persistently abnormal liver function test results. Those patients exhibited a distinct immunoregulatory cytokine pattern, favoring viral persistence and explaining the less aggressive course of this disease entity than chronic HCV infection.
Keywords: Cytokines, Hepatitis C virus, occult
Introduction
Hepatitis C virus (HCV) is a commonly encountered pathogen in medical practice. It is estimated that 2% to 3% of the world is affected by HCV, with a prevalence of 170 million people (3% of the world's population) and an incidence of 3 to 4 million per year.[1] Chronic HCV infection is a progressive disease that may lead to liver cirrhosis and hepatocellular carcinoma. The hallmark of chronic hepatitis C is the presence of anti-HCV and HCV RNA in serum for more than 6 months after acute infection.[2,3]
Occult HCV infection is a new form of HCV infection that has been described and is defined by the presence of HCV-RNA in liver cells with undetectable anti-HCV and serum viral RNA.[4] Histological evaluation of the liver biopsies of patients with occult HCV infection documented different degrees of necroinflammatory activity and fibrosis (including cirrhosis) as reported for chronic hepatitis C.[5] Patients with occult HCV infection present with a milder disease form than patients with chronic hepatitis C infection.[6]
Several studies have suggested that the T-cell immunoregulatory cytokines play a key role in both HCV-related liver damage and the mechanism of viral persistence. Therefore, while some cytokines may exert a proinflammatory activity, such as interleukin (IL)-1, interferon-gamma (IFN-γ), IL-8, tumor necrosis factor-alpha (TNF-α), and IL-2, which can prime T-cells toward a T-helper type 1 (Th-1) immunity, others have a predominantly anti-inflammatory activity, as is the case for IL-4, IL-6, and IL-10, which are involved in Th-2 immunity. Additionally, some of these cytokines may have a fibrogenic (e.g., TGF-β) or an antifibrogenic role (e.g., IFN-γ).[7] Some authors have suggested that a preferential shift towards either Th1 or Th2 response may influence the clinical outcome and disease progression.[8–12]
The aim of this work was to study the Th1/Th2 cytokine levels in patients with occult HCV infection vs those with chronic hepatitis C infection.
Materials and Methods
This study was conducted on 27 consecutive untreated patients with proven occult HCV infection (HCV-RNA in liver specimens as detected by reverse transcriptase-polymerase chain reaction; RT-PCR and by in situ hybridization, with negative anti-HCV antibodies and serum HCV-RNA). Cytokine patterns, biochemical and virological characteristics of these patients were compared with those of a group of 28 untreated anti-HCV- and serum HCV-RNA-positive patients with histopathologically proved chronic hepatitis C infection, equally matched to age, gender, and body mass index (BMI) during the same study period from June 2009 to June 2010.
We started with 108 patients, attending the outpatient clinic of Tropical Medicine Unit, Mansoura University Hospital, with unexplained persistently abnormal liver function test results. Of them, 81 patients were excluded for being negative for HCV-RNA in their liver biopsy specimens.
Exclusion criteria
All known causes of liver diseases were excluded on the basis of analytical, clinical, and epidemiological data: infection by HBV (i.e., subjects were negative for hepatitis B surface antigen and for serum HBV-DNA), autoimmunity, metabolic and genetic disorders, HAV, HEV, CMV, HIV, NASH, alcohol intake, and drug toxicity. This study was conducted following the guidelines of declaration of Helsinki, 1975.
Samples
1. Blood samples for separating peripheral blood mononuclear cells
PBMCs were collected from all patients on the same day that the liver biopsy was performed. PBMCs were obtained by Ficoll-Hypaque density gradient of EDTA anti-coagulated blood according to the manufacturer's instructions (Lymphoflot, Biotest, 63303 Dreleich, Germany). Cells were washed three times with Mg2+ - and Ca2+ -free PBS and resuspended to 1 × 106 cell/ml in PBS.
Cytokines’ assay
From each patient, 2 ml venous blood were collected into clean dry plastic tube and allowed to clot, the yielded serum was used for quantitative detection of IL-2, IL-4, and IFN-γ serum levels which were measured with enzyme-linked immunosorbent assay (ELISA) kits (Bender MedSystems, Vienna, Austria).
2. Liver biopsy
Enrolled patients underwent an ultrasound-guided liver biopsy (using Tru-cut needles) for diagnostic purposes. Liver biopsy specimens were divided into two portions. One portion was fixed in 10% formalin and was paraffin embedded for routine histological diagnosis. Histological evaluation was performed by a pathologist who was blinded with respect to the HCV RNA status of the liver biopsy specimens. Necroinflammatory activity and fibrosis were scored according to the METAVIR score system.[13,14] A minor fragment of the specimen (4–5 mm to make an average weight of 15-20 mg) was immediately immersed in dry ice until extraction of RNA. The extracted RNA was immediately reverse transcribed and stored at -20°C until used for detection of HCV RNA by RT-PCR.
2.1. Preparation of liver cell lysate for total RNA isolation
The amount of tissue was determined by weighing (all samples weighed 20 mg each) according to the recommendation of Norgen Biotek corporation (Canada). Tissues were then slowly ground while still in dry ice. Then, 600 μl of lysis solution was added to the tissue samples and grinding was continued until homogenization of the samples. Homogenization was insured by passing the lysate 5 to 10 times through a 25-gauge needle attached to a syringe. Lysate was then transferred to an RNase-free microfuge tube and spun down for 2 minutes to pellet any cell debris. Supernatant was then transferred to another RNase-free microfuge tube and an equal volume of 70% ethanol was added and vortexed for proper mixing.
2.2. Total RNA extraction and purification
Lysate (600 μl) with ethanol from the previous step was applied to the assembled column and collection tube and centrifuged for 1 minute. The flow-through was discarded and the spin column and collection tube was reassembled and the process was repeated until the lysate finishes. After this, 400 μl of wash solution was added to the column and centrifuged for one minute, the flow-through was discarded and the process repeated twice. Then, RNA was eluted by adding 50 μl of elution buffer to the column and centrifugation for a minute. The purified RNA was immediately reverse transcribed into cDNA and stored at -20°C until used for PCR.
3. Peripheral-blood mononuclear cell lysate for total RNA isolation
Blood samples for separating PBMCs were collected from all patients on the same day that the liver biopsy was performed. PBMCs were obtained by Ficoll-Hypaque density gradient of EDTA anti-coagulated blood according to the manufacturer's instructions (Lymphoflot, Biotest, 63303 Dreleich, Germany). Cells were washed three times with Mg2+ - and Ca2+ -free PBS and resuspended to 1 × 106 cell/ml in PBS. Up to 100 μl of PBMCs suspension in PBS was transferred to an RNase-free microfuge tube. To them was added 350 μl of lysis solution, cells were then lysed by vortexing for 15 seconds (or until the mixture becomes clear). After this, 200 μl of 95% ethanol was added to the lysate and mixed by vortexing for 10 seconds. The lysate was used for RNA extraction following the same protocol for liver cell lysate.
Reverse transcription and nested PCR
The synthesis of cDNA and the two PCR rounds were performed using oligonucleotide primers from the highly conserved 5 untranslated region of the genome; external primers [P1 (sense, -GCGACACTCCACCATAGAT-; nucleotides 10–28) and P4 (antisense, -ACTCGCAAGCACCCTATCA-; nucleotides 303-285)] for the first-round PCR and internal primers [P2 (sense, -CTGTGAGCAACTACTGTCT-; nucleotides 36–55) and P3 (antisense, -CGGTGTACTCACCGGTTCC-; nucleotides 161–143)] for the second-round PCR.[31]
Amplification of the cDNA was performed using 15 μl of the cDNA solution and 50 pmol of the outer primers (P1 and P4). Thirty cycles of DNA amplification were carried out, followed by an extension step for 10 minutes at 72°C. Each cycle of PCR consisted of 95°C for 45 seconds, 50°C for 45 seconds, and 72°C for 45 seconds. The second PCR was carried out in the same way with 5 μl of the first PCR mixture and 50 pmol of each inner primer (P2 and P3). The amplified DNA was visualized by 2% agarose gel electrophoresis and ethidium bromide staining. The size of the second product generated by PCR was 126 base pair. Furthermore, several negative controls (no-RNA) were included in each PCR step, to assure the specificity of the results.
Statistical analysis
The statistical analyses were performed by SPSS software, package release, version 15.0 (Chicago, IL, USA). Comparisons between groups were made by Student's t test (for continuous variables) and by either the χ2 test or Fisher's exact test (for categorical data). Correlations were determined by Spearman's rank correlation test. Epidemiological and clinical data of the patients (gender; age; body-mass index; estimated duration of abnormal liver function test results; previous blood transfusions; levels of AST, ALT, G-GT, cholesterol, and triglycerides; and presence or absence of intrahepatic HCV-RNA) were included in a logistic regression analysis to identify independent factors associated with necroinflammatory activity and fibrosis. A 2-tailed P value <0.05 was considered to denote statistical significance.
Results
Twenty seven - occult HCV patients and 28 patients with chronic HCV infection were included in this study after giving a written, well-informed consent.
Table 1 shows the characteristics of patients with occult HCV infection and those with chronic hepatitis C infection, where no significant difference regarding age, gender, and BMI was observed. Significant increase was noted in classic HCV infection than occult HCV infection regarding bilirubin (P<0.001), ALT (P = 0.009), AST (P = 0.013), AFP (P<0.001), while serum albumin was significantly higher in occult HCV cases than those with chronic HCV infection (P<0.001).
Table 1.
Clinical and laboratory characteristics of in the studied groups
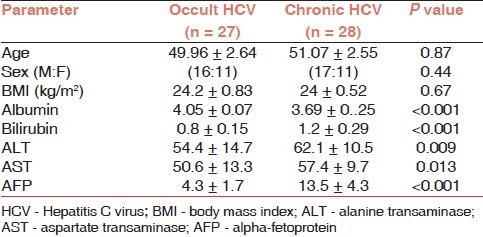
Table 2 shows the histological characteristics of both studied groups. Hepatic necroinflammation (P<0.001), fibrosis (P<0.001), and cirrhosis (P = 0.03) were significantly observed in patients with chronic HCV infection than those with occult HCV.
Table 2.
Histopathological characteristics of the studied groups
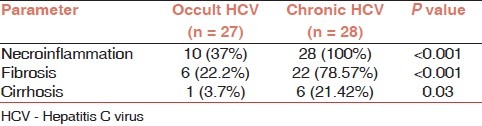
Table 3 shows the Th1/Th2 cytokine patterns of the studied patients’ groups. The levels of the Th1 cytokines, IL-2, and IFN-γ were increased in patients with chronic HCV infection (P<0.001), vs those with occult HCV infection. The Th2-cytokine; IL-4 was significantly increased in patients with occult HCV infection (P<0.001) vs those with chronic HCV infection. These results indicated that Th1 cytokines are predominant in patients with chronic HCV infection than those with occult HCV infection, whereas Th2 cytokine; IL 4 is predominant in patients with occult HCV infection than those with chronic HCV infection.
Table 3.
Th1/Th2 cytokine patterns in the studied groups

Discussion
Epidemiological studies of HCV infection have suggested that the Nile-Delta region of Egypt has the highest prevalence rates of HCV infection in the world, with a seroprevalence rate of 20% to 30% in villagers above the age of 30 years. Women had a similar or a slightly lower prevalence of antibodies to HCV than men.[15]
In this work, the prevalence of occult HCV infection was (27/108) 25% among patients with unexplained abnormal liver function test results. This finding would alert clinicians about this serious underestimated hidden liver viral pathogen and may re-categorize the etiologic diagnostic possibilities of unexplained persistent liver biochemistry abnormalities; this would be probably applicable after large epidemiological studies to clarify the approximate prevalence of occult HCV infection among the Egyptians.
There is suggestive evidence that T-cell immunoregulatory cytokines may play a key role in the etiopathogenesis of occult HCV infection. Activated CD4+ T cells can be divided into two subsets based on their cytokine secretion profiles.[16–18] (12-14) The Th1 subset produces IFN-γ, TNF-α, and IL-2, and participates in cell-mediated immune responses,[17,18] while the Th2 subset produces IL-4 and IL-10, and mediates humoral immune responses.[19] The Th1/Th2 cytokine balance is likely important in determining the rate of HCV chronicity as well as the pattern and extent of HCV-induced liver injury.[20,21]
In this study, there was a significant increase in Th1 cytokines; IL-2 and IFN-γ in patients with chronic HCV infection (193.6 ± 15.4 and 64.8 ± 6.76, respectively) compared with those with occult HCV infection (102.1 ± 6.7 and 28.6 ± 4.2, respectively) (P<0.001), as shown in Table 3.
This significant shift of cytokine pattern in chronic HCV infection toward Th1 pattern more than occult HCV infection may suggest that the clinical and histological differences observed between the studied groups are a consequence of the hosts’ immunological system responses and their derived cytokines as the cytokine shift toward Th1 is associated with more disease progression. These data are supported by the previous research findings claiming that IFN-γ expression is clearly augmented in the serum of chronic hepatitis C patients,[22–24] and its increase has been correlated with an increase in the disease severity.[25]
IL-2 is considered to be a Th1-type cytokine and is involved in enhancing the proliferation and activation of most T lymphocytes, NK cells, and B-lymphocytes. Liver sinusoidal and inflammatory cells have been reported to be sources of IL-2 and no consensus exists on its predictive value. However, it is apparent that the expression of IL-2 is associated with a more advanced stage of disease.[26–28] Napoli et al. in 1996 demonstrated that as liver injury worsens, there is an increase in intrahepatic Th1-like cytokine m-RNA levels. In particular, there was a positive correlation between IFN-γ and IL-2 m-RNA expression and the severity of both the inflammatory and fibrotic components in the portal tracts.[8]
Our data showed a significant increase in serum IL-4 in patients with occult HCV infection in comparison with those with chronic HCV infection (P<0.001). IL-4 has been shown to regulate a wide spectrum of function of B cells, monocytes/macrophages, and other non-hematopoietic cells.[29] Increased level of Th2, cytokine-IL-4, may be responsible for the decrease in IFN-γ and IL-2 production among occult HCV infection in the present study. This finding is concordant with another study stating that Th2 cytokines regulate the antibody secretion by B cells and have suppressor functions.[30] Additionally, the increased levels of Th2 cytokines, IL-4, and IL-10, may be a responsible factor for the decrease in IFN-γ production.[8]
Patients with occult HCV infection have abnormal liver function tests and 35% of them have histological damage, including liver cirrhosis.[4] However, patients with occult HCV infection presents a milder disease form than those with chronic hepatitis C infection.[6] The low number of infected hepatocytes found in patients with occult HCV infection may be related with less liver damage. Furthermore, the immune response of these patients may be fine-tuned better than that of patients with chronic hepatitis C, leading to a more effective control of the infection.[6] There is suggestive evidence that T-cell immunoregulatory cytokines may play a key role in the persistence of HCV infection and influencing the extent of liver damage.[31–35]
Despite the less aggressive course of occult HCV and the absence of HCV-RNA in peripheral blood, the body immune system cannot eradicate the infection. This could be, possibly, due to the predominant Th2; IL-4 in occult HCV that may be responsible for persistence of infection in these patients, agreeing with reports from other authors.[6,27] It has been proposed that the inability to terminate HCV infection may also result from the inappropriate release of some T-cell and monocyte-macrophage-derived cytokines, such as IL-4 and IL-10.[36,37] These cytokines may inhibit cellular-mediated antiviral responses by interfering with T-cell activation and function.[38–41] These data probably, expressing the situation in our work, where undetectable serum HCV-RNA in case of occult HCV infection, can explain the low level of Th1 cytokines in this study, among such patients than those with chronic HCV infection. The existence of a relationship between chronic HCV replication and Th1 predominance was further supported by the finding of marked decrease in the Th1 response in patients in whom the HCV-RNA titer dropped to undetectable levels following in vivo IFN-γ parenteral manipulations.[42,43]
Conclusion
Our data revealed a high prevalence of occult HCV infection (25%) in patients presented with unexplained persistently abnormal liver function test results. Those patients exhibited a distinct immunoregulatory cytokine profile, with a predominant Th2 cytokine response favoring viral persistence in the liver tissue, despite its absence from peripheral blood. Lack of Th1 cytokine response in occult HCV infection could explain the less aggressive course of this disease entity compared with those with chronic HCV infection.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
References
- 1.Antonelli A, Ferri C, Galeazzi M, Giannitti C, Manno D, Mieli-Vergani G, et al. HCV infection: pathogenesis, clinical manifestations, and therapy. Clin Exp Rheumatol. 2008;26(Suppl 48):S39–47. [PubMed] [Google Scholar]
- 2.Chen SL, Morgan TR. The natural history of hepatitis C virus infection. Int J Med Sci. 2006;3:47–52. doi: 10.7150/ijms.3.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36(Suppl 1):S35–46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 4.Castillo I, Pardo M, Bartolomeé J, Ortiz-Movilla N, Rodríguez-Iñigo E, de Lucas S, et al. Occult hepatitis C virus infection in patients in whom the etiology of persistently abnormal results of liver-function tests is unknown. J Infect Dis. 2004;189:7–14. doi: 10.1086/380202. [DOI] [PubMed] [Google Scholar]
- 5.Yano M, Kumada H, Kage M, Ikeda K, Shimamatsu K, Inoue O, et al. The long-term pathological evolution of chronic hepatitis C. Hepatology. 1996;23:1334–40. doi: 10.1002/hep.510230607. [DOI] [PubMed] [Google Scholar]
- 6.Pardo M, López-Alcorocho JM, Rodríguez-Iñigo E, Castillo I, Carreño V. Comparative study between occult hepatitis C virus infection and chronic hepatitis C. J Viral Hepat. 2007;14:36–40. doi: 10.1111/j.1365-2893.2006.00783.x. [DOI] [PubMed] [Google Scholar]
- 7.Tilg H, Kaser A, Moschen AR. How to modulate inflammatory cytokines in liver diseases. Liver Int. 2006;26:1029–39. doi: 10.1111/j.1478-3231.2006.01339.x. [DOI] [PubMed] [Google Scholar]
- 8.Napoli J, Bishop A, Mc Guinness PH, Painter DM, McCaughan GW. Progressive liver injury in chronic hepatitis C infection correlates with increased intra-hepatic expression of Th1-associated cytokines. Hepatology. 1996;24:759–65. doi: 10.1002/hep.510240402. [DOI] [PubMed] [Google Scholar]
- 9.Tsai SL, Liaw YF, Chen MH, Huang CY, Kuo GC. Detection of type 2-like helper cells in hepatitis C virus infection: implications for hepatitis C virus chronicity. Hepatology. 1997;25:449–57. doi: 10.1002/hep.510250233. [DOI] [PubMed] [Google Scholar]
- 10.Sarih M, Bouchrit N, Bebslimane A. Different cytokine profiles of peripheral blood mononuclear cells from patients with persistent and self-limited hepatitis C virus infection. Immunol Lett. 2000;74:117–20. doi: 10.1016/s0165-2478(00)00210-8. [DOI] [PubMed] [Google Scholar]
- 11.Sobue S, Nomura T, Ishikawa T, Ito S, Saso K, Ohara H, et al. Th1/Th2 cytokine profiles and their relationship to clinical features in patients with chronic hepatitis C virus infection. J Gastroenterol. 2001;36:544–51. doi: 10.1007/s005350170057. [DOI] [PubMed] [Google Scholar]
- 12.Yamaki K, Uchida H, Li X, Yanagisawa R, Takano H, Hayashi H, et al. Effect of varying types of anti-arthritic drugs on Th1 and Th2 immune responses in mice. Int J Immunopathol Pharmacol. 2005;18:133–44. doi: 10.1177/039463200501800114. [DOI] [PubMed] [Google Scholar]
- 13.Bedossa P METAVIR Cooperative Study Group. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology. 1994;20:15–20. [PubMed] [Google Scholar]
- 14.Bedossa P, Poynard T The METAVIR Cooperative Study Group. An algorithm for the grading of activity in chronic hepatitis C. Hepatology. 1996;24:289–93. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 15.Frank C, Mohamed MK, Strickland GT, Lavanchy D, Arthur RR, Magder LS, et al. The role of parenteral antischistosomal therapy in the spread of Hepatitis C Virus in Egypt. Lancet. 2000;355:887–91. doi: 10.1016/s0140-6736(99)06527-7. [DOI] [PubMed] [Google Scholar]
- 16.Masmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 17.Gioia C, Horejsh D, Agrati C, Martini F, Capobianchi MR, Ippolito G, et al. T-Cell response profiling to biological threat agents including the SARS coronavirus. Int J Immunopathol Pharmacol. 2005;18:525–30. doi: 10.1177/039463200501800312. [DOI] [PubMed] [Google Scholar]
- 18.Lanzilli G, Falchetti R, Tricarico M, Ungheri D, Fuggetta MP. In vitro effects of an immunostimulating bacterial lysate on human lymphocyte function. Int J Immunopathol Pharmacol. 2005;18:245–54. doi: 10.1177/039463200501800207. [DOI] [PubMed] [Google Scholar]
- 19.Clerici M, Shearer GM. The Th1-Th2 hypothesis of HIV infection: New insights. Immunol Today. 1994;15:575–81. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 20.Vecchiet J, Dalessandro M, Travasi F, Falasca K, Di Iorio A, Schiavone C, et al. Interleukin-4 and interferon-gamma production during HIV-1 infection and changes induced by anti-retroviral therapy. Int J Immunopathol Pharmacol. 2003;16:157–66. doi: 10.1177/039463200301600210. [DOI] [PubMed] [Google Scholar]
- 21.Perrella A, Borgia G, Borrelli F, Di Sirio S, Gnarini M, Grattacaso S, et al. TNF-alpha serum level elevations in chronic hepatitis C patients with diabetes mellitus. Int J Immunopathol Pharmacol. 2005;18:189–93. doi: 10.1177/039463200501800120. [DOI] [PubMed] [Google Scholar]
- 22.Tilg H, Wilmer A, Vogel W, Herold M, Nölchen B, Judmaier G, et al. Serun levels of cytokynes in chronic liver diseases. Gastroenterology. 1992;103:264–74. doi: 10.1016/0016-5085(92)91122-k. [DOI] [PubMed] [Google Scholar]
- 23.Cacciarelli TV, Martinez OM, Gish RG, Villanueva JC, Krams SM. Immunoregulatory cytokines in chronic hepatitis C virus infection: pre- and post-treatment with interferon alfa. Hepatology. 1996;24:6–9. doi: 10.1002/hep.510240102. [DOI] [PubMed] [Google Scholar]
- 24.Kamal SM, Graham CS, He Q, Bianchi L, Tawil AA, Rasenack JW, et al. Kinetics of intrahepatic hepatitis C virus (HCV)-specific CD4+ T cell responser in HCV and Schistosoma mansoni coinfection: relation to progression of liver fibrosis. J Infect Dis. 2004;189:1140–50. doi: 10.1086/382278. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Peralta RP, Fang JW, Davis GL, Gish R, Tsukiyama-Kohara K, Kohara M, et al. Optimization for the detection of hepatitis C virus antigens in the liver. J Hepatol. 1994;20:143–7. doi: 10.1016/s0168-8278(05)80481-7. [DOI] [PubMed] [Google Scholar]
- 26.Makris M, Preston FE, Ralph S. Increased soluble IL-2 receptor levels in HCV-infected haemophiliacs: A possible indicator of liver disease severity. Br J Haematol. 1994;87:419–21. doi: 10.1111/j.1365-2141.1994.tb04936.x. [DOI] [PubMed] [Google Scholar]
- 27.Bozkaya H, Bozdayi AM, Aslan N, Türkay C, Sarioglu M, Cetinkaya H, et al. Circulating IL-2 and IL-10 in chronic active hepatitis C with respect to the response to IFN treatment. Infection. 2000;28:309–13. doi: 10.1007/s150100070025. [DOI] [PubMed] [Google Scholar]
- 28.Gramenzi A, Andreone P, Loggi E, Foschi FG, Cursaro C, Margotti M, et al. Cytokine profile of peripheral blood mononuclear cells from patients with different outcomes of hepatitis C virus infection. J Viral Hepat. 2005;12:525–30. doi: 10.1111/j.1365-2893.2005.00634.x. [DOI] [PubMed] [Google Scholar]
- 29.Paul WE. Interleukin4: A prototypic immunoregulatory lymphokine. Blood. 1991;77:1859–70. [PubMed] [Google Scholar]
- 30.Bahri A, Abdullah C, Hikmet A. Serum profile of T helper 1 and T helper 2 cytokines in patients with chronic hepatitis C virus infection. Turk J Gastroenterol. 2003;14:7–11. [PubMed] [Google Scholar]
- 31.Koziel MJ. Cytokines in viral hepatitis. Semin Liver Dis. 1999;2:157–68. doi: 10.1055/s-2007-1007107. [DOI] [PubMed] [Google Scholar]
- 32.Gramenzi A, Andreone P, Loggi E, Foschi FG, Cursaro C, Margotti M, et al. Cytokine profile of peripheral blood mononuclear cell from patients with different outcomes of hepatitis C virus infection. J Viral Hepat. 2005;12:525–30. doi: 10.1111/j.1365-2893.2005.00634.x. [DOI] [PubMed] [Google Scholar]
- 33.Kempuraj D, Donelan J, Frydas S, Iezzi T, Conti F, Boucher W, et al. Interleukin-28 and 29 (IL-28 and IL-29): New cytokines with anti-viral activities. Int J Immunopathol Pharmacol. 2004;17:103–6. doi: 10.1177/039463200401700201. [DOI] [PubMed] [Google Scholar]
- 34.Petrarca C, Frydas S, Donelan J, Boucher W, Papadopoulou N, Cao J, et al. Interleukin 27 (IL-27): A novel pleiotropic cytokine involved in T cell differentiation and T cell response modulation. Int J Immunopathol Pharmacol. 2005;18:191–4. doi: 10.1177/039463200501800201. [DOI] [PubMed] [Google Scholar]
- 35.Huang SH, Frydas S, Conti P, Kempuraj D, Barbacane RC, Grilli A, et al. Interleukin-17: A revisited study. Int J Immunopathol Pharmacol. 2004;17:1–4. doi: 10.1177/039463200401700101. [DOI] [PubMed] [Google Scholar]
- 36.Salgame P, Abrams JS, Clayberger C, Goldstein H, Convit J, Modlin RL, et al. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991;254:279–82. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- 37.Mosmann TR, Subash S. The expanding universe of T cell subsets.Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 38.Sher A, Gazzinelli RT, Oswald IP, Clerici M, Kullberg M, Pearce EJ, et al. Role of T-cell derived cytokines in the down regulation of immune responses in parasitic and retroviral infection. Immunol Rev. 1992;127:183–204. doi: 10.1111/j.1600-065x.1992.tb01414.x. [DOI] [PubMed] [Google Scholar]
- 39.Scott P, Kaufmann SH. The role of T-cell subsets and cytokines in the regulation of infection. Immunol Today. 1991;12:346–8. doi: 10.1016/0167-5699(91)90063-Y. [DOI] [PubMed] [Google Scholar]
- 40.Ada GL, Blanden RV. Immunity and cytokine regulation in viral infection. Res Immunol. 1994;145:625–9. doi: 10.1016/s0923-2494(05)80044-6. [DOI] [PubMed] [Google Scholar]
- 41.Del Prete G, Maggi E, Romagnani S. Th1 and Th2 cells: functional properties, mechanisms of regulation, and role in diseases. Lab Invest. 1994;70:299–306. [PubMed] [Google Scholar]
- 42.Brinkmann V, Geiger T, Alkan S, Heusser CH. Interferon α increases the frequency of interferon gamma-producing CD4+ T Cells. J Exp Med. 1993;178:1655–62. doi: 10.1084/jem.178.5.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parronchi P, De Carli M, Manetti R, Simonelli C, Sampognaro S, Piccinni MP, et al. IL-4 and IFN-gamma exert opposite regulatory effects on the development of cytolytic potential by Th1 or Th2 human T cell clones. J Immunol. 1992;149:2977–83. [PubMed] [Google Scholar]


