Abstract
Background:
Preterm neonates comprise the most heavily transfused group of patients, and about 85% of extremely low birth weight newborns receive a transfusion by the end of their hospital stay. The aim of this study was to assess the possible metabolic effects of RBC transfusion on preterm infants, especially during the first 2 weeks of life, and its relation to blood volume.
Materials and Methods:
This study was conducted on 40 preterm neonates with gestational age of less than or equal to 34 weeks. They received RBCs transfusion during first 2 weeks of life. Venous blood samples of infants were collected 2 to 4 hours before and 1 hour after the end of transfusion to evaluate hemoglobin (Hb) level, hematocrit, acid-base, electrolytes, and glucose status. Then, infants were classified into two main groups: those who received RBCs volume less than or 20 ml/kg and those who received RBCs volume more than 20 ml/kg.
Results:
Infants received a mean volume of 20.38 ± 3.2 ml/kg RBCs (range, 10.9 - 26.6 ml/kg) at a median age of 9.8 ± 3.6 days. After transfusion, a significant increase of mean Hb (P<0.001), mean Hct (P<0.001), pH (P<0.001), pO2 (P<0.05), and a significant decrease of the pCO2 (41.46 ± 8.8torr vs 35.4 ± 9.34 torr; P<0.001) were observed. In addition, there was a significant increase of serum K+ (P<0.001), and a significant decrease of Ca+2 (P<0.001). A positive correlation was found between the K+ intake and the changes of kalemia (r = 0.99; P = 0.00). Furthermore, we observed an inverse correlation between the patients’ calcium intake and the changes of calcemia (r = -0.35; P = 0.02). On comparing the changes in clinical and biochemical variables between two groups after transfusion, we observed a significant increase in mean Hb and Hct associated with a significant decrease in mean serum Ca+2 (P<0.001) in the group receiving the larger blood volume.
Conclusion:
RBC transfusion was effective in improving anemia, oxygenation, increasing pH, and decreasing CO2 and Ca+2. However, from a more clinically relevant point of view, we demonstrated the development of hyperkalemia, especially in infants with a previously borderline hyperkalemia.
Keywords: Blood transfusion, electrolytes, preterm infants
Introduction
Preterm infants are often anemic and typically experience heavy blood losses from frequent laboratory testing in the first few weeks of life. Although their anemia is multifactorial, repeated blood sampling and reduced erythropoiesis with extremely low serum levels of erythropoietin are major determining factors.[1–3] Therefore, preterm neonates comprise the most heavily transfused group of patients, and about 85% of extremely low birth weight newborns receive a transfusion by the end of their hospital stay.[4,5] Approximately one half of all RBC transfusions administered to extremely low birth weight (ELBW) infants before discharge are given in the first 2 weeks of life, when neonatal cardiorespiratory illness is most severe and laboratory blood testing is greatest.[6]
When deciding to transfuse, it is important to note that despite the advances made in blood transfusion practices, complications still exist. Potential risks of RBC transfusions include infection, metabolic and cardiovascular complications, hypothermia, iron overload, and graft-versus-host disease.[7]
Metabolic complications of transfusion therapy include citrate toxicity, hyperkalemia, and hypothermia. These complications are most commonly observed during large-volume infusions.[8]
Sodium citrate is the substance used to prevent blood coagulation during blood collection. The liver, under normal conditions, can rapidly metabolize sodium citrate; however, the metabolic capacity of the liver can be exceeded when large volumes of blood are transfused. This may result in an increased level of citrate and may induce hypocalcemia and hypomagnesemia with clinical symptoms such as paresthesia, tetany, and arrhythmia.[9]
In addition, during storage, RBCs leak potassium into the plasma or additive solution. At their outdate, extracellular (plasma) potassium levels in a RBC unit approximates 0.05 mEq/ml. This relatively small potassium load rarely causes problems in small volume transfusions, because of post-transfusion rapid dilution and redistribution into cells. However, rapid infusion of large volumes of RBCs into neonates or patients with cardiac, hepatic, or renal dysfunction mandates close monitoring.[10,11]
The recent observation in our unit of a life-threatening case of hyperkalemia in a preterm infant after RBC transfusion (unreported data) stimulated us to review the literature on this issue. To our knowledge, only a few studies[12,13] have examined the possible adverse metabolic effects of blood transfusion in neonates.
Our goal was to determine the possible relationships between RBC transfusions and the onset of adverse metabolic effects in preterm infants. In particular, we hypothesized that RBC transfusions during the first two weeks of life may induce significant changes of blood acid-base, electrolytes, and glucose status in preterm infants and that these changes might be dependent on the volume of transfused blood.
Materials and Methods
Study design and ethical considerations
We conducted this prospective study in the Neonatal Intensive Care unit (NICUs) of Cairo Pediatric University Hospitals, between May 2010 and January 2011. The study was approved by the ethical committee at Cairo University.
Patients
The study included 40 preterm neonates who were eligible for the study according to the following inclusion criteria: (i) preterm infants less than or equal to 34 weeks of gestational age; (ii) those who were in need for RBCs transfusion during the first two weeks of life. We chose the first two weeks because of the increased need for transfusion during this period.
Infants who presented with major congenital anomalies, inherited metabolic disorders, acute renal failure, systemic hypotension and shock were excluded from our study. Furthermore, to evaluate the effects of transfusions on PO2 and PCO2, we excluded those infants whose FiO 2 and/or ventilator settings were changed during the transfusion.
Fluid and Electrolyte Intake
The volume of fluids given to our infants during the first week of life was started based on the infant's weight and depended on plasma electrolyte concentrations. It was increased in increments of 20 to 40 ml/kg/day until they reached 150 ml/kg at the end of the first week of life. We added 2 to 4 mmol/kg/day for sodium, 1 to 2 mmol/kg/day for potassium, and 1.5 to 2.2 mmol/kg/day for calcium. Sodium and potassium were given after the first day of life.
Potassium was not added until good urine output was established and good renal function was ensured.
Transfusions
(I) Infants underwent transfusion according to the following guidelines[14]
Asymptomatic infants with Hct <21% and reticulocytes <100 000/Ul (2%)
-
Infants with Hct <31% and one of the following:
- Hood O2 <36%
- Mean airway pressure <6 cm H2O by continuous positive airway pressure (CPAP) or intermittent mandatory ventilation (IMV)
- >9 apneic and bradycardic episodes per 12h or 2/24 h requiring bag and mask ventilation while on adequate methylxanthine therapy
- HR> 180/min
- RR>80/min sustained for 24 hours
- Weight gain of <10g/d for 4 days on 100 Kcal/kg/d
Infants with Hct <36% and requiring >35% O2 or mean airway pressure 6 to 8 cm H2O by CPAP or IMV
RBC preparation
All RBC units were fresh (less than 1 week old). Adult RBCs which were used for transfusion were stored in citrate phosphate; dextrose and adenine anticoagulant preservative (CPDA-1); citric acid anhydrous, 0.299 g/l; sodium citrate (dehydrate), 2.63 g/l; monophasic sodium phosphate monohydrate, 0.222g/l; dextrose monohydrate, 3.19 g/l; adenine, 0.0275 g/l.
Volume of transfused blood
The volume of RBCs needed by each infant was calculated using the following formula:[15]
[80 × weight (kg) × (desired Hct-current Hct)/Hct of donor unit]
Patients were classified according to the volume of transfused blood into two main groups: Preterm neonates who received RBCs volume less than or 20 ml/kg and preterm neonates who received RBCs volume more than 20 ml/kg.
Blood transfusion was given through a peripheral vein at the infusion rate of 5 ml/kg/hour. All enteral and parenteral feeding were withheld during the transfusion.
Baseline data collection
At the time of recruitment into the study, baseline clinical data from the medical charts were reviewed regarding birth weight, gestational age, prenatal complications, main pathologies, age of transfusion, and amount of transfused RBCs.
Laboratory workup
Venous Blood samples of infants were collected 2 to 4 hours before and 1 hour after the end of transfusion.
Conventional laboratory blood analyses of pH, blood gases, and electrolyte levels (Na+, K+, Ca+2) were performed with benchtop analyzers (GEM 3500 PREMIER). In addition, a complete blood count was performed to show hemoglobin (Hb) and Hct and blood glucose was done using a glucometer.
Every RBC unit was studied for its acid-base status, electrolytes, and glucose status as well as its Hb and Hct.
Statistical analysis
The clinical data were described in terms of range, mean ± standard deviation (± SD), frequencies (number of cases), and percentages when appropriate. Comparison of quantitative variables between the study groups was done using Mann Whitney U test for independent samples. Comparison between pretreatment and post-treatment values was done using paired t test for paired (matched) samples. Correlation between various variables was done using Pearson moment correlation equation for linear relation. A probability value (P value) less than 0.05 was considered statistically significant. All statistical calculations were done using computer programs SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) version 15 for Microsoft Windows and Stats Direct statistical software version 2.7.2 for MS Windows, Stats Direct Ltd., Cheshire, UK.
To better evaluate the potential risk of hyperkalemia after RBC transfusion, we calculated the K+ intake (RBC unit [K+] × volume/kg transfused RBCs). Moreover, to exclude the potential confounder effect of the different concentration of K+ in each donor's unit, simple regression analysis was used also for evaluating the potential correlation between Hct of donor units and the concentration of K+ in the supernatant and between Hct of donor units and change of K+ after transfusion.
We calculated the Ca2+ intake (RBC unit [Ca2+] × volume/kg transfused RBCs) and glucose intake (RBC unit [glucose] × volume/kg transfused RBCs).
We used simple regression analysis to assess the correlation between K+, Ca2+, and glucose intake and changes of patient's kalemia, calcemia, and glycemia.
Results
Patients’ characteristics
The present study was conducted on 40 preterm infants with a mean gestational age of 30.9 ± 1.9 weeks (range, 26-34 weeks); their mean birth weight was 1.366 ± 390 g (range, 750-2 010 g). Twenty-two infants were females (55%) and 18 infants were males (45%). Most of our studied infants (67.5%) were affected by respiratory distress syndrome (RDS), 13 infants (32.5%) had patent ductus arteriosus, nine infants (22.5%) had neonatal jaundice, three infants (7.5%) had pneumothorax, while three infants (7.5%) suffered from neonatal sepsis.
Blood transfusion
According to used guidelines, our patients received a mean volume of 20.38 ± 3.2 ml/kg RBCs (range, 10.9 - 26.6 ml/kg) at a median age of 9.8 ± 3.6 days.
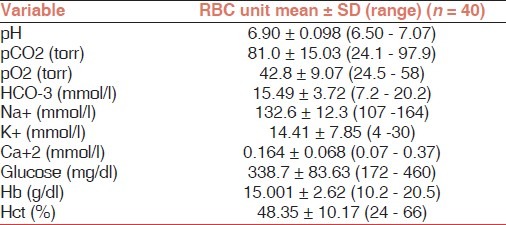
Chemical analysis of RBC donor units revealed that the mean Hb concentration and Hct were 15.001 ± 2.62 g/dl and 48.35 ± 10%, respectively. The rest of the biochemical profile is reported in Table 1. The K+ concentration in the RBC units ranged between 4 to 30 mmol/l and the associated patient intake of K+ was 0.25 + 0.12 mmol/kg. There was no significant correlation between the Hct of the RBC units and the concentration of K+ in the supernatant (P = 0.40) and between Hct of RBC units and patients changes of kalemia (P = 0.43).
Table 1.
The biochemical profile of RBC units
RBC transfusion effects on studied neonates
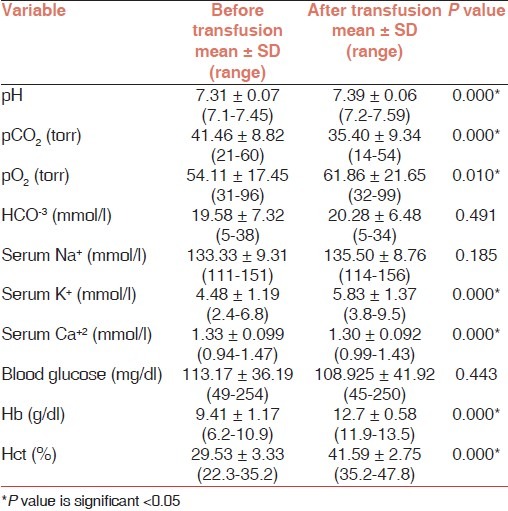
RBC transfusion induced a statistically significant increase of mean Hb (9.41 ± 1.1 g/dl vs 12.7 ± 0.5 g/dl; P<0.001) and mean Hct (29.5 ± 3.3% vs 41.5 ± 2.5%; P<0.001). Regarding the acid-base status, RBC transfusion induced a statistically significant increase of pH (7.31 ± 0.07 vs 7.39 ± 0.06; P<0.001), pO2 (54.1 ± 17.45 torr vs 61.8 ± 21.6 torr; P<0.05) and a statistically significant decrease of the pCO2 (41.46 ± 8.8 torr vs 35.4 ± 9.34 torr; P<0.001).
The serum electrolytes were also affected by the transfusions, there was a statistically significant increase of serum K+ (4.48 ± 1.19 mmol/l vs 5.83 ± 1.37 mmol/l; P<0.001), and a statistically significant decrease of Ca+2 (1.33 ± 0.09 mmol/l vs 1.30 ± 0.09 mmol/l; P<0.001). On the other hand, blood glucose was not affected by RBC transfusion [Table 2].
Table 2.
Comparison of the biochemical parameters of preterm infants before and after RBC transfusion
Six (15%) of our infants developed hyperkalemia following the RBC transfusion. Three cases were an exacerbation of a previous hyperkalemia; the infants had pretransfusion K+ levels of 5.7, 5.8, and 6.5 mmol/l, whilst their post-transfusion K+ levels reached 8.9, 9, and 9.2 mmol/l, respectively. The infants were non-acidotic and their associated K+ intake was 0.416, 0.525, and 0.37 mmol/kg, as they received 18 ml/kg, 23 ml/kg, and 20 ml/kg of packed RBCs, respectively.
However, the remaining three infants had no previous hyperkalemia; the first infant (30 weeks gestation) received 16 ml/kg, had a K+ intake of 0.485 mmol/kg and his post-transfusion K+ level reached 8.5 mmol/l; the second infant (34 weeks) received 18 ml/kg, his K+ intake was 0.445 mmol/kg and his K+ level was 8.8 mmol/kg; the third infant (32 weeks) was on mechanical ventilation, received 14 ml/kg, his K+ level reached 9 mmol/l and his K+ intake was 0.525 mmol/kg.
None of these infants were acidotic, hyperglycemic, or hypocalcemic at the time of transfusion, which are the factors that might lead to hyperkalemia-associated cardiac arrests; however, our infants were treated with intravenous calcium gluconate, insulin, and salbutamol until their K+ level was stabilized.
As regards the Ca+2 level, only one 28 gestational week infant with a borderline hypocalcemia before transfusion of 0.93 had a calcium level of 0.94 after the transfusion.
Simple regression analysis showed a positive relationship between the changes of kalemia and K+ intake, (r = 0.99, P = 0.00) [Figure 1]. We also demonstrated an inverse relationship between the Ca+2 intake mean (0.03±0.001); r (-0.35); P = 0.027 [Figure 2].
Figure 1.
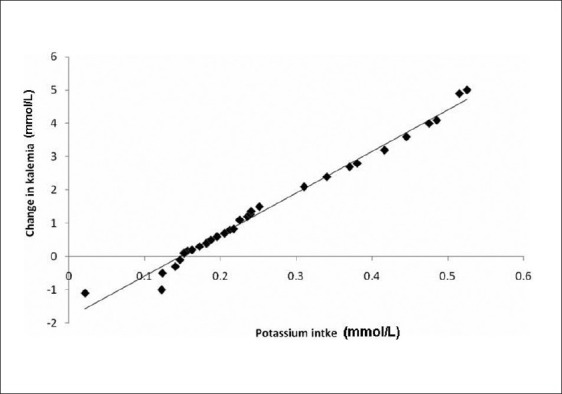
Correlation between potassium intake and change of kalemia among study cases (r = 0.99, P = 0.00)
Figure 2.
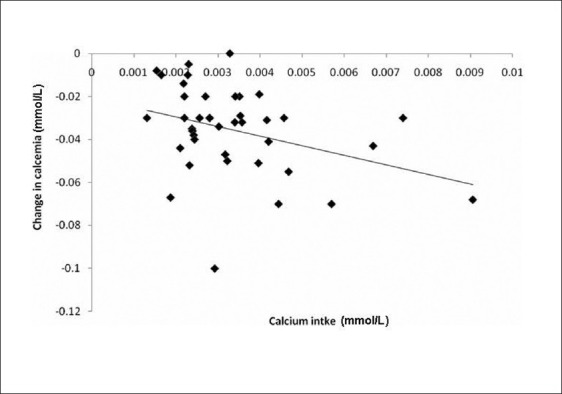
Correlation between Ca intake and changes of calcemia among the study cases (r-0.35); P = 0.027)
The studied infants were divided into 2 groups according to the volume of blood transfused. Infants in group 1 (<20 ml/kg) had a mean gestational age of 31.1 ± 2.31 weeks and mean weight weight of 1416.65 ± 446.1 g, the mean age at transfusion was 9.41 ± 3.84 days, infants in group 2 had a mean gestational age of 30.8 ± 1.71 weeks and a weight of 1329.21 ± 307.34 g and a mean age of transfusion of 10.22 ± 3.52 days, with no statistically significant difference between these parameters.
There was no statistical significant difference between the frequency of medical morbidities, fluid and electrolyte intake between the 2 groups.
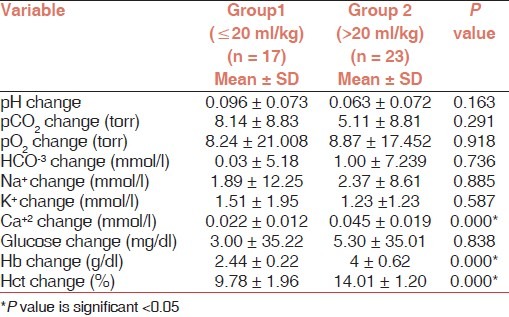
By comparing the changes in clinical or biochemical variables between the two groups, we observed a statistically significant increase in mean Hb and Hct in group 2 more than group 1 and a statistically significant decrease in mean serum Ca+2 in group 2 more than group 1, i.e., more volume of RBC induced more increase in Hb (2.44 ± 0.22 g/dl vs 4 ± 0.62 g/dl; P<0.001) and Hct (9.78 ± 1.96% vs 14.01 ± 1.20%; P<0.001) and induced more decrease in Ca+2 (0.022 ± 0.012 mmol/l vs 0.045 ± 0.019 mmol/l; P<0.001) [Table 3].
Table 3.
Changes of biochemical data before and after RBC transfusion in both groups
Discussion
In this prospective study, we found that packed RBC transfusion in preterm neonates was effective in ameliorating their anemia, and inducing an increase in their pO2 and pH. However, this was associated with a decrease of calcemia and the development of hyperkalemia. Moreover, we observed that with increased volume of transfusion, there was a significant increase in Hb and Hct and a concomitant decrease of calcemia.
In our infants, we found a statistically significant increase of pO2 and pH after RBC transfusion, suggesting that iatrogenic anemia may contribute to the maldistribution of regional blood flow and O2 delivery in neonates with RDS.[16] This agrees with a previous study that suggests that packed RBCs increase regional oxygen delivery and tissue surface pH.[17] This was relevant clinically in our study when we observed a statistically significant decrease in the heart rate of our infants after transfusion, suggesting that this decrease in heart rate was most probably explained by the migitation of tissue hypoxia and concomitant tachycardia.[18]
Unlike previous studies,[13,19] who found a decrease of glycemia following transfusion, we found that there was no change in the blood glucose levels in our preterm infants. This was observed despite of the high blood glucose in the donor units which could lead to a hyperinsulinemia[20] and despite our practice of withholding, all enteral and parenteral nutrition during the transfusion. This could probably be explained by the short duration of withholding of the feeds and the relatively stable blood glucose levels during this period.
As regards the level of ionized calcium, our results demonstrated a statistically significant decrease in the level of ionized calcium after transfusion and this was evident even with the increase in the volume of RBCs. Furthermore, we found an inverse correlation between the calcium intake and the changes of calcemia. This may be due to the low calcium concentration of the donor units and due to the chelating action of the citrate.[19]
Although this finding agrees with previous studies,[13,19] done during the first week of life, the calcium concentration in our study remained within the normal range. From a clinical point of view, none of our patients exhibited clinical signs of hypocalcemia except for one patient with borderline hypocalcemia whose level remained unchanged.
In addition, this study was designed to address the concern that RBC transfusion may be associated with hyperkalemia. Clinical hyperkalemia resulting from RBC transfusion has been recognized as a transfusion complication for decades.[20]
We observed that the K+ level after transfusion increased significantly from 4.48 ± 1.19 to 5.83 ± 1.37, P value, 0.00. We also demonstrated a positive linear relationship between the K+ intake and the change in K+ concentration in the overall population. However, these results were not apparent when the patients were stratified into 2 groups. This was apparent clinically in our study, when six patients developed hyperkalemia, three cases with a life-threatening hyperkalemia requiring treatment; three of those were an exacerbation of a previous hyperkalemia.
Hyperkalemia related to transfusion depends not only on the K+ concentration of the RBCs unit but also on the volume and rate of RBCs transfusion.[21]
In our study, K+ concentration of our RBCs units, stored in CPDA 1 blood was quite variable, ranging from 4 to 30 mmol/l, which was comparable with the levels found in previous studies.[19,22–24]
Virates has shown that the K+ of RBCs might be due to the age and duration of RBCs in the supernatant; K+ is frequently higher than normal levels, especially in units at the end of their storage.[25]
As regards the rate of transfusion, it has been determined that the ratio of the transfusion rate to venous return, defined as the cardiac output plus the transfusion rate, is important in terms of potassium concentration and development of hyperkalemia.[26]
Strauss has shown that small volume transfusions (i.e., 15 ml/kg) of stored red cells out to expiration administered slowly over 3 to 4 hours usually does not pose a problem in relatively healthy infants.[27]
So, when deciding to perform a RBCs transfusion in infants with a borderline plasma potassium level, we have to check the potassium level of preterm neonates as they might experience a further increase of their kalemia. Also, we have to consider this effect in infants who are at risk of hyperkalemia (i.e., infants with kalemia >5 mmol/l, infants with renal failure) and in calculating the remaining daily intake of K+ (the recommended IV intake of K+ is 1 to 2 mmol/kg/day[28] for avoiding an iatrogenic hyperkalemia.
Determination of potassium in both RBCs units and patients’ blood should be routinely considered. Point-of-care laboratory testing may allow for expedited reporting of potassium concentrations to providers. Other measures aimed at preventing the transfusion of hyperkalemic blood products include the preoperative washing of RBC units by transfusion medicine or intraoperative washing of RBC units using cell salvage equipment.[29]
The limitations of our study include its small sample size, the need to know the exact age of the blood units, the duration of the blood transfusion, and the impossibility of measuring exactly the overall electrolyte enteral and/or parenteral intake for these premature infants.
Conclusion
We found that RBCs transfusions in preterm infants were safe and well tolerated. The adverse metabolic effects associated with the transfusion were a decrease of calcemia and hyperkalemia. However, from a more clinically relevant point of view, we demonstrated the development of hyperkalemia, especially in infants with a previously borderline hyperkalemia.
Based on our results, we recommend that liberal packed RBC guidelines placed for neonates, especially preterms should place into account the metabolic derangements that could accompany such transfusions. Hence, when deciding to perform a transfusion, during the first weeks of life, we should have a check for the serum Ca2+ and K+ concentrations prior to transfusion and infants with borderline hypocalcemia and hyperkalemia should be monitored during the procedure.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
References
- 1.Madan A, Kumar R, Adams MM, Benitz WE, Geaghan SM, Widness JA. Reduction in red blood cell transfusions using a bedside analyzer in extremely low birth weight infants. J Perinatol. 2005;25:21–5. doi: 10.1038/sj.jp.7211201. [DOI] [PubMed] [Google Scholar]
- 2.Messer J, Haddad J, Donato L, Astruc D, Matis J. Early treatment of premature infants with recombinant human erythropoietin. Pediatrics. 1993;92:519–23. [PubMed] [Google Scholar]
- 3.Lin JC, Strauss RG, Kulhavy JC, Johnson KJ, Zimmerman MB, Cress GA, et al. Phlebotomy overdraw in the neonatal intensive care nursery. Pediatrics. 2000;106:E19. doi: 10.1542/peds.106.2.e19. [DOI] [PubMed] [Google Scholar]
- 4.Bell EF, Strauss RG, Widness JA, Mahoney LT, Mock DM, Seward VJ, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005;115:1685–91. doi: 10.1542/peds.2004-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohls RJ. Transfusions in the preterm neonates. Neo Reviews. 2007;8:377–86. [Google Scholar]
- 6.Widness JA, Seward VJ, Kromer IJ, Burmeister LF, Bell EF, Strauss RG. Changing patterns of red blood cell transfusion in very low birth weight infants. J Pediatr. 1996;129:680–7. doi: 10.1016/s0022-3476(96)70150-6. [DOI] [PubMed] [Google Scholar]
- 7.Roseff SD, Luban NL, Manno CS. Guidelines for assessing appropriateness of pediatric transfusion. Transfusion. 2002;42:1398–413. doi: 10.1046/j.1537-2995.2002.00208.x. [DOI] [PubMed] [Google Scholar]
- 8.Hendrickson JE, Hillyer CD. Non infectious serious hazards of transfusion. Anesth Analg. 2009;108:759–69. doi: 10.1213/ane.0b013e3181930a6e. [DOI] [PubMed] [Google Scholar]
- 9.American Association of Blood Banks. Technical Manual. Noninfectious Complications of blood transfusion. 1999:577–600. [Google Scholar]
- 10.Carvalho B, Quiney NF. ‘Near-miss’ hyperkalaemic cardiac arrest associated with rapid blood transfusion. Anaesthesia. 1999;54:1094–6. doi: 10.1046/j.1365-2044.1999.01109.x. [DOI] [PubMed] [Google Scholar]
- 11.Murthy BV. Hyperkalaemia and rapid blood transfusion. Anaesthesia. 2000;55:398. doi: 10.1046/j.1365-2044.2000.01378-14.x. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes da Cunha DH, Nunes Dos Santos AM, Kopelman BI, Areco KN, Guinsburg R, de Araújo Peres C, et al. Transfusions of CPDA-1 red blood cells stored for up to 28 days decrease donor exposures in very low-birth-weight premature infants. Transfus Med. 2005;15:467–73. doi: 10.1111/j.1365-3148.2005.00624.x. [DOI] [PubMed] [Google Scholar]
- 13.Tan KL, Wong LY. Effect of blood transfusion on acid base, glucose and electrolyte status in very low birth weight infants. Biol Neonate. 1991;59:373–80. doi: 10.1159/000243374. [DOI] [PubMed] [Google Scholar]
- 14.Straus RG. Erythropoeitin and neonatal anemia. N Engl J Med. 1994;330:1227. doi: 10.1056/NEJM199404283301709. [DOI] [PubMed] [Google Scholar]
- 15.Christou HA, Shannon K, Rowitch DH. Anemia. In: Cloherty JP, Eichenwald EC, Stark AR, editors. Manual of neonatal care. 6th ed. Philadelphia: Lippincott Williams and Wilkins; 2008. pp. 436–4. [Google Scholar]
- 16.Nexo E, Christenson NC, Olesen J. Volume of blood removed for analytical purposes during hospitalization of low birth weight infants. Clin Chem. 1981;27:759–61. [PubMed] [Google Scholar]
- 17.La Gamma EF, Krauss A, Peter AM Auld. Effects of increased red cell mass on subclinical tissue acidosis in hyaline membrane disease. Arch Dis Child. 1996;75:F87–93. doi: 10.1136/fn.75.2.f87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Widness JA, Lowe LS, Bell EF, Burmeister LF, Mock DM, Kistard JA, et al. Adaptive responses during anemia and its correction in lambs. J Appl Physiol. 2000;88:1397–406. doi: 10.1152/jappl.2000.88.4.1397. [DOI] [PubMed] [Google Scholar]
- 19.Dani C, Perugi S, Benuzzi A, Corsini I, Bertini G, Pratesi S, et al. Effects of red blood cell transfusions during the first week of life on acid-base, glucose, and electrolytes in preterm neonates. Transfusion. 2008;48:2302–7. doi: 10.1111/j.1537-2995.2008.01839.x. [DOI] [PubMed] [Google Scholar]
- 20.Smith HM, Farrow SJ, Ackerman JD, Stubbs JR, Sprung J. Cardiac arrests associated with hyperkalemia during red blood cell transfusion: A case series. Anesth Analg. 2008;106:1062–9. doi: 10.1213/ane.0b013e318164f03d. [DOI] [PubMed] [Google Scholar]
- 21.Hall TL, Barnes A, Miller JR, Bethencourt DM, Nestor L. Neonatal mortality following transfusion of red cells with high plasma potassium levels. Transfusion. 1993;33:606–9. doi: 10.1046/j.1537-2995.1993.33793325059.x. [DOI] [PubMed] [Google Scholar]
- 22.Fernandes da Cunha DH, Nunes Dos Santos AM, Kopelman BI, Areco KN, Guinsburg R, de Araújo Peres C, et al. Transfusions of CPDA-1 red blood cells stored for up to 28 days decrease donor exposures in very low-birth-weight premature infants. Transfus Med. 2005;15:467–73. doi: 10.1111/j.1365-3148.2005.00624.x. [DOI] [PubMed] [Google Scholar]
- 23.Batton DG, Maisels MJ, Shulman G. Serum potassium changes following packed red cell transfusions in newborn infants. Transfusion. 1983;23:163–4. doi: 10.1046/j.1537-2995.1983.23283172858.x. [DOI] [PubMed] [Google Scholar]
- 24.Sumpelmann R, Schurholz T, Thorns E, Hausdorfer J. Acidbase, electrolyte and metabolite concentrations in packed red blood cells for major transfusion in infants. Paediatr Anaesth. 2001;11:169–73. doi: 10.1046/j.1460-9592.2001.00637.x. [DOI] [PubMed] [Google Scholar]
- 25.Vraets A, Lin Y, Callum JL. Transfusion-associated hyperkalemia. Transfus Med Rev. 2011;25:184–96. doi: 10.1016/j.tmrv.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Brown KA, Bissonnette B, Mclntyre B. Hyperkalemia during rapid blood transfusion and hypovolemic cardiac arrest in children. Can J Anaesth. 1990;37:747–54. doi: 10.1007/BF03006533. [DOI] [PubMed] [Google Scholar]
- 27.Strauss RG. Data-driven blood banking practices for neonatal RBC transfusions. Transfusion. 2000b;40:1528–40. doi: 10.1046/j.1537-2995.2000.40121528.x. [DOI] [PubMed] [Google Scholar]
- 28.Greene HL, Hambidge KM, Schanler R, Tsang RC. Guidelines or the use of vitamins, trace elements, calcium, magnesium, and phosphorus in infants and children receiving total parenteral nutrition: Report of the Subcommittee on Pediatric Parenteral Nutrient Requirements from the Committee on Clinical Practice Issues of the American Society for Clinical Nutrition. Am J Clin Nutr. 1988;48:1324–42. doi: 10.1093/ajcn/48.5.1324. [DOI] [PubMed] [Google Scholar]
- 29.Smith HM, Farrow SJ, Ackerman JD, Stubbs JR, Sprung J. Cardiac arrests associated with hyperkalemia during red blood cell transfusion: A case series. Anesth Analg. 2008;106:1062–9. doi: 10.1213/ane.0b013e318164f03d. [DOI] [PubMed] [Google Scholar]


