Abstract
Background:
As glass ionomers have the ability to reload fluoride from outside sources, the aim was to compare the recharge pattern of six glass ionomer cements after exposure to fluoride.
Materials and Methods:
Fuji VII, Fuji IX, Riva Pink, Riva Bleach, Ketac Fil and Fuji IX Extra were investigated. The fluoride-containing materials used were tooth paste and mouth wash (Colgate). Specimens of each material (n=15) were immersed separately in deionized water for 59 days. Then the samples of each material were divided into three groups of five each. Two groups were recharged for 2, 20 and 60 min daily during three consecutive weekly intervals and then no treatment for one week. The third group was used as control. Fluoride release measurements (μg/cm2/day) were made in every 24 h. One-way and repeated measures analysis of variance tests were used.
Results:
Tooth paste recharged materials showed higher level of recharge. On day 1, the difference of fluoride release from different treatment groups of different materials except for Fuji IX Extra were not significant (P>0.05). On days 7 and 14, the differences observed were significant (P<0.05) for all materials except for Fuji VII (tooth paste versus mouth wash) and Trial Fuji IX (mouth wash versus control) and on day 14 for Rvia Pink (mouth wash versus control). On days 21 and 28, the differences observed were significant for all the materials (P<0.05) except for Riva Pink (toothpaste versus mouth wash), Riva Bleach, Ketac Fil and Trial FujiI X (mouth wash versus control) on day 28.
Conclusion:
A time tabled schedule of application of fluoride-containing materials could help to achieve high fluoride release.
Keywords: Glass ionomer cements, mouth wash, recharge, toothpaste
INTRODUCTION
Fluoride release ability of glass ionomer cements is well documented.[1–5] Anti-caries property is established by increasing enamel resistance to demineralization[6,7] and enhancing remineralization of the early carious lesion.[8] The majority of fluoride released from glass ionomers occur within first day after the restoration,[9] which may be attributed to high instability and erosion of glass ionomers during the early setting period.[10] After this, there is a rapid decrease over the next few days.[2,11] It is thought that, this fluoride release drop over time restricts the ability of the materials to inhibit secondary caries around restorations. It is because, the low doses of fluoride released may not be at levels which are required for preventive effects.[12] So, the potential for fluoride recharge by a dentifrice or mouth wash is suggested to be more important than fluoride release alone.[13] The exposure of dental materials to topical fluoride creates a fluoride recharge potential in vitro.[13–18] The ability of a restoration to act as a fluoride reservoir is mainly dependent on the kind and permeability of filling material, the frequency of fluoride exposure and the kind and concentration of fluoride agent.[19]
A number of new GIC materials have been introduced which claim to have improved therapeutic properties. The aim of this study was to assess and compare the recharge pattern of six conventional and new aged glass ionomer cements following exposure to a fluoride mouth wash or toothpaste containing sodium fluoride (NaF) over different exposure times (2, 20, 60 min).
MATERIALS AND METHODS
Six glass-ionomer cements were investigated in this study including Fuji VII, Fuji IX, Fuji IX Extra (GC Corporation, Tokyo, Japan), Riva Pink Protect, Riva Bleach Protect (SDI, Australia) and Ketac Fil (3 M, ESPE, USA). The fluoride-containing agents used were toothpaste (Colgate total 1000 ppm F) and mouth wash (Mouth Rinse 220 ppm F, Colgate).
Specimen preparation
Cylindrical cavities (4 mm diameter and 4 mm depth) were prepared in acrylic resin blocks using a drill press (Morgon, Taiwan). Two shallow retentive points were made at the opposite sides of the holes with ½ round bur. The diameter and depth were measured using an electronic digital caliper (Dentagauge 2, Erskine Dental, Private Box 448, 13428 Maxella Ave, Marina Dell Rey CA 90292 USA).
The materials were mixed according to manufacturer's instructions and placed in the prepared cavities. The specimens were covered by a mylar strip and glass slides were allowed to set at room temperature for 10 min. Excess material was removed by pressure applied on the glass slide.
Prior to testing, the specimens were stored in a 95% relative humidity environment at 37°C for 24 h.
Each specimen of each material (n=15) was immersed in 1 ml of deionized water in polyethylene vials and incubated in an incubator (Lindner and May, Australia) at 37°C.
After 24 h, the containers were thoroughly shaken and the samples were removed, rinsed, dried and then reimmersed into a new vial containing 1 ml of deionized water. The procedure was repeated for 17 days. Following this, all samples were immersed in 500 ml of distilled water and on days 24, 31, 38, 45, 52 and 59 changing of water was performed. On day 60 samples were placed into new individual vials containing 1 ml of deionized water and the amount of fluoride released was measured after 24 h. This amount of fluoride release measurement on day 60 was considered as the base measurement of fluoride release after exhaustion of the materials. Then the 15 samples of each material were divided into three groups of five each. The first group used for treatment with fluoride agent (Mouth Rinse 220 ppm, Colgate), the second group for treatment with toothpaste (Colgate total) and the third group with no treatment as control.
The specimens for recharging with mouth rinse were immersed in the mouth rinse (1 ml) for 2 min and then were placed in vials containing 1 ml of deionized water for 24 h. Then the specimens were removed, dried and again were placed in 1 ml of mouth rinse for 2 min and then again were placed in 1 ml of dieonized water for 24 h. The treatment was repeated for one week. Fluoride measurements were made every 24 h. In the second and third weeks, the same procedure was followed by 20 min and 1 h immersion times. In the fourth week, no treatment was applied. In the case of specimens for recharging with toothpaste, the same procedure was performed. The specimens were exposed to 1 ml of toothpaste for 2 min, 20 min and 1 h for three consecutive weeks and then no treatment was perfomed. The specimens were wiped out by tissue before immersing in deionized water in each consequent step. In the control group, deionized water was changed in 24 h intervals and daily fluoride measurements were made.
Fluoride measurements were made using a fluoride ion selective electrode (Ion-Check 45, Radiometer Analytical, USA).
The instrument was calibrated according to manufacturer's instructions using six standard fluoride solutions containing 0.20, 1.00, 2.00, 10.00, 20.00 and 100 ppm F‾, respectively. To provide constant background ionic strength, decomplex fluoride, and adjust the solution pH before measurement, 0.1 ml of TISAB III (Total ionic strength adjustment buffer) (Orion Research Incorporated, USA) was added to each solution. The concentration (ppm) of each solution was recorded for each sample.
The final results were reported as fluoride release rate (μg/cm2/day) by taking into account the surface area and solution volume of each specimen using the following equation,[20] where 0.1256 cm2 is the surface area of the material tested.
μgF/cm2 = ppm F (μgF/ml) ml (Volume of medium at unit time) 1/0.1256 cm2
Statistical analyses
The Software GraphPad InStat version 3.0 Windows (GraphPad Software, Inc. 5755 Oberlin Drive, #10 San Diego, CA 92121 USA) was used for statistical analyses. One-way analysis of variance (ANOVA) and repeated measures ANOVA and Tukey-Kramer post test were performed to compare the amount of fluoride release of different materials in different days and also for the comparison of the change of fluoride release over time respectively. Data collected on first day and the day before the last day of each treatment i.e. days 7, 14, 21 and 28 were compared.
RESULTS
The mean and standard deviation of fluoride released from six glass ionomer cements tested with different treatments are shown in Table 1.
Table 1.
Mean and standard deviation (SD) of fluoride release from the six dental materials studied with different treatments
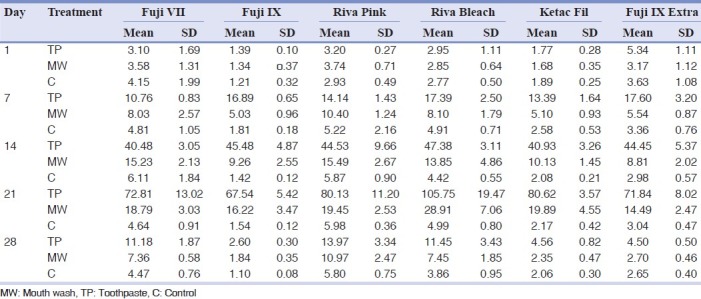
The pattern of fluoride release from different materials after recharging with toothpaste and mouth wash over time and also control are shown in Figures 1–6. Comparison of the pattern of fluoride released from six restoratives in different treatment groups are shown in Figures 7–9. In comparison to control groups, all materials recharged with toothpaste showed higher level of recharge. The pattern of uptake and release of fluoride for all the materials for each treatment were nearly the same. The recharge capability of the materials increased slightly during first week with 2 min treatment time.
Figure 1.
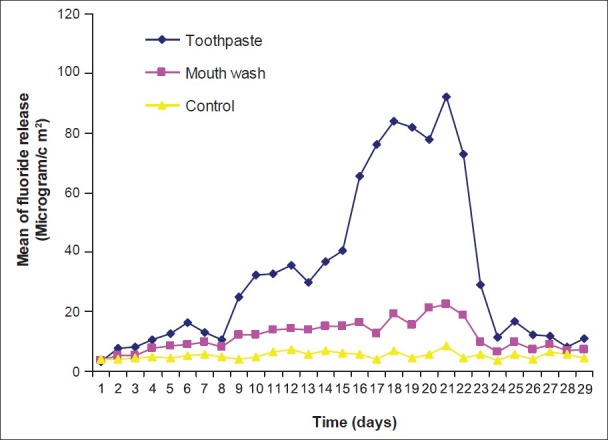
Pattern of fluoride release from Fuji VII after recharging with toothpaste and mouth wash
Figure 6.
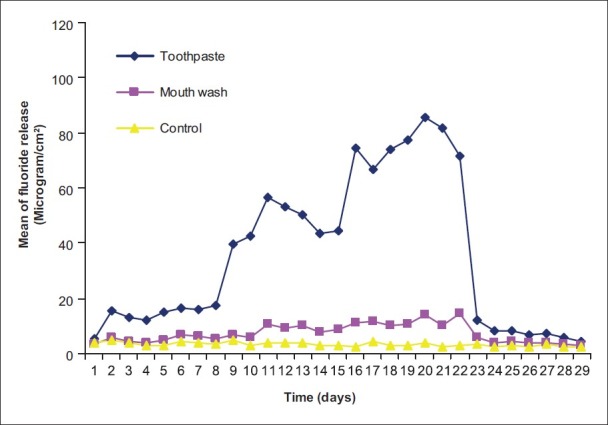
Pattern of fluoride release from Fuji IX Extra after recharging with toothpaste and mouth wash
Figure 7.
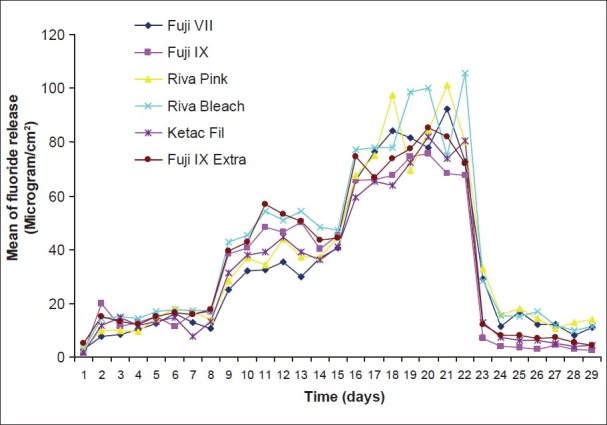
Pattern of fluoride release from six restoratives after recharging with toothpaste
Figure 9.
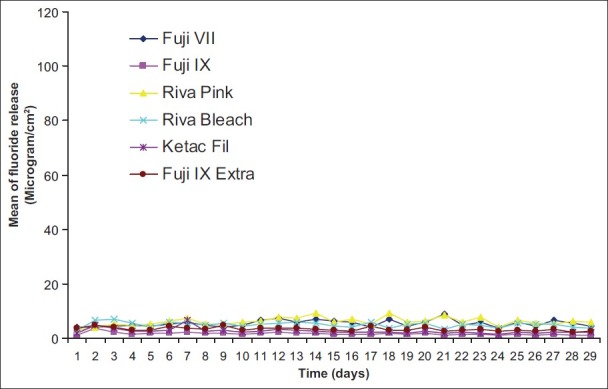
Pattern of fluoride release from six restoratives without treatment (control)
Figure 2.
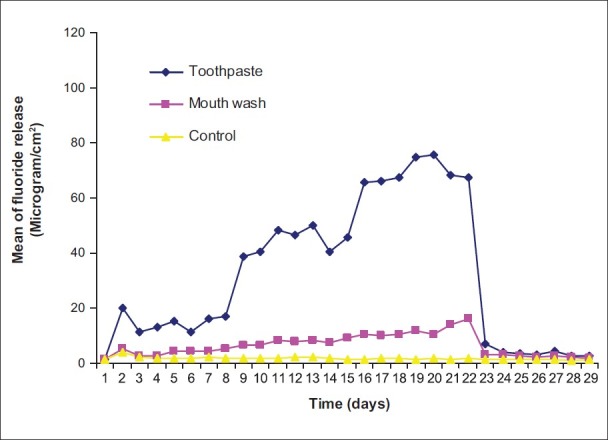
Pattern of fluoride release from Fuji IX after recharging with toothpaste and mouth wash
Figure 3.
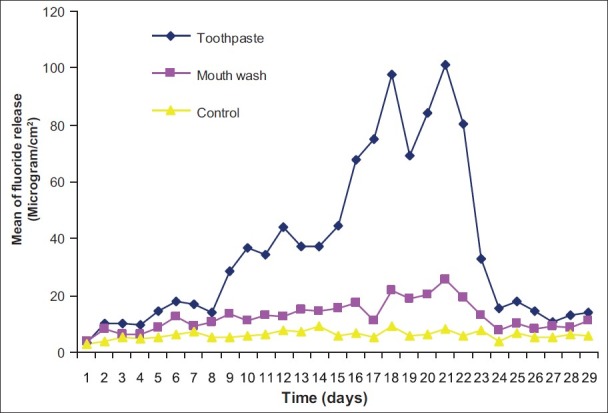
Pattern of fluoride release from Riva Pink after recharging with toothpaste and mouth wash
Figure 4.
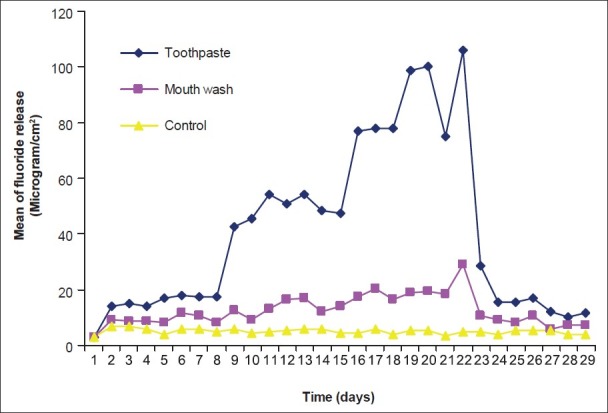
Pattern of fluoride release from Riva Bleach after recharging with toothpaste and mouth wash
Figure 5.
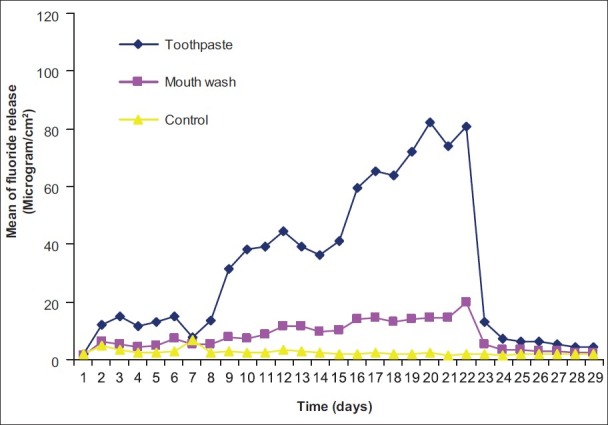
Pattern of fluoride release from Ketac Fil after recharging with toothpaste and mouth wash
Figure 8.
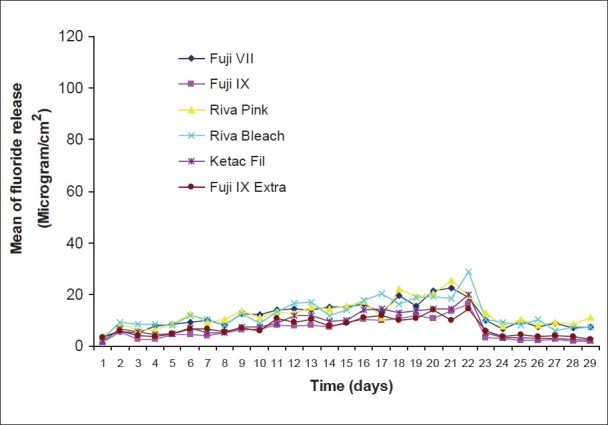
Pattern of fluoride release from six restoratives after recharging with mouth wash
The amount of recharge and consequent fluoride release were substantially increased to 7.7-20.1 and 5.43-9.25 μg/cm2 for toothpaste and mouth wash respectively on day 2 and increased slightly up to 7.61-17.3 and 4.24-10.5 μg/cm2 on day seven for toothpaste and mouth wash treatments respectively. In the second week with changing the treatment time to 20 min, the fluoride recharge and subsequent fluoride release again were sharply increased up to 25.1-42.8 μg/cm2 on day 9 and slightly increased up to day 14 (36.9-48.3 μg/cm2 ) for toothpaste treatment. For mouth wash treatment, the fluoride release after recharging for 20 min increased slightly up to 7-13.8 μg/cm2 on day 9 and slightly increased up to day 14 (7.55-15.1 μg/cm2). After 1 h treatment (third week), a substantial increase of recharge observed for toothpaste treatment while this was slight for mouth wash treatment groups. In the fourth week that no treatment was applied, the fluoride release sharply declined to base line levels after two days and with some fluctuations remained steady during the week for both toothpaste and mouth wash treatment groups. In control groups, the amount of fluoride release remained at base line levels with some fluctuations.
On day 1, the differences of fluoride release from different treatment groups of different materials except for Fuji IX Extra were not significant (P>0.05). In the case of Fuji IX Extra, the differences between mouth wash and toothpaste groups were significant (P<0.05) but their differences with the control group were not significant (P>0.05). On day 7, the differences observed among different treatment groups were significant (P<0.05-P<0.001) for all materials except for Fuji VII (toothpaste versus mouth wash) and Fuji IX Extra (mouth wash versus control). On day 14, the differences observed among different groups were significant (P<0.05-P<0.001) for all materials except for Rvia Pink (mouth wash versus control). On day 21, the difference observed among different treatment groups were significant for all the materials (P<0.05-P<0.001). On day 28, the difference observed among different groups were significant (P<0.05-P<0.001) except for Riva Pink (toothpaste versus mouth wash), Riva Bleach, Ketac Fil and Fuji IX Extra (mouth wash versus control).
The differences observed between day 1 and day 7 for different materials treated with toothpaste were significant for Fuji IX, Ketac Fil and Fuji IX Extra while for mouth wash the differences for Fuji VII, Fuji IX and Riva Pink were significant. In the case of controls, the differences for Fuji IX, Riva Bleach and Ketac Fil were significant. The differences observed between day 7 and day 14 for different materials treated with toothpaste were significant for all materials as well as for mouth wash it was the same except for Riva Bleach. In the case of controls the differences were not significant (P>0.05) except for Fuji IX. The differences observed between day 14 and 21 for different materials treated with toothpaste were significant for all materials while it was the same for mouth wash except for Riva Pink. In the case of controls, the differences were not significant (P>0.05) except for Riva Pink. The differences observed between day 21 and 28 for different materials treated with toothpaste and mouth wash were significant for all materials. In the case of controls, the differences were not significant (P>0.05) except for Fuji IX and Riva Pink. The differences observed between day 1 and 28 for different materials treated with toothpaste were not significant (P>0.05) for all materials while it was the same for mouth wash except for Fuji VII and Riva Pink. In the case of controls, the differences were not significant (P>0.05) except for Riva Pink.
The significant differences among different materials with different treatments in different time intervals (day 1, 7, 14, 21 and 28) are shown in Table 2.
Table 2.
Significant differences of mean of different materials with different treatments in different time intervals (days)
DISCUSSION
The pattern of recharge for the conventional glass ionomers after a sharp rise followed by a rapid decline and then gradual prolonged release which are similar to those reported in other studies[21–24] except for the data reported by Rao et al., indicating no significant rechargability for Fuji VII for up to 30 days after exposure to a 1000 ppm fluorinated dentifrice.[25] All the tested materials reacted positively to exposure to external fluoride source. The exposure did not have any effects on the underlying fluoride release from the cements and the release rates returned to base line within two days after stopping of application of fluoride. It is suggested that fluoride uptake may be more of a surface rather than a bulk diffusion effect and that reexposure of fluoride will enhance fluoride release. It has also been indicated that first exposure modifies cement, making it more susceptible to external fluoride and the possible disruption of the cement matrix on the first exposure and thus opening pathways for fluoride uptake on the second exposure.[21] The results of continuous recharging and release in current experiment and the significant increase of fluoride uptake and release by increasing of exposure time indicate that other factors such as the time of exposure may also be involved.
Mousavinasab and Meyers (2009) also observed higher fluoride release after recharging of glass ionomers with tooth paste. Higher fluoride release after tooth paste application compared to mouth wash was attributed to the retaining of the sticky tooth paste in the superficial pores of the glass ionomers tested.[5] In the current study, compared to mouth wash, higher rechargability was also observed when tooth paste was applied. With increasing of exposure time, the rechargability was increased. As the same methodology was used after immersing of the glass ionomers in the tooth paste at all time intervals, it seems the exposure time and the nature of tooth paste specially containing higher amount of fluoride influence the recharge pattern of glass ionomers with tooth paste rather than its sticky nature. This suggests, if possible, the use of higher concentrations of fluoride in mouth wash or more regular application to brushing with tooth paste.
CONCLUSION
If high fluoride uptake and release is expected for prevention of caries in a long period of time, a time tabled schedule of application of fluoride-containing materials could help to achieve high fluoride release. As the mechanism suggested for the increase in the uptake of fluoride may cause disruption of the cement matrix, the application of this procedure may affect the mechanical properties of the materials; therefore, further investigations on this effect should be considered.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Forsten L. Short and long-trem fluoride release from glass ionomers and other fluoride-containing filling materials in vitro. Scand J Dent Res. 1990;98:179–85. doi: 10.1111/j.1600-0722.1990.tb00958.x. [DOI] [PubMed] [Google Scholar]
- 2.Creanor SL, Carruthers LH, Saunders WP, Strang R, Foye RH. Fluoride uptake and release characteristics of glass ionomer cements. Caries Res. 1994;28:322–8. doi: 10.1159/000261996. [DOI] [PubMed] [Google Scholar]
- 3.De Moor RJ, Verbeeck RM, De Maeyer EA. Fluoride release profiles of glass ionomer formulations. Dent Mater. 1996;12:88–95. doi: 10.1016/S0109-5641(96)80074-1. [DOI] [PubMed] [Google Scholar]
- 4.Mousavinasab SM, Meyers I. Fluoride release by glass ionomer cements, compomer and giomer. Dent Res J (Isfahan) 2009;6:75–81. [PMC free article] [PubMed] [Google Scholar]
- 5.Mousavinasab SM, Meyers I. Fluoride release and uptake by glass ionomer cements, compomer and giomer. Res J Biol Sci. 2009;4:609–16. [PMC free article] [PubMed] [Google Scholar]
- 6.Hattab FN, Mok NYC, Agnew EC. Artificially formed caries-like lesions around restorative materials. J Am Dent Assoc. 1989;118:193–7. doi: 10.14219/jada.archive.1989.0233. [DOI] [PubMed] [Google Scholar]
- 7.Francii C, Deaton TG, Arnold RR, Swift EJ, Jr, Perdigao J, Bawden JW. Fluoride release from restorative materials and its effect on dentine demineralization. J Dent Res. 1999;78:1647–54. doi: 10.1177/00220345990780101001. [DOI] [PubMed] [Google Scholar]
- 8.Hatibovic-Kofman S, Suljak JP, Koch G. Remineralization of natural carious lesions with a glass ionomer cement. Swed Dent J. 1997;21:11–7. [PubMed] [Google Scholar]
- 9.El Mallak BF, Sarker NK. Fluoride release from glass ionomer cements in deionised water and artificial saliva. Dent Mater. 1990;6:118–22. doi: 10.1016/s0109-5641(05)80041-7. [DOI] [PubMed] [Google Scholar]
- 10.DeSchepper EJ, Berr EA, Cailleteau JG, Tate WH. A comparative study of fluoride release from glass ionomer cements. Quintessence Int. 1991;22:215–9. [PubMed] [Google Scholar]
- 11.Preston AJ, Mair LH, Agalamanyi EA, Higham SM. Fluoride release from aesthetic dental materials. J Oral Rehabil. 1999;26:123–29. doi: 10.1046/j.1365-2842.1999.00357.x. [DOI] [PubMed] [Google Scholar]
- 12.Behrend B, Geurtsen W. Long-term effects of four extraction media on the fluoride release from four polyacid-modified composite resins (compomers) and one resin-modified glass-ionomer cement. J Biomed Mater Res. 2001;58:631–7. doi: 10.1002/jbm.1062. [DOI] [PubMed] [Google Scholar]
- 13.Hatibovic-Kofman S, Koch G, Eksrand J. Glass ionomer materials as rechargeable fluoride-release system. Int J Paediater Dent. 1997;7:65–73. doi: 10.1111/j.1365-263x.1997.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 14.Creanor SL, Saunders WP, Carruthers LH, Strang R, Foye RH. Effect of extrinsic fluoride concentration on the uptake and release of fluoride from two glass ionomer cements. Caries Res. 1995;29:424–6. doi: 10.1159/000262103. [DOI] [PubMed] [Google Scholar]
- 15.Diaz-Arnold AM, Holmes DC, Wistrom DW, Swift EJ. Short-term fluoride release/uptake of glass ionomer restoratives. Dent Mater. 1995;11:96–101. doi: 10.1016/0109-5641(95)80041-7. [DOI] [PubMed] [Google Scholar]
- 16.Young A, Frithjof R, von der Fehr TF, Nordbo H. Fluoride release and uptake in vitro from a composite resin and two orthodontic adhesives. Acta Odontol Scand. 1996;54:223–8. doi: 10.3109/00016359609003528. [DOI] [PubMed] [Google Scholar]
- 17.Grobler SR, Roussouw RJ, van Wyk Kotze TJ. A comparison of fluoride release from various dental materials. J Dentist. 1998;26:259–65. doi: 10.1016/s0300-5712(97)00011-0. [DOI] [PubMed] [Google Scholar]
- 18.Perston AJ, Agalamanyi EA, Higham SM, Mair LH. Fluoride recharge of aesthetic dental materials. J Oral Rehabil. 1999;26:936–40. doi: 10.1046/j.1365-2842.1999.00502.x. [DOI] [PubMed] [Google Scholar]
- 19.Wiegand A, Buchalla W, Attin T. Review on fluoride-releasing restorative materials-Fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater. 2007;23:343–62. doi: 10.1016/j.dental.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 20.Hsu H, Huang G, Chang H, Wang Y, Guo M. A continuous flow system for assessing fluoride release/uptake of fluoride-containing restorative materials. Dent Mater. 2004;20:740–9. doi: 10.1016/j.dental.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Rothwell M, Anstice HM, Pearson GJ. The uptake and release of fluoride by ion-leaching cements after exposure to toothpaste. J Dent. 1998;26:591–7. doi: 10.1016/s0300-5712(97)00035-3. [DOI] [PubMed] [Google Scholar]
- 22.Xu X, Burgess JO. Compresive strength, fluoride release and recharge of fluoride releasing materials. Biomaterials. 2003;24:2451–61. doi: 10.1016/s0142-9612(02)00638-5. [DOI] [PubMed] [Google Scholar]
- 23.Itota T, Carrick TE, Yoshiyama M, McCabe JF. Fluoride release and recharge in giomer, compomer and resin composite. Dent Mater. 2004;20:789–95. doi: 10.1016/j.dental.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Yan Z, Sidhu SK, Mahmoud GA, Carrick TE, McCabe JF. Effects of temperature on the fluoride release and recharging ability of glass ionomers. Oper Dent. 2007;32:138–43. doi: 10.2341/06-36. [DOI] [PubMed] [Google Scholar]
- 25.Rao A, Sudha P. Fluoride rechargability of a non-resin auto-cured glass ionomer cement from a fluoridated dentifrice: An in vitro study. J Indian Soc Pedod Prev Dent. 2011;29:202–4. doi: 10.4103/0970-4388.85812. [DOI] [PubMed] [Google Scholar]


