Abstract
Background:
Socket preservation after tooth extraction is one of the indications of bone grafting to enhance preorthodontic condition. The aim of this study is to determine the effects of socket preservation on the immediate tooth movement, alveolar ridge height preservation and orthodontic root resorption.
Materials and Methods:
In a split-mouth technique, twelve sites in three dogs were investigated as an experimental study. Crushed demineralized freeze-dried bone allograft (DFDBA) (CenoBone®) was used as the graft material. The defects were made by the extraction of 3rd premolar. On one side of each jaw, the defects were preserved by DFDBA and defects of the other side left opened as the control group. Simultaneously the teeth adjacent to the defects were pulled together by a NiTi coil spring. After eight weeks, the amount of (OTM), alveolar height, and root resorption were measured. Analysis of variance was used for purpose of comparison.
Results:
There was a slight increase in OTM at grafted sites as they were compared to the control sites (P<0.05). Also a significant bone resorption in control site and successful socket preservation in experimental site were observed. Reduction of root resorption at the augmented site was significant compared to the normal healing site (P<0.05).
Conclusion:
Using socket preservation, tooth movement can be immediately started without waiting for the healing of the recipient site. This can provide some advantages like enhanced rate of OTM, its approved effects on ridge preservation that reduces the chance of dehiscence and the reduction of root resorption.
Keywords: Demineralized freeze-dried bone allograft, orthodontic tooth movement, root resorption, socket preservation
INTRODUCTION
Bone graft is a tissue or material used to repair a defect or deficiency in contour and volume. Bone grafts sources consist of autogenous bone grafting, alloplast substances, xenogenic bone substance, and tissue engineered osteogenic material[1] like osteoinductive agents.[2,3] The most popular sources of graft in maxillofacial grafts are autografts, but its limited availability, increased cost, size mismatch and the need for second surgery in donor site shifted surgeons using other grafting materials whenever possible.
The use of demineralized freeze-dried bone allograft (DFDBA) has been the focus of much attention since Senn used demineralized bone for the first time.[4] But controversy on osteoinductive potential of DFDBA began to emerge with more research.[5–10] The controversy may be because of differences in osteoinductivity potential of commercially available DFDBA, this could be traced as[11] concentration of growth factors, demineralization procedures, age of donor and recipient, storage and sterilization procedures, dietary factors and size of pieces. Reynold et al. showed lower osteoinduction in DFDBA compared to freezed dried bone allograft (FDBA) and suggest that the releasing of bone morphogenetic proteins (BMPs) through demineralization procedures leads to this difference[11] DFDBA has been made in different shapes[12–14] such as chips, powder, crushed, etc. By decreasing the size of the pieces, handling and osteoinductive potential improves.[15,16]
There are many indications of bone graft to enhance preorthodontic condition. One of them is socket preservation after tooth extractions. Tooth extraction may be needed as a result of caries, traumas, periodontal diseases or space needed for orthodontic treatments. These extractions result in resorptive remodeling of alveolar ridge[17,18] especially in buccal aspect.[19] Alveolar bone volume and favorable architecture of the alveolar ridge is needed to avoid fenestration or dehiscence through orthodontic tooth movement (OTM).[20] Today this can be achieved by less traumatic extraction techniques and socket augmentation by graft materials.[21–24,10] More important is buccal plate defects because they will not heal completely without use of grafting techniques.[25–27] Lasella et al. suggested that DFDBA with a collagen membrane can reduce the atrophy after tooth extractions.[23] Preorthodontic bone grafting also has the advantage of easier and less detrimental tooth movement through primary woven bone, and inhibition of gingival invagination formations.[20] Another instance for bone preservation before orthodontic therapy is alveolar cleft defects (ACD). In 1908 Lexar reported the first graft of the ACD.[28] The objectives of graft in ACD are ONF closure, adequate bone support for the dentition, adequate bone volume and favorable ridge architecture,[29] and elimination of mucosal recess.[30] The secondary osteoplasty provides a bone matrix for eruption of the permanent teeth (especially canine) and supports the permanent teeth through OTM.[31]
In 1996, Diedrich[20] succeeded in using guided tissue regeneration to improve periodontal condition to move tooth into infrabony defects. Naaman[32] reported a successful movement of teeth into grafted site three months after bone implantation. In 2007, Oltramari et al.[33] moved tooth into a minipig bone defect, filled with xenogenic graft. They demonstrated the possibility of action and a similar distance of OTM both as in sockets with secondary healing.
Tooth movement and tooth eruption into a graft site is an inevitable phenomenon. More research should be carried out to identify and reduce risks and offer the patient greater safety during orthodontic therapy. The purpose of the present study is to detect the effect of orthodontic socket preservation by DFDBA (CenoBone®) on the amount of OTM and root resorption. Also the effectiveness of this bone substitute (CenoBone®) will be investigated for preserving the alveolar height.
MATERIALS AND METHODS
Twelve sites in three dogs were investigated as an experimental split-mouth study. All of the dogs were males and German race with the age range of 13±1 months and weight range of 20±5 kg. The animals were caged individually and fed with dog food. CenoBone® (Tissue Regeneration Corporation, Kish Island, Iran) crushed was used as the DFDBA graft material.
Sedation was performed by intramuscular injection of 0.27 mg/kg diazepam (Chemi Daru, Tehran, Iran) and 5.5 mg/kg ketamine (Parke-Davis, Detroit, Michigan, USA). The angiocath was placed against cephalic vein and general anesthesia started with 1 gr Nesdonal infusion throughout the surgery. The extraction sites were prepared by the extraction of 3rd premolar. The clinicians tried to perform extractions as low traumatic as possible by forceps and elevators in different sizes and shapes. On one side of each jaw, the defects were preserved as the experimental group by bone substitute i.e., DFDBA (CenoBone®), the other side served as the control group for secondary healing. For placing the bone substitute, crushed DFDBA was packed into the socket area by a cylindrical flat ended instrument 1 mm in diameter. Simultaneously the teeth adjacent to the defects were pulled together by a NiTi-based closed-coil spring (Ormco®, Orange, California, USA). A 0.012 inch soft (dead) stainless steel wire was placed around the cementoenamel junction (CEJ). The wires on collar of the teeth were fixed by a NO-MIX (Dentaurum®, Inc., Ispringen, Germany) composite [Figure 1]. The sites were examined for any appliance dislodgement weekly. Intramuscular injection of antibiotic Cephalosporin (Iran Pharmaceutical Export Company, Tehran, Iran), 30 mg/kg/j was prescribed for 48 h after surgery.
Figure 1.
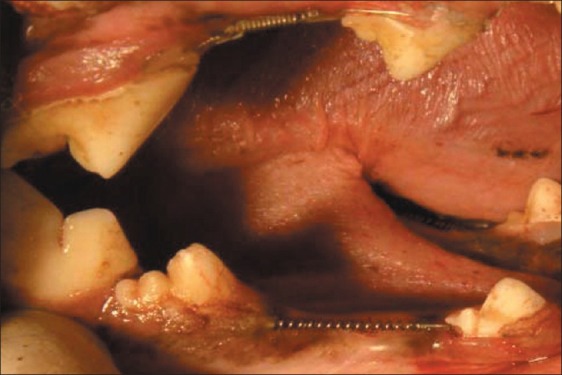
NiTi closed-coil springs attached with wire and bonded with NO-MIX® composite
After eight weeks, radiographs were taken and the amounts of OTM were measured by comparing the changes of distance between the adjacent teeth. The exact amounts of tooth movement were measured clinically by Boley Gauge Caliper with sharp tips located at the CEJ of crowns and then were compared and double checked by radiographs. The distance between the line connecting CEJ of teeth on the both sides of extraction site and the underlying bone was measured radiographically and magnification was considered and corresponding calculations were conducted. The radiographs were studied to evaluate the success of the graft. The experiment was performed according to the Ethics Committee of Shahid Beheshti University of Medical Science.
After that, the animals were sacrificed by Nesdonal overdose. The studying areas were removed from the jaw and fixed in Formalin 40%. The fixed blocks were soaked in Formic acid for 6 months. Decalcified sites were cut into pieces as small as the root and the adjacent bone area. After usual histologic preparations mesiodistal sections were cut with a microtome set at 4 μm.
Quantitative histomorphometric assessments of the coded specimens were made by the same examiner twice. Analysis of variance was performed by statistical package of SPSS (version 15) for purpose of comparison between two groups.
RESULTS
Orthodontic tooth movement
Measurements revealed a slight increase in OTM at grafted sites as they were compared to the control sites on the other side of the jaw (P<0.05). Mean movement of the teeth opposed to graft was 3.7±1.83mm that is about 37% greater than the control group (2.7±1.7 mm). This difference was statically significant. One-Sample Kolmogorov–Smirnov test was used to determine whether the data set is well-modeled by a normal distribution or not. The distribution of vectors was normal.
Vertical bone atrophy
Using one-sample Kolmogorov–Smirnov test, the normal distribution of the measured data was checked. There was statically significant difference (P<0.05) between the grafted sites (1.79±0.4 mm) and control sites (2.67±0.61 mm).The mentioned values determine significant bone resorption in control site and successful socket preservation in experimental site (P<0.05) [Figure 2].
Figure 2.
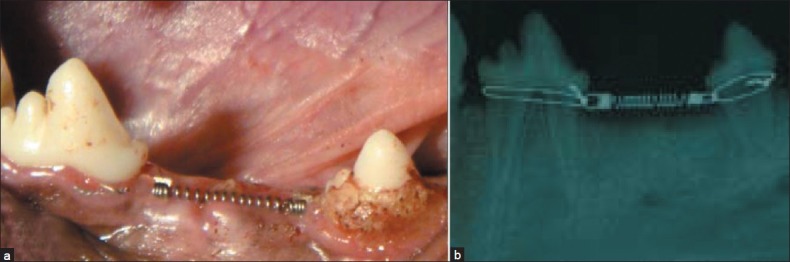
(a) NiTi closed-coil springs in the lower jaw extraction site. (b) A radiograph taken from the same site
Root resorption
The prepared samples were studied by an expert examiner and the amounts of root resorption were categorized in groups named 0 through 8; 0 for the ones with no recognizable resorption lacuna and 8 for the highest amount of resorption, others were divided in levels between them by the amount of their lacunae.
Using Friedman analysis, the level of root resorption was compared between the two groups (i.e. the grafted and the healing site). The resorption level was lower in grafted sites than the normal healing site (P<0.05) [Figure 3].
Figure 3.
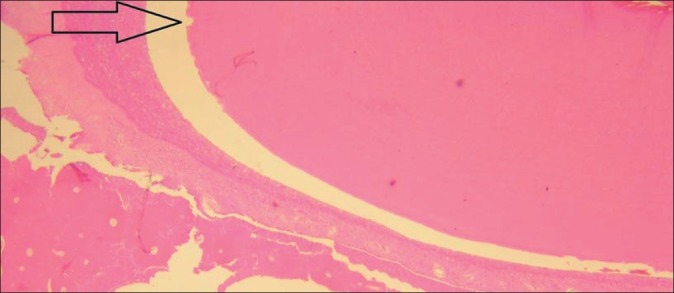
A resorptive lacuna is present near the arrow in the control site
DISCUSSION
This statically significant difference in the amount of tooth movement between the experimental groups means the success of tooth movement into the experiment sites filled with DFDBA with slight increase in OTM rate. This observation is opposite to Oltramary et al.[33] that moved teeth into a defect previously filled with xenograft in test group and filled with blood clots in the control group. They suggested a similar distance of OTM both in test and control group. In their study, orthodontic movement started after 3 months of healing of the grafted site. They allocated a healing period for organization of bone substitute in the alveolar socket. The time taken for the study has been even more in other studies like Kawamoto studies in Japan waiting for 4 months. But the mentioned healing period was eliminated by authors and no clinical change was observed. Yaun et al.[34] suggested that orthodontic retraction into extraction sites should be initiated at an early stage after tooth extraction. They claimed that this could use the advantage of bone remodeling in extraction sites. So they proposed the start of orthodontic movement about a week after extraction without any augmentation in the socket. They also suggested that the tooth on the recent extraction side moved faster than that on the healed side.[35]
BMP molecules that may be present in DFDBA may be one of the reasons for increasing bone turnover rate. By starting orthodontic treatment just after DFDBA grafting, we can use BMPs potentials for increasing the turnover rate at the same time that it helps for bone height preservation.[36]
Eight weeks after implantation of the graft, radiographic linear measurements, comparing the bone level with CEJ of adjacent teeth, revealed statistically significant decrease in the amount of bone loss in grafted sites. This finding suggests that the allograft reduces the bone atrophy at the defects. This capability of preserving greater bone height is in agreement with findings of previous animal experiments.
Rate of alveolar bone atrophy is attributed to a variety of factors which Atwood[37] divided into four categories: anatomic, metabolic, functional and prosthetic. Although other studies agree with decreased alveolar atrophy using DFDBA,[23] but the level of bone regenerated in the extraction sockets will never reach the bone level attached to the tooth surface.[38] This bone loss may be slighter in human experiments because of reduced level of gingival inflammation. This attributes to loss of hygienic functions because of using orthodontic wires and springs in dog. The other suggesting reason is that using human allograft in dogs means losing the osteoinductive potential of the allograft because it functions as a xenograft in dogs (osteoinductive materials can induce differentiation of mesenchymal cells into chondroblasts and osteoblasts and improving bone formation[39] ). Also moving the adjacent teeth through the graft may have deleterious effects on the remodeling of bone.
As it was mentioned, the data is collected at the end of treatment period (after 8 weeks), but alteration of tooth movement during the experiment period is an issue of concern for further investigation. So, further studies are needed to identify any difference in OTM rate within or even after this time period.
Lower root resorption level shows the effectiveness of using DFDBA socket preservation in reducing one of the major side effects of orthodontic therapy.
CONCLUSION
Following socket preservation, OTM can be immediately initiated without waiting for the healing of the recipient site. Using a bone alloplast graft for its approved effects on ridge preservation has become common in today's practice. The possibility of immediate orthodontic treatment helps the patient gain his esthetics and function as soon as possible.
Using DFDBA during orthodontic treatment will increase the rate of OTM. This can also decrease the treatment period.
Using DFDBA at extraction site as a socket preservation method could reduce root resorption after OTM through the extraction site.
Cenobone® DFDBA is approved for its ability in socket height preservation and it could be proposed for clinical applications such as socket preservation and alveolar cleft grafting.
ACKNOWLEDGEMENT
Authors wish to thank Center for Dental Research for their financial support and Tissue Regeneration Corporation. Cenobone® is the product of Tissue Regeneration Corporation (TRC, 3rd industrial park, Kish free zone, Kish Island, Iran) that is linked to Iranian tissue bank to prepare a wide range of machined allografts.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Hibi H, Yamada Y, Ueda M, Endo Y. Alveolar cleft osteoplasty using tissue engineeredosteogenic material. Int J Oral Maxillofac Surg. 2006;35:551–5. doi: 10.1016/j.ijom.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Boyne PJ, Nath R, Nakamura A. Human recombinant BMP-2 in osseous reconstruction of simulated cleft palate defects. Br J Oral Maxillofac Surg. 1998;36:84–90. doi: 10.1016/s0266-4356(98)90173-5. [DOI] [PubMed] [Google Scholar]
- 3.Kawamoto T, Motohashi N, Kitamura A, Baba Y, Suzuki S, Kuroda T. Experimental tooth movement into bone induced by recombinant human bone morphogenetic protein-2. Cleft Palate Craniofac J. 2003;40:538–43. doi: 10.1597/1545-1569_2003_040_0538_etmibi_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 4.Senn N. On the healing of aseptic bone cavities by implantation of aseptic decalcified bone. Am J Med Sci. 1889;18:219–43. doi: 10.1097/00000658-188907000-00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melloning JT, Bowers GM, Bailey RC. Comparison of bone graft materials. Part 1. New bone formation with auto grafts and allografts determined by strontium-85. J Periodontol. 1981;52:291–6. doi: 10.1902/jop.1981.52.6.291. [DOI] [PubMed] [Google Scholar]
- 6.Melloning JT, Bowers GM, Cotton WR. Comparison of bone graft materials. Part 2. New bone formation with autografts and allografts: A histological evaluation. J Periodontol. 1981;52:297–302. doi: 10.1902/jop.1981.52.6.297. [DOI] [PubMed] [Google Scholar]
- 7.Bauer HC, Tonkvist H, Nilsson OS. Effects of biophosphonates on the incorporation of calcium-45 and 3H-proline in orthotopic and in demineralized matrix-induced bone in rats. Bone. 1986;7:129–35. doi: 10.1016/8756-3282(86)90685-x. [DOI] [PubMed] [Google Scholar]
- 8.Paul BF, Horning GM, Hellstein JW, Schafer DR. The osteoinductive potential of demineralized freeze-dried bone allograft in human non-orthotopic sites: A pilot study. J Periodontol. 2001;72:1064–8. doi: 10.1902/jop.2001.72.8.1064. [DOI] [PubMed] [Google Scholar]
- 9.Yukna RA, Vastardis S. Comparative evaluation of decalcified and non-decalcified freeze-dried bone allografts in rhesus monkeys. I. Histologic findings. J Periodontol. 2005;76:57–65. doi: 10.1902/jop.2005.76.1.57. [DOI] [PubMed] [Google Scholar]
- 10.Zubillaga G, Hagen SV, Simon BI, Deasy MJ. Changes in alveolar bone height and width following post-extraction ridge augmentation using a fixed bioabsorbable membrane and demineralized freeze-dried bone osteoinductive graft. J Periodontol. 2003;74:965–75. doi: 10.1902/jop.2003.74.7.965. [DOI] [PubMed] [Google Scholar]
- 11.Solheim E. Osteoinduction by demineralised bone. Int Orthop. 1998;22:335–42. doi: 10.1007/s002640050273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damien C, Parsons J. Bone graft and bone substitutes: A review of current technology and applications. J Appl Biomater. 1991;2:275–88. doi: 10.1002/jab.770020307. [DOI] [PubMed] [Google Scholar]
- 13.DeGroot K. Bioceramics consisting of calcium phosphate salts. Biomaterials. 1980;1:47–50. doi: 10.1016/0142-9612(80)90059-9. [DOI] [PubMed] [Google Scholar]
- 14.Stephan E, Jiang D, Lynch S, Busch P, Dziak R. Anorganic bovine bone supports osteoblastic cell attachment and proliferation. J Periodontol. 1999;70:364–9. doi: 10.1902/jop.1999.70.4.364. [DOI] [PubMed] [Google Scholar]
- 15.Sampath TK, Reddi AH. Importance of geometry of the extracellular matrix in endochondral bone differentiation. J Cell Biol. 1984;98:2192–7. doi: 10.1083/jcb.98.6.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Syftestad G, Urist MR. Degradation of bone matrix morphogenetic activity by pulverization. Clin Orthop. 1979;141:281–5. [PubMed] [Google Scholar]
- 17.Abrams H, Kopczyk R, Kaplan AL. Incidence of anterior ridge deformities in partially edentulous patients. J Prosthet Dent. 1987;57:191–4. doi: 10.1016/0022-3913(87)90145-4. [DOI] [PubMed] [Google Scholar]
- 18.Atwood DA, Coy WA. Clinical, cephalometric, and densitometric study of reduction of residual ridges. J Prosthet Dent. 1971;26:280–95. doi: 10.1016/0022-3913(71)90070-9. [DOI] [PubMed] [Google Scholar]
- 19.Johnson K. A study of the dimensional changes occurring in the maxilla following tooth extraction. Aust Dent J. 1969;14:241–4. doi: 10.1111/j.1834-7819.1969.tb06001.x. [DOI] [PubMed] [Google Scholar]
- 20.Diedrich PR. Guided tissue regeneration associated with orthodontic therapy. Semin Orthod. 1996;2:39–45. doi: 10.1016/s1073-8746(96)80038-7. [DOI] [PubMed] [Google Scholar]
- 21.Fiorellini JP, Howell TH, Cochran D, Malmquist J, Lilly LC, Spagnoli D, et al. Randomized study evaluating recombinant human bone morphogenetic protein-2 for extraction socket augmentation. J Periodontol. 2005;76:605–13. doi: 10.1902/jop.2005.76.4.605. [DOI] [PubMed] [Google Scholar]
- 22.Brugnami F, Then PR, Moroi H, Leone CW. Histologic evaluation of of human extraction sockets treated with demineralized freeze-dried bone allograft (DFDBA) and cell occlusive membrane. J Periodontol. 1996;67:821–5. doi: 10.1902/jop.1996.67.8.821. [DOI] [PubMed] [Google Scholar]
- 23.Iasella J JM, Greenwell H, Miller RL, Hill M, Drisko C, Bohra AA, et al. Ridge preservation with demineralized freeze-dried bone allograft (DFDBA) and a collagen membrane compared to extraction alone for implant site development: A clinical and histologic study in humans. J Periodontol. 2003;74:990–9. doi: 10.1902/jop.2003.74.7.990. [DOI] [PubMed] [Google Scholar]
- 24.Mcallicter BS, Haghighat K. Bone augmentation techniques. J Periodontol. 2007;78:377–96. doi: 10.1902/jop.2007.060048. [DOI] [PubMed] [Google Scholar]
- 25.Hurzeler MB, Kohal RJ, Naghshbandi J, Mota LF, Conradt J, Hutmacher D, et al. Evaluation of a new bioresorbable barrier to facilitate guided bone regeneration around exposed implant treads.An experimental study in the monkey. Int J Oral Maxillofac Surg. 1998;27:315–20. doi: 10.1016/s0901-5027(05)80623-x. [DOI] [PubMed] [Google Scholar]
- 26.Okamoto T, Onofre Da silva A. Histological study on the healing of rat dental sockets after partial removal of the buccal bony plate. J Nihon Univ Sch Dent. 1983;25:202–13. doi: 10.2334/josnusd1959.25.202. [DOI] [PubMed] [Google Scholar]
- 27.Simpson HE. Experimental investigation into the healing of extraction wounds in macacus rhesus monkeys. J Oral Surg Anesth Hosp Dent Serv. 1960;18:391–9. [PubMed] [Google Scholar]
- 28.Lexer E. The use of free bone graft together with experiments on joint stiffness and joint transplantation. Arch Klin Chir. 1908;86:942. [Google Scholar]
- 29.Witsenburg B. The reconstruction of anterior residual bone defects in patients with cleft lip, alveolus and palate–A review. J Maxillofac Surg. 1985;13:197–208. doi: 10.1016/s0301-0503(85)80048-5. [DOI] [PubMed] [Google Scholar]
- 30.Bergland O, Semb G, Abyholm F. Elimination of the residual alveolar cleft by secondary bone grafting and subsequent orthodontic treatment. Cleft Palate J. 1986;23:175–205. [PubMed] [Google Scholar]
- 31.Horswell B, Henderson JM. Secondary Osteoplasty of the Alveolar Cleft Defect. J Oral Maxillofac Surg. 2003;61:1082–90. doi: 10.1016/s0278-2391(03)00322-7. [DOI] [PubMed] [Google Scholar]
- 32.Naaman NB, Chaptini E, Taha H, Mokbel N. Combined bone grafting and orthodontic treatment of an iatrogenic periodontal defect: A case report with clinical reentry. J Periodontol. 2004;75:316–21. doi: 10.1902/jop.2004.75.2.316. [DOI] [PubMed] [Google Scholar]
- 33.Oltramary PV, Navarro RL, Henriques JF, Taga R, Cestari TM, Ceolin DS, et al. Orthodontic movement in bone defects filled with xenogenic graft: An experimental study in minipigs. Am J Orthod Dentofacial Orthop. 2007;131:302.e10–7. doi: 10.1016/j.ajodo.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 34.Yuan X, Luo S, Shen G. Experimental study on selecting optimal time of orthodontic tooth movement into extraction sites. Hua Xi Kou Qiang Yi Xue Za Zhi. 2003;21:311–3. [PubMed] [Google Scholar]
- 35.Yuan X, Cao H, Luo S. Influence of bone remodeling in extraction sites on tooth movement. Hua Xi Kou Qiang Yi Xue Za Zhi. 2003;21:307–10. [PubMed] [Google Scholar]
- 36.Boyan BD, Ranly DM, Schwartz Z. Use of growth factors to modify osteoinductivity of demineralized bone allografts: Lessons for tissue engineering of bone. Dent Clin North Am. 2006;50:217–28. doi: 10.1016/j.cden.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Atwood DA. cephalometric study of the clinical rest position of the mandible. Part II. The variability in the rate of bone loss following the removal of occlusal contacts. J Prosthet Dent. 1957;7:544–52. [Google Scholar]
- 38.Schropp L, Wenzel A, Kostopoulos L, Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003;23:313–23. [PubMed] [Google Scholar]
- 39.Urist M. Bone: Formation by autoinduction. Science. 1965;150:893–9. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]


