Summary
Fragile X syndrome (FXS) is a rare inherited genetic disorder causing severe intellectual disability and autistic-like symptoms. Individuals with FXS, males in particular, often exhibit extreme eye gaze avoidance and hyperarousal when they encounter stressful social situations. We investigated whether oxytocin (OT), a hormone with prosocial and anxiolytic effects, could alleviate symptoms of social anxiety in this population. A randomized double-blind placebo-controlled single-dose trial was performed with intranasal administration of placebo, 24 IU OT and 48 IU OT. Measures of eye gaze frequency, heart rate, respiratory sinus arrhythmia (RSA), heart rate variability (HRV) and salivary cortisol were obtained during a structured social challenge conducted 50 min following OT administration. Ten low-functioning males with FXS (aged 13–28 years) traveled to Stanford for the initial visit: 8 completed the study. Eye gaze frequency improved significantly in response to the 24 IU OT dose and salivary cortisol levels decreased significantly in response to the 48 IU OT dose. There was no effect of OTon heart rate, RSA or HRV although individual plots of the heart rate data suggested that OT increased heart rate in some participants and decreased heart rate in others. These findings suggest that intranasal administration of OT may ameliorate some symptoms of social anxiety in patients with FXS. Further double-blind placebo-controlled studies of OT, conducted in combination with behavioral treatment programs, may be warranted.
Keywords: Fragile X syndrome, Oxytocin, Social anxiety, Cortisol, Eye gaze, Cardiac activity
1. Introduction
Fragile X syndrome (FXS) is a rare inherited genetic disorder occurring in approximately 1 in every 3–4000 live births (Crawford et al., 1999; Turner et al., 1996). The syndrome arises from disruption in expression of the fragile X mental retardation gene 1 (FMR1) caused by amplification of a CGG repeat in the 5′ untranslated region (Verkerk et al., 1991). In normal alleles, a CGG repeat within FMR1 varies from 6 to 50. Expansions of ~50–200 repeats are associated with the “premutation” form of FXS whereas larger expansions (200 to thousands) are considered “full mutations” and are typically associated with excessive methylation of cytosines in the FMR1 promoter. This modification extinguishes transcription of the FMR1 gene into mRNA, stopping translation of the fragile X mental retardation protein (FMRP). FMRP is a messenger RNA-binding brain protein involved in the maturation and elimination of synapses during typical development (O’Donnell and Warren, 2002). Increased FMRP is also observed in association with new learning and response to varying environmental conditions (Irwin et al., 2005). Thus, reduced FMRP in individuals with the full FMR1 mutation significantly increases risk for both long-term neurodevelopmental and real-time neurofunctional abnormalities. To date, FXS is the most common known form of inherited intellectual disability.
Studies from our laboratory and others indicate that the most common problem behaviors observed in FXS consist of hyperarousal, disturbance in language/communication, and social anxiety (Reiss and Hall, 2007). In males, problematic behaviors often take the form of social deficits with peers, social avoidance, gaze aversion, qualitative abnormalities in communication, unusual responses to sensory stimuli, stereotypic behavior, inattention, impulsivity and hyperactivity. FMRP expression (as quantified by immunocytochemistry) has been linked to many of these phenotypic characteristics of FXS, including social withdrawal, anxiety and depression as well as to quantitative measures of brain development and function (Lightbody and Reiss, 2009).
Individuals with FXS, particularly males, often have abnormally strong physiological and behavioral responses to social stimuli, associated with increased levels of arousal and stress reactivity. For example, Miller et al. (1999) used a laboratory paradigm to study electrodermal responses to auditory, visual, touch, vestibular, and olfactory stimuli to assess sympathetic nervous system activity in children and adults with fragile X syndrome. In this study, increased electrodermal response (EDR) to stimulation and lower rates of habituation to stimulation were found in FXS as compared to age and gender matched control subjects. Other investigators studying spectral analysis of heart beat intervals have found that boys with FXS have increased heart rate and lower parasympathetic activity during experimental challenge (Boccia and Roberts, 2000; Hall et al., 2009a). For example, in the study by Hall et al. (2009a), males with FXS (8–20 years of age) had higher heart rate, lower amplitude respiratory sinus arrhythmia (RSA) and lower heart rate variability during both a baseline and social interaction, relative to their typically developing siblings. In another study from our group, we reported that children with FXS, especially males, had higher levels of salivary cortisol compared to their non-FXS siblings. Increased cortisol was significantly associated with behavior problems in boys and girls with FXS but not in their unaffected siblings (Hessl et al., 2002). The finding of abnormal cortisol levels in individuals with FXS is complemented by the discovery that FMRP is involved in regulating the glucocorticoid receptor in the hippocampus (Brown et al., 2001).
There is currently no cure for FXS though initial efforts are now being made to intervene at the level of downstream systems altered by reduced levels of FMRP. Examples of such interventions include the use of agents to reduce metabotropic glutamate activity (Bear et al., 2004; Berry-Kravis et al., 2009; Garber et al., 2006) or to re-regulate the cholinergic system (Kesler et al., 2009). Less specific psychotherapeutic and pharmacological interventions targeting specific behaviors are also often used in the clinical setting for affected individuals (Berry-Kravis and Potanos, 2004). However, studies conducted to date have reported few significant positive effects for these approaches, particularly with respect to increasing appropriate social behavior in individuals with FXS (Hall, 2009).
A potentially promising pharmacotherapy for patients with FXS is the neuropeptide oxytocin (OT), which has a variety of prosocial, antistress, and anxiolytic properties. Briefly, OT is synthesized in the hypothalamus and released into systemic circulation via the posterior pituitary. OT is also released as a neurotransmitter into the central nervous system (CNS), and OT receptors are found in a variety of socially relevant and stress-sensitive neuroanatomical regions. Centrally administered OT facilitates contact-seeking, attachment bond formation, and social memory in rodents (Ferguson et al., 2000; Williams et al., 1992; Witt et al., 1992). Centrally administered OT also diminishes stress-induced adrenocorticotropic hormone (ACTH) and cor-ticosterone release, and inhibits corticotropin releasing factor (CRF) mRNA expression in the hypothalamus during restraint stress in rats (Windle et al., 2004). Similarly, female mice lacking the oxytocin gene exhibit enhanced corticosteroid responses to psychogenic stressors and enhanced anxiety on the elevated plus-maze compared to wildtype controls (Amico et al., 2004; Mantella et al., 2003).
Recently, several investigators have reported that administration of OT can improve social functioning in patients diagnosed with autism. For example, OT administration has been shown to improve social decision-making (Andari et al., 2010; Hollander et al., 2007), reduce repetitive behaviors (Hollander et al., 2003), increase emotion recognition (Guastella et al., 2010), and promote visual scanning of faces (Andari et al., 2010) in youth and adult patients with high functioning autism and Asperger’s syndrome. When administered intranasally, OT also attenuates the neuroendocrine stress response in rodents and monkeys and alters emotion regulation in humans (Heinrichs et al., 2003; Parker et al., 2005; Windle et al., 2004). Effects of intranasal OT have also been demonstrated with functional neuroimaging (Kirsch et al., 2005) as well as with standardized testing, self-report or expert ratings (Kosfeld et al., 2005). CNS penetration following intranasal administration occurs within minutes and delivery to the brain is thought to occur via an extracellular pathway (e.g., patent intercellular clefts in the olfactory epithelium) (Balin et al., 1986), indicating that the observed behavioral effects of intranasally administered OTresult from altered neural function. However, it should be pointed out that while vasopressin has been detected in cerebrospinal fluid (CSF) and plasma within 30 min of intranasal administration (Born et al., 1998, 2002), similar work has not yet been published with intranasally administered OT in humans.
The extant literature strongly suggests that intranasally administered OT is safe and associated with very few adverse effects. Thus, intranasally administered OT is a promising medication to utilize in a clinical trial designed to improve social functioning in persons with FXS. The purpose of the present study was to determine whether administration of intranasal OT could improve socially appropriate behaviors and reduce concomitant social anxiety in males with FXS. Specifically, we hypothesized that individuals with FXS would show significant gains in eye contact frequency, reduced physiological arousal and reduced salivary cortisol during OT administration compared to placebo.
2. Materials and methods
2.1. Subjects
All male adolescent and adult subjects aged 13–28 years with a confirmed genetic diagnosis of FXS (fully methylated full mutation as shown by standard Southern blot analysis) who lived within a 100-mile radius of Stanford University were eligible for inclusion in the study. Exclusion criteria for the study included cardiovascular disease, bradycardia (<60 beats/min) and individuals taking opiates or opiate antagonists, corticosteroids, or typical/atypical antipsychotics. In order to avoid the confounding factors present in other medication studies of FXS, we also attempted to exclude individuals who were taking any other psychoactive medication (e.g., stimulants, antidepressants, anxiolytics). However, the majority of parents we contacted (~25) were unwilling to discontinue their child’s medications. Beginning 2007 and ending in 2009, we were able to enroll ten participants into the study. One subject was excluded from the study because he was taking an antipsychotic medication during the first visit and one subject refused to travel to Stanford following his initial visit and was therefore lost to follow-up. Eight subjects were therefore included in the present study.1 The mean age of the remaining participants was 21.3 years (SD = 5.1), range 13–28 years. All participants obtained an Adaptive Level classification of “Low” on the Vineland-II Adaptive Behavior Scales (Sparrow et al., 2005) with Adaptive Behavior Composite standard scores ranging from 31 to 58 (mean = 43.0, SD = 12.2). Given that Vineland-II standard scores have a population mean of 100 and an SD of 15, the participants in this study were therefore functioning approximately 3–5 SD’s below the norm. All procedures were approved by the local IRB at Stanford University and parental consent and subject assent were obtained in all cases.
2.2. Dosing plan
Each subject was required to visit the lab on three separate occasions, with each visit being spaced 7 days apart. During each visit, subjects were scheduled to receive either placebo, 24 IU Syntocinon® (Novartis, Basel, Switzerland) or 48 IU Syntocinon®. Similar doses of OT have been administered to subjects in previous studies of social functioning (Andari et al., 2010; Feifel et al., 2010; Guastella et al., 2008; Hollander et al., 2007; Ditzen et al., 2009). In order to avoid any subjective substance effects (e.g., olfactory effects) other than those caused by OT, the placebo solution was compounded by a local pharmacy and contained all inactive ingredients except for the neuropeptide. Each dose was administered via two identical spray bottles that were made up by a Stanford pharmacist who also kept the randomization and blinding scheme. On scheduled placebo days, the pharmacist filled both bottles with placebo solution; on scheduled 24 IU days, the pharmacist filled one bottle with placebo and the other bottle with Syntocinon® (4 IU per puff); on scheduled 48 IU days, the pharmacist filled both bottles with Syntocinon® (4 IU per puff). During each visit, at approximately 2–3 pm, subjects received 6 puffs from one bottle (3 puffs per nostril) and 6 puffs (3 puffs per nostril) from the other bottle. Given that the total number of puffs (i.e., 12) was always the same, and not related to dosing, both the subjects and the investigators were therefore blind as to which compound the patient received on a given visit. Subjects received all of the treatments in random order over the 3 visits. The randomization scheme was generated using an online randomization plan generator (http://www.randomization.com) with an estimated sample size of 12. However, given that we were only able to include 8 participants in the data analysis, we confirmed post-study that the participants had been randomized and counterbalanced. On the first visit, participants P4 and P8 received placebo, participants P2, P5 and P7 received 24 IU OT and participants P1, P3 and P6 received 48 IU OT. On the final visit, participants P1, P2 and P6 received placebo, participants P3 and P8 received 24 IU OTand participants P4, P5 and P7 received 48 IU OT. Subjects were therefore successfully randomized and counterbalanced to the conditions. On the day after the final visit, all subjects were subsequently enrolled in a two-week open-label trial of 48 IU OT. The data from the open-label trial will be presented in a separate report.
2.3. Measures
2.3.1. Safety data
During the first visit to the lab, the study physician took vital signs and conducted a brief physical examination of each subject to ensure safety for subsequent dosing. Side effects as a result of dosing, if any, were carefully recorded at each visit by all research investigators who met with the subject. Research physicians monitored each subject during the course of the protocol and recommended appropriate action for adverse re’actions.
2.3.2. Social challenge
During each visit to the lab, a videotaped social challenge lasting 10 min was administered to each subject 50 min following intranasal administration of OT or placebo. For the first 5 min (social proximity), subjects were required to sit quietly in a chair while a female experimenter (AAL), who sat directly opposite the subject with knees almost touching, read a magazine or book. For the second 5-min period (social interaction), the experimenter remained seated and said, “OK, now we are going to have a conversation and I need you to look me in the eyes as much as possible while we are talking”. The experimenter then looked directly at the subject and began asking a series of questions such as “tell me what movies you like to watch”, allowing sufficient gaps of time between each question for the subject to respond. The experimenter also delivered eye gaze prompts (i.e., “remember to look me in the eyes”) every 30-s. The video camera was positioned on a tripod approximately 3 m directly behind the experimenter and 1 m above the experimenter’s head so that the participant’s face could be viewed. A camera operator, who was also present in the room, monitored the experiment and focused the camera so that the participant’s head and shoulders filled the view-screen. The camera operator did not interact with the subject at any time and remained out of the participant’s line of sight throughout the experiment. To measure potential cardiovascular effects, subjects were required to wear a plastic chest belt and a heart-rate monitoring device (Mini-Logger 2000) that was attached to the subject’s belt-loop (see Hall et al., 2009a). A similar social challenge procedure has been conducted in previous studies with individuals with FXS (Hall et al., 2006, 2009a). There did not appear to be any order or practice effects with this paradigm and the same tester.
2.3.3. Salivary cortisol
To evaluate potential alterations in stress responses following OT administration, salivary cortisol samples were collected immediately prior to the social challenge administration, and 20 min following the end of the social challenge. All sample collection and handling procedures were developed in consultation with the fourth author (KJP) to minimize the potential impact of contaminants, and confounding dietary and physiological factors on the assessment (Hessl et al., 2002).
At each sample point, saliva samples were collected and transported using a “Salivette” device (Sarstedt; Newton, NC) that was composed of a cotton roll and two plastic tubes that fit one inside the other. For each sample, subjects were instructed to place the cotton roll in their mouth, and think of their favorite food until the cotton was saturated (~2 min). They were instructed thereafter to replace the cotton roll in the Salivette device. Sample collection occurred between 2 and 4 pm for all subjects to control for circadian variation in cortisol levels (Dorn et al., 2007; Ranjit et al., 2005).
2.3.4. Data analysis
The videotapes for each social challenge was transferred to DVD and subsequently reviewed by the third author (BEM) (who was also blinded). Participant eye gaze was scored in continuous time using a simple paper and pencil recording method. Specifically, whenever the participant moved his eyes to look directly at the experimenter’s face, the observer placed a tally mark (without looking down) onto a recording sheet. The dependent variable was the number of times that eye gaze occurred during the social proximity and social interaction phases. All heart period data obtained during the social challenge were downloaded from the Mini-Logger 2000 device to a PC using Mini-Log 2000W Version 1.2 (Mini-Mitter Co., Inc., 2001). The data were then analyzed using MXedit Version 2.21 (Delta-Biometrics Inc. 1994). The inter-beat interval (IBI) data were first inspected to remove artifacts and to correct errors. In addition to calculating heart rate (beats per minute), MXedit also provides estimates of RSA and heart rate variability (HRV). The software automatically calculates these estimates by (a) converting the heart periods to constant sampling rates by resampling the data at 500-ms intervals, (b) detrending the resampled data with a 21-point moving cubic polynomial, (c) processing the detrended data with a digital band-pass filter (0.12 Hz for the low limit of the bandpass, 0.40 Hz for the high limit of the bandpass), and (d) computing the natural logarithm of the band-passed variance (Byrne, 1993). The dependent variables were the subject’s heart rate, RSA and HRV during the social proximity and social interaction phases. To assess potential reactivity or recovery effects to the social challenge, we also examined change scores for each of these measures.
Saliva samples were placed in a refrigerator and then transported to the laboratory within 2 days of collection. Upon receipt in the laboratory, saliva was separated from the cotton wad by centrifugation at 3000 RPM at 25 °C for 10 min and stored at −80 °C until quantification. Salivary cortisol was measured in duplicate using a commercially prepared radioimmunoassay (Coat-a-Count, Siemens; Los Angeles, CA). Samples were assayed using 200 μl of saliva per tube following manufacturer’s instructions. All samples from a particular subject were analyzed in the same assay run. Intra- and inter-assay coefficients of variation were under 10%, and assay sensitivity was 0.215 nmol/L (0.008 μg/dl). Correlations between duplicate samples within each assay were typically greater than 95%. The dependent variable was cortisol concentration measured prior to and following the social challenge. To assess potential reactivity or recovery effects to the social challenge, we also examined change scores for this measure.
For each dependent variable, we conducted a repeated measures analysis of variance (ANOVA) to evaluate treatment response using SPSS version 18.0 (SPSS Inc., Chicago, IL). Given that there are known developmental changes in cortisol (Kiess et al., 1995) and cardiac activity (Silvetti et al., 2001) across the age range of our subjects (13–28 years), we included age as a covariate in the cortisol and cardiac activity analyses. Age and Vineland-II score were included as covariates in the eye gaze analysis. All post hoc analyses were conducted using Tukey’s LSD where appropriate. The p value for significance was set at 0.05.
3. Results
No adverse events were reported during the study. However, participant P7 developed Bell’s Palsy on one side of his face a few days following his first visit to the lab, and his second visit was therefore delayed by two months as a precautionary measure. All other participants were seen at the planned weekly intervals at the same time each day (2–3 pm).
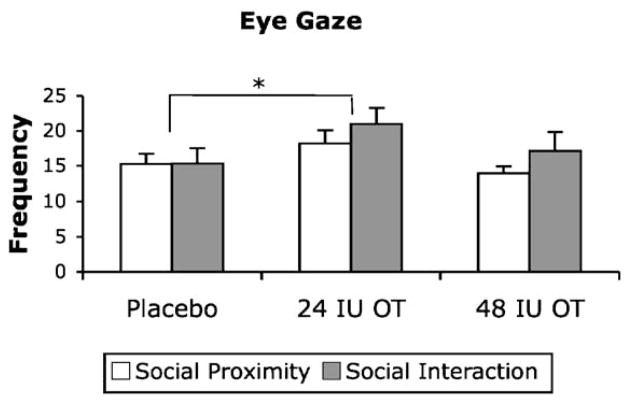
Fig. 1 shows the mean frequency of participant eye gaze observed in the social proximity and social interaction phases of the social challenge following administration of placebo, 24 IU OT and 48 IU OT.
Figure 1.
Eye gaze frequency observed in the social proximity and social interaction phases of the social challenge at each dose. Data are expressed as mean + SEM, n = 8 per group. There was a significant main effect of dose, with eye gaze frequency occurring at higher levels following the 24 IU dose compared to placebo. There was no difference in eye gaze frequency between the social proximity and social interaction phases. *p < 0.05 (ANOVA + LSD test).
A repeated measures ANOVA indicated that there was a significant main effect of OT dose on eye gaze frequency [F(2,10) = 4.82, p = 0.034, partial η2 = .49; Fig. 1]. Post hoc analysis using Tukey’s LSD indicated that eye gaze frequency increased significantly following the 24 IU dose compared to placebo (p = .042). There was no difference in eye gaze frequency between the social proximity and social interaction phases of the social challenge. Analysis of change scores between proximity and interaction phases of the social challenge indicated that there was no effect of OT dose on eye gaze reactivity.
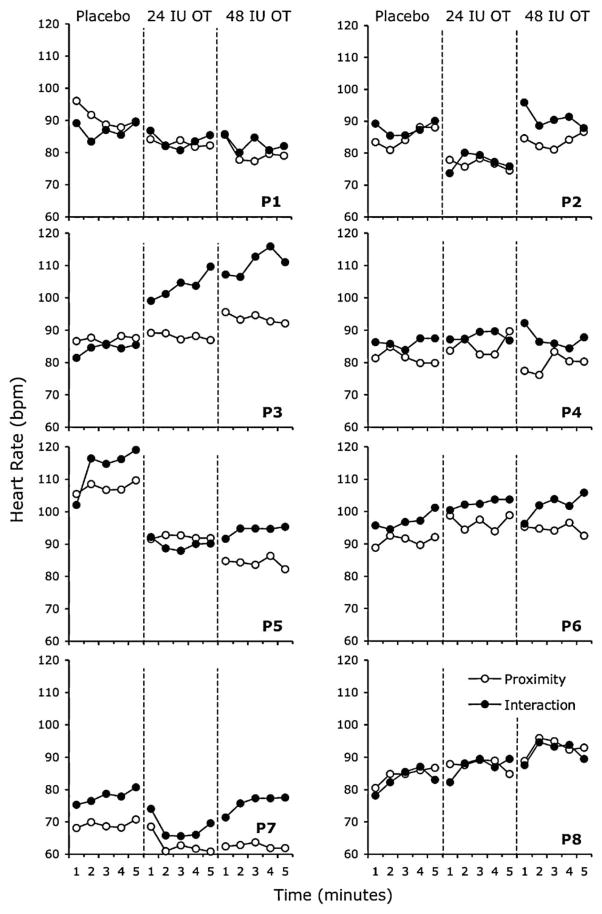
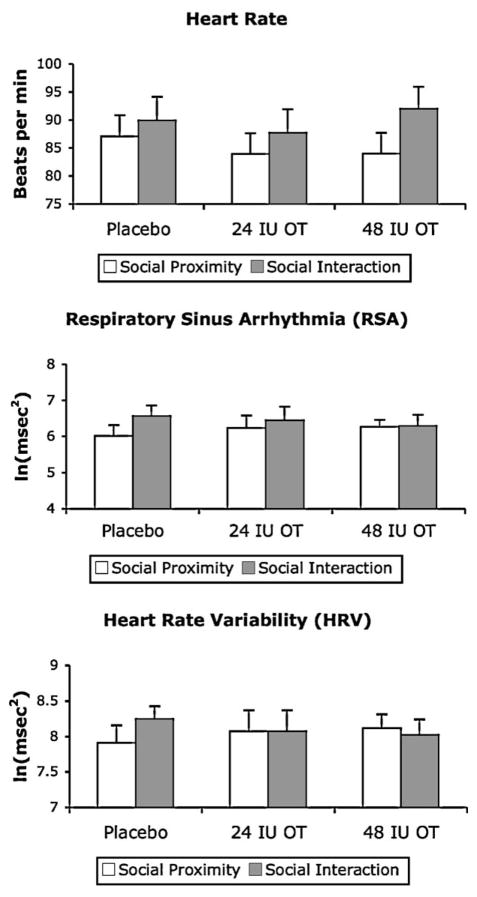
Fig. 2 shows individual plots of heart rate levels obtained for each participant in each phase of the social challenge at each OT dose. Visual inspection of the data suggests that the effect of OT on participant heart rate levels was somewhat variable across participants. For some participants (e.g., P1, P2, P5 and P7), administration of either the 24 IU or 48 IU OT dose appeared to result in decreased heart rates, whereas for other participants (e.g., P3, P6 and P8), OT administration appeared to result in increased heart rates. Fig. 3 (upper panel) shows the mean heart rate levels calculated across participants in each phase of the social challenge at each dose. The corresponding data for RSA (middle panel) and HRV (lower panel) levels are also shown.
Figure 2.
Heart rate levels (beats per minute) recorded for each participant (P). Data are plotted in 1 min bins in the social proximity and social interaction phases of the social challenge at each dose. Open circles represent heart rate levels in the social proximity phase. Filled circles represent heart rate levels in the social interaction phase.
Figure 3.
Mean heart rate, respiratory sinus arrhythmia (RSA) and heart rate variability (HRV) levels recorded at each dose, plotted in the social proximity and social interaction phases of the social challenge. Data are expressed as mean + SEM, n = 8 per group.
A repeated measures ANOVA, conducted for each dependent variable with age as a covariate, indicated that there was no effect of OT dose on heart rate, RSA, or HRV. Analysis of change scores also indicated that there was no effect of OT dose on reactivity to social challenge for the heart rate, RSA, or HRV levels.
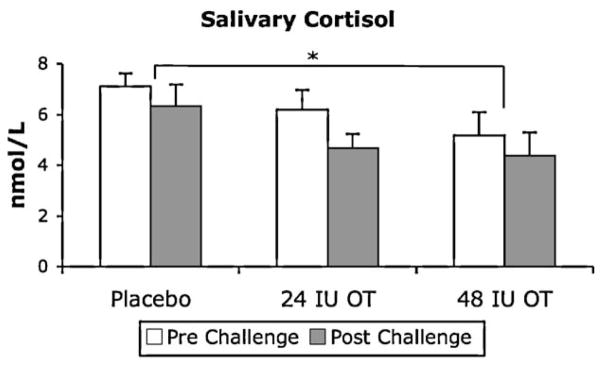
Fig. 4 shows mean salivary cortisol levels plotted prior to and following the social challenge following intranasal administration of placebo, 24 IU OT and 48 IU OT.
Figure 4.
Salivary cortisol levels obtained at each dose, plotted prior to and following the social challenge. Data are expressed as mean + SEM, n = 8 per group. There was a significant main effect of dose, with salivary cortisol occurring at lower levels following the 48 IU dose compared to placebo. There was no difference in salivary cortisol levels prior to and following the social challenge *p < 0.05 (ANOVA + LSD test).
A repeated measures ANOVA, conducted with age as a covariate, indicated that there was a significant main effect of OT dose on salivary cortisol levels [F(2, 12) = 6.78, p = 0.011, partial η2 = .53; Fig. 4]. Post hoc analysis using Tukey’s LSD showed that salivary cortisol levels decreased significantly in response to the 48 IU dose compared to placebo (p = 0.05). There were no differences in salivary cortisol levels prior to and following the social challenge. Analysis of change scores indicated that there was no effect of OT dose on cortisol reactivity to social challenge.
4. Discussion
To date, no pharmacotherapies have been shown to alleviate the debilitating behavioral features of FXS. These features include extreme social anxiety and hyperarousal that occur during social situations, behaviors that are particularly prominent in males. Results from this randomized double-blind single-dose study showed that eye gaze frequency improved following 24 IU administration of OT and that salivary cortisol levels decreased following 48 IU administration of OT. Safety data also showed that OT can be safely administered intranasally and is well tolerated by adolescent and adult male patients with FXS. These data suggest that OT may have beneficial effects on social functioning in patients with FXS.
While we found that cortisol levels decreased following OT administration at the 48 IU dose, we did not find any concomitant changes in heart rate levels, RSA or HRV in patients with FXS. Interestingly, in a recent study involving healthy college-age students, Norman et al. (2011) found that HRV increased significantly following intranasal administration of 20 IU OT, whereas heart rate levels did not change. In the Norman et al. study however, participants were required to read “non-arousing” material following OTadministration rather than engage in a social challenge. It is possible that the different study conditions and populations may have accounted for the difference in findings between the studies. In a previous study that included a social challenge, we found that heart rate levels in males with FXS, aged 8–20 years, averaged approximately 90 bpm at baseline, whereas heart rate levels in their same-gender typically developing siblings averaged approximately 75 bpm (Hall et al., 2009a). In the present study, we found mean heart rate levels in our patients to be approximately 85–90 bpm during social challenge, comparable to our previous report. While we did not include a comparison group of individuals in this study, these data suggest that individuals with FXS have significantly elevated heart rate levels, irrespective of whether or not they are undergoing a social challenge. It is therefore unlikely that we would be able to see changes in heart rate levels or HRV following OT administration simply because our participants were already experiencing chronically high levels of arousal.
Intranasal OT administration in FXS may work mechanistically by dampening amygdala reactivity toward socially threatening and anxiety-provoking stimuli (Kirsch et al., 2005; Petrovic et al., 2008), decreasing HPA axis activation, and increasing social motivation (Witt et al., 1992). Whether or not these presumed deficits in OT biology are directly linked to reduced FMRP in FXS, or whether OT biology is primarily altered as a consequence of the abnormal social development that characterizes FXS, remains unknown. Although the available data do not help differentiate between these two possibilities, several lines of investigation point to dysregulation of the OT system in FXS: (1) mRNAs encoding both FMRP and OT precursor are localized in the dendritic domains of mammalian CNS neurons (Smith, 2004), (2) OT immunoreactive cell numbers are significantly reduced in the brains of male FMR1-knockout mice (Carter, 2008), (3) FMRP and OTare both involved in regulation of the HPA axis (Hessl et al., 2004; Parker et al., 2005), and (4) the non-coding BC1 mRNA transcript and OT occur in axonal terminals of hypothalamo-neurohypophyseal neurons (Tiedge et al., 1993) (BC1 as an important link between FMRP and translational regulation of specific target mRNAs (Steward and Schuman, 2003)). Further studies are clearly needed to carefully elucidate and causally determine the complex links between OT, FMRP, HPA axis physiology, anxiety, and social functioning in individuals with FXS.
To our knowledge, this is the first study to evaluate the effects of administering OT to patients with an established genetic disorder. Previous studies have evaluated OT efficacy on more heterogeneous populations (e.g., autism spectrum disorders, obsessive–compulsive disorders) for which the genetic basis has not been established (Ansseau et al., 1987; den Boer and Westenberg, 1992; Epperson et al., 1996; Guastella et al., 2010; Hollander et al., 2003). In those studies, it is unclear whether variability in outcome following OT administration was associated with differential responsiveness to OT or due to the heterogeneity of the study population itself. In the present study, we administered OT to a group of individuals with a single and well-known genetic basis underlying the FXS pathology. Another important component of the present study is that we evaluated the effects of OT administration on a “real-life” social encounter, i.e., a stressful 10 min social challenge conducted with a female experimenter. Previous studies have employed simulated social games (Andari et al., 2010; Kosfeld et al., 2005), and static social photographs (Guastella et al., 2008) to evaluate the effects of OT on social behaviors. Given that these studies employed simulated aspects of social settings, rather than real-life social encounters, the ecological validity of these tasks could be questioned.
There are a number of limitations associated with the study. First, only a small number of participants could be included in our study. We attempted to study the effects of OT administration in isolation from other medications by recruiting males with FXS (fully methylated full mutation) who were not taking other psychoactive medications. However, as a result, we were only able to obtain a small sample of participants simply because the majority of males with FXS are prescribed psychoactive medications (e.g., antipsychotics, stimulants, and/or antidepressants) (Berry-Kravis and Potanos, 2004), and the parents of these individuals were very reluctant to discontinue these medications. Six of the eight participants included in this study were completely medication-free with one participant remaining on a low dose antidepressant and another taking an antiepileptic medication. However, the two participants who were taking medications did not appear to differ from those who were medication-free in their response to OT.
A second limitation concerns the fact that we administered only a single dose of OT. Given that OT has not been administered to individuals with FXS before, in this study, we wanted to determine whether OT is a safe and tolerable medication for this population, as well as to collect preliminary data on the effects of OTon social functioning in FXS. Further randomized double-blinded studies will therefore be needed to determine whether prolonged administration of OT (e.g., over several days or weeks) could produce additional improvements in social functioning in FXS.
A third limitation concerns the variability and magnitude of the response to OT. While improvements in eye gaze frequency occurred at the 24 IU dose, the overall mean improvement was low (i.e., an average of 6 additional occurrences of eye gaze during social interaction). In a previous study (see Hall et al., 2009a) we found that eye gaze in males with FXS also occurred at extremely low rates – approximately 10% of time on average during social interaction – despite the fact that prompts for eye gaze were repeatedly presented. It is also unclear why eye gaze frequency improved in response to the 24 IU dose, but not in response to the 48 IU dose. Conversely, salivary cortisol levels decreased in response to the 48 IU dose, but not in response to the 24 IU dose. Interestingly, in a study involving couples engaged in a conflict discussion, salivary cortisol levels were also found to decrease following intranasal administration of 40 IU OT (Ditzen et al., 2009). The differential effect seen for eye gaze frequency and cortisol in response to the different doses may be related to a time course effect of dosing. It is possible that cortisol would take longer to change than eye gaze or cardiac activity and the effect may only have been captured with the larger 48 IU dose. However, the majority of studies published to date have evaluated the effects of administering OT at the 24 IU dose only. Further studies will therefore be needed to determine which OT dose level may be the most beneficial to individuals with FXS.
In summary, we found that intranasal administration of OT may ameliorate some of the symptoms of social anxiety in individuals with FXS. While the effect of OT in this single-dose double-blind study was small, it is possible that repeated administration of OT could produce further reductions in social anxiety over time. In a previous study, we found that eye gaze in FXS could be significantly improved following the application of behavioral (non-pharmacological) reinforcement techniques conducted over several days (Hall et al., 2009b). Again, however, improvement in the mean duration of eye gaze was small (e.g., 2–4 s) even after 200 trials of behavioral training were conducted. It seems likely that a single medication (or behavioral intervention) would not be completely successful in treating the behavioral symptoms of FXS, simply because many of these behaviors appear to result from complex interactions between biological and environmental factors (Hall, 2009). For example, eye gaze avoidance could initially result from factors associated with the FMR1 gene. However, once eye gaze avoidance has occurred, negative reinforcement processes operating in the child’s natural environment could then quickly serve to shape and maintain eye gaze avoidance in the child’s repertoire. For example, the child could quickly learn that eye gaze avoidance often results in the removal of parental social demands or other aversive tasks (Hall et al., 2009a). Future studies should therefore begin to examine whether a combination of pharmacological and behavioral treatment approaches could be the most effective strategy for reducing the behavioral symptoms of social anxiety in individuals with FXS.
Acknowledgments
We thank Iris Bichsel of Internationale Apotheke (Bern, Swit-zerland) for supplying the Syntocinon® and Erin Falconer of Advantage Pharmaceuticals Inc. (Rocklin, CA) for compounding the placebo. We thank Dr. David Hessl for his assistance with our recruitment efforts, Marty Hamilton for conducting the randomization and blinding, Dr. Thalia Robakis for assistance with the physician checks, and Ms. Shellie Hyde for conducting the cortisol radioimmunoassays.
Role of funding source
This research was supported by a gift from the Lynda and Scott Canel Fragile X Research Fund. The funding source had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. We also acknowledge support from NIH grants K08MH081998 (Hall), R01MH050047 (Reiss) and a grant from the Simons Foundation Autism Research Initiative (Parker).
Footnotes
Participant P5 continued to take an antiepileptic medication (phenytoin), and participant P8 continued to take a low dose anti-depressant medication (10 mg fluoxetine) at the time of the study. Participant P4 had discontinued buspirone and nefazodone and participant P5 had discontinued risperidone two weeks prior to participating. Participant P8 had discontinued methylphenidate 1 month prior to participating in the study.
Contributors
Dr. Allan Reiss conceived and designed the study, conducted the physician checks, and contributed to the writing of the manuscript. Dr. Scott Hall recruited the participants, administered the oxytocin, videotaped the social challenge, analyzed the results, and drafted the manuscript. Dr. Amy Lightbody conducted the social challenge and scored the behavioral assessments. Brigid McCarthy conducted the analysis of the videotapes and the heart rate data. Dr. Karen Parker conducted the cortisol analyses and contributed to the writing of the manuscript.
Conflict of interest
Dr. Allan Reiss is currently a consultant for Novartis though was not associated with the company at the time of the study. No funding or other support was received from Novartis for the design or implementation of the study, analysis of the data, or manuscript preparation.
References
- Amico JA, Mantella RC, Vollmer RR, Li X. Anxiety and stress responses in female oxytocin deficient mice. J Neuroendocrinol. 2004;16 (4):319–324. doi: 10.1111/j.0953-8194.2004.01161.x. [DOI] [PubMed] [Google Scholar]
- Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci USA. 2010;107 (9):4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansseau M, Legros JJ, Mormont C, Cerfontaine JL, Papart P, Geenen V, Adam F, Franck G. Intranasal oxytocin in obsessive-compulsive disorder. Psychoneuroendocrinology. 1987;12 (3):231–236. doi: 10.1016/0306-4530(87)90009-6. [DOI] [PubMed] [Google Scholar]
- Balin BJ, Broadwell RD, Salcman M, el-Kalliny M. Avenues for entry of peripherally administered protein to the central nervous system in mouse, rat, and squirrel monkey. J Comp Neurol. 1986;251 (2):260–280. doi: 10.1002/cne.902510209. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27 (7):370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Hessl D, Coffey S, Hervey C, Schneider A, Yuhas J, Hutchison J, Snape M, Tranfaglia M, Nguyen DV, Hagerman R. A pilot open label, single dose trial of fenobam in adults with fragile X syndrome. J Med Genet. 2009;46 (4):266–271. doi: 10.1136/jmg.2008.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis E, Potanos K. Psychopharmacology in fragile X syndrome – present and future. Ment Retard Dev Disabil Res Rev. 2004;10 (1):42–48. doi: 10.1002/mrdd.20007. [DOI] [PubMed] [Google Scholar]
- Boccia ML, Roberts JE. Behavior and autonomic nervous system function assessed via heart period measures: the case of hyperarousal in boys with fragile X syndrome. Behav Res Methods Instrum Comput. 2000;32 (1):5–10. doi: 10.3758/bf03200783. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5 (6):514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Born J, Pietrowsky R, Fehm HL. Neuropsychological effects of vasopressin in healthy humans. Progr Brain Res. 1998;119:619–643. doi: 10.1016/s0079-6123(08)61595-2. [DOI] [PubMed] [Google Scholar]
- Brown V, Jin P, Ceman S, Darnell JC, O’Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, Darnell RB, Warren ST. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107 (4):477–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- Byrne EA. Unpublished Manual. University of Maryland; 1993. Inter-Beat-Interval Editing for Heart Period Variability Analysis: An Integrated Training Program with Standards for Reliability Assessment. [Google Scholar]
- Carter S. Sex differences in the developmental consequences of oxytocin and vasopressin: implications for autism?. Paper Presented at the American College of Neuropsychopharmacology; Chicago, IL. Chicago: University of Illinois; 2008. [Google Scholar]
- Crawford DC, Meadows KL, Newman JL, Taft LF, Stanfeild ML, Holmgreen P, Yeargin-Alsopp M, Boyle C, Sherman SL. Prevalence and phenotype consequence of FRAXA and FRAXE alleles in a large, ethnically diverse, special education-needs population. Am J Hum Genet. 1999;64:495–507. doi: 10.1086/302260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Boer JA, Westenberg HGM. Oxytocin in obsessive compulsive disorder. Peptides. 1992;13:1083–1085. doi: 10.1016/0196-9781(92)90010-z. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol Psychiatry. 2009;65 (9):728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Lucke JF, Loucks TL, Berga SL. Salivary cortisol reflects serum cortisol: analysis of circadian profiles. Ann Clin Biochem. 2007;44 (Pt 3):281–284. doi: 10.1258/000456307780480954. [DOI] [PubMed] [Google Scholar]
- Epperson CN, McDougle CJ, Price LH. Intranasal oxytocin in obsessive-compulsive disorder. Biol Psychiatry. 1996;40 (6):547–549. doi: 10.1016/0006-3223(96)00120-5. [DOI] [PubMed] [Google Scholar]
- Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galan-gue B, Minassian A, Becker O, Cooper J, Perry W, Lefebvre M, Gonzales J, Hadley A. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol Psychiatry. 2010;68 (7):678–680. doi: 10.1016/j.biopsych.2010.04.039. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25 (3):284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Garber K, Smith KT, Reines D, Warren ST. Transcription, translation and fragile X syndrome. Curr Opin Genet Dev. 2006;16 (3):270–275. doi: 10.1016/j.gde.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, Hickie IB. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67 (7):692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry. 2008;63 (1):3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Hall SS. Treatments for fragile X syndrome: a closer look at the data. Dev Disabil Res Rev. 2009;15 (4):353–360. doi: 10.1002/ddrr.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, DeBernardis M, Reiss A. Social escape behaviors in children with fragile X syndrome. J Autism Dev Disorders. 2006;36 (7):935–947. doi: 10.1007/s10803-006-0132-z. [DOI] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, Huffman LC, Lazzeroni LC, Reiss AL. Physiological correlates of social avoidance behavior in children and adolescents with fragile X syndrome. J Am Acad Child Adolesc Psychiatry. 2009a;48 (3):320–329. doi: 10.1097/CHI.0b013e318195bd15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Maynes NP, Reiss AL. Using percentile schedules to increase eye contact in children with fragile X syndrome. J Appl Behav Anal. 2009b;42 (1):171–176. doi: 10.1901/jaba.2009.42-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54 (12):1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Hessl D, Glaser B, Dyer-Friedman J, Blasey C, Hastie T, Gunnar M, Reiss AL. Cortisol and behavior in fragile X syndrome. Psychoneuroendocrinology. 2002;27 (7):855–872. doi: 10.1016/s0306-4530(01)00087-7. [DOI] [PubMed] [Google Scholar]
- Hessl D, Rivera SM, Reiss AL. The neuroanatomy and neuroendocrinology of fragile X syndrome. Ment Retard Dev Disabil Res Rev. 2004;10 (1):17–24. doi: 10.1002/mrdd.20004. [DOI] [PubMed] [Google Scholar]
- Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, Anagnostou E, Wasserman S. Oxytocin increases retention of social cognition in autism. Biol Psychiatry. 2007;61 (4):498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria CM, Aronowitz BR, Mosovich S. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger’s disorders. Neuropsychopharmacology. 2003;28 (1):193–198. doi: 10.1038/sj.npp.1300021. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Christmon CA, Grossman AW, Galvez R, Kim SH, DeGrush BJ, Weiler IJ, Greenough WT. Fragile X mental retardation protein levels increase following complex environment exposure in rat brain regions undergoing active synaptogenesis. Neurobiol Learn Mem. 2005;83 (3):180–187. doi: 10.1016/j.nlm.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Kesler SR, Lightbody AA, Reiss AL. Cholinergic dysfunction in fragile X syndrome and potential intervention: a preliminary 1H MRS study. Am J Med Genet. 2009;149A (3):403–407. doi: 10.1002/ajmg.a.32697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiess W, Meidert A, Dressendorfer RA, Schriever K, Kessler U, Konig A, Schwarz HP, Strasburger CJ. Salivary cortisol levels throughout childhood and adolescence: relation with age, pubertal stage, and weight. Pediatr Res. 1995;37(4 Pt 1):502–506. doi: 10.1203/00006450-199504000-00020. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25 (49):11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435 (7042):673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Lightbody AA, Reiss AL. Gene, brain, and behavior relationships in fragile X syndrome: evidence from neuroimaging studies. Dev Disabil Res Rev. 2009;15 (4):343–352. doi: 10.1002/ddrr.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantella RC, Vollmer RR, Li X, Amico JA. Female oxytocin-deficient mice display enhanced anxiety-related behavior. Endocrinology. 2003;144 (6):2291–2296. doi: 10.1210/en.2002-0197. [DOI] [PubMed] [Google Scholar]
- Miller LJ, McIntosh DN, McGrath J, Shyu V, Lampe M, Taylor AK, Tassone F, Neitzel K, Stackhouse T, Hagerman RJ. Electrodermal responses to sensory stimuli in individuals with fragile X syndrome: a preliminary report. Am J Med Genet. 1999;83 (4):268–279. [PubMed] [Google Scholar]
- Norman GJ, Cacioppo JT, Morris JS, Malarkey WB, Berntson GG, Devries AC. Oxytocin increases autonomic cardiac control: moderation by loneliness. Biol Psychol. 2011;86 (3):174–180. doi: 10.1016/j.biopsycho.2010.11.006. [DOI] [PubMed] [Google Scholar]
- O’Donnell WT, Warren ST. A decade of molecular studies of fragile X syndrome. Annu Rev Neurosci. 2002;25:315–338. doi: 10.1146/annurev.neuro.25.112701.142909. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Intranasal oxytocin administration attenuates the ACTH stress response in monkeys. Psychoneuroendocrinology. 2005;30 (9):924–929. doi: 10.1016/j.psyneuen.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J Neurosci. 2008;28 (26):6607–6615. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjit N, Young EA, Raghunathan TE, Kaplan GA. Modeling cortisol rhythms in a population-based study. Psychoneuroendocrinology. 2005;30 (7):615–624. doi: 10.1016/j.psyneuen.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Hall SS. Fragile X syndrome: assessment and treatment implications. Child Adolesc Psychiatr Clin North Am. 2007;16 (3):663–675. doi: 10.1016/j.chc.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Silvetti MS, Drago F, Ragonese P. Heart rate variability in healthy children and adolescents is partially related to age and gender. Int J Cardiol. 2001;81 (2–3):169–174. doi: 10.1016/s0167-5273(01)00537-x. [DOI] [PubMed] [Google Scholar]
- Smith R. Moving molecules: mRNA trafficking in mammalian oligodendrocytes and neurons. Neuroscientist. 2004;10 (6):495–500. doi: 10.1177/1073858404266759. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. Parent/Caregiver Rating Form. AGS Publishing; Circle Pines, MN: 2005. Vineland Adaptive Behavior Scales (Vineland-II) [Google Scholar]
- Steward O, Schuman EM. Compartmentalized synthesis and degradation of proteins in neurons. Neuron. 2003;40 (2):347–359. doi: 10.1016/s0896-6273(03)00635-4. [DOI] [PubMed] [Google Scholar]
- Tiedge H, Zhou A, Thorn NA, Brosius J. Transport of BC1 RNA in hypothalamo-neurohypophyseal axons. J Neurosci. 1993;13 (10):4214–4219. doi: 10.1523/JNEUROSCI.13-10-04214.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner G, Webb T, Wake S, Robinson H. Prevalence of fragile X syndrome. Am J Med Genet. 1996;64 (1):196–197. doi: 10.1002/(SICI)1096-8628(19960712)64:1<196::AID-AJMG35>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65 (5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Williams JR, Carter CS, Insel T. Partner preference development in female prairie voles is facilitated by mating or the central infusion of oxytocin. Ann N Y Acad Sci. 1992;652:487–489. doi: 10.1111/j.1749-6632.1992.tb34393.x. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo–pituitary–adrenal activity. J Neurosci. 2004;24 (12):2974–2982. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt DM, Winslow JT, Insel TR. Enhanced social interactions in rats following chronic, centrally infused oxytocin. Pharmacol Biochem Behav. 1992;43 (3):855–861. doi: 10.1016/0091-3057(92)90418-f. [DOI] [PubMed] [Google Scholar]