Abstract
Expression of the epithelial sodium channel (ENaC) at the apical membrane of cortical collecting duct (CCD) principal cells is modulated by regulated trafficking mediated by vesicle insertion and retrieval. Small GTPases are known to facilitate vesicle trafficking, recycling, and membrane fusion events; however, little is known about the specific Rab family members that modify ENaC surface density. Using a mouse CCD cell line that endogenously expresses ENaC (mpkCCD), the channel was localized to both Rab11a- and Rab11b-positive endosomes by immunoisolation and confocal fluorescent microscopy. Expression of a dominant negative (DN) form of Rab11a or Rab11b significantly reduced the basal and cAMP-stimulated ENaC-dependent sodium (Na+) transport. The greatest reduction in Na+ transport was observed with the expression of DN-Rab11b. Furthermore, small interfering RNA-mediated knockdown of each Rab11 isoform demonstrated the requirement for Rab11b in ENaC surface expression. These data indicate that Rab11b, and to a lesser extent Rab11a, is involved in establishing the constitutive and cAMP-stimulated Na+ transport in mpkCCD cells.
Keywords: small GTPase, siRNA, dominant-negative Rab
the epithelial sodium channel (ENaC) constitutes the rate-limiting entry step in Na+ reabsorption across several epithelia including the kidney, lung, colon, salivary glands and sweat glands (8, 27, 45). ENaC-mediated transport is an essential component underlying salt and water homeostasis. Knockout of the ENaC α-subunit in mice is lethal as offspring fail to clear airway fluid at birth (32). Abnormal regulation of the channel that results in either gain or loss of function has been implicated in the pathogenesis of several disease states, including forms of salt-sensitive hypertension, salt wasting (pseudohypoaldosteronism type II), and is thought to contribute to the progression of pulmonary disease in cystic fibrosis (28, 33, 43, 51, 63).
The apical membrane abundance and open probability of ENaC are altered by a wide variety of hormonal and cellular effectors (6, 7, 30, 42, 48, 52). The hormone vasopressin acutely increases Na+ transport by increasing the apical surface density (channel number) of ENaC (9, 12, 15, 21, 22, 39, 66). These channels are transported from subapical vesicles and inserted into the apical membrane by exocytosis (9, 11). To regulate the surface ENaC density, ubiquitination of ENaC at the apical surface acts as a signal for channel endocytosis (1, 19, 24, 28, 34, 37). We previously demonstrated the involvement of endocytic adaptor epsin in the clathrin-mediated retrieval of ubiquitinated ENaC from the apical membrane (68). These endocytosed channels traffic through EEA1-positive early endosomes (68). Ubiquitinated cargo such as ENaC is recruited to early endosomes by the ubiquitin-interacting motifs on hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) and other components of the endosomal-sorting complexes required for transport (ESCRT-0) complex (17). Hrs has recently been shown to be critical to ENaC recycling (72). Retrieved channels in EEA1 compartments are only free to recycle back to the apical cell surface once the ubiquitin has been removed by specific deubiquitinating proteins (DUBs). Two DUBs have been implicated in this process to date (10, 23), but it is likely that more DUBs will be implicated in this regulation. Our previous work demonstrated that the DUB UCH-L3 appears to act on ENaC in the early endocytic pathway to facilitate its recycling. The itinerary of ENaC trafficking through intracellular vesicle compartments following the fate decision in early endosomes has yet to be mapped, but from a growing body of work it is likely that the small GTPases will be important mediators of ENaC's intracellular trafficking.
The Rab-GTPase family of proteins is a subset of the larger Ras superfamily of G proteins and comprises >70 family members (26, 61). In the process of transitioning between donor and acceptor membrane compartments, Rab proteins switch between two conformations, an inactive, GDP-bound and an active, GTP-bound form (47). An exchange factor (GEF) catalyzes the GDP-GTP transition while GTP hydrolysis to GDP is catalyzed by a GTPase-activating protein (GAP) (26, 29). Rab proteins facilitate steps in vesicle regulation, including vesicle formation, trafficking, and fusion. They are often linked to cytoskeletal motor proteins to regulate vesicle movement (18, 29, 46). It is possible to alter the intrinsic activity of Rabs by introducing mutations that lock the GTPase in the GTP-bound (constitutively active, CA) or the GDP-bound (dominant negative; DN) confirmation.
A number of epithelial channels have been shown to be regulated by Rab proteins, providing precedence for a role of Rabs in channel regulation by membrane trafficking (14, 49, 53–58, 65, 71). As ENaC recycles in polarized epithelial cells, it is likely that the channel traverses a number of Rab-dependent compartments en route to the apical membrane. Prior studies have demonstrated the involvement of Rab 4, 11, and 27 in ENaC regulation (38, 53, 54, 58). These Rabs have been previously shown to regulate exocytosis of recycling and biosynthetic cargoes (20, 25, 35, 36, 50, 59, 60, 70).
The two related isoforms of the GTPase Rab11, Rab11a and Rab11b, share ∼90% amino acid homology, with the least similarity found in their membrane-binding C termini (40). Rab11a localizes to the apical recycling endosome (ARE) in polarized epithelial cells, where it regulates the apical recycling and exocytic insertion of membrane proteins (13, 16). Rab11b localizes to apical vesicles distinct from a Rab11a compartment in polarized MDCK and gastric parietal cells (41). While a recent publication implicated Rab11a in the regulation of ENaC ectopically expressed in Chinese hamster ovary (CHO) cells (38), no studies have reported a role for Rab11b in apical recycling of ENaC, and we sought to clarify the involvement of each Rab11 isoform in a cell line in which ENaC is endogenously expressed and regulated (62, 65).
Disruption of Rab11 activity with DN mutants led to a reduction in ENaC-mediated Na+ transport. Specific knockdown of Rab11a using small interfering (si) RNAs produced a small decrease in ENaC surface expression and no significant decrease in ENaC-mediated Na+ transport. A much larger impact on ENaC regulation was obtained when Rab11b activity was altered. With both the expression of a DN-Rab11b mutant or knockdown of endogenous Rab11b, basal, unstimulated Na+ transport was reduced and acute ENaC trafficking and recycling to the apical surface were significantly impaired. These findings indicate the absolute requirement for Rab11b to deliver ENaC to the apical membrane.
MATERIALS AND METHODS
Reagents and antibodies.
All reagents were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. The anti-Rab11b and α1-Na-K-ATPase polyclonal antibodies were obtained from Cell Signaling Technology (Beverly, MA). Other antibodies used included anti-Rab11a (BD Biosciences, San Jose, CA), anti-GFP (Abcam, Cambridge, MA), anti-actin (Sigma), monoclonal anti-β-ENaC (Santa Cruz Biotechnology, Santa Cruz, CA), anti-γ-ENaC antibody (StressMarq, Victoria, BC), and fluorescently tagged phalloidin-Alexa 633 (Invitrogen, Carlsbad, CA).
ENaC antibody characterization.
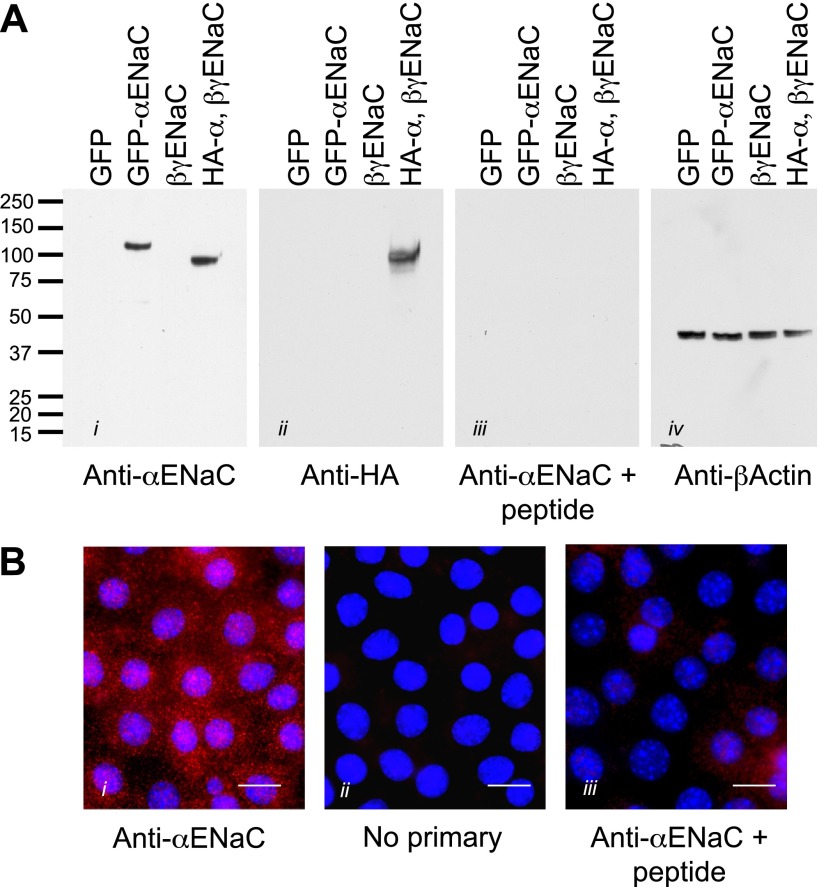
An affinity- purified rabbit polyclonal antibody was raised against α-ENaC using a 14-amino acid antigen specific for mouse α-ENaC. The sequence (PMQGLGKGDKREEQ) was conjugated to KHL by the addition of a terminal cystine and antibodies raised and purified commercially by GenScript (Piscataway, NJ). To verify the specificity of a newly developed anti-α-ENaC antibody, Fisher rat thyroid (FRT) epithelial cells were transiently transfected with epitope, and fluorescently tagged ENaC and untagged ENaC [plasmids for green fluorescent protein (GFP)-ENaC and double-tagged hemagglutinin (HA)-ENaC-V5 were kindly provided by Drs. J. Stockand, UTHSCSA, San Antonio, TX and T. Kleyman (Renal-Electrolyte Division, School of Medicine, University of Pittsburgh), respectively, and have been previously characterized (31, 64)]. FRT cells were transfected with ENaC using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. As a control, an eYFP-membrane reporter plasmid was used (Clontech, Mountain View, CA). Cell lysates of transfected and control FRT cells were obtained (as described below), and proteins were resolved by SDS-PAGE to test the specificity of the antibody in standard Western blots. In addition, mpkCCD cells mounted on filters were prepared for immunofluorescent imaging, biotinylation, and Western blotting (see below). Controls included incubation of the primary antibody with the immunizing peptide (1 mg/ml) for 1 h at 37°C before incubation with the sample and the use of preimmune serum to check for a specific signal (Fig. 1). Bands observed on Western blots using the anti-α-ENaC antibody were verified by incubation with an anti-HA antibody against the epitope-tagged α-ENaC. We were unable to detect cleaved forms of α-ENaC in these overexpressing FRT cells by using either the anti-ENaC antibody or the HA-tagged form of the channel.
Fig. 1.
Characterization of a novel anti-α-epithelial sodium channel (ENaC) antibody. A: to test the specificity of the affinity-purified antibody, Fisher rat thyroid (FRT) cells were transiently transfected with the following constructs: green fluorescent protein (GFP) alone, GFP-tagged mouse α-ENaC, untagged mouse β,γ-ENaC only, and hemagglutinin (HA)-tagged mouse α-ENaC with untagged β,γ-ENaC. Whole cell lysates from these samples were loaded onto gels, and proteins were resolved by SDS-PAGE. Blots were probed with i) the α-ENaC antibody (1:1,000), ii) anti-HA antibody (1:1,000), iii) the α-ENaC antibody preincubated with the immunizing peptide (1 μg/μl), and iv) anti-β actin (1:1,000) as a loading control. From the detected bands, it is clear that the antibody recognizes the full-length α-ENaC and not β- or γ-ENaC. The addition of a GFP tag shifted the apparent weight up by ∼20 kDa as expected, and use of the anti-HA antibody produced a band at the same weight, indicating that the antibody is recognizing α-ENaC specifically. In addition, preincubation with the immunizing peptide eliminated the specific signal. It is therefore likely that this antibody is specific for α-ENaC in the mpkCCD cells. B: to demonstrate the specificity of fluorescent immunolabeling, mpkCCD cells cultured on filters were fixed (as described in materials and methods) and permeabilized, and indirect immunofluorescent labeling was performed using an Alex 568 anti-rabbit secondary antibody (Invitrogen) at 1:2,000 dilution with the following conditions: i) α-ENaC as the primary antibody, ii) no primary antibody (to test for nonspecific secondary labeling), and iii) α-ENaC antibody preincubated with immunizing peptide (as for the Western blots in A). Bars = 10 μm.
Cell culture.
The mpkCCDc14 cells (provided by A. Vandewalle and M. Bens, Institut National de la Santé et de la Recherche Médicale, Paris, France) were grown in flasks (passage 30–40) in defined medium as described previously (9, 67). Growth medium was composed of equal volumes of DMEM and Ham's F12 supplemented with 60 nM sodium selenate, 5 mg/ml transferrin, 2 mM glutamine, 50 nM dexamethasone, 1 nM triiodothyronine, 10 ng/ml epidermal growth factor, 5 g/ml insulin, 20 mM d-glucose, 2% vol/vol FCS, and 20 mM HEPES (Invitrogen, Sigma), pH 7.4, at 37°C in 5% CO2-95% air atmosphere. The medium was changed every second day. For experiments, the mpkCCD cells were subcultured onto permeable filter supports (0.4-μm pore size, 0.33- and 75-cm2 surface area; Transwell, Corning, Lowell, MA). Cells were cultured for at least 7 days in defined medium after which a confluent transporting cell monolayer had developed that could be assessed by recording open-circuit voltage and transepithelial resistance using “chopstick” electrodes (Millipore, Billerica, MA). Typically, 24 h before use in any investigation, medium incubating cells on filters was replaced with a minimal medium (without drugs or hormones) that contained only DMEM and Ham's F12.
Immunoisolation of Rab11-positive endosomes.
The immunoisolation method used was described previously (62). Briefly, mpkCCD cells cultured on 75-mm-diameter filters, washed twice with PBS at 4°C, and then scraped in 300 μl of homogenization buffer which contained 3 mM imidazole, pH 7.4, 250 mM sucrose, 0.5 mM EDTA, and Complete EDTA-free Protease Inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Cells were homogenized by 20 strokes of a Dounce homogenizer and centrifuged for 10 min at 3,000 g. The resulting postnuclear supernatant (PNS) was adjusted to a 40% (wt/vol) sucrose solution. The PNS was placed in 12-ml capacity polyclear centrifuge tubes (Sorvall, Newtown, CT) and overlaid with 6 ml of 35% (wt/vol) sucrose and 4 ml of 25% (wt/vol) sucrose, then centrifuged in a TH-641 rotor at 108,000 g for 3 h at 4°C. The endosome-enriched fraction containing vesicles positive for markers of the early and recycling endosomes at the 25%/35% sucrose interface was collected, diluted threefold with PBS, and spun at 108,000 g for 30 min at 4°C. Pelleted endosomes were resuspended in 0.1% BSA/PBS. Rabbit anti-Rab11a, anti-Rab11b, Na-K-ATPase, or a nonspecific rabbit IgG was added to apportioned samples and incubated with the isolated endosomes overnight at 4°C with rotation. During this period, sheep anti-rabbit magnetic Dynabeads (Invitrogen) were washed with 1% BSA/PBS three times and incubated with 1 ml 1% BSA/PBS overnight at 4°C. Following washing, the beads were recovered with a magnet and resuspended in 50 μl of 1% BSA/PBS. The blocked and washed beads were then added to the samples and incubated with each of the antibody-endosome fractions for 6 h at 4°C with rotation. The bead-antibody-endosome complexes were collected with a magnet, washed twice with 1% BSA/PBS, once with 0.1% BSA/PBS, and then once with PBS. Laemmli sample buffer was added to the immunoisolated endosomes, and samples were resolved on 6–18% SDS-PAGE, transferred to polyvinylidene difluoride (1 h at 100 V), and blotted for proteins of interest.
Short-circuit current and membrane capacitance recordings.
Cells cultured on filter supports were mounted in modified Ussing chambers (Harvard Apparatus, Holliston, MA), and the cultures were continuously short circuited with an automatic voltage clamp in a system that permitted simultaneous detection of short-circuit current (ISC) and total membrane capacitance (CT) to be performed (designed and manufactured by W. Van Driesche, Leuven, Belgium), using our previously described methods (9–11). The bathing Ringer solution was composed of 120 mM NaCl, 25 mM NaHCO3, 3.3 mM KH2PO4, 0.8 mM K2HPO4, 1.2 mM MgCl2, 1.2 mM CaCl2, and 10 mM glucose. Chambers were constantly gassed with a mixture of 95% O2-5% CO2 at 37°C, which maintained the pH at 7.4. Chamber washes were carried out by a fivefold volume exchange (25–30 ml) with 37°C Ringer solution to wash out each chamber's basolateral bathing solution. As the cells were sensitive to changes in pressure, flow, and temperature, the chamber solutions were only exchanged during wash periods with no flow at steady state. A typical stimulation protocol involved the addition of 10 μM forskolin (Fisher Scientific, Pittsburgh, PA) basolaterally, which produced a maximum ISC stimulation after 20–30 min; forskolin was washed from the basolateral side of the chamber, and current declined back to basal levels within 30 min. To determine the net Na+ transport through ENaC, 10 μM amiloride (Sigma) was added to the apical cell surface at the end of each experiment.
DNA constructs: Rab11a- and b-S25N adenoviruses.
Generation of the adenoviral constructs was recently described in detail (62). GFP-tagged Rab11a S25N and Q70L constructs were obtained from Dr. M. Zerial (Max Planck Institute, Dresden, Germany). Amplified human Rab11a and Rab11b were TOPO-cloned into the pCR2.1 vector (Invitrogen) and then sequenced using M13 primers (Invitrogen). The S25N and Q70L point mutations were introduced into the wild-type GFP-Rab11b construct using a Quik-Change Site-directed Mutagenesis kit (Qiagen, Valencia, CA) following the manufacturer's instructions. All constructs were sequenced before use. Recombinant adenovirus-expressing GFP-tagged Rab11a-S25N (pAdTet-GFPRab11aS25N) and adenovirus-expressing GFP-Rab11b-S25N was created using the ViraPower Adenoviral Expression System (Invitrogen) per the manufacturer's instructions as described previously (O'Brien Center, Vector Core, University of Pittsburgh, PA) (62).
Immunofluorescence labeling.
Routine immunofluorescent labeling of samples was performed as follows. Unless described otherwise, all steps were performed at 4°C. Polarized, filter-grown mpkCCD cells were washed three times with PBS containing 0.1 mM CaCl2 and 1 mM MgCl2 (PBS+CM) and fixed with 4% paraformaldehyde in PBS+CM for 30 min. Following three PBS+CM washes, the cells were permeabilized with 0.1% Triton X-100 and 0.1% NP-40 in PBS+CM for 20 min for samples requiring antibody labeling. Cells were labeled in blocking buffer consisting of 10% normal goat serum, 10% dry nonfat milk, and 0.05% Triton X-100 in PBS+CM overnight at 4°C. Unbound primary antibody was removed by four washes with PBS+CM. Primary antibodies were labeled with corresponding fluorescence-conjugated secondary antibodies in blocking buffer for 2 h at 37°C. For cells expressing GFP-tagged constructs, no antibody labeling was required and following counterstaining with 4,6-dimidino-2-phenylindole cells were washed again and mounted on coverslips in fluoromount-G (SouthernBiotech, Birmingham, AL) for imaging. Images were captured using an Olympus IX81 fluorescent microscope (Olympus, Center Valley, PA) fitted with a DSU spinning disk and 300-W fluorescent light source using either a ×60, 1.4-numerical aperture (NA) oil or ×20, 0.45-NA long working distance air objective. Single fluorescent images were captured using a Retiga cooled CCD camera (QImaging, Surrey, BC) at 1,024 × 1,024 resolution using SlideBook (Olympus). Linear adjustments of brightness and contrast were made offline in MetaMorph (Molecular Devices, Downingtown, PA).
For dual-label colocalization studies, filter-grown cells were fixed and processed using a pH-shift protocol as described previously (2). When antibodies of the same species were used, a sequential staining protocol (adapted from one provided by Jackson ImmunoResearch) was employed. Following fixation, unreacted paraformaldehyde was quenched with PBS containing 20 mM glycine, pH 8.0 and 75 mM NH4Cl for 10 min at room temperature. Cells were then incubated for 30 min at room temperature in a blocking buffer containing 0.025% (wt/vol) saponin and 8.5 mg/ml of fish skin gelatin dissolved in PBS. The cells were incubated with primary antibody, diluted in blocking buffer for 16 h at 4°C, and then washed three times with blocking buffer for 5 min each. The samples were then incubated for 16 h at 4°C with 13 μg/ml goat-anti-rabbit IgG Fab fragments, washed three times with blocking solution, and then incubated for 1 h at room temperature with secondary antibody (donkey anti-goat Cy3 diluted 1:3,000). Following washes with blocking buffer and PBS, the cells were postfixed with 4% paraformaldehyde in 100 mM sodium cacodylate (pH 7.4) for 5 min at room temperature. The unreacted paraformaldehyde was quenched for 5 min at room temperature with the quenching buffer described above. The cells were incubated with blocking solution for 30 min at room temperature and then incubated with the next rabbit primary antibody for 2 h at room temperature. After three 5-min washes with blocking buffer, the cells were incubated with a tertiary antibody (donkey anti-rabbit FITC, diluted 1:200) for 1 h at room temperature. The cells were rinsed with blocking buffer and then PBS, post-fixed, and then mounted as described previously (2). In control reactions, the Fab fragments or individual primary antibodies were left out of the incubations (not shown).
Imaging was performed using a TCS-SL confocal microscope (Leica, Dearfield, IL) equipped with argon, green helium-neon, and red helium-neon lasers. Images were acquired using a ×100 1.4-NA oil objective. Photomultipliers were set to 600–900 V and zoom at ×4.0. Images were collected every 0.30 μm and averaged four times. All pixel values fell within the 8-bit range of the captured image files. The images (512 × 512 pixels) were saved in a TIFF format, contrast was corrected in Photoshop, and electronic files were generated in Adobe Illustrator.
Stacks of dual-labeled confocal sections were imported into Volocity (PerkinElmer, Waltham, MA), background noise was removed using the fine (3 × 3) median noise reduction filter, and a scatter plot of voxel intensities for each of the markers was generated using the colocalization function. The images were thresholded using a fixed value of 15, and Mander colocalization coefficients for each of two markers (Mx and My, respectively) were calculated for the entire three-dimensional image using the following equations (1)
| 1 |
where X1 is equal to the intensity of marker X at a given voxel and Xi, colocalized = Xi if the associated intensity of the other marker (Yi) is above the threshold value and therefore colocalizes. When Xi, colocalized = 0, it indicates that Yi is below the threshold value and does not colocalize. Yi and Yi, colocalized are similarly defined. An Mx or My value of 1.0 indicates 100% colocalization, while a value of 0.0 indicates no colocalization. The values for Mx and My can be similar or not, as one marker may have a broader distribution than the other in the sampled region of the tissue.
siRNA.
To knock down the expression of Rab11a or Rab11b, siRNAs specific for each mouse isoform (4 constructs/isoform) were commercially obtained (Dharmacon/Thermo Fisher Scientific, On-Target Plus) and introduced into the mpkCCD cells using Lipofectamine 2000 at a concentration of 50 nM as described previously (10). The target sequences for Rab11a and Rab11b were as follows: 1) GUACAGGGCUAUAACGUCU, UAAGAGUGAUUUACGUCAU, GCGACGACGAGUACGACUA, and UAACAGAGAUAUACCGCAU for Rab11a; and, 2) GGGACGACGAGUACGAUUA, GCAUUCAGGUGGACGGCAA, UGACAUAUGAGAACGUGGA, and CAUCUUGACAGGAUAGGGA for Rab11b. Cells were seeded onto filter supports and allowed to polarize over 72 h before use in electrophysiological experiments. Following ISC and CT measurements, the cells were harvested from the filter supports in lysis buffer containing protease inhibitors (as above) and proteins were resolved by SDS-PAGE to determine the extent of protein knockdown.
Surface biotinylation.
To demonstrate the change in ENaC surface expression following siRNA knockdown of Rab11 isoforms, we performed surface biotinylation using a modified protocol similar to the approach we described before in these cells (11). Basically, cells were transfected with siRNA as described above and seeded onto 6.5-mm-diameter filter inserts (Transwell, Corning Costar). There were three groups of cells: control (nontargeting) siRNA, Rab11a, and Rab11b siRNA-transfected cells. A total of 12 filters were used for each group and pooled following biotinylation to obtain 1 sample. The inserts were first washed five times with ice-cold PBS containing Mg2+ and Ca2+ to remove medium containing FBS. The cells were biotinylated at 4°C in borate buffer (85 mM NaCl, 4 mM KCl, 15 mM Na2B4O7, 375 μg biotin at pH 9) on the apical surface with the basolateral side of the monolayer bathed in medium containing FBS to prevent basolateral biotinylation. After 20 min, basolateral and apical sides were aspirated and medium containing FBS was placed on the cells to quench the signal. Monolayers were washed five times with ice-cold PBS with agitation, and the cells were harvested by scraping in PBS using a 200-μl pipette tip. The cell homogenate was obtained by lysing cells in lysis buffer (see above) and then centrifugation for 5 min at 5,000 rpm. The cell homogenate was assayed for protein concentration, and a small (20 μl) aliquot of the total lysate was removed to be used as whole cell lysate to probe for intracellular proteins and as a loading control. For the biotinylated sample, 300 μg of protein was incubated with 150 μl avidin bead slurry as previously described (11). Samples were heated to 95°C for 8 min and separated on a 10% SDS-PAGE. Samples were transferred to nitrocellulose membranes (Millipore) using a Trans-Blot SD Semi-Dry Transfer Cell (Bio-Rad, Hercules, CA) following the manufacturer's protocol. Nitrocellulose was blocked in 5% skim milk constituted in PBS for 3 h. The membrane was transferred to 1% skim milk-PBS containing antibodies (1:1,000 α-ENaC, 1:250 β,γ-ENaC) at 4°C overnight. Following antibody incubation, incubation blots were washed four times in PBS. Horseradish peroxidase-conjugated secondary antibodies (KPL, Gaithersburg, MD) were diluted 1:5,000 in 1% skim milk-PBS. Membranes were incubated with secondary antibody for 1 h at room temperature. The membrane was washed twice for 30 s in PBS followed by one 15-min wash and four 5-min washes. Reactive proteins were visualized with enhanced chemiluminescence (PerkinElmer Life Sciences, Wellesley, MA). Western blots were quantified after digital capture (scanning) using Adobe Photoshop CS. Band intensities were normalized to actin (WCL), corrected for background, and expressed as a percentage of the control siRNA levels (n = 2).
Statistics.
All data were analyzed using SigmaPlot (Systat, Chicago, IL). Summarized data were evaluated for normality and equal variance, and t-tests were carried out to determine whether differences were statistically different from each other. For any difference in the mean values, P < 0.05 was considered significantly different.
RESULTS
Characterizing a new anti-mouse α-ENaC antibody.
Due to previous difficulties in obtaining reliable ENaC antibodies for use with mouse tissue, we had a purified rabbit polyclonal antibody manufactured to specifically recognize the mouse α-ENaC subunit. Characterization of this antibody involved transiently overexpressing tagged versions of mouse ENaC in FRT cells and running the whole cell lysate on SDS-PAGE. The antibody specifically identified the full-length form of the mouse α-ENaC subunit and did not cross react with either β- or γ-ENaC (Fig. 1A). In addition, the shift in apparent molecular weight was observed in the GFP-tagged ENaC construct compared with the HA-tagged version of the expression plasmid. Along with specific recognition of α-ENaC by Western blotting, the antibody could also be used for immunofluorescent labeling (Fig. 1B). Preimmune bleeds and peptide competition experiments also verified the specificity of this new ENaC antibody.
ENaC shows greater colocalization with Rab11b than Rab11a.
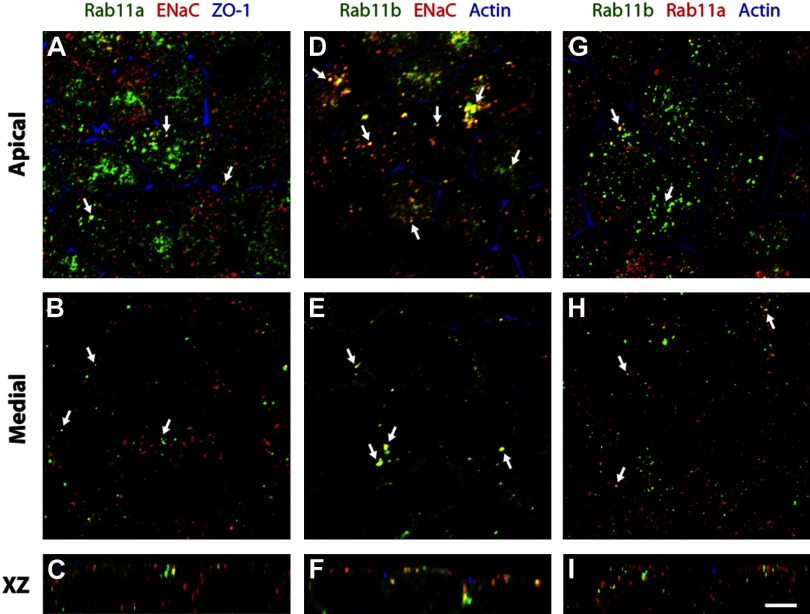
Previous studies have identified the expression of Rab11 in rat kidney collecting duct tissue (4). However, there is little data concerning the isoform-specific expression of Rab11 in kidney cells and tissues. There is also a lack of information about whether ENaC is localized in a Rab11 isoform-selective manner in kidney epithelial cells. Using specific Rab11a and Rab11b antibodies (see Fig. 5), we confirmed that both isoforms were expressed in mpkCCD cells (Fig. 2). Each protein showed a punctate, vesicular distribution, and most of the punctae concentrated at the apical pole of the cells; however, punctae were also observed along the lateral margins of the cell (Fig. 2, B, E, and H). Consistent with a previous analysis in Madin-Darby canine kidney and gastric parietal cells (13, 41), we observed only a small degree of colocalization between these two isoforms (colocalization coefficient of Rab11a vs. Rab11b = 0.16; Rab11b vs. Rab11a = 0.22; Table 1; Fig. 2, G–I).
Fig. 5.
Adenoviral overexpression of Rab11. A: to reliably overexpress mutants of Rab11, mpkCCD cells were infected using adenoviral constructs containing either DN-Rab11a-GFP, DN-Rab11b-GFP, or GFP alone as a control. Expression of each Rab11 isoform was determined by Western blotting using isoform-specific antibodies (or an anti-GFP antibody as a control) to demonstrate overexpression of the DN-Rab11 constructs. B: as the constructs were GFP tagged, it was possible to monitor the level of overexpression by live cell fluorescent microscopy. An example of adenoviral-infected mpkCCD cells cultured on filter supports is presented for each of the constructs. Scale bars = 50 μm.
Fig. 2.
Distribution of Rab11 and ENaC in mpkCCD cells. A–C: distribution of Rab11a (green), α-ENaC (red), and the tight junction-associated protein zonula occludens (ZO)-1 (blue). D–F: distribution of Rab11b (green), α-ENaC (red), and actin (blue), which is concentrated at the apicolateral junction of the cells. Actin is also found in the cortical region along the basolateral surfaces of the cell (and associated with microvilli), but this staining is not apparent in these images. G–I: localization of Rab11b (green), Rab11a (red), and actin (blue) in mpkCCD cells. Images were acquired with a confocal microscope, and projections of 3 optical sections from the apical or medial planes of the monolayers or individual xz sections are shown. Scale bar = 5 μm.
Table 1.
Colocalization coefficients
| Means ± SE (n) | |
|---|---|
| ENaC vs. Rab11a | 0.281 ± 0.070 (5) |
| *Rab11a vs. ENaC | 0.213 ± 0.010 (5) |
| ENaC vs. Rab11b | 0.516 ± 0.043 (5) |
| Rab11b vs. ENaC | 0.296 ± 0.031 (5) |
| Rab11a vs. Rab11b | 0.160 ± 0.020 (3) |
| Rab11b vs. Rab11a | 0.228 ± 0.012 (3) |
The proportion of colocalized immunofluorescent signal for labeled Rab11a, Rab11b, and epithelial sodium channel (ENaC) or Rab11a and Rab11b is presented as a mean of >3 different experiments (n). A significantly (*) greater proportion of ENaC signal was localized with Rab11b than Rab11a (P < 0.05).
Next, we used an α-ENaC antibody (Fig. 1) and a sequential labeling protocol to examine the localization of ENaC with both Rab11 isoforms. Like Rab11, α-ENaC was found in small punctate vesicular elements that concentrated at the apical pole of the cells. We observed that α-ENaC colocalized with Rab11a (colocalization coefficient of 0.28; Table 1) (Fig. 2, A–C); however, a significantly greater pool of α-ENaC colocalized with Rab11b (colocalization coefficient of 0.51; Table 1; P < 0.05) (Fig. 2, D–F). These results indicate that mpkCCD cells express both Rab11 isoforms and that ENaC may be preferentially associated with the Rab11b pool of vesicles.
ENaC is present in immunoisolated Rab11 vesicles.
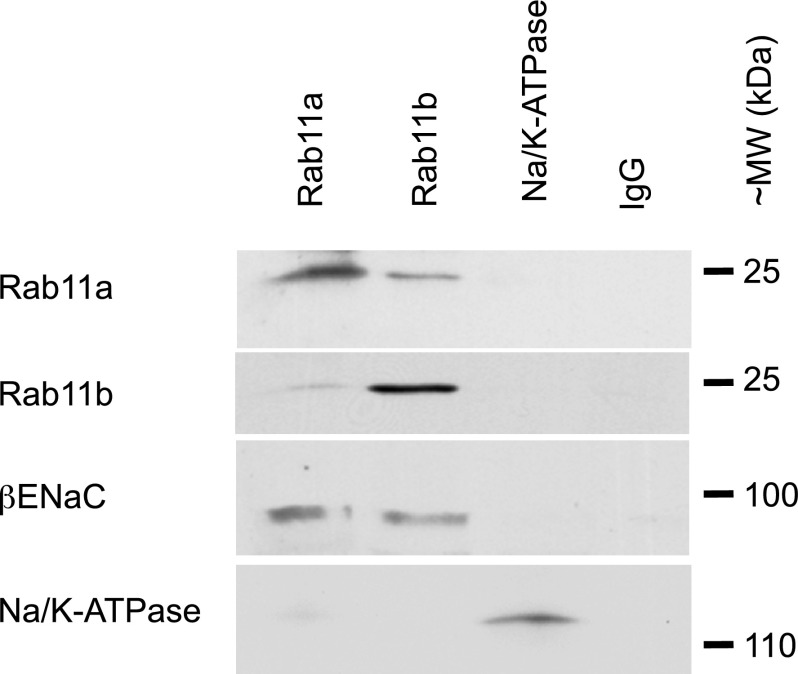
A magnetic bead immunoisolation technique was employed to verify that ENaC was colocalized in Rab11-positive vesicles. Unlike immunoprecipitation techniques, immunoisolation does not require that proteins physically interact. Endosomal vesicles were isolated from filter-grown mpkCCD cells by discontinuous sucrose gradient centrifugation. This technique has been employed previously by us to isolate the chloride channel CFTR in Rab11-positive vesicles (62). The isolated vesicles were incubated with antibodies that recognize either Rab11a, Rab11b, or control antibodies and separated magnetically by incubation with secondary antibodies attached to magnetic beads. The α-subunit of Na-K-ATPase and nonspecific preimmune IgG were used as negative controls. Isolated samples were resolved on gradient SDS-PAGE immunoblots probed and blotted with antibodies against β-ENaC, Rab11a, Rab11b, or the Na-K-ATPase. In agreement with the immunofluorescent studies presented above, we observed small amounts of Rab11b in the Rab11a-immunoisolated endosomes and vice versa (Fig. 3). This technique also confirmed β-ENaC was present in both Rab11a- and Rab11b-positive endosomes. Control immunoisolations using the Na-K-ATPase or control antibody showed little detectable amounts of Rab11 or ENaC. These studies confirm that ENaC is associated with both Rab11a- and Rab11b-positive pools of endosomes.
Fig. 3.
ENaC localized in immunoisolated Rab11-positive vesicles. Following isolation of Rab11a- and Rab11b-specific vesicles, samples were resolved by SDS-PAGE. As controls, the α-subunit of the Na-K-ATPase (localized to the basolateral membrane) and nonspecific IgG antibodies were used to verify the specificity of ENaC isolation. Blots were sequentially probed using Rab11a, Rab11b, β-ENaC, and the Na-K-ATPase antibodies. ENaC was localized to both Rab11a- and Rab11b-positive vesicles, but was not detected in the control isolations. A portion of Rab11a was detected in the Rab11b-positive samples and vice versa. Blots are representative of 2 similar experiments.
DN-Rab11 expression reduces ENaC currents and prevents ENaC recycling.
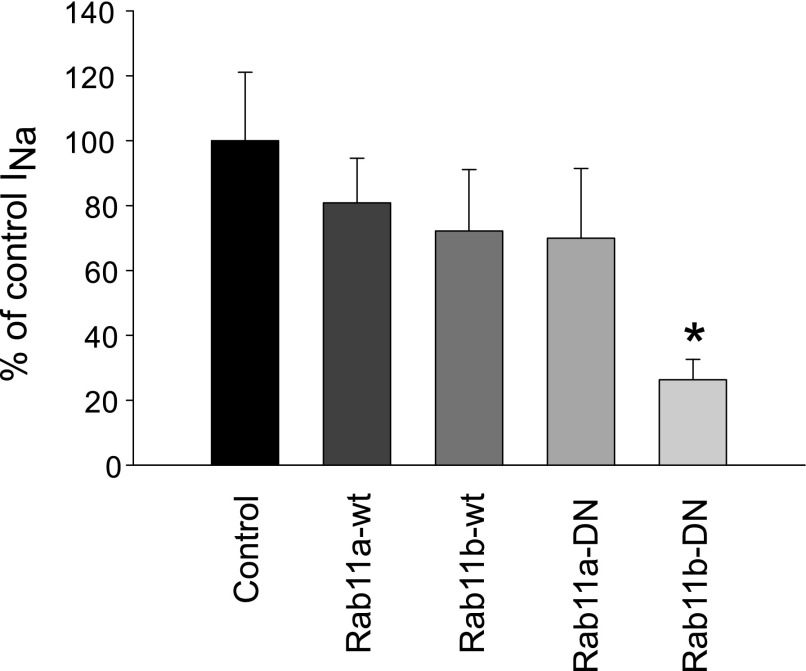
As indicated in the introduction, mutations in Rab-GTPases are able to lock the proteins into either the active, GTP-bound, or inactive, GDP-bound state. Following transient transfection of wild-type (wt) Rab11a and wtRab11b or the DN-Rab11 forms, cells were seeded onto filter supports to record ENaC-mediated ISC in Ussing chambers. A significant reduction in cAMP-stimulated ENaC current was observed in cells transiently transfected with DN-Rab11b (Fig. 4). However, no significant change in ENaC currents was observed in cells overexpressing Rab11a-DN, wt-Rab11a, or wtRab11b constructs. The lack of a significant response following DN-Rab11a overexpression could be due to low transfection efficiency (∼50%), and we therefore developed adenoviral DN-Rab11 constructs to permit a more acute and titratable expression of the DN-Rab11 in mpkCCD cells.
Fig. 4.
Amiloride-sensitive short-circuit current (ISC) in Rab11-transfected cells. Rab11 plasmids were transient transfected into mpkCCD cells, which were seeded onto permeable filter supports for electrophysiological measurements. The forskolin-stimulated, amiloride-sensitive ISC in either Rab11-WT or dominant negative (DN) constructs were normalized to control ISC values. Overexpression of DN-Rab11b produced a significant reduction in stimulated ISC, while the other constructs had no significant impact compared with GFP-transfected controls (n = 5, *P < 0.05).
Representative fluorescent images of mpkCCD cells expressing GFP-DN-Rab11 constructs are presented in Fig. 5. Addition of the GFP tag increased the apparent molecular weight of the expressed mutant Rab11 proteins, making them readily detectable over endogenous Rab11 isoforms by Western blotting. The viral particle load and protein expression were determined for each virus to ensure comparable levels of overexpression of Rab11 were achieved (∼95% transfection efficiency). Expression of GFP-Rab11a or GFP-Rab11b resulted in a diffuse cytoplasmic localization of the GFP-tagged Rab11.
Electrophysiological recordings were performed 24 h after viral infection to assess the impact on ENaC-mediated current and changes in membrane capacitance (CT) following cAMP stimulation. We demonstrated previously that CT recordings constitute a reliable readout of membrane surface area (9). The changes in CT in the mpkCCD cells are due to delivery or removal of membrane vesicles during vesicle-trafficking events at the apical surface. When vesicles are delivered to and fuse with the apical membrane during exocytosis, there is a corresponding increase in CT. Conversely, when vesicles are endocytically retrieved from the membrane there is a decline membrane surface area. A change in CT is apparent under non-steady-state conditions, for example, when a pool of ENaC-containing vesicles are induced to fuse with the apical surface following cAMP stimulation. By repeatedly simulating cells with cAMP, we can induce rounds of insertion and retrieval to monitor vesicle-trafficking events.
Sample traces of both ISC and CT measurements are presented in Fig. 6. Expression of DN-Rab11b reduced both the basal and cAMP-stimulated ENaC activity. In conjunction with this loss in ENaC-mediated Na+ transport, there was a significant reduction in the basal and stimulated membrane capacitance (summarized in Fig. 6, C and D).
Fig. 6.
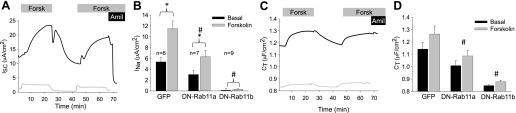
DN-Rab11 expression reduces ENaC-mediated Na+ transport and membrane trafficking. A: representative ISC recordings from mpkCCD cells expressing either GFP (black trace) as a control or DN-Rab11b-GFP (grey trace). Cells were stimulated with 10 μM forskolin (Forsk) during the indicated periods (bar above trace) to induce ENaC trafficking to the apical membrane. At the end of the recording, the measured ISC was inhibited with 10 μM amiloride (Amil) to block ENaC transport. Both the basal and cAMP-stimulated ENaC-dependent ISC were significantly inhibited by overexpression of DN-Rab11b compared with control. B: summarized data from a number of experiments similar to those presented in A for GFP-, DN-Rab11a-, and DN-Rab11b-expressing mpkCCD cells *Significant difference, P < 0.01 from unstimulated ISC. #Significant difference from GFP control. C: simultaneous membrane capacitance (CT) recordings taken in conjunction with the ISC recordings in A are presented. Both the basal and cAMP-stimulated CT of cells expressing DN-Rab11b were significantly reduced compared with GFP-expressing control cells. The data suggest that loss in ENaC ISC is likely the result of the inability to deliver ENaC to the apical membrane in the DN-Rab11b-expressing cells. D: summarized data from a number of experiments similar to that presented in C for the change in CT following forskolin stimulation for cells expressing GFP, DN-Rab11a, and DN-Rab11b. A significant reduction in cAMP-stimulated CT was observed for both DN-Rab11 isoforms compared with controls, suggesting inhibition of ENaC-containing vesicle exocytosis. The number of observations for each group is the same as in B. #Significant difference (P < 0.01) from GFP control.
Not only was there a reduction in absolute CT values in DN-Rab11-expressing cells, the response to forskolin stimulation was also significantly reduced. The increase in CT following cAMP stimulation is a measure of the number of vesicles that traffic to the surface in response to this agonist. The ΔCT change in control cells (GFP alone infected) was 0.12 ± 0.013 μF/cm2 compared with DN-Rab11a with 0.08 ± 0.008 μF/cm2 and DN-Rab11b with a ΔCT of 0.03 ± 0.005 μF/cm2 (n = 9, P < 0.01 for control vs. DN-Rab11a or -b). The reduction of the cAMP response was likely due to an inability of the DN-Rab11-expressing cells to deliver ENaC-containing vesicles to the surface.
It is possible to estimate the number of vesicles fusing with the apical surface that result in the observed CT increase (see Ref. 5 for details). If the specific capacitance of a biological membrane is assumed to be 1 μF/cm2, then we can estimate a value of 9.6 × 107 vesicles/cm2 are exocytosed following cAMP stimulation in control mpkCCD cells and 2.4 × 107/cm2 for DN-Rab11b-expressing cells. Taking this one step further by estimating the cell density for mpkCCD cells cultured on filter supports (∼2.5 × 105/cm2), it equates to ∼375 vesicles/cell in control cells compared with ∼95 vesicles/cell in the DN-Rab11b-expressing cells. Even without the final estimation of vesicles per cell, there is approximately four times the number of vesicles fusing with the apical membrane in control cells compared with the DN-Rab11b-expressing cells. While the expression of DN-Rab11a reduced both ISC and a CT, it was not to the same extent as DN-Rab11b when protein levels of the DN constructs of the two isoforms were similarly expressed.
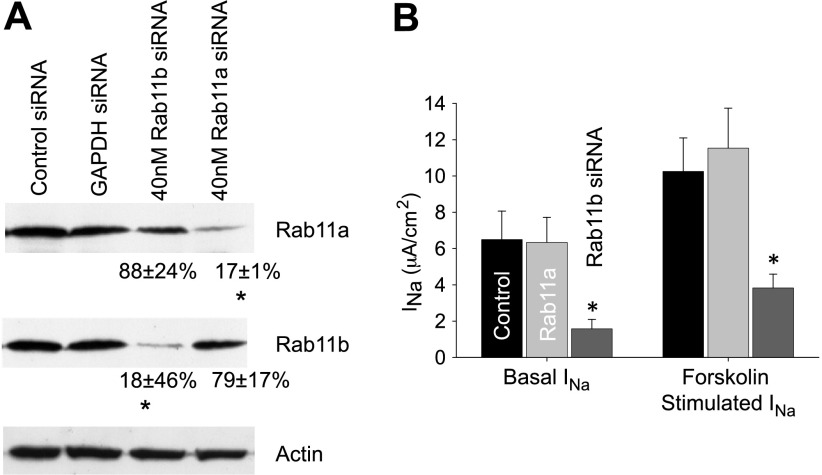
Rab11 knockdown.
With expression of DN-Rab11 resulting in a significant reduction in ENaC-mediated Na+ transport, there was still the possibility of a nonspecific, off-target effect of DN-Rab11b expression causing the reduction in ENaC currents. Similarly, as the two isoforms of Rab11 share significant homology, it is possible that the high expression of DN-Rab11a may nonspecifically interact with GEFs that would normally associate with Rab11b. Therefore, to address the roles of each Rab11 isoform in ENaC regulation directly, the protein levels Rab11a and Rab11b were specifically knocked down using a siRNA approach. The isoform-specific knockdown of Rab11 was confirmed by Western blotting with a >80% decrease in protein expression obtained for each isoform (Fig. 7). No off-target effects were noted with control knockdown of GAPDH or a scrambled siRNA construct. Following siRNA transfection, cells were seeded onto filter supports and allowed to polarize for 48 h, before being mounted in Ussing chambers for electrophysiological measurements. The basal and forskolin-stimulated amiloride-sensitive ISC was significantly reduced in the Rab11b knockdown cells, but not reduced in cells with Rab11a knockdown compared with controls (Fig. 7B). In summary, these results indicate that both steady-state ENaC surface expression and cAMP-mediated ENaC trafficking are likely regulated by Rab11b, and not Rab11a.
Fig. 7.
Rab11b knockdown reduces ENaC-mediated Na+ transport. A: to directly investigate the role of each Rab11 isoform in ENaC regulation, small interfering (si) RNA was used to specifically knock down the expression of Rab11a and Rab11b in the mpkCCD cells. Specific knockdown was confirmed by Western blotting (scrambled siRNA or GAPDH knockdown acted as controls). Quantification of the apparent band intensities as a percentage of control siRNA (corrected for background and normalized to actin loading control) is presented for each Rab11 isoform beneath the relevant band (n = 2–4, P < 0.01). *Significant reduction from control. B: the amiloride-sensitive ENaC current from control siRNA (n = 7), Rab11a (n = 6), and Rab11b (n = 17) knockdown in mpkCCD cells is summarized. A significant reduction in ENaC-mediated ISC under basal and forskolin-stimulated (10 μM for 30 min) conditions was only observed in Rab11b knockdown cells compared with scrambled controls (P < 0.05), and there was no significant difference in Rab11a siRNA knockdown cells compared with controls.
ENaC surface biotinylation.
To demonstrate that the reduction in measured ISC following Rab11b knockdown was due to a change in channel surface density, we performed surface biotinylation on filter-cultured mpkCCD cells where each Rab11 isoform was knocked down separately (as above). There was a small (∼30%) reduction in surface expression of each ENaC subunit following Rab11a knockdown and a greater loss of surface ENaC expression (∼80%) following Rab11b knockdown. These data indicate that the loss of ENaC current with Rab11b knockdown was likely the result of inability of the mpkCCD cells to deliver ENaC to the apical surface, and not due to changes in channel activity. The reduction in surface expression was also not due to a loss of whole-cell ENaC expression as evident from the quantification of whole cell levels presented in Fig. 8B.
Fig. 8.
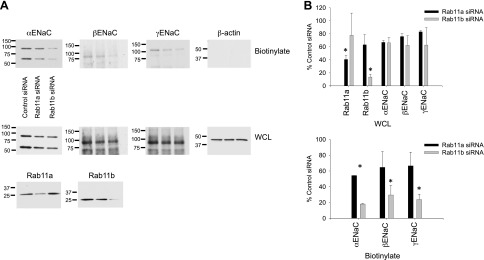
Surface biotinylation demonstrates reduced ENaC expression after Rab11b knockdown. A: surface biotinylation was performed on filter-cultured mpkCCD cells transfected with control, Rab11a, or Rab11b siRNA. Apical surface expression of the 3 ENaC subunits is presented in the top blots, with an actin control to demonstrate the specificity of the biotin labeling. Whole-cell lysate (WCL) blots are presented at the bottom to probe for whole cell levels of ENaC and Rab11. B: quantification of band intensities for n > = 2 experiments are presented for all proteins. *Significant reduction (P < 0.01) in expression compared with control siRNA cells.
DISCUSSION
Following clathrin- and epsin-dependent apical endocytosis, ENaC can be recycled constitutively or via a regulated pathway that is stimulated by mediators that increase cAMP production (8–10). The nature of these recycling pathways are unknown, but it is likely that ENaC recycling involves the passage through one or more endocytic compartments in a process facilitated by members of the Rab family of small GTPases. Among the specific Rabs that are localized to endocytic compartments, Rab11 is a key regulator of apical recycling pathways in polarized epithelial cells (13, 16, 38, 41, 62). It is known to facilitate the recycling of a number of receptors and transporters including IK channels, CFTR, Kv1.5, aquaporin 2, ClC-2, and ENaC (26, 38, 56, 62, 65). Our previous work identified delivery of ENaC into early endosomal compartments via clathrin-mediated endocytosis following its removal from the apical membrane (68). For ENaC to be returned to the apical surface following endocytosis, it most likely passes through several vesicle compartments in a process facilitated by members of the Rab family of small GTPases.
The current results, and our recent discovery of a role for Rab11b in CFTR recycling (62), indicate that Rab11b may have an important role in the regulation of epithelial channel recycling and surface expression. We confirmed the role for Rab11b in ENaC surface delivery by several investigations. First, we observed that there was a large degree of colocalization between ENaC and Rab11b in immunolocalization studies. Second, ENaC was found in immunoisolated Rab11b endosomes. Third, expression of DN-Rab11b dramatically inhibited ENaC activity, which capacitance measurements indicate results from a large decrease in vesicle exocytosis and a reduction in surface expression of ENaC. Fourth, downregulation of Rab11b expression by siRNA resulted in a dramatic loss of ENaC activity. Finally, surface biotinylation confirmed the loss of ENaC surface expression following a reduction in Rab11b levels. It is unclear why there was little loss of ENaC activity or surface expression when Rab11a expression was decreased by siRNA, as a previous study demonstrated a role for Rab11a in exocytosis of ENaC in CHO cells (38). Expression of the DN-Rab11a construct produced an intermediate reduction in ENaC current and CT compared with expression of DN-Rab11b.
The open question that remains, however, is why ENaC is found in both Rab11a and Rab11b endosomes. One possibility is that ENaC may sequentially pass through both Rab11a and Rab11b compartments at some point in its lifetime. Alternatively, there may be distinct pools of ENaC that are recycling through different populations of endosomes. An additional possibility is that the Rab11a- and Rab11b-localized channels have different fates. For example, ENaC in Rab11b endosomes may recycle relatively rapidly, whereas that in Rab11a endosomes may be undergoing slow recycling to the Golgi/trans-Golgi network, a process that is known to be regulated by Rab11a (44, 69). We have also not explored the possibility that the state of proteolytic cleavage may alter the fate of recycling ENaC.
In addition to Rab11, prior studies have linked Rab4 and Rab27 to ENaC regulation (3, 54, 58), demonstrating that the Rab GTPases are important mediators of ENaC trafficking and that trafficking plays a key role in determining ENaC density at the membrane surface. Our data further establish Rab11b as an essential component of the trafficking pathway regulating ENaC surface density in mpkCCD cells. Further investigation will be required to define the conditions under which the channel enters each Rab11-positive compartment, and the role of each in determining ENaC surface expression.
GRANTS
This work was supported by the following: National Institutes of Health (NIH) Grants K99DK078917 to M. B. Butterworth, R37DK54425 and R01DK077777 to G. Apodaca; DK057718 and DK047874 to J. P. Johnson, and DK54814 to R. A. Fizzell, and by Cystic Fibrosis Foundation Grant BUTTER06G0 to M. B. Butterworth. This work was also supported by the Pittsburgh Center for Kidney Research (P30DK079307).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.B.B., R.S.E., and M.R.S. provided conception and design of research; M.B.B., R.S.E., M.R.S., L.I.G., and X.L. performed experiments; M.B.B., R.S.E., L.I.G., X.L., and G.A. analyzed data; M.B.B., R.S.E., M.R.S., G.A., and R.A.F. interpreted results of experiments; M.B.B., L.I.G., and X.L. prepared figures; M.B.B. drafted manuscript; M.B.B., R.S.E., M.R.S., G.A., R.A.F., and J.P.J. edited and revised manuscript; M.B.B. and J.P.J. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of M. R. Silvis: Fred Hutchinson Cancer Research Center, Seattle, WA.
REFERENCES
- 1.Abriel H, Staub O. Ubiquitylation of ion channels. Physiology 20: 398–407, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Apodaca G, Katz LA, Mostov KE. Receptor-mediated transcytosis of IgA in MDCK cells is via apical recycling endosomes. J Cell Biol 125: 67–86, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagnasco SM. Role and regulation of urea transporters. Pflügers Arch 450: 217–226, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Barile M, Pisitkun T, Yu MJ, Chou CL, Verbalis MJ, Shen RF, Knepper MA. Large scale protein identification in intracellular aquaporin-2 vesicles from renal inner medullary collecting duct. Mol Cell Proteomics 4: 1095–1106, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertrand CA, Frizzell RA. The role of regulated CFTR trafficking in epithelial secretion. Am J Physiol Cell Physiol 285: C1–C18, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Bhalla V, Soundararajan R, Pao AC, Li H, Pearce D. Disinhibitory pathways for control of sodium transport: regulation of ENaC by SGK1 and GILZ. Am J Physiol Renal Physiol 291: F714–F721, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Blazer-Yost BL, Liu X, Helman SI. Hormonal regulation of ENaCs: insulin and aldosterone. Am J Physiol Cell Physiol 274: C1373–C1379, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Butterworth MB, Edinger RS, Frizzell RA, Johnson JP. Regulation of the epithelial sodium channel by membrane trafficking. Am J Physiol Renal Physiol 296: F10–F24, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butterworth MB, Edinger RS, Johnson JP, Frizzell RA. Acute ENaC stimulation by cAMP in a kidney cell line is mediated by exocytic insertion from a recycling channel pool. J Gen Physiol 125: 81–101, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butterworth MB, Edinger RS, Ovaa H, Burg D, Johnson JP, Frizzell RA. The deubiquitinating enzyme UCH-L3 regulates the apical membrane recycling of the epithelial sodium channel. J Biol Chem 282: 37885–37893, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Butterworth MB, Frizzell RA, Johnson JP, Peters KW, Edinger RS. PKA-dependent ENaC trafficking requires the SNARE-binding protein complexin. Am J Physiol Renal Physiol 289: F969–F977, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Butterworth MB, Helman SI, Els WJ. cAMP-sensitive endocytic trafficking in A6 epithelia. Am J Physiol Cell Physiol 280: C752–C762, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Casanova JE, Wang X, Kumar R, Bhartur SG, Navarre J, Woodrum JE, Altschuler Y, Ray GS, Goldenring JR. Association of rab25 and rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol Biol Cell 10: 47–61, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cayouette S, Boulay G. Intracellular trafficking of TRP channels. Cell Calcium 42: 225–232, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Chalfant ML, Coupaye-Gerard B, Kleyman TR. Distinct regulation of Na+ reabsorption and Cl− secretion by arginine vasopressin in the amphibian cell line A6. Am J Physiol Cell Physiol 264: C1480–C1488, 1993. [DOI] [PubMed] [Google Scholar]
- 16.Chen W, Feng Y, Chen D, Wandinger-Ness A. Rab11 is required for trans-Golgi network to plasma membrane transport and a preferential target for GDP dissociation inhibitor. Mol Biol Cell 9: 3241–3257, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clague MJ, Urbe S. Endocytosis: the DUB version. Trends Cell Biol 16: 551–559, 2006. [DOI] [PubMed] [Google Scholar]
- 18.D'Arrigo A, Bucci C, Toh BH, Stenmark H. Microtubules are involved in bafilomycin A1-induced tubulation and Rab5-dependent vacuolation of early endosomes. Eur J Cell Biol 72: 95–103, 1997. [PubMed] [Google Scholar]
- 19.Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Munster C, Chraibi A, Pratt JH, Horisberger JD, Pearce D, Loffing J, Staub O. Phosphorylation of Nedd4–2 by Sgk1 regulates epithelial Na+ channel cell surface expression. EMBO J 20: 7052–7059, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eathiraj S, Mishra A, Prekeris R, Lambright DG. Structural basis for Rab11-mediated recruitment of FIP3 to recycling endosomes. J Mol Biol 364: 121–135, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Ecelbarger CA, Kim GH, Terris J, Masilamani S, Mitchell C, Reyes I, Verbalis JG, Knepper MA. Vasopressin-mediated regulation of epithelial sodium channel abundance in rat kidney. Am J Physiol Renal Physiol 279: F46–F53, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Edinger RS, Rokaw MD, Johnson JP. Vasopressin stimulates sodium transport in A6 cells via a phosphatidylinositide 3-kinase-dependent pathway. Am J Physiol Renal Physiol 277: F575–F579, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Fakitsas P, Adam G, Daidie D, van Bemmelen MX, Fouladkou F, Patrignani A, Wagner U, Warth R, Camargo SM, Staub O, Verrey F. Early aldosterone-induced gene product regulates the epithelial sodium channel by deubiquitylation. J Am Soc Nephrol 18: 1084–1092, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Flores SY, Debonneville C, Staub O. The role of Nedd4/Nedd4-like dependant ubiquitylation in epithelial transport processes. Pflügers Arch 446: 334–338, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Fukuda M. Versatile role of Rab27 in membrane trafficking: focus on the Rab27 effector families. J Biochem 137: 9–16, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Fukuda M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol Life Sci 65: 2801–2813, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Phys Rev 77: 359–396, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Gormley K, Dong Y, Sagnella GA. Regulation of the epithelial sodium channel by accessory proteins. Biochem J 371: 1–14, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA 103: 11821–11827, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill WG, Butterworth MB, Wang H, Edinger RS, Lebowitz J, Peters KW, Frizzell RA, Johnson JP. The epithelial sodium channel (ENaC) traffics to apical membrane in lipid rafts in mouse cortical collecting duct cells. J Biol Chem 282: 37402–37411, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Hughey RP, Mueller GM, Bruns JB, Kinlough CL, Poland PA, Harkleroad KL, Carattino MD, Kleyman TR. Maturation of the epithelial Na+ channel involves proteolytic processing of the α- and γ-subunits. J Biol Chem 278: 37073–37082, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, Rossier BC. Early death due to defective neonatal lung liquid clearance in a-ENaC-deficient mice. Nature Genet 12: 325–328, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Hummler E, Horisberger JD. Genetic disorders of membrane transport. V. The epithelial sodium channel and its implication in human diseases. Am J Physiol Gastrointest Liver Physiol 276: G567–G571, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Itani OA, Stokes JB, Thomas CP. Nedd4–2 isoforms differentially associate with ENaC and regulate its activity. Am J Physiol Renal Physiol 289: F334–F346, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Jing J, Prekeris R. Polarized endocytic transport: the roles of Rab11 and Rab11-FIPs in regulating cell polarity. Histol Histopathol 24: 1171–1180, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jing J, Tarbutton E, Wilson G, Prekeris R. Rab11-FIP3 is a Rab11-binding protein that regulates breast cancer cell motility by modulating the actin cytoskeleton. Eur J Cell Biol 88: 325–341, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kabra R, Knight KK, Zhou R, Snyder PM. Nedd4–2 induces endocytosis and degradation of proteolytically cleaved epithelial Na+ channels. J Biol Chem 283: 6033–6039, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Karpushev AV, Levchenko V, Pavlov TS, Lam V, Vinnakota KC, Vandewalle A, Wakatsuki T, Staruschenko A. Regulation of ENaC expression at the cell surface by Rab11. Biochem Biophys Res Commun 377: 521–525, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kleyman TR, Ernst SA, Coupaye-Gerard B. Arginine vasopressin and forskolin regulate apical cell surface expression of epithelial Na+ channels in A6 cells. Am J Physiol Renal Fluid Electrolyte Physiol 266: F506–F511, 1994. [DOI] [PubMed] [Google Scholar]
- 40.Lai F, Stubbs L, Artzt K. Molecular analysis of mouse Rab11b: a new type of mammalian YPT/Rab protein. Genomics 22: 610–616, 1994. [DOI] [PubMed] [Google Scholar]
- 41.Lapierre LA, Dorn MC, Zimmerman CF, Navarre J, Burnette JO, Goldenring JR. Rab11b resides in a vesicular compartment distinct from Rab11a in parietal cells and other epithelial cells. Exp Cell Res 290: 322–331, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Lu C, Pribanic S, Debonneville A, Jiang C, Rotin D. The PY motif of ENaC, mutated in Liddle syndrome, regulates channel internalization, sorting and mobilization from subapical pool. Traffic 8: 1246–1264, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med 10: 487–493, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Mallet WG, Maxfield FR. Chimeric forms of furin and TGN38 are transported with the plasma membrane in the trans-Golgi network via distinct endosomal pathways. J Cell Biol 146: 345–359, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matalon S, Lazrak A, Jain L, Eaton DC. Invited review: biophysical properties of sodium channels in lung alveolar epithelial cells. J Appl Physiol 93: 1852–1859, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Peranen J, Auvinen P, Virta H, Wepf R, Simons K. Rab8 promotes polarized membrane transport through reorganization of actin and microtubules in fibroblasts. J Cell Biol 135: 153–167, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pfeffer S, Aivazian D. Targeting Rab GTPases to distinct membrane compartments. Nat Rev Mol Cell Biol 5: 886–896, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Pochynyuk O, Tong Q, Staruschenko A, Ma HP, Stockand JD. Regulation of the epithelial Na+ channel (ENaC) by phosphatidylinositides. Am J Physiol Renal Physiol 290: F949–F957, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Pochynyuk O, Stockand JD, Staruschenko A. Ion channel regulation by Ras, Rho, and Rab small GTPases. Exp Biol Med 232: 1258–1265, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Prekeris R. Rabs, Rips, FIPs, and endocytic membrane traffic. ScientificWorldJournal 3: 870–880, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossier BC, Pradervand S, Schild L, Hummler E. Epithelial sodium channel and the control of sodium balance: interaction between genetic and environmental factors. Annu Rev Physiol 64: 877–897, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Rotin D. Regulation of the epithelial sodium channel (ENaC) by accessory proteins. Curr Opin Nephrol Hypertens 9: 529–534, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Saxena S, Singh M, Engisch K, Fukuda M, Kaur S. Rab proteins regulate epithelial sodium channel activity in colonic epithelial HT-29 cells. Biochem Biophys Res Commun 337: 1219–1223, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Saxena SK, Horiuchi H, Fukuda M. Rab27a regulates epithelial sodium channel (ENaC) activity through synaptotagmin-like protein (SLP-5) and Munc13–4 effector mechanism. Biochem Biophys Res Commun 344: 651–657, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Saxena SK, Kaur S. Rab27a negatively regulates CFTR chloride channel function in colonic epithelia: involvement of the effector proteins in the regulatory mechanism. Biochem Biophys Res Commun 346: 259–267, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Saxena SK, Kaur S. Regulation of epithelial ion channels by Rab GTPases. Biochem Biophys Res Commun 351: 582–587, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Saxena SK, Kaur S, George C. Rab4GTPase modulates CFTR function by impairing channel expression at plasma membrane. Biochem Biophys Res Commun 341: 184–191, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Saxena SK, Singh M, Shibata H, Kaur S, George C. Rab4 GTP/GDP modulates amiloride-sensitive sodium channel (ENaC) function in colonic epithelia. Biochem Biophys Res Commun 340: 726–733, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Schonteich E, Wilson GM, Burden J, Hopkins CR, Anderson K, Goldenring JR, Prekeris R. The Rip11/Rab11-FIP5 and kinesin II complex regulates endocytic protein recycling. J Cell Sci 121: 3824–3833, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seachrist JL, Ferguson SS. Regulation of G protein-coupled receptor endocytosis and trafficking by Rab GTPases. Life Sci 74: 225–235, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Segev N. Ypt/Rab GTPases: regulators of protein trafficking. Sci STKE 2001: re11, 2001. [DOI] [PubMed] [Google Scholar]
- 62.Silvis MR, Bertrand CA, Ameen N, Golin-Bisello F, Butterworth MB, Frizzell RA, Bradbury NA. Rab11b regulates the apical recycling of the cystic fibrosis transmembrane conductance regulator in polarized intestinal epithelial cells. Mol Biol Cell 20: 2337–2350, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Snyder PM. The epithelial Na+ channel: cell surface insertion and retrieval in Na+ homeostasis and hypertension. Endocr Rev 23: 258–275, 2002. [DOI] [PubMed] [Google Scholar]
- 64.Staruschenko A, Adams E, Booth RE, Stockand JD. Epithelial Na+ channel subunit stoichiometry. Biophys J 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swiatecka-Urban A, Talebian L, Kanno E, Moreau-Marquis S, Coutermarsh B, Hansen K, Karlson KH, Barnaby R, Cheney RE, Langford GM, Fukuda M, Stanton BA. Myosin Vb is required for trafficking of the cystic fibrosis transmembrane conductance regulator in Rab11a-specific apical recycling endosomes in polarized human airway epithelial cells. J Biol Chem 282: 23725–23736, 2007. [DOI] [PubMed] [Google Scholar]
- 66.Verrey F, Groscurth P, Bolliger U. Cytoskeletal disruption in A6 kidney cells: impact on endo/exocytosis and NaCl transport regulation by antidiuretic hormone. J Membr Biol 145: 193–204, 1995. [DOI] [PubMed] [Google Scholar]
- 67.Vinciguerra M, Deschenes G, Hasler U, Mordasini D, Rousselot M, Doucet A, Vandewalle A, Martin PY, Feraille E. Intracellular Na+ controls cell surface expression of Na,K-ATPase via a cAMP-independent PKA pathway in mammalian kidney collecting duct cells. Mol Biol Cell 14: 2677–2688, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang H, Traub LM, Weixel KM, Hawryluk MJ, Shah N, Edinger RS, Perry CJ, Kester L, Butterworth MB, Peters KW, Kleyman TR, Frizzell RA, Johnson JP. Clathrin-mediated endocytosis of the epithelial sodium channel. Role of epsin. J Biol Chem 281: 14129–14135, 2006. [DOI] [PubMed] [Google Scholar]
- 69.Wilcke M, Johannes L, Galli T, Mayau V, Goud B, Salamero J. Rab11 regulates the compartmentalization of early endosomes required for efficient transport from early endosomes to the trans-Golgi network. J Cell Biol 151: 1207–1220, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yudowski GA, Puthenveedu MA, Henry AG, von ZM. Cargo-mediated regulation of a rapid Rab4-dependent recycling pathway. Mol Biol Cell 20: 2774–2784, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zacchi P, Murphy C, Mellman I, Zerial M. A role for rab17 in polarised membrane traffic. Mol Biol Cell Suppl 7: 591a, 1996. [Google Scholar]
- 72.Zhou R, Kabra R, Olson DR, Piper RC, Snyder PM. Hrs controls sorting of the epithelial Na+ channel between endosomal degradation and recycling pathways. J Biol Chem 285: 30523–30530, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]