Abstract
Background:
Oral lichen planus (OLP) is a chronic mucocutaneous lesion with unknown etiology. Oral lichenoid lesions (OLL) comprise a family of lesions with different etiologies. Both lesions have similar clinical and histopathologic characteristics although their management is different. Differential diagnosis between OLP and OLL has always been a major challenge.
Materials and Methods:
In this prospective analytical study, the role of mast cells in pathogenesis of these lesions was investigated by evaluation of 52 patients with clinical and histopathological diagnosis of OLP (26 cases) and OLL (26 cases) based on WHO criteria, and by applying a more accessible staining methods, Hematoxylin and Eosin, toluidine blue (histochemistry) and Periodic Acid Schiff staining. In order to distinguish these two lesions, number of mast cells and thickness of epithelium and basement membrane were measured using light microscopy. Data were analyzed by SPSS software using t-test method (P<0.001).
Results:
No significant difference was observed between the total numbers of mast cells of two groups (P=0.148), but a statistically significant difference was detected between degranulated mast cells in two groups (P<0.001). A significant difference was also observed between the thickness of epithelium in two groups (P<0.001), although no difference was seen between basement membrane thickness in these lesions.
Conclusion:
Number of degranulated mast cells in reticular layer of corium in lichenoid lesions was more than that of OLP. This implies that despite the increase in number of these cells, in both groups of diseases, the role of these cells has not been the same in pathogenesis of the diseases. Moreover, the epithelium thickness was lower in lesions of OLP compared to lesions of oral lichenoid, so this parameter may be a useful criterion together with other histopathological and clinical finding to discriminate these lesions. However, discrepancy of basement membrane thickness can not be a reliable criterion. Finally we suggest more accessible staining methods which are reliable for differentiation of these two lesions.
Keywords: Differential diagnosis, histochemistry, mast cells, oral lichen planus, oral lichenoid lesion
INTRODUCTION
Oral lichen planus (OLP) and oral mucosal lichenoid Lesions are among the lesions, which are causing confusion and lacking consensus among clinicians and pathologists. OLP is a mucocutaneous lesion developed as a result of failure in immunology system. Considering the clinical and histopathological aspects, these lesions are typically comparable to OLP; however, in most cases, an association with drug or allergic (sensitivity) reactions has been observed. Differentiation between these two lesions is a subject of attention and importance in order to avoid inappropriate treatments and superfluous expenditures. Particular clinical and histopathologic criteria were introduced by WHO in order to make a precise diagnosis.[1] Histopathologic features of lichen planus are not unique for the lesions because several other lesions such as lichenoid reactions to drug or restorative material, lupus erythematosus, graft versus host disease and chronic ulcerative stomatitis may also characterize or overlap similar histopathologic features.[2] Dubreuil[3] described microscopic characteristics of lichen planus in 1906 for the first time. Subsequently, Shaklar[4] presented following features for lichen planus’ microscopic view:
Sub epithelial dense infiltrations of lymphohistiocytic cells
Vacuole formation, edema and degeneration of hydropic keratinocytes of basal lamina
Rupture of epithelium's basement membrane
Augment in thickness of epithelium
Lichenoid lesions are similar to lichen planus; however, it may have deeper infiltrations of inflammatory cells containing eosinophils, plasma cells, and neutrophils instead of band like infiltrations of lymphocytes.[5,6]
Lichen planus is a lesion due to immunology system imbalance in which distinguished, specific, and nonspecific immunologic mechanisms interfere. Specific procedure occurs during cytotoxic T cells of CD8+ activation via antigen expression by keratinocytes of basal lamina. Nonspecific procedure takes place via degranulation of mast cells and matrix metalloproteinase activity.
These mechanisms generally include following steps:
Aggregation of T lymphocytes cells in superficial lamina properia
Destruction of basement membrane
Intra epithelial migration of T lymphocytes
Demolition of kertinocytes by apoptosis process.
Exact mechanisms of apoptosis and demolition of basal lamina cells are not identified yet. Infiltration of TNF-α and its attachment to the surface of keratinocytes by means of molecules of Fas ligand (CD95L) and Fas (CD95), activating Caspase cascade may be the reactor of apoptosis.[7–10]
Scores of endeavors have been made so far to come across histopathologic findings facilitating diagnosis and several other factors have been studied such as eosinophils aggregation,[11] number of Langerhans cells,[12] markers of CD4+, CD8+, CD1+, HLA-DR, Lichen planus specific Antigen and S100,[13–15] but none has lead to comprehensible results. Immunofluorescence is another method to distinguish these two lesions. This method is not applicable due to high expenses, difficulty in access and counterfeit negative results.[16] In the previous study of the author, there was an attempt to make a discrimination between these two lesions by immunohistochemistry using antitryptase, revealed reliable results.[17] In the present study, based on the role of mast cells in pathogenesis of these lesions, Toluidine Blue (TB) and periodic acid schiff (PAS) staining which are less expensive and more accessible are used to achieve findings in order to differentiate these two lesions, based on number of granulated mast cells and mean of epithelium and basement membrane thickness, we also reevaluated findings of previous study by applying a more accessible method. Moreover, possibility of any relationship between number of these cells and thickness of PAS-positive basement membrane and epithelium thickness in these lesions was investigated.
MATERIALS AND METHODS
Study population for this prospective analytical study are selected from patients referring to Isfahan school of dentistry, clinics affiliated to this school and dental offices in Isfahan city with potential diagnosis of lichen planus and oral lichenoid lesions (OLL).
Including criteria
Sufficient epithelium and band like inflammatory infiltration.
Sufficient connective tissue.
Excluding criteria
Dysplasia in ephithelium
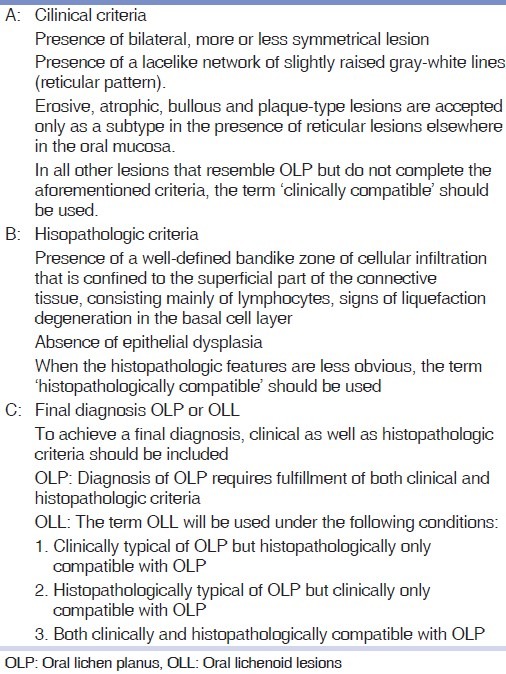
Same as our previous study,[17] specific form [Table 1] for clinical and histopathologic study of the patients was applied based on WHO criteria which contains 3 parts: “Section A” clinical criteria, “Section B” histopathologic criteria”, and “Section C” analysis of the criteria and final diagnosis. After clinical examination of patients (Section A) was completed then biopsy samples were taken from appropriate area of affected region. In order to avoid autolysis and degranulation of mast cells’ cytoplasm, samples were immediately placed in 10% formalin-buffered solution. Passing laboratory steps, samples were stained through H and E method and after microscopic study, “Section B”, i.e., histopathologic study form was completed. Clinical and histopathologic study form was arranged based on WHO criteria which were revised in year 2003. Last of all, considering data attained from sections “A and B”, section C was completed and the final diagnosis was signified.
Table 1.
Modified world health organization diagnostic criteria of OLP and OLL
Easy sampling method was chosen for the study and the sample volume was 26 for each group. Investigating the samples, mean of the epithelium thickness in H and E stained slide was determined. For this purpose, epithelium thickness was measured in two specified areas (maximum and minimum) using a graded eyepiece reticle. Measurement was performed from the surface of epithelium to deepest areas of basal cells next to lymphocyte band at a total magnification of ×100 and the mean of these two numbers was calculated at μm.
After that, mean of basement membrane thickness in each slide stained with PAS was determined. So, the width of basement membrane of positive PAS was measured in 3 identified areas using graded eyepiece reticle at magnification of ×400 and finally the mean of these 3 numbers was calculated at μm. Then, total number of mast cells and also degranulated mast cells was counted in 3 separated areas beneath the lymphocyte band in slides, stained with TB. After that, mean of these three numbers and the ratio of degranulated to total number of mast cells was calculated. Graded eyepiece reticle was utilized because of calibration of microscope field and also for avoiding areas of overlap. Data analyzed by using t-test and Pearson correlation test.
RESULTS
specimen stained with TB, mast cells were mostly observed beneath band like lymphocytic infiltration in reticular layer of connective tissue in an outspread manner or attached to vessels and less in papillary layer of corium. These cells were clearly identifiable with their metachromatically stained granules.
A difference was observed between the morphology of mast cells in this area. A number of cells demonstrated altered stainability trait signifying degranulation with cytoplasm lightening of the cell and distinct core or free granules around these cells.
In some cases, rupture in cytoplasmic membrane of the cell was clearly observed. These cells were considered as degranulated mast cells [Figure 1].
Figure 1.
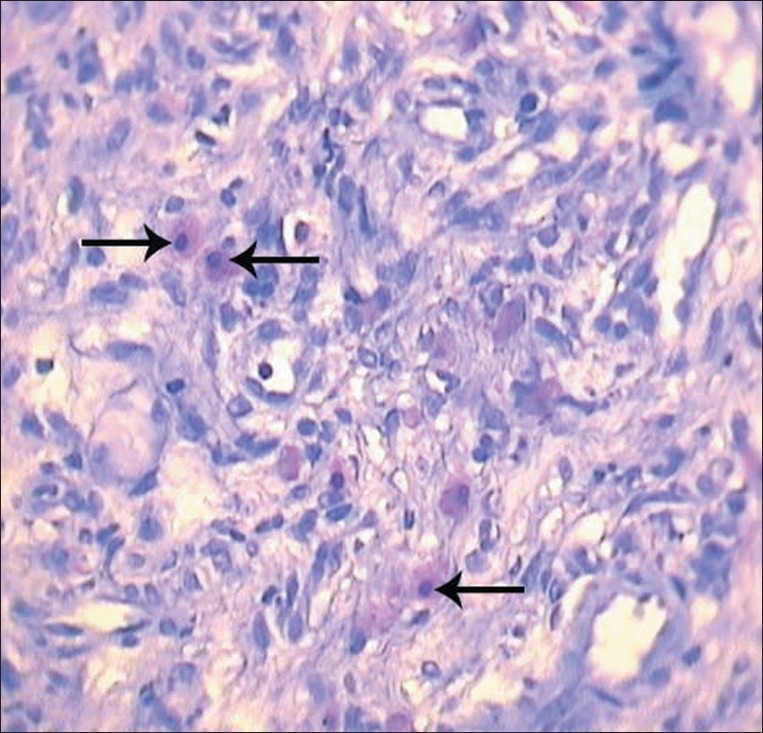
Degranulated mast cells (arrows) in reticular layer of corium (magnification of 40×10, TB staining)
In intact mast cells group, cytoplasm showed high density and stainibility, as the core was not visible and no free granule could be noticed around the cell. These mast cells were regarded as intact cells [Figure 2].
Figure 2.
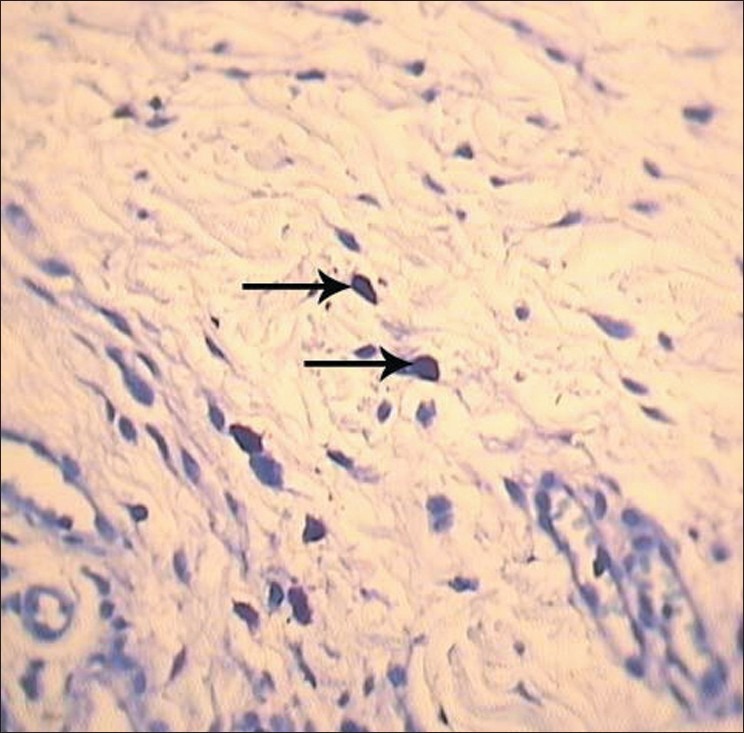
Intact mast cells (arrows) in reticular layer of corium (magnification of 40×10, TB staining)
Total number of mast cells stained with TB including both degranulated and intact ones, showed no statistically significant difference (P=0/148) between two groups of OLP (mean±SD=30.96±3.87) and OLL (mean±SD=29.07±5.26).
Comparing degranulated mast cells of OLP group (mean±SD=19.61±2.13) to that of OLL group (mean±SD=22.38±3.57), a statistically significant difference P=0.001 was obtained. This implies that degranulated mast cells in reticular layer are more in OLL group compared to OLPgroup.
In comparison of degranulated cells to total number of mast cells in two groups of OLP (mean±SD=0.62±0.05) and OLL (mean±SD=0.75±0.08), a statistically significant difference P<0.001 was observed. This ratio was higher in OLL group.
Comparing mean of epithelium thickness in two groups of OLP (mean±SD=263.84±67.06) and OLL (mean±SD=442.69±121.52), a statistically significant difference P<0.001 was observed which shows that mean of epithelium thickness in lesions of OLP is less than OLL.
In comparison between means of basement membrane thickness of two groups of OLP (mean±SD=41.69±26.59) and OLL (mean±SD=35.96±27.65), no statistically significant difference was observed, (P=0.45).
In addition, a statistically significant inverse relationship was observed between the ratio of degranulated mast cells with mean of basement membrane thickness in OLP group (P=0.003 and r=–0.55) that means, as much as this ratio is increased, the basement membrane thickness will be reduced.
DISCUSSION
As mentioned, the aim of this study was to compare OLP with OLL especially considering the number of degranulated mast cells and thickness of epithelium and basement membrane. In two groups of OLP and OLL, no statistically significant difference was observed between total number of mast cells, both in degranulated and intact cells.
In our previous study, similar results were also attained in which number of degranulated mast cells of reticular layer of corium in OLL were more than that of OLP.[17] In present study, mast cells’ counting was performed in a larger population samples. In addition, the mean value of epithelium thickness and basement membrane thickness was measured and comparisons were made. Another advantage of this study compared to previous study was utilization of cheaper and more accessible staining methods (PAS and TB).
On the basis of studies by Laine,[18] Thornhill,[19] and Eversol,[20] pathogenesis of OLL which is in fact a type of sensitivity reaction could be justified because, chemicals in pharmaceutical compounds or tooth restorations, are the agent of stimulation and degranulation of mast cells. They lead to delayed hypersensitivity reactions in OLL, so these lesions could disappear after removal of stimulating agent such as amalgam restorations if patch test is positive.[18–20]
Hence, degranulation of mast cells in OLL could be regarded as a primary reaction in the early stages of lesion trend, while in OLP this occurs in final phases of the event induced by T cells.
Another objective in this study was to compare the means of epithelium thickness and basement membrane in two lesions. In this study, epithelium thickness in lichen planus was significantly lower (P<0.001) than that of oral lichenoid. This finding is similar to the results of study of Juneja who suggested that, the reason is immature and abnormal distinction of keratinocytes in OLP and stated that they are susceptible to apoptosis. Also it could be assumed that immunity reaction of the cell in lichen planus is the cause of apoptosis in majority of keratinocytes in this lesion, compared to OLL which leads to reduction of epithelium thickness in OLP.
In this study, no significant difference was observed between basement membrane thickness of two groups of OLP and OLL (P=0.45) but in Juneja’ work, basement membrane in lichen planus lesions showed a higher thickness compared to that of oral mucosal lichenoid.[21]
Zhou et al.,[22] stated that in demolition area of basement membrane of lichen planus, majority of mast cells were degranulated which signifies the function of degranulated mast cells in basement membrane's reduction. In accordance to this finding we also found an inverse relationship in the ratio of degranulated mast cells with mean of basement membrane thickness in OLP group. As much as this ratio is increased, the basement membrane thickness got reduced.
CONCLUSION
The observed difference in degranulated mast cell population and epithelium thickness which were obtained by using more available staining procedures may be a useful method for histopathologic distinction between OLP and OLL. This may also explain the different therapeutic approaches toward these two lesions. Further studies with a larger sample size and evaluation of drugs which act on mast cells, population and function are recommended. Study about basement membrane's shape should also be done.
ACKNOWLEDGMENT
This study was supported by vice chancellor for research of Isfahan University of Medical Sciences. We thank Miss F. Mahmoody for technical assistance and Dr. B. Soleymani for statistical analysis.
Footnotes
Source of Support: This report is based on a thesis which was submitted to the School of Dentistry, Isfahan University of Medical Sciences, in partial fulfillment of the requirements for the M. Sc degree in Pathology (#388458). This study was financially supported and approved by Isfahan University of Medical Sciences, Isfahan, Iran
Conflict of Interest: None declared.
REFERENCES
- 1.Rad M, Hashemipoor MA, Mogtahedi A, Zarei MR, Chamani G, Kakoei S, et al. Correlation between clinical and histopathologic diagnoses of oral lichen planus based on modified WHO diagnostic criteria. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:796–800. doi: 10.1016/j.tripleo.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 2.Neville B, Damm D, Allen C, Boquot J. 3rd ed. Philadelphia: WB Saunders; 2009. Oral and maxillofacial pathology; pp. 782–8. [Google Scholar]
- 3.Ismail SB, Kumar SK, Zain RB. Oral lichen planus and lichenoid reactions: Etiopathogenesis, diagnosis, management and malignant transformation. J Oral Sci. 2007;44:89–106. doi: 10.2334/josnusd.49.89. [DOI] [PubMed] [Google Scholar]
- 4.Shaklar G. Lichen planus as an oral ulcerative lesion. J Oral Surg Oral Med Oral Pathol. 1972;33:376–88. doi: 10.1016/0030-4220(72)90467-7. [DOI] [PubMed] [Google Scholar]
- 5.Jahanshahi GH. 3rd ed. Vol. 1. Isfahan: Isfahan university of medical sciences; 2006. A guid book for differential diagnosis of oral lesion; pp. 19–25. [Google Scholar]
- 6.Lynch M, Brightman Greenberg M. 10th ed. Piladelphia: J.B. Lipincott Company; 2003. Burket's oral Medicine, Diagnosis and treatment; pp. 77–107. [Google Scholar]
- 7.Sugerman PB, Savage NW, Walsh LJ, Zhao ZZ, Zhouxy XJ, Khan A, et al. The pathogenesis of oral lichen planus. Crit Rev Oral Biol Med. 2002;13:350–65. doi: 10.1177/154411130201300405. [DOI] [PubMed] [Google Scholar]
- 8.Khan A, Farah C, Savage NW, Walsh L, Harbrow D, Sugerman P. Cytokines in oral lichen planus. J Oral Pathol Med. 2003;32:77–83. doi: 10.1034/j.1600-0714.2003.00077.x. [DOI] [PubMed] [Google Scholar]
- 9.Sugerman P, Savage N, Seymour G, Walsh L. Is there a role for tumor necrosis factor alpha (TNF-a) in oral lichen planus? J Oral Pathol Med. 1996;25:219–24. doi: 10.1111/j.1600-0714.1996.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 10.Simark Mattsson C, Jontell M, Bergenholtz G, Heyden M, Dahlgren UI. Distribution of interferon – gamma mRNA positive cells in oral lichen planus lesions. J Oral Pathol Med. 1998;27:483–8. doi: 10.1111/j.1600-0714.1998.tb01917.x. [DOI] [PubMed] [Google Scholar]
- 11.Firth AN, Reade PC. Comparison of eosinophil densities in oral mucosal lichen planus and lichenoid drug reaction. J Oral Pathol Med. 1990;19:86–8. doi: 10.1111/j.1600-0714.1990.tb00802.x. [DOI] [PubMed] [Google Scholar]
- 12.Perieto VG, Gasal M, McNutt NS. Immunohistochemistry detects differences between lichen planus like keratosis lichen planus and lichenoid actinic keratosis. J Cutan Pathol. 1993;20:143–7. doi: 10.1111/j.1600-0560.1993.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 13.Mc Carten BE, Lomey PJ. Expression of CD1, and HLA-DR by langerhans cells in oral lichenoid drug eruption and idiopathic Oral lichen plunus. J Oral Pathol Med. 1997;26:176–80. doi: 10.1111/j.1600-0714.1997.tb00454.x. [DOI] [PubMed] [Google Scholar]
- 14.Jahanshahi GH, Negintaji A. Comparative study of number and distribution of S 100+, CD4+ and CD8+ cells in oral lichen planus and oral lichenoid reactions by immunohistochemical staining. J Esf Dent Sch. 2005;2:21–8. [Google Scholar]
- 15.MeCarten BC, Lamey P. Lichen planus specific antigen in oral lichen planus and oral lichenoid drug eruptions. Oral Surg Oral Med Pathol Oral Radiol Endod. 2000;81:585–7. doi: 10.1067/moe.2000.103885. [DOI] [PubMed] [Google Scholar]
- 16.Sugerman P, Savage N. Oral lichen planus causes, diagnostic and management. J Aust Dent. 2002;47:240–97. doi: 10.1111/j.1834-7819.2002.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 17.Jahanshahi GH, Aminzadeh A. A histochemical and immunohistochemical study of mast cells differentiating oral lichen planus from oral lichenoid reactions. Quintessence Int. 2010;41:221–7. [PubMed] [Google Scholar]
- 18.Lain J, Konttinen T, Bliar N, Hapoonen P. Immunocompatent cells in amalgam associated oral lichenoid contact lesions. J Oral Pathol Med. 1999;28:117–21. doi: 10.1111/j.1600-0714.1999.tb02008.x. [DOI] [PubMed] [Google Scholar]
- 19.Thornhill MH, Pember MN, Simmon RK, Tneaker ED. Amalgam – contact hypersensitivity lesions and Oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:291–9. doi: 10.1067/moe.2003.115. [DOI] [PubMed] [Google Scholar]
- 20.Eversol LR. Immunopathology of oral mucosal ulcerative, desquamative and bullous disease.Selective review of the literature. Oral Surg Oral Med Oral Pathol. 1994;77:555–710. doi: 10.1016/0030-4220(94)90312-3. [DOI] [PubMed] [Google Scholar]
- 21.Juneja M, Mahajan S, Rao N, George T, Boaz K. Histochemical analysis of pathological alterations in oral lichen planus and oral lichenoid lesions. J Oral Sci. 2006;48:185–93. doi: 10.2334/josnusd.48.185. [DOI] [PubMed] [Google Scholar]
- 22.Zhou XJ, Sugerman PB, Savage NW, Walsh LJ, Seymour GJ. Intra- epithlelial CD8+ T cells and basement membrane disruption in oral lichen planus. J Oral Pathol Med. 2002;31:23–7. doi: 10.1046/j.0904-2512.2001.10063.x. [DOI] [PubMed] [Google Scholar]


