Abstract
Purpose
Benign prostatic hyperplasia and hypogonadism are common disorders in aging men. There is concern that androgen replacement in older men may increase prostate size and symptoms of benign prostatic hyperplasia. We examined whether combining dutasteride, which inhibits testosterone to dihydrotestosterone conversion, with testosterone treatment in older hypogonadal men with benign prostatic hyperplasia reduces androgenic stimulation of the prostate compared to testosterone alone.
Materials and Methods
We conducted a double-blind, placebo controlled trial of 53 men 51 to 82 years old with symptomatic benign prostatic hyperplasia, prostate volume 30 cc or greater and serum total testosterone less than 280 ng/dl (less than 9.7 nmol/l). Subjects were randomized to daily transdermal 1% T gel plus oral placebo or dutasteride for 6 months. Testosterone dosing was adjusted to a serum testosterone of 500 to 1,000 ng/dl. The primary outcomes were prostate volume measured by magnetic resonance imaging, serum prostate specific antigen and androgen levels.
Results
A total of 46 subjects completed all procedures. Serum testosterone increased similarly into the mid-normal range in both groups. Serum dihydrotestosterone increased in the testosterone only but decreased in the testosterone plus dutasteride group. In the testosterone plus dutasteride group prostate volume and prostate specific antigen (mean ± SEM) decreased 12% ± 2.5% and 35% ± 5%, respectively, compared to the testosterone only group in which prostate volume and prostate specific antigen increased 7.5% ± 3.3% and 19% ± 7% (p = 0.03 and p = 0.008), respectively, after 6 months of treatment. Prostate symptom scores improved in both groups.
Conclusions
Combined treatment with testosterone plus dutasteride reduces prostate volume and prostate specific antigen compared to testosterone only. Coadministration of a 5α-reductase inhibitor with testosterone appears to spare the prostate from androgenic stimulation during testosterone replacement in older, hypogonadal men with symptomatic benign prostatic hyperplasia.
Keywords: testosterone, prostate, 5-alpha reductase inhibitors, dihydrotestosterone
Benign prostatic hyperplasia and hypogonadism are common in the aging male, present alone or in combination in nearly 40% of men more than 50 years old.1,2 BPH can contribute to lower urinary tract symptoms and urinary retention.2 Hypogonadism is associated with loss of energy, decreased muscle bone mass and sexual dysfunction.3 While many symptoms of hypogonadism respond to testosterone replacement,4 symptomatic BPH is considered a relative contraindication to testosterone treatment due to concerns that testosterone might increase prostate size and exacerbate LUTS.4,5
The prostate is an androgen responsive organ. Testosterone and its more potent androgen metabolite, DHT, are required for normal prostate development. Compared to eugonadal men, age matched hypogonadal men have lower prostate volume,6,7 and testosterone replacement in the latter group mildly increases prostate volume and serum PSA.7,8 BPH results from prostate growth under the influence of androgens. BPH and low testosterone levels increase in prevalence with aging, and 15% to 25% of men with BPH also have symptomatic androgen deficiency.1,9
DHT is produced from the reduction of testosterone by the enzymes 5α-reductase types I and II. In eugonadal men with BPH, 5αRI treatment decreases serum and intraprostatic DHT levels, prostate volume, PSA and LUTS,10 and prostate volume and PSA reductions may be greater in men with lower testosterone levels.9 Moreover 5αRIs may decrease the risk of prostate cancer.11,12 Combining androgens with a 5αRI has been proposed as a prostate sparing androgen replacement regimen.13,14 To our knowledge the impact of this combination specifically on the prostate in hypogonadal men with BPH has not been tested.
We hypothesized that treatment with testosterone combined with the potent type I and II 5αRI, dutasteride, would increase serum testosterone levels in hypogonadal men with BPH without increasing prostate size or serum PSA, or worsening of LUTS that might occur with testosterone therapy alone. We conducted a double-blind, placebo controlled trial comparing changes in prostate size, PSA and LUTS after 6 months of testosterone plus dutasteride vs testosterone alone in hypogonadal men with an enlarged prostate and moderate LUTS.
MATERIALS AND METHODS
Subjects
Subjects were recruited from the VAPSHCS, Seattle, Washington using weekly reports from the Veterans Affairs central laboratory. A total of 102 subjects were screened and all had low testosterone and more than 1 symptom of androgen deficiency.5 The study inclusion criteria were age 50 years old or older, morning serum total testosterone less than 280 ng/dl (less than 9.7 nmol/l) or less than 300 ng/dl on 2 separate mornings, prostate volume 30 cc or greater by MRI, PSA 1.5 to 10 ng/ml, I-PSS 8 to 20, PVR urinary volume 200 ml or less, and a maximum urinary flow rate 10 ml per second or greater. For subjects with a PSA greater than 4.0 to 10.0 ng/ml a pretreatment prostate biopsy was required within the 6 months preceding randomization.
Exclusion criteria were history of prostate or breast cancer or prostatic intraepithelial neoplasia, acute urinary retention within 3 months of screening, previous 5αRI treatment, invasive therapy for BPH, severe acute or chronic systemic illness, α-blocker use within the last month, bleeding disorder, androgen or antiandrogen use within the last year, active alcohol or drug abuse, untreated obstructive sleep apnea, hematocrit greater than 52%, weight greater than 300 lbs or a skin condition which might interfere with transdermal testosterone absorption. The study was conducted from March 2005 to March 2009, was monitored throughout by an independent safety officer and was approved by the institutional review board of the VAPSHCS. Subjects gave written informed consent before screening.
Study Medications
Subjects were randomized to receive transdermal 1% T gel 7.5 gm (AndroGel®) plus oral placebo daily (T only) or 0.5 mg dutasteride (GlaxoSmithKline, Philadelphia, Pennsylvania) daily (T+D). On days 7 and 14 of treatment the T gel dose was adjusted to achieve a level of 500 to 1,000 ng/dl using 2.5 gm increments of T gel.
Study Design
In this 6-month, randomized, double-blind, placebo controlled, single site study a 2:2 block computer randomization was used. The primary outcome, prostate volume, was measured by MRI at baseline and during the final week of treatment month 6. Secondary outcomes (hormones, PSA, PVR, maximum urinary flow rate, I-PSS) were measured at baseline, and at months 3 and 6 when the medical history and clinical symptoms were assessed, and a physical examination was performed.
For safety monitoring serum PSA was evaluated at the month 3 visit by nonblinded study personnel. If the PSA increased by 1 ng/ml or greater above baseline, it was repeated within 2 weeks. If the repeat value remained 1 ng/ml or greater above baseline, the subject chart was reviewed by the safety officer. Subjects were withdrawn for drug noncompliance (less than 80% of study drugs taken) or temporarily for a hematocrit greater than 54% until clinical reevaluation and resolution with testosterone dose adjustment.
Hormone Assays and Safety Laboratory Tests
Serum androgens were quantified by liquid chromatography-tandem mass spectrometry on a Waters Acquity UPLC® coupled with a Micromass Premiere™ XE tandem quadrupole mass spectrometer as described previously using deuterated internal standards.15 Intra-assay coefficients were testosterone 4.9% and DHT 4.4%. Serum LH, FSH and SHBG were quantified by immunofluorometric assay.15 Samples for all subjects were measured in 1 assay. Free testosterone was calculated using the Södergard equation. PSA, chemistry studies and complete blood counts were measured in the VAPSHCS clinical laboratory.
Prostate MRI
Prostate MRI was performed on a 1.5 Tesla MRI machine (Signa HDx, GE Medical Systems, Milwaukee, Wisconsin). All images were read by a single radiologist blinded to subject identifiers and randomization status. Noncontrast T2-weighted images were obtained in 2 planes (axial and sagittal) with a slice thickness of 3 mm with a gap of 1 mm between slices. The region of interest was drawn on each slice in the axial plane and the prostate volume was calculated as the sum of the area on all slices (cm2) × 0.4 (accounting for the slice thickness and slice gap).
Statistical Analyses
As no data were available regarding the effect of T+D on prostate volume, sample size estimates were based on the effect size of 17% with a standard deviation of 20% for dutasteride alone.16 We estimated a sample size of 22 subjects per group needed for 80% power at α = 0.05.
Intent to treat analysis was performed (in 53) with the last observation carried forward in all cases. There were no differences in the conclusions using intent to treat or completers only (46) analyses. Due to nonnormality hormone data were log transformed before analysis with paired t tests and 2-sample t tests for baseline and between group comparisons, respectively, using STATA® version 10.0 with α = 0.05 considered significant.
RESULTS
Study Population
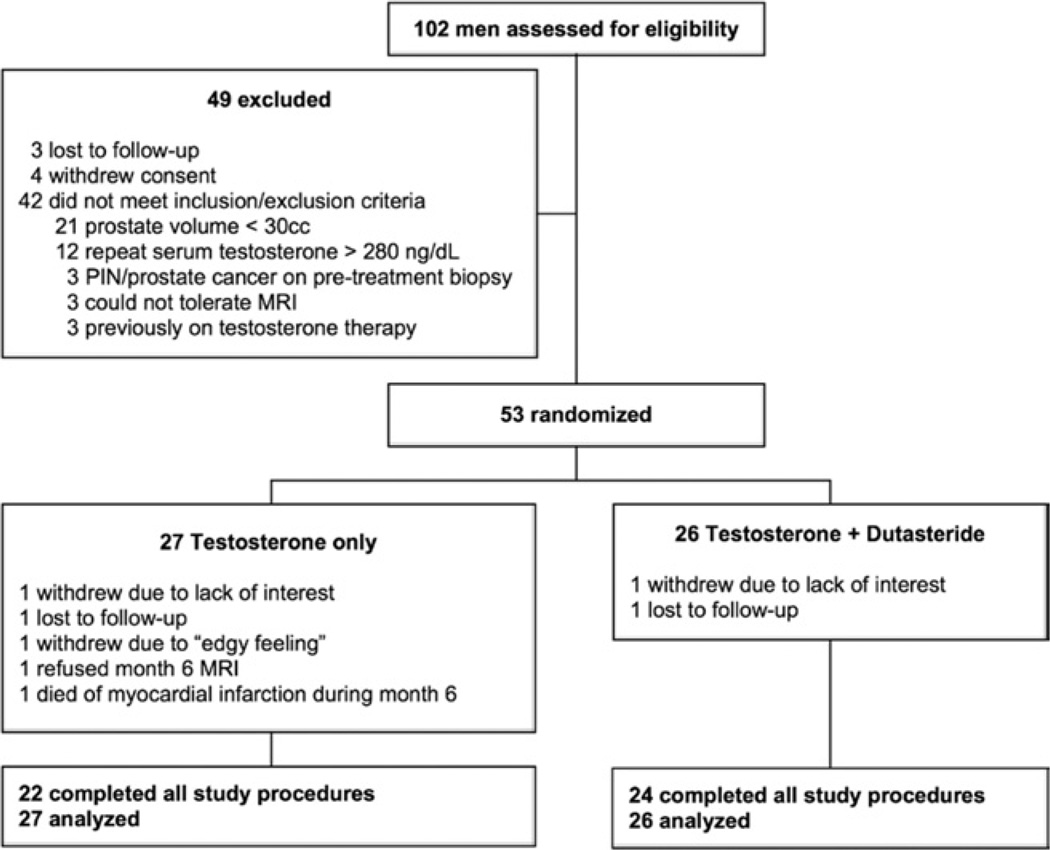
Study enrollment and randomization are depicted in figure 1. There were no significant differences between subjects in the 2 treatment groups at baseline (table 1). Of the subjects 31 required testosterone dose adjustment during the first month to achieve the target testosterone concentration of 500 to 1,000 ng/dl. Eight subjects required an adjustment down to 5 or 2.5 mg/day, 5 and 3 in the T only and T+D groups, respectively. There were 23 subjects who required increases to 10 or 12.5 mg daily, 12 and 11 in the T only and T+D groups, respectively.
Figure 1.
Profile of study enrollment, randomization and completion. PIN, prostatic intraepithelial neoplasia.
Table 1.
Baseline characteristics for all subjects enrolled
| Mean ± SD T Only (27) |
Mean ± SD T + D (26) |
p Value | |
|---|---|---|---|
| Age | 63.5 ± 8.0 | 63.6 ± 5.5 | 0.60 |
| Wt (kg) | 103.5 ± 18.1 | 104.1 ± 16.6 | 0.90 |
| Body mass index (kg/m2) | 32.9 ± 5.7 | 32.2 ± 4.1 | 0.60 |
| Total T (ng/dl) | 206 ± 109 | 213 ± 68 | 0.51 |
| DHT (ng/dl) | 47 ± 94 | 28 ± 15 | 0.58 |
| PSA (ng/ml) | 2.9 ± 2.9 | 2.1 ± 1.3 | 0.78 |
| Prostate vol (cc) | 54.3 ± 38.1 | 44.4 ± 19.8 | 0.44 |
| I-PSS | 13.5 ± 2.7 | 13.3 ± 3.1 | 0.68 |
| Uroflow (cc/sec) | 13.8 ± 3.0 | 13.4 ± 3.5 | 0.64 |
| PVR (cc) | 43 ± 44 | 48 ± 55 | 0.49 |
For conversion from ng/dl to nmol/l multiply by 0.03467.
Serum Testosterone and DHT Concentrations
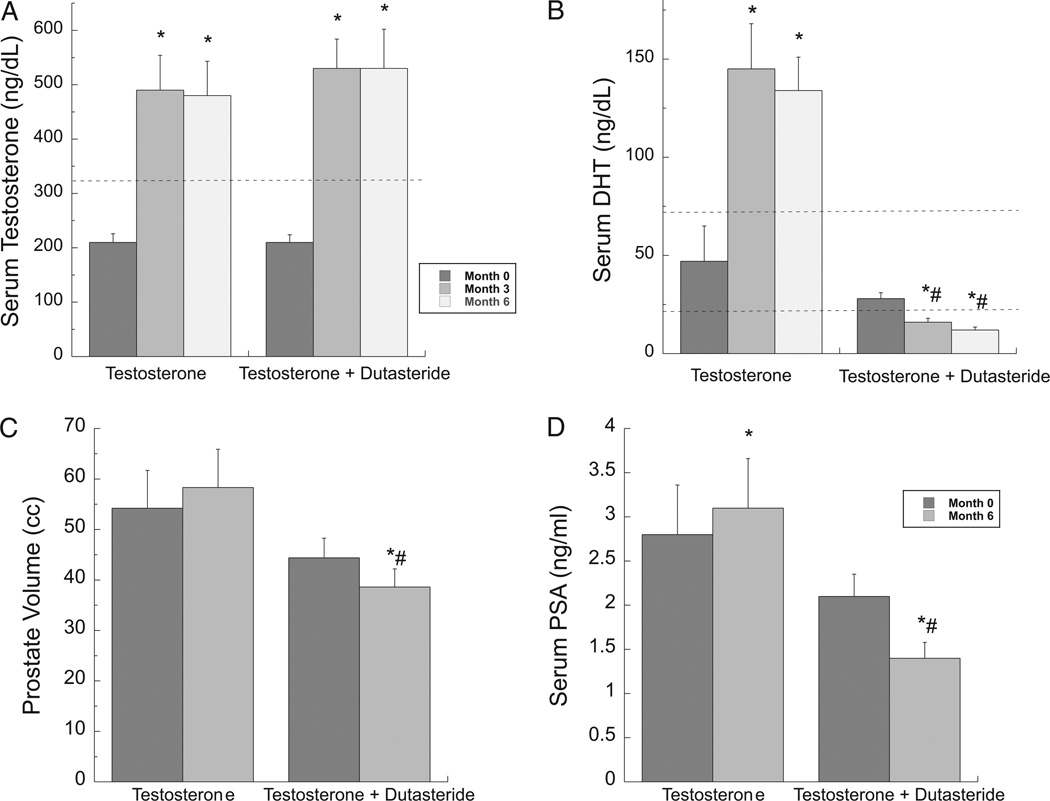
Both treatment groups had significant, approximately 2.5-fold, increases in serum testosterone compared to baseline, achieving concentrations within the normal range (fig. 2, A and table 2). There were no significant differences in serum total and free testosterone between the treatment groups. Serum DHT significantly increased compared to baseline in the T only group (fig. 2, B). In contrast, subjects in the T+D group had a significant decrease in serum DHT compared to baseline, resulting in significant differences between the 2 groups during treatment.
Figure 2.
Serum androgen concentrations, PSA and prostate volume in older, hypogonadal men with BPH treated with T only or T+D for 6 months. Serum was collected 12 to 32 hours after most recent testosterone gel application. A, serum T. B, serum DHT. C, prostate volume measured by MRI. D, serum PSA. Error bars are ± SEM. Broken lines denote lower end (for T) or normal range (for DHT) for circulating levels in healthy young men. Asterisk indicates p <0.05 vs baseline. Pound sign indicates p <0.05 vs T only. For conversion to nmol/l multiply by 3.467.
Table 2.
Serum hormone levels
| Mean ± SD T Only |
Mean ± SD T + D |
|||||
|---|---|---|---|---|---|---|
| Day 0 | Mo 3 | Mo 6 | Day 0 | Mo 3 | Mo 6 | |
| Total testosterone (ng/dl) | 206 ± 109 | 494 ± 331* | 481 ± 329* | 213 ± 68 | 525 ± 268* | 534 ± 360* |
| DHT (ng/dl) | 47 ± 94 | 145 ± 120* | 134 ± 87* | 28 ± 15 | 16 ± 11*,† | 12 ± 7*,† |
| Free testosterone (ng/dl) | 4.2 ± 2.0 | 11.3 ± 6.8* | 11.4 ± 11.1* | 4.5 ± 1.8 | 12.0 ± 6.1* | 12.3 ± 9.6* |
| Estradiol (pg/ml) | 12.7 ± 8.1 | 21.4 ± 13.3* | 19.7 ± 13.9* | 17.1 ± 11.2 | 28.3 ± 17.7* | 39.3 ± 23.8* |
| DHEA (ng/dl) | 72 ± 42 | 98 ± 92 | 97 ± 86 | 99 ± 68 | 109 ± 93 | 111 ± 90 |
| Androstenedione (ng/dl) | 45 ± 21 | 99 ± 72* | 100 ± 57* | 47 ± 28 | 140 ± 60* | 123 ± 61* |
| LH (IU/l) | 4.0 ± 3.3 | 1.0 ± 1.5* | 1.4 ± 1.7* | 4.5 ± 2.3 | 1.4 ± 1.7* | 2.4 ± 2.9* |
| FSH (IU/l) | 7.1 ± 6.3 | 1.8 ± 2.5* | 4.0 ± 6.1* | 6.9 ± 5.9 | 2.4 ± 2.3* | 4.0 ± 5.6* |
| SHBG (µg/ml) | 3.8 ± 1.9 | 3.6 ± 2.0 | 4.1 ± 2.4 | 3.6 ± 2.5 | 3.6 ± 2.6 | 3.9 ± 2.8 |
For conversion from ng/dl to nmol/l multiply by 0.03467.
p <0.05 vs baseline.
p <0.05 vs T only.
Prostate Volume
After 6 months of testosterone replacement, subjects in the T+D group had a significantly smaller prostate volume compared to those in the T only group (p = 0.03; fig. 2, C; table 3). In the T only group there was a small increase in prostate volume from baseline that did not reach significance (7.5% ± 3.3%, p = 0.07). In contrast, in the T+D group there was a significant decrease in prostate volume from baseline (−12.0% ± 2.6%, p <0.005).
Table 3.
Prostate dynamic measures and symptom scores
| Mean ± SD T Only |
Mean ± SD T + D |
|||||
|---|---|---|---|---|---|---|
| Day 0 | Mo 3 | Mo 6 | Day 0 | Mo 3 | Mo 6 | |
| Prostate vol (cc) | 54.2 ± 38.1 | — | 58.3 ± 38.7 | 44.4 ± 19.8 | — | 38.6 ± 18.4*,† |
| PSA (ng/ml) | 2.8 ± 2.9 | — | 3.1 ± 2.9* | 2.1 ± 1.3 | — | 1.4 ± 1.2*,† |
| I-PSS | 13.5 ± 2.7 | 11.6 ± 5.0* | 11.1 ± 5.2* | 13.3 ± 3.1 | 10.2 ± 5.4* | 10.3 ± 6.6* |
| Uroflow (cc/sec) | 13.8 ± 3.0 | 12.7 ± 3.4* | 13.8 ± 5.1 | 13.4 ± 3.5 | 13.2 ± 5.8 | 14.6 ± 6.7 |
| PVR (cc) | 43 ± 44 | 36 ± 36 | 39 ± 45 | 48 ± 55 | 41 ± 42 | 32 ± 36 |
p <0.05 vs baseline.
p <0.05 vs T only.
Serum PSA and Other Prostate Related Outcomes
Serum PSA increased slightly but significantly during the treatment period vs baseline in the T only group (19% ± 36%, p = 0.008; fig. 2, D). In contrast, subjects in the T+D group had a significant decrease in serum PSA (−35% ± 26%, p = 0.0006), resulting in a significant difference in PSA between the groups at month 6 (p = 0.008). Changes in prostate volume and in PSA correlated with the changes in serum DHT but not with changes in other androgen concentrations (r = 0.41, p = 0.005 for change in prostate volume; r = 0.72, p <0.001 for change in PSA). As expected, the change in PSA correlated well with the change in prostate volume (r = 0.37, p = 0.01).
The I-PSS score improved slightly in both treatment groups (table 3). Urinary flow rates worsened transiently in the T only group, but otherwise there were no changes in urinary flow rates or PVRs in either group, or differences between the groups in these parameters at any point.
Other Hormones
In both groups serum estradiol increased significantly, and LH and FSH were similarly suppressed with treatment (table 2). Serum concentrations of SHBG and the adrenal androgen DHEA were not affected by treatment. However, levels of androstenedione, which is a testosterone precursor and metabolite,17 were markedly increased in both groups.
Adverse Events
In each group 2 men noticed mild breast tenderness. There were no significant changes or differences between the groups in weight (data not shown). There were no reported adverse effects on libido or mood (which tended to improve in both groups), or energy level. In the T+D group 1 subject had an exacerbation of preexisting colitis and another of eczema. In the T only group 2 men had concerning increases in PSA, with 1 that prompted a prostate biopsy that was negative and another that returned to baseline with a reduction in testosterone dose. In the T only group 2 subjects had an increased hematocrit (greater than 52%) which responded to T dose adjustment. A transient rash developed in 1 subject at the gel application site.
There were 2 serious adverse events in individuals in the T only group. One subject died of a myocardial infarction during month 6 and a second, who completed the study, had a non-ST segment myocardial infarction during month 4. Both subjects had a history of cardiovascular disease before randomization and a normal hematocrit.
DISCUSSION
We found that T alone and T+D effectively increased serum testosterone similarly into the mid-normal range in hypogonadal men with prostatic enlargement and moderate symptomatic BPH. In contrast to T alone that slightly, although not significantly, increased prostate size and significantly increased PSA, T+D decreased prostate volume and serum PSA. These findings suggest that the combination of T+D might be a safe treatment for hypogonadism in older men with BPH that has less stimulatory effect on the prostate gland compared with testosterone treatment alone.
Men with symptomatic BPH have been specifically excluded from most trials of testosterone replacement reported to date due to concerns regarding symptom exacerbation. Thus, this trial is unique in studying the effect of testosterone replacement and coadministration of a potent 5αRI, dutasteride, in hypogonadal men with documented prostatic enlargement and moderate LUTS. We demonstrated previously that the combination of testosterone plus a type II specific 5αRI, finasteride, prevented increases in prostate volume and PSA observed in older men with low to low-normal testosterone levels but without BPH.8 Moreover in that study a less sensitive measure of prostate size was used (transrectal ultrasound).
In this study LUTS improved slightly without alteration in measures of urinary tract physiology in both treatment groups. While these findings may represent regression to the mean, others have observed improvement in LUTS with testosterone therapy.18 Our results suggest that preexisting prostatic enlargement and symptomatic BPH should not necessarily preclude the treatment of hypogonadal men with testosterone. The incidence of urinary obstruction and retention requiring intervention (both rare events) as well as BPH progression are strongly related to prostate volume and PSA.19–21 Therefore, the differences in prostate size between the T only and T+D groups may be clinically relevant, especially over years of treatment. A longer, larger, trial is needed to determine whether there are significant differences in BPH complications between T only vs T+D treatment because our study was not adequately powered to assess these clinical end points.
Older men are at significant risk for the development of prostate cancer. Small trials have not demonstrated an increased risk of prostate disease with testosterone treatment and high endogenous androgen levels are not associated with an increased prostate cancer risk.22 Our findings are timely given recent chemoprevention trials that demonstrated a 25% reduction in the risk of developing prostate cancer in men treated with 5αRIs and a decreased risk of urinary obstruction.11,12 The combination of testosterone plus a 5αRI may be an effective way for hypogonadal men with BPH to achieve the symptomatic improvements in androgen deficiency without increasing their risk of prostate cancer. Moreover recent data suggest that low T concentrations are associated with earlier mortality.23 Whether T replacement decreases mortality in hypogonadal men has not been tested, but our results suggest that men with preexisting prostatic enlargement should not be excluded from long-term trials of androgen replacement designed to assess important clinical risks and benefits.
The mechanism whereby T+D reduces prostate volume is likely attributable to changes in the intraprostatic hormonal milieu. In our study changes in prostate volume correlated with changes in serum DHT but not T, DHEA or androstenedione, highlighting the importance of DHT in prostate growth. 5αRIs decrease serum and prostate DHT concentrations24 but since DHT is the predominant androgen within the prostate (but not in serum), 5αRIs appear to have relatively prostate selective physiological effects. Thus, combining physiological doses of testosterone with a 5αRI might decrease prostate androgen concentrations and action with relative preservation of the beneficial end organ effects of testosterone,8 including increased bone mineral density and muscle mass,8,25 a goal of nonsteroidal selective androgen receptor modulator development and perhaps prostate cancer chemoprevention. Whether current standards for monitoring PSA in the setting of testosterone treatment or 5αRI administration5 should apply to men treated with the combination will need to be addressed in future studies.
Of note, 2 subjects in the T only group had significant cardiovascular events during treatment. This study was not sufficiently powered to assess cardiovascular end points and, thus, caution should be taken in interpreting this observation. However, in light of a recent study of frail, mobility impaired older men which demonstrated an increase in adverse cardiovascular events in men treated with testosterone vs placebo,26 patients with preexisting cardiovascular disease should be closely monitored while on testosterone therapy.
CONCLUSIONS
We have demonstrated that the combination of T+D effectively decreases prostate volume and PSA in men with hypogonadism and BPH compared to T only, but does not impact the achievement of therapeutic serum T levels. T+D is a promising strategy for the treatment of hypogonadism in men with BPH. Larger studies are needed to compare the impacts of these therapies on urological symptoms and complications.
ACKNOWLEDGMENTS
Supported by an investigator initiated grant from GlaxoSmithKline.
Study received institutional review board approval.
Ms. Kathy Winter (VAPSHCS, Seattle, Washington) coordinated this research study. T gel was provided at no cost by Solvay Pharmaceuticals.
Abbreviations and Acronyms
- 5αRI
5α-reductase inhibitor
- BPH
benign prostatic hyperplasia
- D
dutasteride
- DHEA
dehydroepiandrosterone
- DHT
dihydrotestosterone
- FSH
follicle-stimulating hormone
- I-PSS
International Prostate Symptom Score
- LH
luteinizing hormone
- LUTS
lower urinary tract symptoms
- MRI
magnetic resonance imaging
- PSA
prostate specific antigen
- PVR
post-void residual
- SHBG
sex hormone-binding globulin
- T
testosterone
- T + D
testosterone gel plus dutasteride
- VAPSHCS
Veterans Affairs Puget Sound Health Care System
Footnotes
Recipient of Grant K23 AG027238 from the National Institute on Aging, a Division of the National Institutes of Health, and a Clinical Research Award from the Endocrine Society.
Supported by the Veterans Affairs Special Fellowship Program in Advanced Geriatrics.
Nothing to disclose.
Supported by the Department of Veterans Affairs.
REFERENCES
- 1.Schatzl G, Brössner C, Schmid S, et al. Endocrine status in elderly men with lower urinary tract symptoms: correlation of age, hormonal status, and lower urinary tract function. The Prostate Study Group of the Austrian Society of Urology. Urology. 2000;55:397. doi: 10.1016/s0090-4295(99)00473-2. [DOI] [PubMed] [Google Scholar]
- 2.Garraway WM, Collins GN, Lee RJ. High prevalence of benign prostatic hypertrophy in the community. Lancet. 1991;338:469. doi: 10.1016/0140-6736(91)90543-x. [DOI] [PubMed] [Google Scholar]
- 3.Gruenewald DA, Matsumoto AM. Testosterone supplementation therapy for older men: potential benefits and risks. J Am Geriatr Soc. 2003;51:101. doi: 10.1034/j.1601-5215.2002.51018.x. [DOI] [PubMed] [Google Scholar]
- 4.Beg S, Al-Khoury L, Cunningham GR. Testosterone replacement in men. Curr Opin Endocrinol Diabetes Obes. 2008;15:364. doi: 10.1097/MED.0b013e328305081a. [DOI] [PubMed] [Google Scholar]
- 5.Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 6.Jin B, Conway AJ, Handelsman DJ. Effects of androgen deficiency and replacement on prostate zonal volumes. Clin Endocrinol (Oxf) 2001;54:437. doi: 10.1046/j.1365-2265.2001.01240.x. [DOI] [PubMed] [Google Scholar]
- 7.Behre HM, Bohmeyer J, Nieschlag E. Prostate volume in testosterone-treated and untreated hypogonadal men in comparison to age-matched normal controls. Clin Endocrinol (Oxf) 1994;40:341. doi: 10.1111/j.1365-2265.1994.tb03929.x. [DOI] [PubMed] [Google Scholar]
- 8.Amory JK, Watts NB, Easley KA, et al. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab. 2004;89:503. doi: 10.1210/jc.2003-031110. [DOI] [PubMed] [Google Scholar]
- 9.Marberger M, Roehrborn CG, Marks LS, et al. Relationship among serum testosterone, sexual function, and response to treatment in men receiving dutasteride for benign prostatic hyperplasia. J Clin Endocrinol Metab. 2006;91:1323. doi: 10.1210/jc.2005-1947. [DOI] [PubMed] [Google Scholar]
- 10.Marberger M. Drug Insight: 5alpha-reductase inhibitors for the treatment of benign prostatic hyperplasia. Nat Clin Pract Urol. 2006;3:495. doi: 10.1038/ncpuro0577. [DOI] [PubMed] [Google Scholar]
- 11.Andriole GL, Bostwick DG, Brawley OW, et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362:1192. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 12.Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 13.Marks LS, Kaplan SA. Should testosterone replacement be given to men with large prostates? J Urol. 2009;182:2109. doi: 10.1016/j.juro.2009.08.101. [DOI] [PubMed] [Google Scholar]
- 14.Gao W, Dalton JT. Ockham’s razor and selective androgen receptor modulators (SARMs): are we overlooking the role of 5alpha-reductase? Mol Interv. 2007;7:10. doi: 10.1124/mi.7.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roth MY, Page ST, Lin K, et al. Dose-dependent increase in intratesticular testosterone by very low-dose human chorionic gonadotropin in normal men with experimental gonadotropin deficiency. J Clin Endocrinol Metab. 2010;95:3806. doi: 10.1210/jc.2010-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Debruyne F, Barkin J, van Erps P, et al. Efficacy and safety of long-term treatment with the dual 5 alpha-reductase inhibitor dutasteride in men with symptomatic benign prostatic hyperplasia. Eur Urol. 2004;46:488. doi: 10.1016/j.eururo.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Luu-The V, Bélanger A, Labrie F. Androgen biosynthetic pathways in the human prostate. Best Pract Res Clin Endocrinol Metab. 2008;22:207. doi: 10.1016/j.beem.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Kalinchenko S, Vishnevskiy EL, Koval AN, et al. Beneficial effects of testosterone administration on symptoms of the lower urinary tract in men with late-onset hypogonadism: a pilot study. Aging Male. 2008;11:57. doi: 10.1080/13685530801953994. [DOI] [PubMed] [Google Scholar]
- 19.Roehrborn CG, McConnell JD, Saltzman B, et al. Storage (irritative) and voiding (obstructive) symptoms as predictors of benign prostatic hyperplasia progression and related outcomes. Eur Urol. 2002;42:1. doi: 10.1016/s0302-2838(02)00210-5. [DOI] [PubMed] [Google Scholar]
- 20.Crawford ED, Wilson SS, McConnell JD, et al. Baseline factors as predictors of clinical progression of benign prostatic hyperplasia in men treated with placebo. J Urol. 2006;175:1422. doi: 10.1016/S0022-5347(05)00708-1. [DOI] [PubMed] [Google Scholar]
- 21.Roehrborn CG, McConnell JD, Lieber M, et al. Serum prostate-specific antigen concentration is a powerful predictor of acute urinary retention and need for surgery in men with clinical benign prostatic hyperplasia. PLESS Study Group. Urology. 1999;53:473. doi: 10.1016/s0090-4295(98)00654-2. [DOI] [PubMed] [Google Scholar]
- 22.Roddam AW, Allen NE, Appleby P, et al. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008;100:170. doi: 10.1093/jnci/djm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93:68. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rittmaster R, Hahn RG, Ray P, et al. Effect of dutasteride on intraprostatic androgen levels in men with benign prostatic hyperplasia or prostate cancer. Urology. 2008;72:808. doi: 10.1016/j.urology.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 25.Page ST, Amory JK, Bowman FD, et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005;90:1502. doi: 10.1210/jc.2004-1933. [DOI] [PubMed] [Google Scholar]
- 26.Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]