Abstract
Background
Transplanted nephron mass is an important determinant of long-term allograft survival, but accurate assessment before organ retrieval is challenging. Newer radiologic imaging techniques allow for better determination of total kidney and cortical volumes.
Methods
Using volume measurements reconstructed from magnetic resonance or computed tomography imaging from living donor candidates, we characterized total kidney (n = 312) and cortical volumes (n = 236) according to sex, age, weight, height, body mass index (BMI), and body surface area (BSA).
Results
The mean cortical volume was 204 mL (range 105–355 mL) with no significant differences between left and right cortical volumes. The degree to which existing anthropomorphic surrogates predict nephron mass was quantified, and a diligent attempt was made to derive a better surrogate model for nephron mass. Cortical volumes were strongly associated with sex and BSA, but not with weight, height, or BMI. Four prediction models for cortical volume constructed using combinations of age, sex, race, weight, and height were compared with models including either BSA or BMI.
Conclusions
Among existing surrogate measures, BSA was superior to BMI in predicting renal cortical volume. We were able to construct a statistically superior proxy for cortical volume, but whether relevant improvements in predictive accuracy could be gained needs further evaluation in a larger population.
Keywords: Kidney volume, Living donor, BSA
The implications of glomerular number, or “functional nephron mass,” are widespread, particularly in the context of transplantation, where the usual receipt of one, rather than two kidneys leaves the transplant recipient with a relative deficit, typically exacerbated by ischemic or traumatic injury when the organ is harvested from deceased donors. Obtaining an accurate estimate of glomerular number is, therefore, of substantial clinical value, and in humans, this has been most accurately estimated using stereologic techniques in kidney samples taken from autopsies (1–3). In these studies, there was considerable person-to-person variation, with a range of 210,000 to 1,800,000 glomeruli per kidney. Glomerular number tends to decline with advancing age and is considerably lower in persons with hypertension (2) and chronic kidney disease (4).
Several proxies for glomerular number or functional nephron mass have been used in epidemiological studies, including kidney weight and kidney size. These measures correlate with posttransplant renal function (5); however, they are difficult to obtain before procurement of the donor kidney, particularly in the setting of deceased donor transplantation. More practically, on a population basis, donor height, weight, and derivations thereof, including DuBois and DuBois’ (6) body surface area (BSA) and Quetélet’s body mass index (BMI) (7) have been used. Multiple observational studies have demonstrated an independent association between larger donor body size and allograft survival (8, 9), an advantage attributed to a larger “dose” of transplanted nephrons. Other studies have demonstrated an independent association between younger donor age and allograft survival, an advantage also attributed to the transplantation of more functional nephrons. These observations have fueled a debate wherein investigators and clinicians have disputed the relative importance of traditional immunological factors (e.g., human leukocyte antigen mismatch and degree of sensitization as expressed by panel reactive antibody) and other “non-immunological” factors (e.g., body size and cold ischemia time) in determining long-term allograft survival (10, 11).
We have recently reported the estimated number of functioning glomeruli at the time of transplantation in deceased (12) and living (13) kidney donors and showed a significant reduction of glomerular number in older deceased donor kidneys. Giral et al. (10) showed that a lower ratio of donor kidney weight to recipient body weight increased the risk of allograft failure as early as 2 years after transplantation. However, clinical applicability of these findings is limited by the lack of reliable surrogates for functional nephron number that can be used for organ allocation. To address these issues, we examined the ability of existing anthropomorphic surrogates of kidney size to predict cortical volume as measured by magnetic resonance angiography (MRA) or computed tomography angiography (CTA). We aimed to establish a better proxy for functional nephron mass by deriving and validating a model to predict cortical volume from commonly measured anthropometric and demographic factors.
RESULTS
Relation Between Cortical Volumes and Mate Kidneys
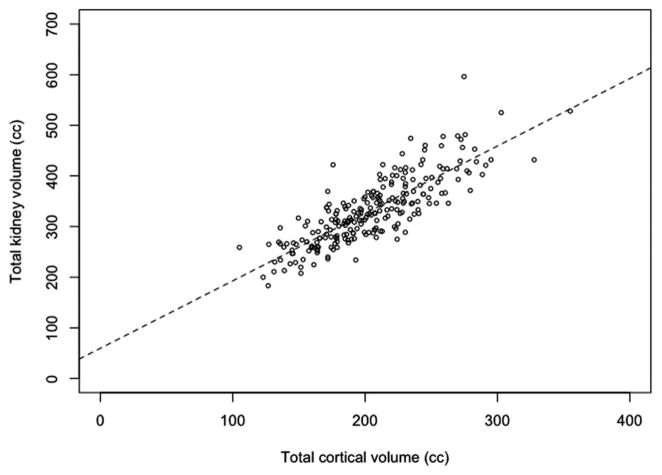
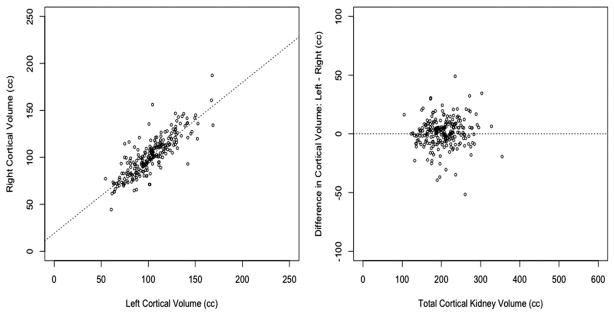
The clinical characteristics of subjects included in this study are listed in Table 1. The mean age was 41 years with a range of 18 to 68 years. Half of the study population was women (53%). The mean cortical and total kidney volumes were 204.2 ± 40.9 mL and 335.7 ± 66.9 mL, respectively. Cortical volumes were 61% of total kidney volumes. Figure 1 illustrates the relation between total (left+right) cortical volume and total kidney volumes. Cortical and total volumes are strongly and directly correlated (R2 = 0.67), with increasing variability in total kidney volumes for larger cortical volumes, as expected. Left and right cortical volumes were virtually identical with only a few outliers (Figure 2). A paired t test did not indicate significant differences between cortical volumes of mate kidneys (P = 0.20).
TABLE 1.
Study population characteristics by sex
| Population characteristics | Male | Female |
|---|---|---|
| Sample size | 145 | 167 |
| Race | ||
| Other | 6 | 5 |
| Asian | 35 | 43 |
| White | 62 | 75 |
| Black | 11 | 3 |
| Hispanic | 31 | 41 |
| Mean (SD) | Minimum, maximum | Mean (SD) | Minimum, maximum | |
|---|---|---|---|---|
| Age (yr) | 39.9 (12.6) | 18.0, 68.0 | 41.7 (11.0) | 18.0, 67.0 |
| Creatininea (mg/dL) | 1.0 (0.14) | 0.6, 1.4 | 0.8 (0.1) | 0.5, 1.2 |
| Heighta (cm) | 175.8 (8.4) | 147.3, 198.1 | 162.6 (8.6) | 137.2, 195.6 |
| Weighta (kg) | 82.2 (11.8) | 54.4, 117.9 | 67.9 (12.6) | 41.7, 105.7 |
| Body mass indexb (kg/m2) | 26.6 (3.3) | 18.2, 36.9 | 25.6 (4.3) | 16.8, 42.6 |
| Body surface areaa (m2) | 2.0 (0.2) | 1.5, 2.5 | 1.7 (0.2) | 1.3, 2.3 |
For categorical variables, the sample size is given.
t test P =< 0.001.
t test P = 0.03.
SD, standard deviation.
FIGURE 1.
Total and cortical volumes.
FIGURE 2.
Right vs. left cortical volumes.
Relations Among Kidney Volume and Age, Sex, Body Size, and Serum Creatinine
The relations among kidney volumes and anthropomorphic measures were analyzed using combined left and right cortical and total kidney volumes. Men had significantly larger cortical volumes than women (Table 2). Similarly, total kidney volumes of men were larger (t test P < 0.001). BSA differed between men and women (2.00 ± 0.17 vs. 1.74 ± 0.19 m2; t test P < 0.001) as did BMI (26.6 ± 3.3 vs. 25.6 ± 4.3 kg/m2; t test P = 0.03). After adjusting for BSA, sex was significantly associated with cortical volumes (lower volumes in women compared with men, parameter estimate 11.8 mL, F test P = 0.04); there was no significant interaction between sex and BSA on cortical volume. Expected mean cortical volumes for men and women at BSA 1.6 m2 were 188.9 and 177.1 mL, respectively, and at 2.0 m2 were 223.7 and 211.9 mL, respectively.
TABLE 2.
Kidney size parameters by sex
| Mean (SD) | Minimum, maximum | Mean (SD) | (Minimum, maximum) | |
|---|---|---|---|---|
| Cortical volumea (mL) | ||||
| Sample size | 101 | 135 | ||
| Right | 112.2 (19.2) | 77.3, 187.2 | 93.7 (18.5) | 44.4, 160.7 |
| Left | 112.1 (19.7) | 54.5, 167.8 | 95.5 (20.7) | 60.7, 168.7 |
| % difference | −0.1 (5.3) | −19.8, 12.0 | 0.8 (6.4) | −20.8, 20.9 |
| Kidney volumea (mL) | ||||
| Sample Size | 145 | 167 | ||
| Right | 181.8 (32.4) | 113.0, 304.1 | 149.8 (28.2) | 90.4, 244.3 |
| Left | 186.8 (32.8) | 102.0, 292.2 | 157.3 (29.9) | 92.8, 280.7 |
| % difference | 1.4 (4.8) | −23.4, 16.0 | 2.4 (5.1) | −26.9, 16.0 |
t test P =< 0.001.
t test P = 0.03.
SD, standard deviation.
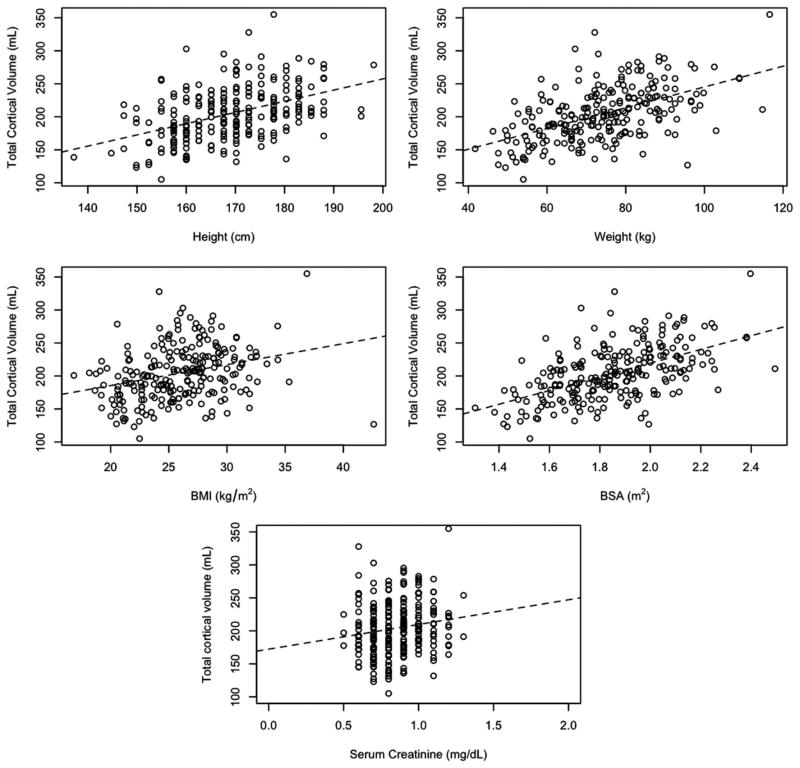
Cortical volumes were compared against common anthropomorphic measures (Figure 3). There were direct but relatively poor correlations among cortical volume and weight, height, and BMI. Similarly, there was a poor correlation between cortical volume and serum creatinine (R2 = 0.02; Fig. 3) as well as estimated glomerular filtration rate (R2 = 0.13). This likely reflects the narrow range of serum creatinine and poor discriminating power of creatinine-based equations in this population. Total cortical volume was directly correlated with BSA (R2 = 0.30; F test P < 0.001). Similar results were observed when examining total kidney, rather than cortical volume.
FIGURE 3.
Relations between total cortical volume and anthropomorphic measures.
A Derived Estimate of Cortical Volume
To predict total cortical volume, we considered candidate predictors (sex, age, race, height and weight, polynomials of height and weight, and derived variables [BMI and BSA]), which were then arranged into the different sets as listed in Table 3. Among a given set, the variables that were most predictive of total cortical volume were selected (see subsequent sections). Table 4 summarizes the variables chosen in each model along with the corresponding prediction errors and confidence intervals.
TABLE 3.
Sets of candidate predictors
| Set 1 | Set 2 | Set 3 | Set 4 | Set 5 | Set 6 | |
|---|---|---|---|---|---|---|
| Candidate predictors | Age | Age | Age | Age | ||
| Sex | Sex | Sex | Sex | |||
| Race | Race | Race | Race | |||
| Height | Height | Height | Height | |||
| Weight | Weight | Weight | Weight | |||
| Height2 | Height2 | |||||
| Weight2 | Weight2 | |||||
| [sqrt]Height | [sqrt]Height | |||||
| [sqrt]Weight | [sqrt]Weight | |||||
| BSA | BSA | BSA | ||||
| BMI | BMI | BMI | ||||
| All pairwise | All pairwise | All pairwise | All pairwise | |||
| Interactions | Interactions | Interactions | Interactions |
TABLE 4.
Variables chosen in model using total cortical volume as the dependent variable
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
|---|---|---|---|---|---|---|
| Variables selected | Sex | Sex | Sex | Sex | ||
| Race | Race | Race | Race | |||
| Weight | Age | [sqrt]Weight | Age | |||
| Weight × age | BSA | [sqrt]Weight × age | BSA | BSA | BMI | |
| BSA × age | BSA × age | |||||
| BSA × race | BSA × race | |||||
| Average prediction error (SD) | 1076.1 (147.9) | 1068.1 (163.4) | 1073.5 (148.4) | 1068.9 (162.0) | 1173.6 (144.5) | 1531.4 (185.2) |
| 95% CI | 1073.2–1079.0 | 1064.9–1071.3 | 1070.6–1076.4 | 1065.7–1072.1 | 1170.8–1176.4 | 1527.8–1535.0 |
Model selection with forward stepwise regression using AIC was performed. Average prediction errors are based on 10,000 replications from cross-validation.
BSA, body surface area; BMI, body mass index; SD, standard deviation; CI, confidence interval.
The criterion used to determine the model most predictive of total cortical volume was lowest average prediction error as described in Materials and Methods. Average prediction errors across the six models were comparable, but model 2 yielded the lowest error (1068.1, standard deviation = 163.4). Model 2 contained BSA, age, race, and interaction terms between BSA and age and race. BSA alone (model 5) had an average prediction error that was only modestly higher than model 2 (1173.6, standard deviation = 144.5). For descriptive purposes, we note that models 1 through 4 had R2 values of 0.37, 0.41, 0.37, and 0.41, respectively; the model with BSA alone had a R2 value of 0.30 and with BMI alone, 0.09. Although the R2 statistic is not appropriate for model selection, its value provides a measure of the proportion of variability in total cortical volume explained by each model, indicating the poor performance of BMI as a metric for cortical volume.
DISCUSSION
The theoretical number of functional nephrons transplanted is increasingly being considered as an important non-immunological determinant of long-term allograft function. Anthropomorphic measures such as BMI and BSA have been used in large registry data analyses as estimates of donor nephron mass (8, 9). In a study of over 1000 deceased-donor kidney transplants, Giral et al. (14) have shown that a donor kidney weight to recipient weight less than 2.3 g/kg was associated with a 55% increased risk for allograft failure at 2 years. They also showed that recipients with a lower donor kidney weight to recipient body weight ratio had an increased risk of developing proteinuria, hypertension, and glomerulosclerosis. This pattern of injury is consistent with the well-described “remnant kidney” phenomenon in rats, in which removal of 5/6th of the total kidney mass leads to a progressive sclerosing glomerulopathy (15). Although the results of Giral et al. provide support for a similar process in humans, translation of these results to donor selection in clinical kidney transplantation is limited by the relatively poor performance of existing surrogates for cortical volume and functional nephron number/mass. The poorer the correlation between any proxy and the true nephron mass (e.g., what might be learned from a large wedge biopsy), the more likely there will be misclassification and thus poorer discrimination when evaluating the association between the “nephron dose” and the eventual performance of the allograft.
Among noninvasive measurements of the kidney in a potential donor, cortical volume would be the best measure of functional nephron mass. Recent improvements in radiologic imaging techniques allow for accurate determination of cortical volume before organ retrieval. We showed in this study that correlations among cortical volume and height, weight, and BMI were relatively poor. Of the existing surrogate measures, BSA had the highest correlation with cortical (R2 = 0.29) and kidney volumes (R2 = 0.37). Other common assumptions of kidney size were tested and quantified. For example, the majority of subjects had symmetric kidney volumes, with 90.7% of the population having right and left kidney volumes that differed less than 10%. Although the assumption of equivalent size implicit in studies using mate-kidney data of transplant registries is reasonable, the cohort in this study represents a healthy segment of the population; a larger degree of asymmetry may exist in the general population. We also showed that men have larger cortical volumes than women (224 ± 37 mL vs. 189 ± 37 mL; t test P < 0.001). Our results are consistent with autopsy studies, which have shown that women have 15% fewer glomeruli than males, and similarly, kidney weighs 16% lower (1, 3). Larger cortical volumes in men could not be entirely attributed to their larger body size. In contrast to previous studies, we did not find a significant correlation between cortical or total kidney volume and age. In general, kidney volume and functional nephron mass are expected to decline with advancing age; however, the process of donor selection may have restricted our older donor sample to only the most robust.
The range of cortical volume in this population was large (105–355 mL), representing a threefold difference. This range is smaller than the reported fourfold to ninefold range of nephron number in autopsy studies of normal humans (1, 3). Again, the narrower range may be due to the selection process of living kidney donors, as those with lower glomerular filtration rates would not have proceeded to this final stage of living donor image studies. Yet, even in this selected population of subjects, the range of nephron mass, as estimated by cortical volume, was large enough for the “remnant kidney” phenomenon to be plausible. For example, transplantation of a single kidney from the smallest individual (44 mL) to the largest individual (with native two-kidney cortical volumes of 355 mL) would be physiologically similar to the loss of more than 5/6th of original nephron mass. In recipients of living donor kidneys, higher allograft weight/recipient body weight (16) and higher donor kidney volume/recipient BSA (5, 17, 18) have been shown to correlate with better outcomes after transplantation.
This study has several strengths. First, the sample was relatively large, with broad racial/ethnic diversity, including a large fraction of donors of minority backgrounds. To our knowledge, it is the largest series of cortical volumes reported to date. Second, we measured cortical volumes in addition to total kidney volumes and used 3-dimensional (3D) reconstruction algorithms that have been validated in animal models. Previous autopsy reports demonstrated a direct correlation between BSA and kidney weight, but sample sizes for these studies were small (n = 39 and 78) (1, 3). Their measurements also differed from ours in that total kidney weight (which contains the medulla and ureter in addition to cortex) was used for analysis, and it was assessed ex vivo. A recent study using radiologic imaging focused on total kidney volume and approximated the kidney to be a prolate ellipsoid to estimate the volume (5). Finally, in developing models to predict cortical and total kidney volume from routinely collected demographic and anthropometric data, we used traditional least squares regression techniques, along with other techniques that may offer better discrimination in the face of biological variability. Although we toiled diligently and creatively to derive an equation that might serve as a better surrogate of cortical volume, our best model showed a marginal improvement in its predictive power compared with the widely used BSA. Future studies using larger samples with greater variability in age, weight, and height might lead us toward a better method for estimating functional nephron mass. It is also noteworthy that BMI, which has been frequently used in studies as a proxy for body size, is a poor surrogate for cortical volume.
There are several limitations to this study. There were no septuagenarians or octogenarians in our donor pool. Thus, although we cannot extrapolate our results to elderly prospective donors, the data derived in this study are reasonably representative of the living donor population. Whether extrapolation of these findings to donors is valid is unknown. Although we believe that cortical volume may be the best proxy of functional nephron mass among healthy donors, we cannot be certain that intact cortex accurately represents functional tissue among the deceased donor population.
In summary, we examined a large cohort of living kidney donors using MRA or CTA to measure cortical and total kidney volume as a proxy for functional nephron mass. We found a high degree of symmetry between right and left kidneys and confirmed the expected associations among sex and body size and the volume parameters. Using multiple analytic techniques, we constructed equations to predict cortical and total kidney volumes from routinely collected demographic and anthropometric data. Although we were able to identify models with better predictive power, we concluded that the improvement was not substantial enough to recommend replacing BSA given its simplicity for practical application. At least among selected living donors, BSA seems to be a reasonably good proxy of functional nephron mass. Conducting a similar exercise among less selected populations more reflective of the deceased donor pool would be a logical next step. A better understanding of the role of the transplanted nephron mass and how best to measure it will be required to optimize organ allocation and maximize allograft survival under conditions of organ scarcity.
MATERIALS AND METHODS
Study Population
Living donor candidates at our institution are required to be in essentially good health, with normal basic blood counts and chemistries, and no significant abnormality on abdominal ultrasound. Since November 2000, all candidates so selected then underwent MRA or CTA as part of their routine donor workup, and these individuals comprised our study population. We obtained records of living donor candidates from November 2000 to June 2010 for inclusion in this study. Total kidney volumes were recorded in 312 subjects; cortical volumes were available in 236 of these subjects (as these data were collected beginning in 2002). As cortical volume better reflects nephron mass than kidney volume, we focused our analyses on cortical volume. We obtained approval by the Institutional Review Board at Stanford University School of Medicine for this study.
Measurement of Total Kidney and Cortical Volumes
Total volumes of the left and right kidneys, and corresponding cortical volumes, were measured by the 3D reconstruction laboratory at our center (19). During 2000 to 2003, we used an ultrafast gadolinium-enhanced magnetic resonance imaging technique. Because of concerns about gadolinium toxicity (20), we modified the technique thereafter by using a spiral computed tomography scan with conventional radiocontrast. Both techniques use a 3D acquisition strategy that is capable of acquiring angiographic images of the artery and vein within 30 seconds and with resolution in the range of 1 mm. The contrast-enhanced ultrafast sequence permits cortical volume to be determined accurately by providing information with high spatial resolution and contrast in 3D fashion. The images are ideal for computerized determination of the cortical volume, thereby circumventing more subjective assessment of boundaries obligated by other techniques. An automated 3D computation of cortical and total kidney volumes was performed with a thresholding algorithm for volume determination based on the Cavalieri method and implemented by means of Advantage Windows TM 2.0 software from General Electric Corporation. The accuracy of cortical volume by this method has been validated in pigs by comparing the in vivo to ex vivo values after removal of the kidney (19, 21). In six transplant donors, volumes were obtained by both MRA and CTA images, and the results were nearly identical (within 5 mL) with each technique (data not shown).
Statistical Analysis
To model total cortical volume, six sets of candidate predictors were constructed, where each set gave rise to a competing model. The six sets of candidate predictors included the following main effects or first-order terms: age, sex, race/ethnicity (white, black, Asian, and Hispanic), height, weight, BSA (6), and BMI (7). Pairwise interactions were considered, with the exception of interaction terms that created additional higher order terms of a main effect (i.e., an interaction between height [2] and age was considered, while an interaction between height [2] and height was not). Sets 1 to 4 were created to determine whether models using first- and higher order variables of height and weight performed better than models using derived variables of height and weight, namely BSA and BMI; sets 5 and 6 were, respectively, composed of BSA and BMI alone.
For each set, forward stepwise regression was applied using the Akaike Information Criterion (AIC) statistic as a criterion for variable selection. To determine the prediction error of the forward stepwise regression procedure corresponding to each set, the process was cross-validated using a training set to build the model (two-thirds of the data set randomly selected) and a test set (the remaining one-third of the data) for validation. This process was repeated 10,000 times, and the prediction error was averaged over the replications. The model corresponding to the set of candidate predictors with the lowest average predictive error was considered the best performing model across the six competing models.
Acknowledgments
This work was supported by NIH K23DK087937 (J.C.T.), NIH K24DK085446 (G.M.C. and M.D.), and the John and Abby Sobrato Foundation (J.L.).
Footnotes
The authors declare no conflicts of interest.
J.C.T and J.P. contributed equally to this manuscript; J.C.T. and J.P. participated in research design and writing of the manuscript, reviewed, edited, and approved the final version of the manuscript; G.M.C., F.C.G., and M.D. participated in research design and performance of research, reviewed, edited, and approved the final version of the manuscript; and J.L. and S.B. participated in the performance of the research, reviewed, edited, and approved the final version of the manuscript.
References
- 1.Hoy WE, Douglas-Denton RN, Hughson MD, et al. A stereological study of glomerular number and volume: Preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int Suppl. 2003:S31. doi: 10.1046/j.1523-1755.63.s83.8.x. [DOI] [PubMed] [Google Scholar]
- 2.Keller G, Zimmer G, Mall G, et al. Nephron number in patients with primary hypertension. N Engl J Med. 2003;348:101. doi: 10.1056/NEJMoa020549. [DOI] [PubMed] [Google Scholar]
- 3.Nyengaard JR, Bendtsen TF. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec. 1992;232:194. doi: 10.1002/ar.1092320205. [DOI] [PubMed] [Google Scholar]
- 4.Luyckx VA, Brenner BM. The clinical importance of nephron mass. J Am Soc Nephrol. 2010;21:898. doi: 10.1681/ASN.2009121248. [DOI] [PubMed] [Google Scholar]
- 5.Poggio ED, Hila S, Stephany B, et al. Donor kidney volume and outcomes following live donor kidney transplantation. Am J Transplant. 2006;6:616. doi: 10.1111/j.1600-6143.2005.01225.x. [DOI] [PubMed] [Google Scholar]
- 6.DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Int Med. 1916;XVII:863. [Google Scholar]
- 7.Eknoyan G. Adolphe Quetelet (1796–1874)—the average man and indices of obesity. Nephrol Dial Transplant. 2008;23:47. doi: 10.1093/ndt/gfm517. [DOI] [PubMed] [Google Scholar]
- 8.Chertow GM, Milford EL, Mackenzie HS, et al. Antigen-independent determinants of cadaveric kidney transplant failure. JAMA. 1996;276:1732. [PubMed] [Google Scholar]
- 9.Kasiske BL, Snyder JJ, Gilbertson D. Inadequate donor size in cadaver kidney transplantation. J Am Soc Nephrol. 2002;13:2152. doi: 10.1097/01.asn.0000024564.22119.3d. [DOI] [PubMed] [Google Scholar]
- 10.Giral M, Nguyen JM, Karam G, et al. Impact of graft mass on the clinical outcome of kidney transplants. J Am Soc Nephrol. 2005;16:261. doi: 10.1681/ASN.2004030209. [DOI] [PubMed] [Google Scholar]
- 11.Chertow GM, Brenner BM, Mackenzie HS, et al. Non-immunologic predictors of chronic renal allograft failure: Data from the United Network of Organ Sharing. Kidney Int Suppl. 1995;52:S48. [PubMed] [Google Scholar]
- 12.Tan JC, Workeneh B, Busque S, et al. Glomerular function, structure, and number in renal allografts from older deceased donors. J Am Soc Nephrol. 2009;20:181. doi: 10.1681/ASN.2008030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan JC, Busque S, Workeneh B, et al. Effects of aging on glomerular function and number in living kidney donors. Kidney Int. 2010;78:686. doi: 10.1038/ki.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giral M, Foucher Y, Karam G, et al. Kidney and recipient weight incompatibility reduces long-term graft survival. J Am Soc Nephrol. 2010;21:1022. doi: 10.1681/ASN.2009121296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hostetter TH, Olson JL, Rennke HG, et al. Hyperfiltration in remnant nephrons: A potentially adverse response to renal ablation. J Am Soc Nephrol. 2001;12:1315. doi: 10.1681/ASN.V1261315. [DOI] [PubMed] [Google Scholar]
- 16.Seun Kim Y, Soo Kim M, Suk Han D, et al. Evidence that the ratio of donor kidney weight to recipient body weight, donor age, and episodes of acute rejection correlate independently with live-donor graft function. Transplantation. 2002;74:280. doi: 10.1097/00007890-200207270-00021. [DOI] [PubMed] [Google Scholar]
- 17.Hugen CM, Polcari AJ, Farooq AV, et al. Size does matter: Donor renal volume predicts recipient function following live donor renal transplantation. J Urol. 2011;185:605. doi: 10.1016/j.juro.2010.09.098. [DOI] [PubMed] [Google Scholar]
- 18.Saxena AB, Busque S, Arjane P, et al. Preoperative renal volumes as a predictor of graft function in living donor transplantation. Am J Kidney Dis. 2004;44:877. [PubMed] [Google Scholar]
- 19.Coulam CH, Bouley DM, Sommer FG. Measurement of renal volumes with contrast-enhanced MRI. J Magn Reson Imaging. 2002;15:174. doi: 10.1002/jmri.10058. [DOI] [PubMed] [Google Scholar]
- 20.Perazella MA. Nephrogenic systemic fibrosis, kidney disease, and gadolinium: Is there a link? Clin J Am Soc Nephrol. 2007;2:200. doi: 10.2215/CJN.00030107. [DOI] [PubMed] [Google Scholar]
- 21.Sommer G, Bouley D, Frisoli J, et al. Determination of 3-dimensional zonal renal volumes using contrast-enhanced computed tomography. J Comput Assist Tomogr. 2007;31:209. doi: 10.1097/01.rct.0000236423.12890.d6. [DOI] [PubMed] [Google Scholar]