Abstract
WNK [with no lysine (K)] kinase is a subfamily of serine/threonine kinases. Mutations in two members of this family (WNK1 and WNK4) cause pseudohypoaldosteronism type II featuring hypertension, hyperkalemia, and metabolic acidosis. WNK1 and WNK4 were shown to regulate sodium chloride cotransporter (NCC) activity through phosphorylating SPAK and OSR1. Previous studies including ours have also shown that WNK4 inhibits NCC function and its protein expression. A recent study reported that a phorbol ester inhibits NCC function via activation of extracellular signal-regulated kinase (ERK) 1/2 kinase. In the current study, we investigated whether WNK4 affects NCC via the MAPK ERK1/2 signaling pathway. We found that WNK4 increased ERK1/2 phosphorylation in a dose-dependent manner in mouse distal convoluted tubule (mDCT) cells, whereas WNK4 mutants with the PHA II mutations (E562K and R1185C) lost the ability to increase the ERK1/2 phosphorylation. Hypertonicity significantly increased ERK1/2 phosphorylation in mDCT cells. Knock-down of WNK4 expression by siRNA resulted in a decrease of ERK1/2 phosphorylation. We further showed that WNK4 knock-down significantly increases the cell surface and total NCC protein expressions and ERK1/2 knock-down also significantly increases cell surface and total NCC expression. These data suggest that WNK4 inhibits NCC through activating the MAPK ERK1/2 signaling pathway.
Keywords: hypertonicity, phosphorylation
wnk kinase is a family of atypical serine/threonine kinases in which members lack a lysine residue that is invariably present in sheet β3 of the catalytical domain of other kinases (30). Mutations in WNK1 and WNK4 have been shown to result in pseudohypoaldosteronism type II (PHA II) (28), featuring hypertension, hyperkalemia, and metabolic acidosis. Sodium chloride cotransporter (NCC) is mostly expressed in the distal convoluted tubule (DCT) and responsible for 5–10% sodium reabsorption in the kidney (6). NCC is normally localized in the apical membrane of DCT where it transports Na+ into the cell, down its chemical gradient maintained by the basolateral Na+-K+-ATPase. Alteration in NCC activity or cell surface expression level affects NaCl reabsorption and ultimately blood pressure.
Studies have shown that wild-type WNK4 inhibits sodium uptake by reducing the surface expression of NCC in Xenopus laevis oocytes, whereas PHA II-causing WNK4 mutant, E562K, loses its inhibitory effect on NCC activity and protein expression (29, 32). We previously demonstrated that overexpression of WNK4 in Cos-7 cells inhibits NCC surface protein expression by enhancing the degradation of NCC through a lysosomal pathway (1), involving sortilin, a lysosomal targeting receptor (35). SPAK/OSR1 have been shown to act as downstream effectors of WNKs (27). WNK1 and WNK4 both activate SPAK/OSR1 by directly phosphorylating certain serine/threonine residues in SPAK/OSR1 (20, 21, 26). Activated SPAK/OSR1 has been demonstrated to phosphorylate specific serine/threonine residues located in the NH2 terminus of NCC and thus, to increase NCC transporter activity (15, 21, 23). However, it remains to be determined whether WNK4 modulates NCC activity and protein expression through an alternative signaling pathway besides WNK4-SPAK/OSR1-NCC signaling pathway, directly leading to suppressed NCC activity and protein expression. Furthermore, beside SPAK as an immediate downstream effector involving stimulatory pathway on NCC, the additional immediate downstream effectors of WNK4 that are responsible for the inhibitory effect on NCC remain largely elusive.
A previous study showed that overexpression of WNK4 increases the phosphorylation of extracellular signal-regulated kinase (ERK) 1/2 in response to epidermal growth factor or hypertonic stimulation in HEK 293 cells (24). Ko and colleagues (10) reported that phorbol ester treatment downregulates cell surface level of NCC via a Ras/Raf/MEK1/2/ERK1/2 signaling pathway in mouse DCT (mDCT) cells. Taking all these data together, it is likely that, other than SPAK/OSR1, ERK1/2 may be one of the effectors situated immediately or intermediately downstream of WNK4. In this study, we identified a novel role of WNK4-ERK1/2 signaling in the regulation of NCC. WNK4 increases the phosphorylation of ERK1/2 in mDCT cells, whereas WNK4 PHA II-causing mutants, E562K and R1185C, lose the ability to phosphorylate ERK1/2. Hypertonicity stimulates ERK1/2 phosphorylation, and knock-down of WNK4 by siRNA reduced the hypertonic stress-induced ERK1/2 phosphorylation. We also showed that knock-down of WNK4 decreased ERK1/2 phosphorylation and increased total and surface endogenous NCC protein expression in mDCT cells. We further showed that knock-down of ERK1/2 increases total and surface endogenous NCC protein expression. These data suggest that WNK4 inhibits NCC expression likely via enhancing WNK4-ERK1/2 signaling pathway.
MATERIALS AND METHODS
Cell culture and transfection.
mDCT cells were obtained from Dr. Peter Friedman (University of Pittsburg). The cells were maintained in DMEM/ F12 (1:1; Invitrogen) supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), and 5% fetal bovine serum. Lipofectamine 2000 (Invitrogen) was used for transfection of plasmids into mDCT cells according to the manufacturer's instructions. Opti-MEM medium was obtained from Invitrogen. Forty-eight hours after transfection, cell lysates were used for Western blot. Lipofectamine RNAiMax (Invitrogen) was used for transfection of WNK4 siRNA into mDCT cells. The sequences of siRNAs used for the WNK4 knock-down include sense: 5′-CGG GCA CGC UCA AGA CGU AUU, and anti-sense: 5′-P UAC GUC UUG AGC GUG CCC GUU (13). The synthetic siRNAs were obtained from Invitrogen. For transfection, 5 μl 20 mM scramble siRNA or WNK4 siRNA duplexes were added into 0.65 ml Opti-MEM and mixed gently. Ten microliters Lipofectamine RNAiMAX were added into the diluted siRNAs solutions, and mixed gently, incubated at room temperature for 15∼20 min to allow siRNAs-liposome complex to form. The OptiMem solution containing siRNA liposome complex was added to a 3.35-ml mDCT cell suspension and the mixture was plated in 60-mm dishes. Twenty-four hours after transfection, cells were reached to 60∼70% confluences; additional plasmid will be transfected into the mDCT cells if needed.
Plasmid, antibody, isotonic and hypertonic solutions.
Hemagglutinin (HA)-tagged WNK4 wild-type (WT) plasmid construct and its two PHA II-causing mutants, HA-tagged WNK4 E562K and R1185C, were used in the study. Phospho-ERK1/2 and SAPK/JNK antibodies were obtained from Cell Signaling Technologies. WNK4 antibody was purchased from Millipore. NCC antibody was a generous gift from Drs. Mark Knepper and Robert Hoover. Isotonic ND96 solution consists of 130 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1.0 mM MgCl2, and 5.0 mM HEPES/Tris, pH 7.4, (280 mosmol/kgH2O). Hypertonic solution contains 240 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1.0 mM MgCl2, and 5.0 mM HEPES/Tris, pH 7.4 (500 mosmol/kgH2O).
Western blotting analysis.
The Western blot analysis was performed as previously described (1, 10). Briefly, mDCT cells were transiently transfected with various plasmid DNA constructs or WNK4 siRNAs as indicated and then lysed in a buffer containing 20 mM HEPES, pH 7.5, 120 mM NaCl, 5.0 mM EDTA, 1.0% Triton X-100, 0.5 mM dithiothreitol, 1.0 mM phenylmethylsulfonyl fluoride and complete protease inhibitor (Roche Diagnotics, Mannheim, Germany, 1 tablet per 50-ml solution). The lysates were spun at 6,000 g for 5 min to pellet the insoluble materials. The proteins from supernatant were quantified using a Pierce BCA Protein Assay kit (Pierce, Rockford, IL). The protein sample was separated by 7.5% SDS-PAGE and transferred to polyvinylidene difluoride membranes (Amersham Biosciences, Piscataway, NJ). The membranes were probed with specific antibodies and subsequently detected with ECL plus system (Amersham Biosciences) or super signal (Pierce).
Cell surface biotinylation.
The cell surface biotinylation was performed as previously described (1). In brief, 48 h after transfection, mDCT cells were serum-starved overnight and cell surface proteins were biotinylated. Cells were then lysed, and cell lysates were briefly sonicated. Biotinylated membrane proteins were bound to neutravidin beads overnight at 4°C. After washes and elution, biotinlyated proteins were subjected to SDS-PAGE and followed by Western blot analysis. NCC was subsequently detected using the NCC antibody.
RT-PCR.
RT-PCR was performed as previously described (25). The mRNA was extracted from mDCT cells using a TRIzol (Invitrogen) based procedure. Reverse transcription was performed using a reverse transcription kit (Invitrogen). The primer sequences for WNK4 are forward primer TGA TGC TAG ACT GGC ACC TAT ATC TGA and reverse primer TCC TTC TTC TGT AGT GTC TGT AGT GC. PCR was performed in the following conditions: 95°C for 3 min, 95°C for 1 min, 59°C for 45 s, 72°C for 1 min for total 35 cycles, and then 72°C for 10 min. PCR products were run on 1.2% agarose gel and were visualized under the ultraviolet light.
Statistical analysis.
The data are presented as means ± SE. For Western blot analysis, we used the percentage change in ratio of p-ERK1/2 over t-ERK1/2 when compared experimental group with the control group. Statistical significance was determined using either a Student's t-test when two groups were compared or by a one-way ANOVA, followed by Bonferroni's post hoc tests when multiple groups were compared. Statistical significance is defined as P value <0.05.
RESULTS
WNK4 wild-type but not its PHA II mutants, E562K or R1185C, increased the phosphorylation of ERK1/2 in cultured mDCT cells.
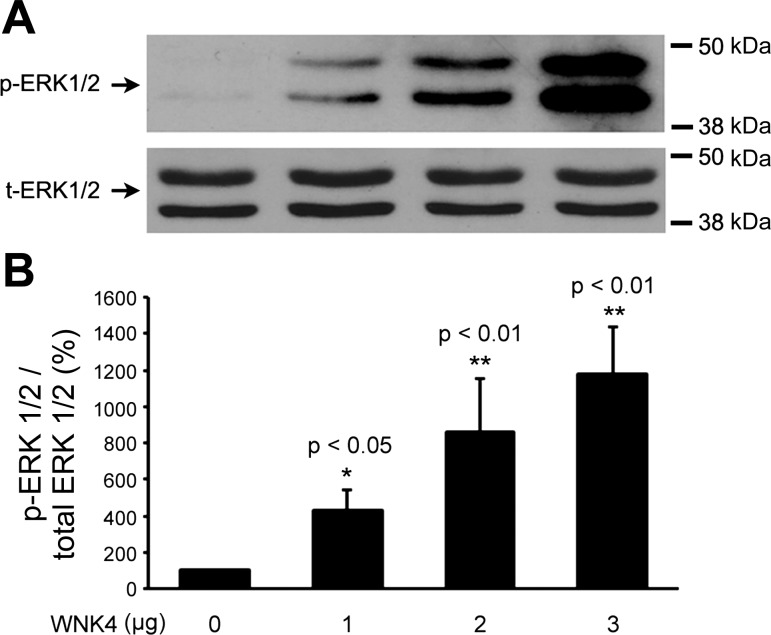
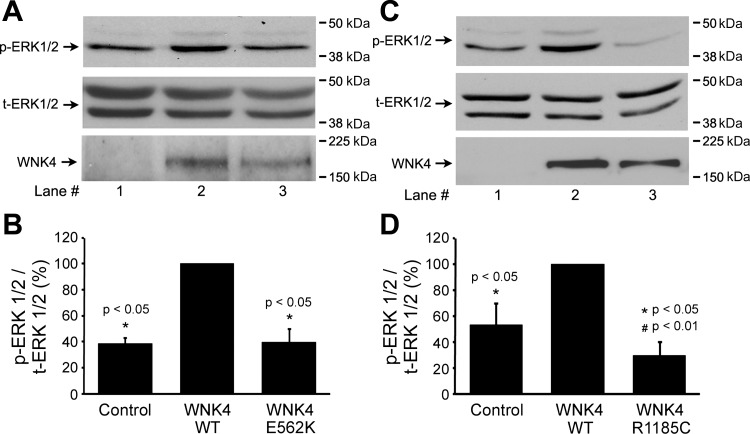
To explore the effect of WNK4 on the phosphorylation of ERK1/2, mDCT cells were transfected with HA-WNK4 WT. As shown in Fig. 1, overexpression of WNK4 WT enhanced ERK1/2 phosphorylation in a dose-dependent manner (100.0 ± 0.0%, 430.6 ± 65.3%, 863.1 ± 167.6%, 1,181.0 ± 147.0%, n = 3). Our previous data showed that the WNK4 PHA II-causing mutants, E562K and R1185C, affect NCC protein expression differently from WNK4 WT, as WNK4 WT enhanced NCC degradation, while its PHA II mutants did not (1). Thus, we next investigated the effects of WNK4 PHA II mutants on ERK1/2 phosphorylation. As shown in Fig. 2, WNK4 enhanced ERK1/2 phosphorylation, whereas WNK4 PHA II mutant E562K lost its ability to enhance ERK1/2 phosphorylation while another WNK4 PHA II mutant R1185C even reduced the phosphorylation level of ERK1/2 compared with the control group. Taking all the data together, these findings suggest that WNK4 WT and its PHA II mutants affect NCC protein expressions differently through the alteration of ERK1/2 phosphorylation.
Fig. 1.
WNK4 enhances extracellular signal-regulated kinase (ERK)1/2 phosphorylation. Mouse distal convoluted tubule (mDCT) cells were transfected with wild-type WNK4. Forty-eight hours after transfection, cell lysates were subjected to SDS-PAGE and immunoblotted with anti-ERK1/2 or anti-phospho-ERK1/2 antibodies. A: representative immunoblot for phosphor-ERK1/2 and total ERK1/2. B: bar graph representing the summarized data of total 3 experiments. The ratio of phosphor (p)-ERK1/2 over total (t) ERK1/2 in WNK4 0 μg group (control) is normalized to 100%. The ratio of the p-ERK1/2 over t-ERK1/2 in the other WNK4 groups is presented as the percentage changes from WNK4 0 μg control group. In WNK4 1 μg group, the ratio of p-ERK1/2 over t-ERK1/2 is 430.6%, indicating a 4.3-fold increase in ERK1/2 phosphorylation compared with the control group. The molecular weight markers are indicated at right side of the blots. *,**Significant changes compared with the control group (P < 0.05 and P < 0.01, respectively).
Fig. 2.
WNK4 PHA II-causing mutants, E562K and R1185C, do not increase ERK1/2 phosphorylation. mDCT cells were transfected with or without WNK4 WT or E562K (A, B) and WNK4 WT or R1185C (C, D) as indicated. Phospho-ERK1/2 levels were detected by anti-phosph-ERK1/2 antibody. A and C: representative immunoblots. B and D: bar graphs representing the summary of 3 independent experiments. These data showed that WNK4 WT increases p-ERK1/2 level, whereas either WNK4 E562K loses its ability to phosphorylate ERK1/2 (B) or WNK4 R1185C (D) even inhibits ERK1/2 phosphorylation compared with control group. The molecular weight markers are indicated at the right side of the blots. *P < 0.05 compared with WNK4 WT group (n = 3). #P < 0.01 compared with the control group.
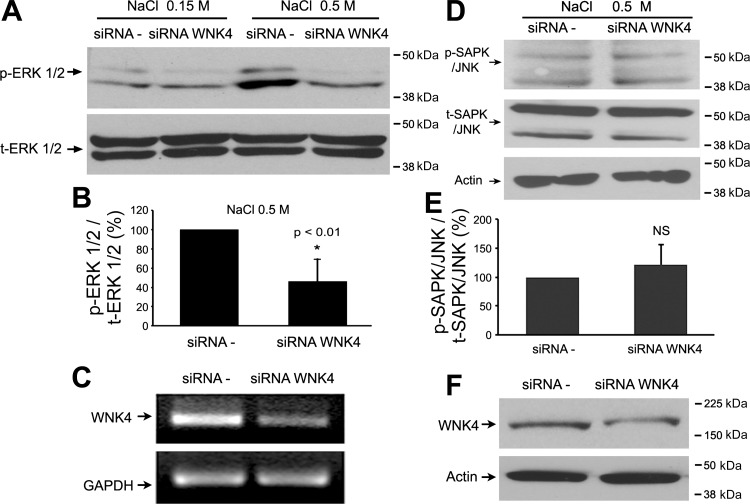
Knock-down of endogenous WNK4 attenuates hypertonic stress-induced ERK1/2 phosphorylation in mDCT cells.
Hypertonicity was shown to increase ERK1/2 phosphorylation (24). To examine whether WNK4 involves hypertonicity-induced ERK1/2 phosphorylation, the endogenous WNK4 was knocked-down by small interfering RNAs in mDCT cells. As shown in Fig. 3, A and B, hypertonicity stimulation using 0.5 M NaCl incubation for 30 min markedly enhanced ERK1/2 phosphorylation (Fig. 3A, lane 3), whereas knocking down of WNK4 significantly reduced hypertonicity-induced ERK1/2 phosphorylation (46.3 ± 11.4% in siRNA WNK4 group vs. 100% in control group, P < 0.01, n = 6). To demonstrate specificity of this effect, knock-down of WNK4 expression did not affect the phosphorylation of SAPK/JNK (121.8 ± 33.3% in siRNA WNK4 group vs. 100.0 ± 0.0% in control group, P = 0.56, n = 4) as shown in Fig. 3, D and E. After mDCT cells were transfected with the specific WNK4 siRNA, WNK4 mRNA expression and protein expression were remarkably decreased by 60 and 55%, respectively, as shown in Fig. 3, C and F. These data provide further evidence that WNK4 lies upstream of ERK1/2 and leads to ERK1/2 phosphorylation and activation.
Fig. 3.
Knock-down WNK4 expression decreased the hypertonicity-induced ERK1/2 phosphorylation, but not SAPK/JNK phosphorylation. mDCT cells were transfected with scramble siRNA (siRNA −) or siRNA WNK4. Forty-eight hours after transfection, cells were treated with either PBS or 0.5 M NaCl for 30 min. Phospho-ERK1/2 and phospho-SAPK/JNK levels were detected by anti-phospho-ERK1/2 and anti-SAPK/JNK antibodies. A: representative immunoblot for ERK1/2 levels. B: bar graph represents the summary data of change of ERK1/2 phosphorylation in both siRNA − and siRNA WNK4 groups under the hypertonic stimulation. *P < 0.01 while compared siRNA WNK4 group to siRNA − control group (n = 6). C: WNK4 mRNA levels were determined by RT-PCR in both siRNA − and siRNA WNK4 groups. D: representative immunoblot for SAPK/JNK level. E: bar graph represents the summary data of 3 independent experiments. There was no significant difference in phospho-SAPK/JNK level between siRNA − and siRNA WNK4 groups. F: WNK4 protein levels were determined by Western blot analysis in both siRNA − and siRNA WNK4 groups. The molecular weight markers are indicated at right side of the blots.
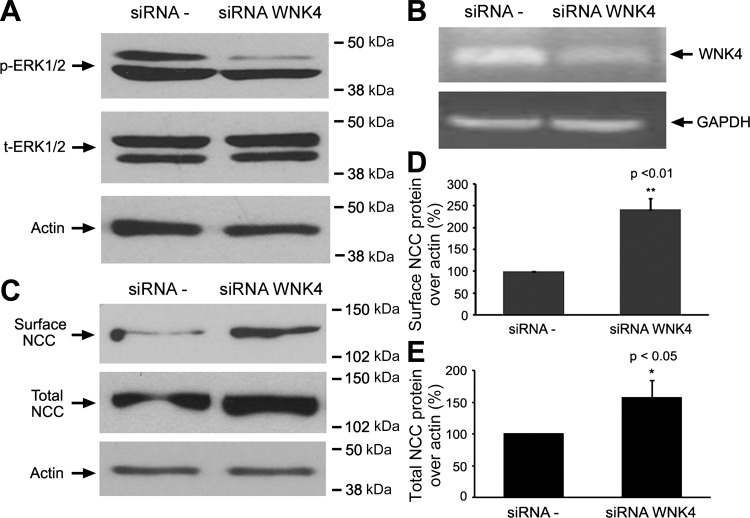
Knock-down of endogenous WNK4 expression increases total and surface NCC protein expression in mDCT cells.
To investigate whether altering the WNK4-ERK1/2 signaling pathway affects NCC protein expression, endogenous WNK4 expression was suppressed by WNK-specific siRNA in mDCT cells (Fig. 4B) and the NCC protein expression was examined by cell surface biotinylation and Western blot analysis. As shown in Fig. 4, in the siRNA WNK4 group, ERK1/2 phosphorylation level was significantly reduced (62 ± 7.1% in siRNA WNK4 group vs. 100% in siRNA-control group, P < 0.01, n = 8), whereas total ERK1/2 level was not significantly changed (96 ± 12.4% in siRNA WNK4 group vs. 100% in siRNA-control group, P = NS, n = 8) in mDCT cells (Fig. 4A). In addition, knock-down of endogenous WNK4 in mDCT cells significantly increased the steady-state levels of total NCC (158.2 ± 13.2 vs. 100 ± 0%, P < 0.05, n = 4; Fig. 4, C and E) and its surface NCC protein expression (241.9 ± 24.9 vs. 100 ± 0%, P < 0.01, n = 4; Fig. 4, C and D). These results suggest that the WNK4-ERK1/2 signaling pathway is responsible for the WNK4-mediated inhibitory effect on NCC expression.
Fig. 4.
Knock-down WNK4 expression decreased ERK1/2 phosphorylation and increased sodium chloride cotransporter (NCC) total and surface protein expressions. mDCT cells were transfected with scramble siRNAs (siRNA −) or siRNA WNK4. Cell surface biotinylations and Western blot were subsequently performed. A: representative immunoblots are shown for total ERK1/2 and p-ERK1/2. B: RT-PCR shows reduced WNK4 mRNA expression in siRNA WNK4 compared with siRNA − group. C: representative immunoblots are shown for cell surface and total NCC protein expressions. D and E: bar graphs represent the summary data of 8 independent experiments for cell surface NCC (D) and total NCC (E) levels. Cell surface and total NCC levels were significantly increased in siRNA the WNK4 group compared with the siRNA − control group. The molecular weight markers are indicated at right side of the blots. *P < 0.05 and **P < 0.01, n = 8.
Knock-down of ERK1/2 also increases total and surface NCC protein expression in mDCT cells.
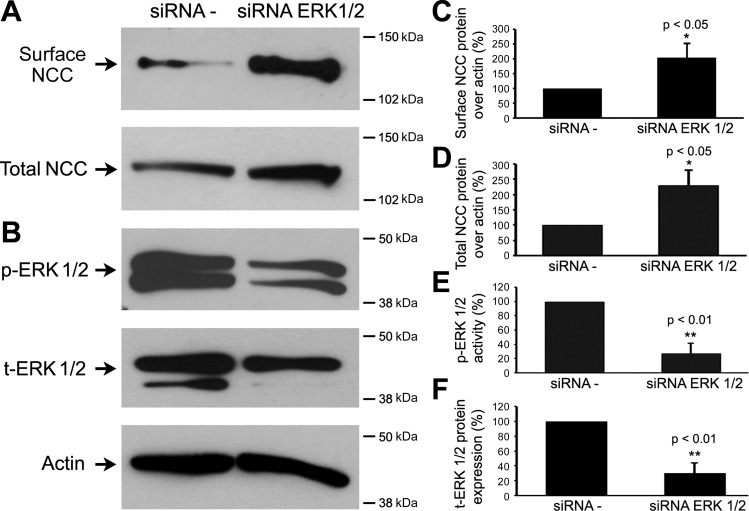
To further confirm that ERK1/2 phosphorylation is critical in the WNK4-mediated inhibitory effect on NCC protein expression, we next knocked-down ERK1/2 expression in mDCT cells and examined NCC protein expression. As shown in Fig. 5, the total ERK1/2 level (30.5 ± 13.7 vs. 100 ± 0%, P < 0.01, n = 4; Fig. 5, B and F) and phosphorylated-ERK1/2 level (27.1 ± 12.5 vs. 100 ± 0.0%, P < 0.01, n = 4; Fig. 5, B and E) were significantly decreased, respectively, in the knock-down group compared with the control group. Silencing ERK1/2 led to an increase in both total and cell surface NCC expression (229.9 ± 51.2 vs. 100 ± 0%, P < 0.05, n = 4; Fig. 5, A and D, and 203.8 ± 47.9 vs. 100 ± 0%, P < 0.05, n = 4; Fig. 5, A and C, respectively). These data further demonstrate that ERK1/2 mediates the inhibitory effect of WNK4 on NCC protein expression.
Fig. 5.
Knock-down ERK1/2 expression increased NCC total and surface protein expressions. mDCT cells were transfected with scramble siRNAs (siRNA −) or siRNA ERK1/2. Cell surface biotinylations and Western blot were subsequently performed. A and B: representative immunoblots are shown for surface and total NCC protein levels (A) and phospho-ERK1/2 and total-ERK1/2 (B). C and D: bar graphs represent the summary data for 3 independent experiments for surface and total NCC protein levels, respectively. E and F: bar graphs represent the summary data of 3 independent experiments for phosphor-ERK1/2 and total ERK1/2 levels, respectively. Cell surface and total NCC levels were significantly increased in the siRNA ERK1/2 group compared with the siRNA − control group. Phospho- and total ERK1/2 levels were significantly decreased in the siRNA ERK1/2 group compared with the siRNA − group. The molecular weight markers are indicated at the right side of the blots. *P < 0.05 and **P < 0.01, n = 4.
DISCUSSION
WNK kinases play the important roles in the regulation of homeostasis of ion channels and transporters (7). Mutations of WNK1 or WNK4 lead to either upregulation of NCC, due to impaired inhibition of NCC expression by WNK4, or to increased WNK1 activation of NCC (8). Although recent studies showed that SPAK/OSR1 kinases act as downstream substrates and effectors of WNK1 and WNK4 (2, 4, 21), the inhibitory effect of WNK4 on NCC cell surface expression likely utilizes an alternative regulatory pathway. In the present study, we established that the WNK4-ERK1/2 signaling pathway is responsible for WNK4-mediated inhibitory effect on NCC protein expression, ultimately NCC function.
As a member of mitogen-activated protein kinases, ERK1/2 is usually phosphorylated and activated as part of the Ras/Raf/MEK1/2/ERK1/2 signaling cascade upon stimulation of cells by various cytokines, growth factors, and extracellular stresses (16, 19). In this study, we found that overexpression of WNK4 enhances ERK1/2 phosphorylation in cultured mouse DCT cells, whereas WNK4 PHA II-causing mutants, E562K and R1185C, failed to enhance or even reduce ERK1/2 phosphorylation. Our data suggested that WNK4 kinase acts upstream of the MAP-ERK1/2 kinase signaling pathway. These findings further support the notion that WNK kinase family is closely intermingled with MAP kinase signaling networks since other WNK family members have already been shown to activate MAP kinase signaling pathways. Indeed, WNK1 was reported to activate ERK5 via the MEK5 (31). Similarly, WNK2 has been shown to activate ERK1/2 through Raf/MEK1/2 (14). These findings suggest that the WNK kinases can be viewed as part of the MAPK signaling cascades, even though WNK kinases do not share the common catalytic lysine residue in their kinase domain (30). Since PHA II mutations in WNK4 prevent increased ERK1/2 phosphorylation, downstream WNK4 signaling events are affected including the regulation of NCC. The precise mechanism by which WNK4 WT and its PHAII mutants affect ERK1/2 phosphorylation remains to be elucidated.
Change in osmolarity affects cellular physiologies, morphologies, and signaling pathways. In the present study, we showed that hypertonic stress stimulates ERK1/2 phosphorylation, an effect that is greatly attenuated upon knocking down endogenous WNK4 expression. Our data demonstrate the involvement of WNK4 in the hypertonicity-induced ERK1/2 phosphorylation and agree with a previous study that showed WNK4 enhancing ERK1/2 phosphorylation in response to hypertonic stimulation (24). These data further confirm that WNK4 is in the upstream of MAPK ERK1/2 signaling pathway. Cation-chloride cotransporter (CCC) is a superfamily consisting of sodium-potassium chloride cotransporter (NKCC), NCC, and potassium chloride cotransporter (KCC). These cotransporters, which are involved in the regulation of cell volume and/or intracellular chloride concentration, are themselves regulated through phosphorylation/dephosphorylation events (5). Changes in tonicity, intracellular Cl−, and cell volume affect CCC functions (5). Hypotonic stimulation and low intracellular Cl− were shown to activate NKCC and inhibit KCC activity by phosphorylating the cotransporters (3, 12). Low Cl− hypotonic stress was also shown to increase WNK1 and SPAK kinase activity, leading to enhanced NCC phosphorylation at Thr53, Thr58, and Ser71(15), suggesting that change in tonicity and intracellular Cl− affects NCC activity through the WNK1-SPAK signaling pathway. Hypotonic low Cl− was also shown to activate NCC by increased phosphorylation of NCC at Thr53 and Thr58 (17). We and other investigators previously showed that WNK4 inhibits NCC (1, 9). It has been reported that hypertonicity enhances ERK1/2 phosphorylation (0.5 M NaCl) and WNK4 enhances the hypertonicity-mediated ERK1/2 phosphorylation (24). Whether the high intracellular Cl− concentration resulting from NaCl hypertonicity is responsible for regulating NCC function remains to be determined. Since tonicity change alters ERK1/2 as well as NCC phosphorylation, WNK4 could be involved in the regulation of NCC through ERK1/2 signaling pathway in response to tonicity change. Further investigation on this potentially complicated issue is needed to be explored in the future study.
WNK4 WT was previously shown to inhibit NCC activity and NCC protein expression, whereas WNK4 the PHA II-causing mutant (E562K) had lost its inhibitory effect on NCC expression (1, 9, 32). We now show that WNK4 increased ERK1/2 phosphorylation, while WNK4 PHA II-causing mutants, E562K and R1185C, lost their ability to phosphorylate ERK1/2. The knock-down of WNK4 expression decreased ERK1/2 phosphorylation, but did not affect SAPK/JNK phosphorylation, indicating that WNK4 is part of the specific MAP-ERK1/2 signaling pathway, but not other MAPK pathways. We showed that the knock-down of WNK4 expression led to increased total and cell surface NCC expression. We also found that knock-down of ERK1/2 led to a similar increase in the total and cell surface expression of the cotransporter, again confirming the link between WNK4, ERK1/2, and NCC. In agreement with previous work, enhanced ERK1/2 phosphorylation by phorbol ester treatment also resulted in the downregulation of the NaCl cotransporter (10), although the mechanism might be different from our present study. Indeed, the phorbol ester-increased ERK1/2 phosphorylation and subsequent reduction in NCC cell surface expression, but not total NCC protein expression, likely occurred via an increase in NCC ubiquitination and degradation (11). In this study, we showed that WNK4, through ERK1/2, not only reduces cell surface expression, but also reduced total NCC expression. These data suggest that WNK4 and phorbol ester might have their own unique signaling cascades affecting the regulation of NCC protein expression, even though they share the same downstream ERK1/2 component.
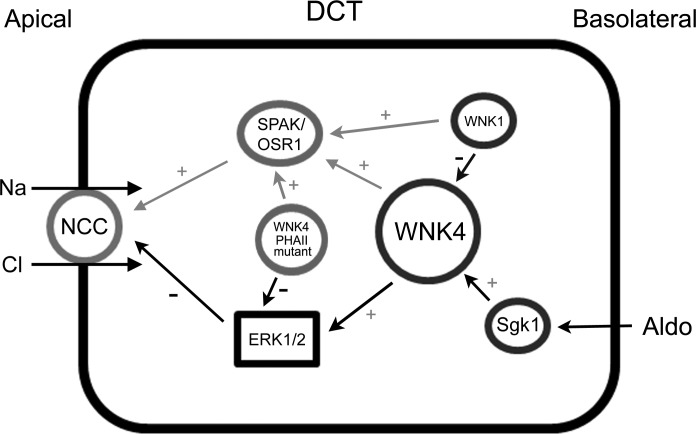
WNK1 and WNK4 have been shown to modulate NCC activity through STE20-related kinases, SPAK/OSR1 (18, 20, 21, 27). A recent study reveals that angiotensin II stimulates NCC activity through WNK4 and SPAK kinases (23), indicating that WNK4-SPAK signaling stimulates NCC activity. In addition, aldosterone has been implicated in activation of NCC through an SGK1 and WNK4 signaling pathway in vitro (22). In combination with findings from our present data, we proposed a working model of WNK4 in the regulation of NCC (Fig. 6), in which WNK4-ERK1/2 signaling pathway is likely to be essential in maintaining the basal inhibitory role in NCC expression and function. Mutations, such as those observed in PHA II, result in a WNK4 kinase that either lost its ability to phosphorylate ERK1/2 or to a WNK4 kinase that inhibits ERK1/2 phosphorylation. Subsequently, mutations in WNK4 lead to increased NCC protein expression and increased sodium reabsorption in the DCT. On the other hand, the WNK4-SPAK/OSR1 signaling pathway may be responsible for altering NCC function in response to dietary salt change (2, 21). As SPAK/OSR1 phosphorylation is increased in D561A knock-in mice (34), the activity of the cotransporter is also increased. In SPAK knockout mice, total NCC protein expression and phosphorylations of NCC at Thr-53, Thr-58, and Ser-71 residues were also significantly reduced (33). When coupled to an increase in cell surface expression due to diminished ERK1/2 phosphorylation, this increase in transport activity accounts for the increased sodium reasborption in the DCT and increase in blood pressure. This model is especially attractive in that it offers reconciliation in the conflicting interpretations of WNK4-SPAK/OSR1 stimulatory role vs. WNK4-ERK1/2 inhibitory role in the regulation of NCC. Future characterization of WNK4 upstream regulators in these two complementary signaling pathways, especially in an animal model, will further clarify the mechanism mediating WNK4 regulation of NCC. Taken all together, we conclude that WNK4-ERK1/2 signaling pathway plays an important role in WNK4-mediated inhibitory regulation of NCC.
Fig. 6.
Proposed model of WNK4 signaling pathway mediating NCC regulation in DCT. WNK1 and WNK4 were shown to phosphorylate SPAK/OSR1 and subsequently phosphorylate NCC to activate NCC, whereas our present data also showed that WNK4 also phosphorylates ERK1/2 and subsequently decreases NCC protein expression, suggesting that WNK4-ERK1/2 signaling pathway is inhibitory in NCC regulation. Aldo, aldosterone; SGK1, serum glucocorticoid-regulated kinase 1; WNK4 PHA II mutant indicates either E562K or R1185C; ERK1/2 is one component of MAPK signaling pathway. Gray black lines indicate the known stimulatory signaling pathways. Thick black lines indicate the novel inhibitory signaling pathway. + Sign indicates a stimulatory effect and − sign indicates an inhibitory effect.
GRANTS
This work is supported by National Institutes of Health Grants DK068226 (to H. Cai), DK068226–06S1 (to H. Cai), and Norman S. Coplon Satellite grant (to H. Cai).
Part of this work has been presented in the 2009 annual ASN meeting.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.Z., D.W., X.F., Y.Z., Y.W., J.Z., and X.Z. performed experiments; B.Z., D.W., X.F., Y.Z., Y.W., J.Z., X.Z., G.C., E.D., D.G., and H.C. analyzed data; B.Z., D.W., X.F., X.Z., and H.C. prepared figures; B.Z. and H.C. drafted manuscript; D.W., X.F., J.Z., G.C., E.D., D.G., and H.C. edited and revised manuscript; X.F., G.C., E.D., D.G., and H.C. interpreted results of experiments; H.C. conception and design of research; H.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Janet Klein and Jing Lin for critical reading and suggestions for this manuscript.
REFERENCES
- 1. Cai H, Cebotaru V, Wang YH, Zhang XM, Cebotaru L, Guggino SE, Guggino WB. WNK4 kinase regulates surface expression of the human sodium chloride cotransporter in mammalian cells. Kidney Int 69: 2162– 2170, 2006. [DOI] [PubMed] [Google Scholar]
- 2. Chiga M, Rai T, Yang SS, Ohta A, Takizawa T, Sasaki S, Uchida S. Dietary salt regulates the phosphorylation of OSR1/SPAK kinases and the sodium chloride cotransporter through aldosterone. Kidney Int 74: 1403– 1409, 2008. [DOI] [PubMed] [Google Scholar]
- 3. Flatman PW. Regulation of Na-K-2Cl cotransport by phosphorylation and protein-protein interactions. Biochim Biophys Acta 1566: 140– 151, 2002. [DOI] [PubMed] [Google Scholar]
- 4. Gagnon KB, England R, Delpire E. Volume sensitivity of cation-Cl− cotransporters is modulated by the interaction of two kinases: Ste20-related proline-alanine-rich kinase and WNK4. Am J Physiol Cell Physiol 290: C134– C142, 2006. [DOI] [PubMed] [Google Scholar]
- 5. Gamba G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev 85: 423– 493, 2005. [DOI] [PubMed] [Google Scholar]
- 6. Gamba G. The thiazide-sensitive Na+-Cl− cotransporter: molecular biology, functional properties, and regulation by WNKs. Am J Physiol Renal Physiol 297: F838– F848, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kahle KT, Ring AM, Lifton RP. Molecular physiology of the WNK kinases. Annu Rev Physiol 70: 329– 355, 2008. [DOI] [PubMed] [Google Scholar]
- 8. Kahle KT, Wilson FH, Lalioti M, Toka H, Qin H, Lifton RP. WNK kinases: molecular regulators of integrated epithelial ion transport. Curr Opin Nephrol Hypertens 13: 557– 562, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Kahle KT, Wilson FH, Leng Q, Lalioti MD, O'Connell AD, Dong K, Rapson AK, MacGregor GG, Giebisch G, Hebert SC, Lifton RP. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet 35: 372– 376, 2003. [DOI] [PubMed] [Google Scholar]
- 10. Ko B, Joshi LM, Cooke LL, Vazquez N, Musch MW, Hebert SC, Gamba G, Hoover RS. Phorbol ester stimulation of RasGRP1 regulates the sodium-chloride cotransporter by a PKC-independent pathway. Proc Natl Acad Sci USA 104: 20120– 20125, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ko B, Kamsteeg EJ, Cooke LL, Moddes LN, Deen PM, Hoover RS. RasGRP1 stimulation enhances ubiquitination and endocytosis of the sodium-chloride cotransporter. Am J Physiol Renal Physiol 299: F300– F309, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lauf PK, Adragna NC. K-Cl cotransport: properties and molecular mechanism. Cell Physiol Biochem 10: 341– 354, 2000. [DOI] [PubMed] [Google Scholar]
- 13. Lazrak A, Liu Z, Huang CL. Antagonistic regulation of ROMK by long and kidney-specific WNK1 isoforms. Proc Natl Acad Sci USA 103: 1615– 1620, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moniz S, Verissimo F, Matos P, Brazao R, Silva E, Kotelevets L, Chastre E, Gespach C, Jordan P. Protein kinase WNK2 inhibits cell proliferation by negatively modulating the activation of MEK1/ERK1/2. Oncogene 26: 6071– 6081, 2007. [DOI] [PubMed] [Google Scholar]
- 15. Moriguchi T, Urushiyama S, Hisamoto N, Iemura S, Uchida S, Natsume T, Matsumoto K, Shibuya H. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem 280: 42685– 42693, 2005. [DOI] [PubMed] [Google Scholar]
- 16. Nishimoto S, Nishida E. MAPK signalling: ERK5 versus ERK1/2. EMBO Rep 7: 782– 786, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pacheco-Alvarez D, Cristobal PS, Meade P, Moreno E, Vazquez N, Munoz E, Diaz A, Juarez ME, Gimenez I, Gamba G. The Na+:Cl− cotransporter is activated and phosphorylated at the amino-terminal domain upon intracellular chloride depletion. J Biol Chem 281: 28755– 28763, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Rafiqi FH, Zuber AM, Glover M, Richardson C, Fleming S, Jovanovic S, Jovanovic A, O'shaughnessy KM, Alessi DR. Role of the WNK-activated SPAK kinase in regulating blood pressure. EMBO Mol Med 2: 63– 75, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene 26: 3100– 3112, 2007. [DOI] [PubMed] [Google Scholar]
- 20. Richardson C, Alessi DR. The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1 signalling pathway. J Cell Sci 121: 3293– 3304, 2008. [DOI] [PubMed] [Google Scholar]
- 21. Richardson C, Rafiqi FH, Karlsson HK, Moleleki N, Vandewalle A, Campbell DG, Morrice NA, Alessi DR. Activation of the thiazide-sensitive Na+-Cl− cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci 121: 675– 684, 2008. [DOI] [PubMed] [Google Scholar]
- 22. Rozansky DJ, Cornwall T, Subramanya AR, Rogers S, Yang YF, David LL, Zhu X, Yang CL, Ellison DH. Aldosterone mediates activation of the thiazide-sensitive Na-Cl cotransporter through an SGK1 and WNK4 signaling pathway. J Clin Invest 119: 2601– 2612, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. San-Cristobal P, Pacheco-Alvarez D, Richardson C, Ring AM, Vazquez N, Rafiqi FH, Chari D, Kahle KT, Leng Q, Bobadilla NA, Hebert SC, Alessi DR, Lifton RP, Gamba G. Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc Natl Acad Sci USA 106: 4384– 4389, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shaharabany M, Holtzman EJ, Mayan H, Hirschberg K, Seger R, Farfel Z. Distinct pathways for the involvement of WNK4 in the signaling of hypertonicity and EGF. FEBS J 275: 1631– 1642, 2008. [DOI] [PubMed] [Google Scholar]
- 25. Uvarov P, Ludwig A, Markkanen M, Pruunsild P, Kaila K, Delpire E, Timmusk T, Rivera C, Airaksinen MS. A novel N-terminal isoform of the neuron-specific K-Cl cotransporter KCC2. J Biol Chem 282: 30570– 30576, 2007. [DOI] [PubMed] [Google Scholar]
- 26. Vitari AC, Deak M, Morrice NA, Alessi DR. The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem J 391: 17– 24, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Welling PA, Chang YP, Delpire E, Wade JB. Multigene kinase network, kidney transport, and salt in essential hypertension. Kidney Int 77: 1063– 1069, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 293: 1107– 1112, 2001. [DOI] [PubMed] [Google Scholar]
- 29. Wilson FH, Kahle KT, Sabath E, Lalioti MD, Rapson AK, Hoover RS, Hebert SC, Gamba G, Lifton RP. Molecular pathogenesis of inherited hypertension with hyperkalemia: the Na-Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc Natl Acad Sci USA 100: 680– 684, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem 275: 16795– 16801, 2000. [DOI] [PubMed] [Google Scholar]
- 31. Xu BE, Stippec S, Lenertz L, Lee BH, Zhang W, Lee YK, Cobb MH. WNK1 activates ERK5 by an MEKK2/3-dependent mechanism. J Biol Chem 279: 7826– 7831, 2004. [DOI] [PubMed] [Google Scholar]
- 32. Yang CL, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 111: 1039– 1045, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang SS, Lo YF, Wu CC, Lin SW, Yeh CJ, Chu P, Sytwu HK, Uchida S, Sasaki S, Lin SH. SPAK-knockout mice manifest Gitelman syndrome and impaired vasoconstriction. J Am Soc Nephrol 21: 1868– 1877, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang SS, Morimoto T, Rai T, Chiga M, Sohara E, Ohno M, Uchida K, Lin SH, Moriguchi T, Shibuya H, Kondo Y, Sasaki S, Uchida S. Molecular pathogenesis of pseudohypoaldosteronism type II: generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metab 5: 331– 344, 2007. [DOI] [PubMed] [Google Scholar]
- 35. Zhou B, Zhuang J, Gu D, Wang H, Cebotaru L, Guggino WB, Cai H. WNK4 enhances the degradation of NCC through a sortilin-mediated lysosomal pathway. J Am Soc Nephrol 21: 82– 92, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]