Abstract
Introduction
Little evidence is available on the natural course of osteoarthritis development and the genes that protect and predispose individuals to it. This study was designed to compare strain-dependent development of osteoarthritis and its association with tissue regeneration in mice. Two recombinant inbred lines LGXSM-6 and LGXSM-33 generated from LG/J and SM/J intercross were used. Previous studies have indicated that LGXSM-6 can regenerate both articular cartilage and ear hole punch while LGXSM-33 cannot.
Methods
Transection of the medial meniscotibial ligament was performed on 10-week-old male mice to induce osteoarthritis. Cartilage damage was analyzed by histology and bone morphology was evaluated using micro-CT. Ear punches were performed and evaluated by measurement of residual hole diameter.
Results
Cartilage analysis showed that LGXSM-33 developed a significantly higher grade of osteoarthritis than LGXSM-6. Bone analysis showed that LGXSM-33 had substantial subchondral bone and trabecular bone thickening 8 weeks post-surgery, while LGXSM-6 showed bone loss over time. We also confirmed that LGXSM-6 can heal ear tissues significantly better than LGXSM-33.
Conclusions
Osteoarthritis was found to be negatively correlated with the degree of tissue regeneration. LGXSM-33, a poor healer of ear tissues (and articular cartilage), developed more osteoarthritis compared to LGXSM-6, which had better regenerative ability for ear tissues and articular cartilage. While these lines have different distribution of the alleles, we assume that the phenotypic differences observed here are due to genetic differences further suggesting that similar sets of physiological processes and gene variants may mediate variation in human osteoarthritis development and tissue regeneration.
INTRODUCTION
Osteoarthritis (OA) is a degenerative joint disease resulting in articular cartilage fibrillation and loss and is estimated to affect 70–90% of the population aged 60 years and older [1]. Knee OA is thought to be dependent on multiple factors with mechanical overload, obesity and trauma being the most prominent risk factors (reviewed in [2, 3]). The variation in OA susceptibility may be due to a variation in responsiveness to these factors. Emerging evidence indicates that 50–75% of variation in OA in humans is genetic, however, little evidence is available on the genes that cause and protect individuals from OA [4–6]. Evidence from genome-wide association studies in humans has shown that OA appears to be highly polygenic with multiple risk alleles conferring small effects [6].
Clinical options for patients with cartilage defects and OA are limited to symptomatic treatment, unless the patient is qualified for osteotomy or total joint replacement. In recent years, treatments that attempt to repair or restore the cartilage lesions have been developed [7, 8] with limited success. Thus, the knowledge of the genetic contribution to the susceptibility or protection from OA would greatly contribute to our understanding of OA and would provide not only potential treatment strategies but may also help predict individuals who are at risk for developing OA.
Recently mouse experimental models of OA have demonstrated that certain strains of mice are substantially protected from developing OA [9, 10]. For example, MRL/MpJ and DBA/1 mice are protected from post-traumatic arthritis and exhibited significantly less OA compared with C57BL/6 [9]. These protected strains also have the ability to repair full-thickness articular cartilage defects [10, 11]. While these strains are outbred and therefore have different genetic backgrounds, these results support the hypothesis that specific regions of genome may contribute to the initiation and progression of OA and tissue regeneration.
In order to begin to examine the genetic contribution to OA in more detail, recombinant inbred strains of LG/J (healer of ear-wounds) and SM/J (non-healer) mice were used [12]. The LG/J strain is the parent line of the “super healer” MRL/MpJ strain [13] and shares 75% of its genome identical-by-descent [13]. We have recently used these recombinant inbred lines to explore heritability for two traits: articular cartilage regeneration and ear wound healing [14]. In this study, we found that these traits are statistically heritable and a strong correlation between the phenotypes exists. While each recombinant inbred strain is a different recombination of the parental genotypes, so we can expect their outcomes to vary depending on the genetic differences that they inherited from parental strains.
In order to evaluate the OA progression in recombinant inbred lines, the destabilization of medial meniscus (DMM) model was used as has been previously reported [15]. This model provides several advantages over other models such as high reproducibility and relatively slow progression of disease and allows examination of changes in the entire joint over time.
In the disease process of OA, damage occurs not only to the cartilage but also to other joint tissues such as the synovium, ligaments, and bone. The boney changes, including osteophyte formation and subchondral bone sclerosis are considered radiological hallmarks of OA [16]. In addition, it has been shown that bone mineral density in patients with the radiographic features of OA is higher than in patients with normal joint morphology [17]. It was hypothesized that osseous changes are considered necessary for the progression of OA, however, whether the bone changes precede or follow cartilage degeneration is unknown [18]. The correlation between cartilage degeneration and bone changes from the onset of OA to advanced stages is not well understood, and further investigation of changes in bone architecture during OA development is required. While not possible to study in humans particularly early OA, in the present study, we were able to monitor osseous changes over the time of induction and progression of OA in mice.
Here we present a study of OA development in selected LGXSM recombinant inbred lines, LGXSM-6 and LGXSM-33 chosen because they represent respectively good and poor extremes in ear hole healing and articular cartilage regeneration [14, 19]. The aim of this study was to compare the strain-dependent development of OA following DMM surgery and to anticipate that these phenotypic differences could be attributed to genetic differences. This data combined with our previous data on genetics of articular cartilage regeneration and ear wound healing allows lines to be drawn between the ability to heal tissues and susceptibility to OA.
MATERIALS AND METHODS
All studies were performed following approval from the Animal Studies Committee of Washington University.
Ear punch procedure
LGXSM-6 and LGXSM-33 mice were evaluated for their tissue regeneration potential with ear punch closure. In 6 week old mice, 2 mm holes were surgically produced in each ear as previously described [20]. The diameters of the holes at 15 and 30 days after ear punching were measured and recorded. All ear punches were performed by the same person and read by two independent observers.
OA induction by DMM surgery
Only male mice were used for this study. Mice were anesthetized using an intraperitoneal injection of ketamine (100 mg/kg), xylazine (20 mg/kg), and acepromazine (10 mg/kg) at 10 weeks of age. DMM was induced in the right knee joint by transection of the anterior attachment of medial meniscotibial ligament (MMTL) described previously [15]. Briefly, the joint capsule was opened with an incision just medial to the patellar tendon and the MMTL was sectioned with micro-surgical scissors. For control, surgery was performed on left knee joints but the ligaments were left intact and termed ‘sham joints’. All mice were weight-bearing following recovery from anesthesia. Mice were sacrificed at 2, 4, and 8 weeks after DMM surgery and subjected to a micro-computed tomography (micro-CT) and histological analyses. The number of mice (N) analyzed for each parameter and time points are indicated in the graphs.
Histological evaluation for cartilage degeneration
Knee joints were fixed in 10% neutral buffered formalin, decalcified with 10% formic acid and embedded in paraffin Coronal histological sections were taken through the joint at 80 μm intervals, stained with toluidine blue, and cartilage damage was scored by two observers (SH, MFR) blinded to sample identity using a published scoring system (Table 1) by Glasson and colleagues [21]. Histological scores were measured in four quadrants (medial femoral condyle, medial tibial plateau, lateral femoral condyle, and lateral tibial plateau) of both knee joints at all sectioned levels (8 sections per knee), to obtain summed and maximum OA scores. Summed score was calculated from all four quadrants for all sections which represented whole joint changes. The maximum score was the highest score within all sectioned levels for a given knee.
Table 1.
Scoring system used to evaluate cartilage degeneration for summed and maximum OA score
| Grade | Osteoarthritis damage |
|---|---|
| 0 | Normal |
| 0.5 | Loss of staining without structural change |
| 1 | Roughened articular surface and small fibrillations |
| 2 | Fibrillation down to the layer immediately below the superficial layer and some loss of surface lamina |
| 3 | Fibrillation/erosion to the calcified cartilage extending to <25% of the articular surface |
| 4 | Fibrillation/erosion to the calcified cartilage extending to 25–50% of the articular surface |
| 5 | Fibrillation/erosion to the calcified cartilage extending to 50–75% of the articular surface |
| 6 | Fibrillation/erosion to the calcified cartilage extending to >75% of the articular surface |
Micro-CT scanning and quantification of bone morphometric parameters
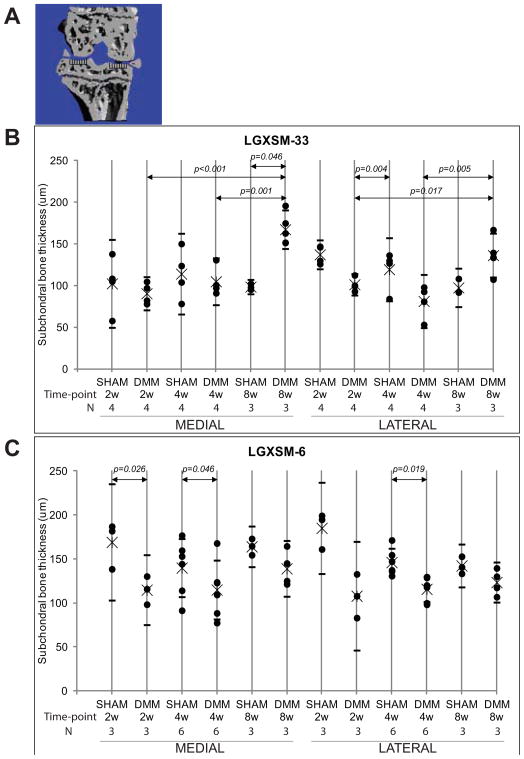
Prior to sectioning, knee joints were scanned using micro-CT scanner (vivaCT 40, SCANCO MEDICAL) for analysis of 3-dimentional structure and bone morphometric parameters. Two-dimensional coronal images of the weight-bearing region of the joint were generated by AMIRA system (Visage Imagings Inc, CA) and used to measure the subchondral bone plate thickness for the medial and lateral tibial plateau. The AMIRA system first produces 3-dimentional images from micro-CT data and then reconstructs the 2-dimentional images. The outline of coronal images reflected mineralized tissues of knee joints. In each region, 8 points were quantified from the top of mineralized articular surface to the beginning of trabecular bone, and average was calculated (Figure 4A). In order to analyze subchondral bone changes, the epiphysis of the tibia was chosen as the region of interest. The outline of the epiphysis was manually selected and care was taken not to select any outgrowing osteophyte. The following morphometric parameters of the tibial subchondral plate were calculated for trabecular compartments: trabecular bone volume fraction (BV/TV), i.e. the ratio of trabecular bone volume over endocortical total volume, trabecular thickness, and connectivity density index, that calculates the number of trabecular connections per unit volume [22].
Figure 4. Changes in subchondral bone thickness in LGXSM-33 and LGX-SM-6 strains.
Coronal sectional images were used for measurement of subchondral bone thickness in the medial and lateral tibial plateau (A). Subchondral bone thickness on medial and lateral sides of LGXSM-33 after 2, 4, and 8 weeks of DMM surgery showed that there were significant differences in thickness of subchondral bone between sham and DMM on medial side after 8 weeks. Similarly, the DMM knees at 8 weeks from both medial and lateral sides were significantly higher than 2 and 4 weeks. In contrast, in LGXSM-6 mice the differences between subchondral bone thickness in sham and DMM knee varied significantly at 2 and 4 weeks on medial side and only at 4 weeks on lateral side. The overall data from A and B shows that LGXSM-33 develops more subchondral bone thickness than LGXSM-6.
Statistical analysis
Non parametric tests were used for histological scores and bone parameter differences between operated and sham knee joints. Analysis of variance (ANOVA) was used to see strain and time point differences. Differences were considered statistically significant when P was <0.05 and results are indicated as data points with mean and upper and lower limits of 95% confidence interval (CI) unless indicated otherwise.
RESULTS
Although little difference in gross appearance of LGXSM-6 and LGXSM-33 mice, the average bodyweight of LGXSM-6 mice was greater than LGXSM-33 mice (at 10 weeks of age, LGXSM-6: 32.93g ± 2.11; LGXSM-33: 25.57g ± 2.14, p<0.001, at 18 weeks of age, LGXSM-6: 35.96g ± 1.47; LGXSM-33: 30.06g ± 2.69, p=0.002).
Ear punch closure
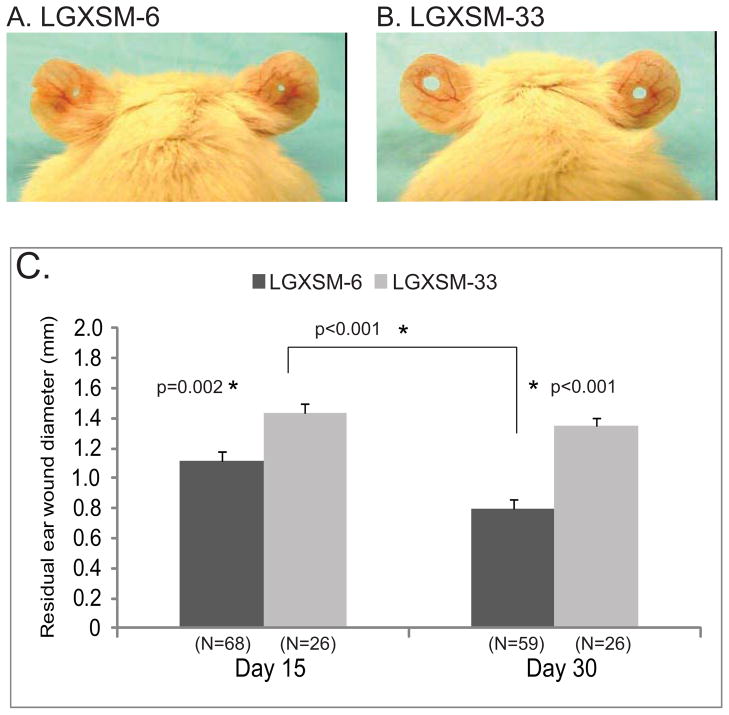
The LGXSM-6 mice were able to significantly heal their ear wounds (Figure 1A) compared to LGXSM-33 mice (Figure 1B). LGXSM-6 mice showed a 45% reduction in ear hole diameter by 15+ days post-procedure and a 60% reduction by 30 days (p<0.001). In contrast, LGXSM-33 mice had only a 30% wound closure by 15 days and no further healing occurred up to 30 days (p=0.40). The average residual wound size after 30 days was 0.80 mm (95% CI 0.68–0.91) and 1.35 mm (95% CI 1.22–1.47) in LGXSM-6 and LGXSM-33 mice, respectively (Figure 1C).
Figure 1. Ear wound healing in LGXSM-6 and LGXSM-33 strains.
Through-and-through ear puncture wounds (2-mm in diameter) on external ears of LGXSM-6 and LGXSM-33 strains were created at 6 weeks of age using standard method. Gross appearance of ear hole in LGXSM-6 (A) and LGXSM-33 (B) mice after 30 days of ear punch is shown. A large ear hole retained in LGXSM-33 strain indicates a failure of healing as compared to LGXSM-6 strain. A graphical representation of residual ear wound diameter (C) after 15- and 30-days of ear punch showed that LGXSM-6 mice significantly healed their ears at both day 15 and day 30 compared to LGXSM-33 mice.
Strain dependent induction of OA
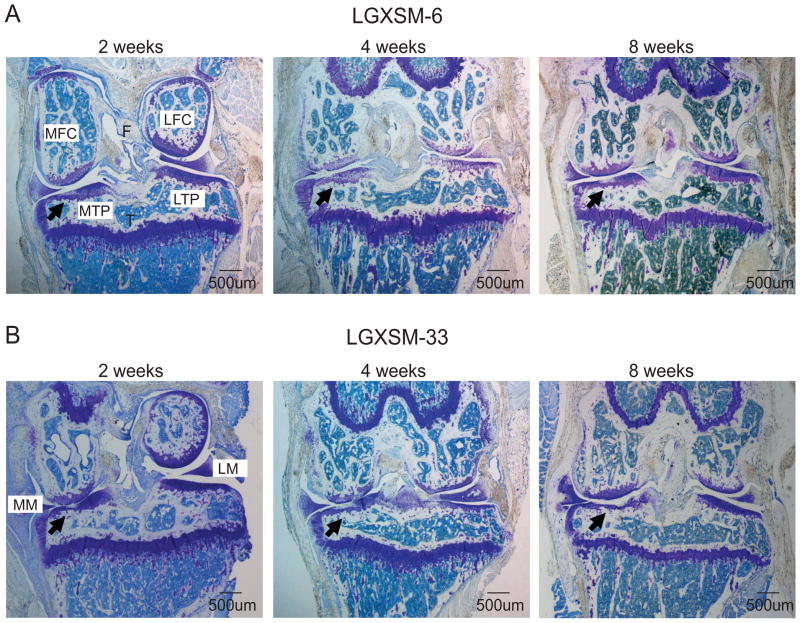
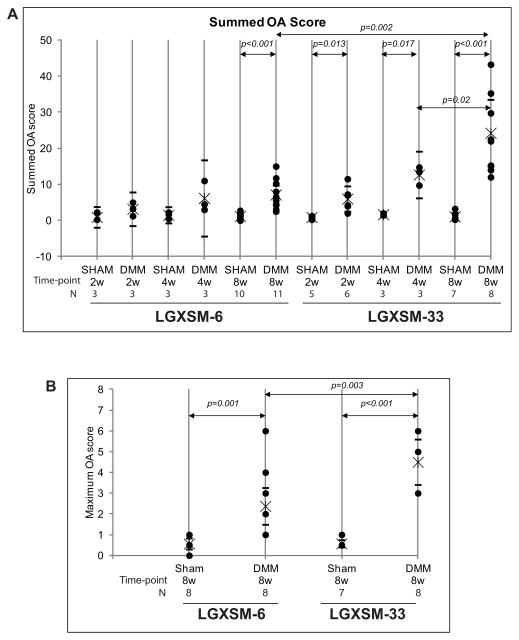
Histological sections showed more cartilage degeneration after induction of OA in LGXSM-33 than in LGXSM-6 mice at 8 weeks post DMM surgery. Histological grading showed that LGXSM-33 mice demonstrated loss of proteoglycan staining with articular fibrillation at 2 weeks and loss of hyaline cartilage, proteoglycan staining, and the lesions extended to calcified cartilage at 4 and 8 weeks after surgery (Figure 2B). In contrast, LGXSM-6 mice showed a focal loss of proteoglycan staining without severe cartilage loss at all time points (Figure 2A). The extent of OA was determined by scoring for specific parameters of OA and is presented as a summed (Figure 3A) and a maximum score (for any single location in one of the 8 sections) (Figure 3B). Histological scores demonstrated that LGXSM-6 mice have a lower summed OA score (7.13; 95% CI 4.56–9.71) than LGXSM-33 mice (24.24; 95% CI 15.00–33.49) on 8 weeks after DMM surgery (Figure 3A). For the summed OA score, LGXSM-33 mice developed OA in a time-dependent manner and to a significantly higher grade of OA than LGXSM-6 mice after 8 weeks of DMM surgery (p=0.002, Figure 3A). For the maximum OA score, LGXSM-33 mice had higher score than LGXSM-6 mice only in the medial side of the joint (p=0.003, Figure 3B).
Figure 2. Histological evaluation for OA development.
Representative histological sections of LGXSM-6 (A) and LGXSM-33 (B) mice after 2, 4 and 8 weeks of DMM surgery (x40) are shown. Black arrows show cartilage damage in medial tibial plateau. MFC = medial femoral condyle, LFC = lateral femoral condyle, MTP = medial tibial plateau, LTP = lateral tibial plateau, MM = medial meniscus, LM = lateral meniscus.
Figure 3. OA score for LGXSM-6 and LGXSM-33.
The sum OA score (A) from all the four quadrants and 8 sections from each knee was based on cartilage damage and shows that LGXSM-33 strain overall develop more OA compared to LGXSM-6 after 8 weeks of surgery. A maximum score (B) representing highest score from medial compartment of LGXSM-6 and LGXSM-33 indicates that DMM knees had significant more OA score in both strains compared to sham knee. It also shows that LGXSM-33 has significantly higher grade of OA than LGXSM-6 at 8 weeks post-surgery.
Quantification of bone morphometric parameters
Bone morphometric parameters were examined on the epiphysis of tibia at 2, 4, and 8 weeks after surgery. Differences in subchondral bone plate thickness were observed over time, between strains and between medial and lateral compartments. LGXSM-33 showed subchondral bone plate thinning at early time points (2 and 4 weeks), however the subchondral bone plate thickened significantly between 4 and 8 weeks on both medial and lateral tibial plateau (medial: p=0.001, lateral: p=0.005, Figure 4B), and when at borderline significance compared to sham knee joints at 8 weeks after surgery (medial: p=0.046, lateral: p=0.005). On the other hand, LGXSM-6 mice showed thinning at all time points on both sides but statistical significant differences were seen between sham and operated knees on medial side at 2 weeks (p=0.026) and 4 weeks (p= 0.046) and on lateral side only at 4 weeks (p=0.019) time points (Figure 4C).
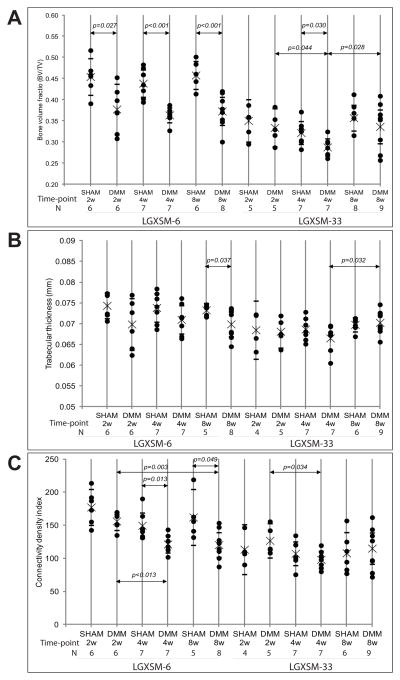
At all time points, LGXSM-6 mice showed significantly lower bone volume fraction (BV/TV) on the operated knee than sham (2 weeks, p=0.027; 4 weeks, p<0.001); 8 weeks, p<0.001), while in LGXSM-33 mice bone volume fraction decreased significantly at 4 weeks (p=0.030) compared to sham and then increased significantly at 8 weeks (p=0.028) compared to at 4 weeks in operated knee. (Figure 5A). Trabecular bone thickness on the operated joint was less than the sham in LGXSM-6 mice at all time points but this difference was significant only at 8 weeks (p=0.037), but in LGXSM-33 mice trabecular thickness did not show significant differences between operated and sham joints overtime. Although it decreased initially at 4 weeks, trabecular thickness was close to that of sham at 8 weeks (p=0.032) (Figure 5B). The trabecular connective density index, which calculates the number of trabecular bone connections, showed that LGXSM-33 mice lost trabecular bone at 4 weeks (p=0.034) and thickened by 8 weeks after surgery, while in the LGXSM-6 mice significant trabecular bone loss was observed at 4 weeks (p=0.013) and 8 weeks (p=0.049) Figure 5C).
Figure 5. Trabecular bone parameters for LGXSM-6 and 33 strains.
All parameters were derived from trabecular bone of the entire epiphysis of DMM and sham in both strains. Bone volume fraction (BV/TV) (A) trabecular thickness (B) and connectivity density index (C) are shown for LGXSM-6 and LGXSM-33 strains.
DISCUSSION
Recent studies have convincingly demonstrated that LGXSM-6 and LGXSM-33 recombinant inbred lines from LG/J and SM/J intercross differ significantly in their capacity to heal through-and-through ear wounds as well as knee articular cartilage [14, 19]. The most significant finding of the present study is that these strains also have different susceptibilities to OA development and progression following transection of the MMTL (the DMM model). In addition, the susceptibility to OA appears to be correlated with the ability to heal ear tissue: i.e. more tissue regeneration, less OA and vice versa. These findings are in agreement with those of others [10, 11], which have suggested that the ability to heal ear tissues and ability to regenerate articular cartilage co-occur with resistance to cartilage degeneration and protection from OA in mice. As it is generally thought that OA initiates when the cells are stimulated to a higher level of metabolic activity with catabolic effects out-striping anabolic effects [23, 24], the simplest interpretation of our results would be that a greater anabolic capacity leads to a better repair response and subsequently less OA development. While these two strains of mice (LGXSM-6 and LGXSM-33) differ in their phenotypic properties, we studied the natural course of development and progression of OA in these strains to see how the changes in cartilage and bone develop over time. The cartilage changes, represented by summed and maximum OA score, show that the LGXSM-33 strain has significantly higher OA score in operated knee compared to sham knee. Similarly this strain has significantly higher summed score in response to DMM at 4 and 8 weeks time points compared to strain LGXSM-6. The summed OA score implies that if all cartilage is degraded it would be 192 (6-max score × 8 slides × 4 quadrants). However, by convention, the OA score is determined at eight weeks after DMM surgery. The scores at this time, range from 20 to 50 depending on the strain (common or genetically engineered strains) [15].
We observed a bone loss following transection of MMTL in both strains although LGXSM-6 consistently showed a greater bone loss at each time point. We also found an increase in thickness of the subchondral bone plate which occurs only concomitant with significant cartilage loss on the articulating surface. As the strain that has insignificant OA (LGXSM-6) does not demonstrate subchondral bone plate thickening, it appears that the subchondral bone plate thickening may be the result of cartilage loss. By comparing the differences in bone and cartilage changes over time we have attempted to close the gap between early and late changes in OA development.
Bone morphologic changes are thought to be an important factor in OA development. Micro-CT has been commonly applied to study osseous changes in various animal models of OA [25, 26] as is evident by the following studies: (i) In a murine OA model where overexpression of Smad3 induced cartilage degeneration, characteristic bone morphometric phenotype were observed by micro-CT including osteophyte formation, subchondral sclerosis, and increased mineralization of meniscus [27]. (ii) STR/ort mice, which develop OA spontaneously, also showed subchondral sclerosis [28]. (iii) In a collagenase-injected murine model of OA, trabecular bone was shown to become thinner and with loss of connections between trabecular bones in the early phases of OA [29]. (iv) The late phase of OA development was examined in a DMM model of ADAMTS-5 null mice wherein the removal of ADAMTS-5 gene protected cartilage from degeneration [30]. Our studies also clearly show that early bone loss is a reaction to the injury, and is followed by subchondral bone thickening only when significant cartilage degeneration has occurred. These results are supported by the numerous other studies where bone loss has been documented in dogs [31] and humans [32–34] following a ligament injury. Subchondral bone changes have also been reported as one of the important morphologic phenotypes correlated with OA progression. For examples, a rat model of OA showed subchondral bone changes following traumatic anterior cruciate ligament transection with bone resorption at early time points and sclerosis in late phase [35]. Similarly, in human OA patients, the femoral heads exhibited trabecular bone thickening exclusively when the cartilage was lost [36] indicating that the subchondral bone changes are secondary to cartilage loss rather than a consequence of direct mechanism of OA.
The baseline measurements of subchondral bone plate thickness are different in these two strains. As such the baseline measurement for subchondral bone thickness in LGXSM-33 strain on both medial and lateral sides was less than LGXSM-6 strain at all time points. Although there was an initial thinning of subchondral bone in both strains in response to DMM surgery, the remarkable differences in thickening of subchondral bone occurred at 8 weeks only in LGXSM-33 strain showing that the response of LGXSM-33 to develop OA is more than LGXSM-6 strain. So the differences in thickness of subchondral bone in LGXSM-33 in response to DMM surgery are likely more important than the absolute baseline values. In other words, it is LGXSM-33 that shows a trend for increased bone thickness at 8 weeks going beyond the baseline value and not LGXSM-6. Other studies have demonstrated differences in subchondral bone thickness between mouse strains, even with knock out of the ADAMTS-5 gene, the subchondral bone thickness is altered [30]. For the recombinant inbred lines used here, one of us [37] has studied bone characteristics and found that LGxSM-6 has higher bone density than LGXSM-33. Future studies will examine all the molecular mechanisms that contribute to genetic differences in bone parameters.
Ear hole closure is very rare in mammals. MRL/MpJ strain was among the first to be recognized to demonstrate an accelerated repair of all tissues of the ear similar to an epimorphic regeneration seen in lower vertebrates [20] and this phenomenon has been shown to be genetically controlled [38]. MRL/MpJ is also known to possess an increased intrinsic resistance to post-traumatic OA [9, 11]. As mentioned above, one of the parent strains of LGXSM-6 and LGXSM-33, LG/J, is also the parent line of MRL/MpJ. Thus, it is tempting to believe that many of the same alleles present in MRL/MpJ are also carried by LG/J and, in turn, by LGXSM-6 and LGXSM-33. The different outcome of ear punch healing in each strain may be informative regarding the regenerative ability in mammals.
Our study is the first to demonstrate a natural course of cartilage and bone changes over time with different susceptibilities to OA development in genetically related strains. The phenotypic difference could be correlated with the rearrangement of parental (LG/J and SM/J) genomes with different distribution of alleles. In other word, the differential phenotypes observed in this study can be attributed to a restricted set of genes according to the way they have been inherited from the parental lines. The use of recombinant inbred lines is very practical in that genotyping of each strain is needed only once since each strain is isogenic and multiple phenotypes from each strain can be obtained on each line, therefore different outcomes from recombinant inbred lines are due to genetic differences. Several of the same recombinant inbred lines of LG/J and SM/J were used to identify specific regions of the genome that participate in growth [39], long bone length [40], bone cross-sectional morphology and strength [37], obesity [41], and response to dietary fat [42, 43].
Besides the genetic information that can be inferred from the present findings, our results also have some interesting molecular and phenotypic interpretations. For instance we found that LGXSM-33 mice, the poor healing strain, develop OA, while LGXSM-6 mice, a good healing strain, show insignificant OA development. This indicates that tissue regeneration in ear (cartilage, blood vessels, nerves, skin) and articular cartilage may involve some of the same physiological processes as resistance to OA. Another aspect of our findings is that regeneration of tissues (ear and articular cartilage) and development of OA are strain dependent and are interlinked with each other as has been previously suggested [14].
In summary, the natural course of OA development shows that LGXSM-6 mice are resistant to OA and demonstrate regeneration of ear tissues as well as articular cartilage while LGXSM-33 mice develop OA with subchondral bone thickening, and are unable to substantially regenerate articular cartilage or ear tissues (Table 2). These results demonstrate that there are potentially related genetic factors responsible for OA development, tissue regeneration, bone response and cartilage regeneration in the LG/J by SM/J intercross populations. These studies provide the proof of principle that genetically different recombinant inbred lines also differ in their susceptibility to OA providing the opportunity to identify specific regions of genome that contribute to variation in OA development and cartilage regeneration in the LGXSM intercross.
Table 2.
Summary of phenotypic characteristics of LGXSM-6 and LGXSM-33 strains
| Property | LGXSM-6 | LGXSM-33 |
|---|---|---|
| Osteoarthritis | Mild | Severe |
| Healing ear punch | Yes | No |
| Bone loss in early phase | Yes | No |
| Bone thickening in late phase | No | Yes |
| Cartilage repair | Yes | No |
Acknowledgments
Funding source
The project was supported by an ARRA Grand Opportunity Grant, Award Number RC2 AR058978; the micro-CT and histological analysis were supported by the Musculoskeletal Research Center, Grant Award Number P30 AR057235, from the National Institute of Arthritis, Musculoskeletal and Skin Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Arthritis, Musculoskeletal and Skin Diseases or the National Institutes of Health.
The authors would like to thank Drs. Rick Wright, Jamie Fitzgerald, and Sonya Glasson for mouse model assistance. The authors would also like to thank Crystal Idleburg, Joseph Futhey, Tarpit Patel, and Timothy Morris for valuable technical assistance, and Dr. Debabrata Patra for critical reading of the manuscript.
Footnotes
Contributions
All authors contributed to the conception and design of the study, analysis and interpretation of data, and preparation and approval of the manuscript. In addition, SH, KLJ and MFR contributed to data acquisition, SH, MFR, JMC and LJS wrote the manuscript, and SH, JMC and LJS take responsibility for the integrity of the work.
Conflict of interest
The authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hinton R, Moody RL, Davis AW, Thomas SF. Osteoarthritis: diagnosis and therapeutic considerations. Am Fam Physician. 2002;65:841–8. [PubMed] [Google Scholar]
- 2.Rai MF, Sandell LJ. Inflammatory mediators: tracing links between obesity and osteoarthritis. Crit Rev Eukaryot Gene Expr. 2011;21:131–42. doi: 10.1615/critreveukargeneexpr.v21.i2.30. [DOI] [PubMed] [Google Scholar]
- 3.Buckwalter JA, Brown TD. Joint injury, repair, and remodeling: roles in post-traumatic osteoarthritis. Clin Orthop Relat Res. 2004:7–16. [PubMed] [Google Scholar]
- 4.Hunter DJ, Snieder H, March L, Sambrook PN. Genetic contribution to cartilage volume in women: a classical twin study. Rheumatology (Oxford) 2003;42:1495–500. doi: 10.1093/rheumatology/keg400. [DOI] [PubMed] [Google Scholar]
- 5.Spector TD, Cicuttini F, Baker J, Loughlin J, Hart D. Genetic influences on osteoarthritis in women: a twin study. BMJ. 1996;312:940–3. doi: 10.1136/bmj.312.7036.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meulenbelt I, Kraus VB, Sandell LJ, Loughlin J. Summary of the OA biomarkers workshop 2010 - genetics and genomics: new targets in OA. Osteoarthritis Cartilage. 2011;19:1091–4. doi: 10.1016/j.joca.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cain EL, Clancy WG. Treatment algorithm for osteochondral injuries of the knee. Clin Sports Med. 2001;20:321–42. doi: 10.1016/s0278-5919(05)70309-4. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert JE. Current treatment options for the restoration of articular cartilage. Am J Knee Surg. 1998;11:42–6. [PubMed] [Google Scholar]
- 9.Ward BD, Furman BD, Huebner JL, Kraus VB, Guilak F, Olson SA. Absence of posttraumatic arthritis following intraarticular fracture in the MRL/MpJ mouse. Arthritis Rheum. 2008;58:744–53. doi: 10.1002/art.23288. [DOI] [PubMed] [Google Scholar]
- 10.Eltawil NM, De Bari C, Achan P, Pitzalis C, Dell’accio F. A novel in vivo murine model of cartilage regeneration. Age and strain-dependent outcome after joint surface injury. Osteoarthritis Cartilage. 2009;17:695–704. doi: 10.1016/j.joca.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzgerald J, Rich C, Burkhardt D, Allen J, Herzka AS, Little CB. Evidence for articular cartilage regeneration in MRL/MpJ mice. Osteoarthritis Cartilage. 2008;16:1319–26. doi: 10.1016/j.joca.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Blankenhorn EP, Bryan G, Kossenkov AV, Clark LD, Zhang XM, Chang C, et al. Genetic loci that regulate healing and regeneration in LG/J and SM/J mice. Mamm Genome. 2009;20:720–33. doi: 10.1007/s00335-009-9216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy ED, Roths JB. Autoimmunity and Lymphoproliferation. Genetic Control of Autoimmune Disease. 1978:207. [Google Scholar]
- 14.Rai MF, Hashimoto S, Johnson EE, Janiszak KL, Fitzgerald J, Heber-Katz E, et al. Heritability of articular cartilage regeneeartion and its association with ear-wound healing. Arthritis Rheum. 2012 doi: 10.1002/art.34396. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glasson SS. In vivo osteoarthritis target validation utilizing genetically-modified mice. Curr Drug Targets. 2007;8:367–76. doi: 10.2174/138945007779940061. [DOI] [PubMed] [Google Scholar]
- 16.Swagerty DL, Jr, Hellinger D. Radiographic assessment of osteoarthritis. Am Fam Physician. 2001;64:279–86. [PubMed] [Google Scholar]
- 17.Nevitt MC, Lane NE, Scott JC, Hochberg MC, Pressman AR, Genant HK, et al. Radiographic osteoarthritis of the hip and bone mineral density. The Study of Osteoporotic Fractures Research Group. Arthritis Rheum. 1995;38:907–16. doi: 10.1002/art.1780380706. [DOI] [PubMed] [Google Scholar]
- 18.Burr DB. The importance of subchondral bone in the progression of osteoarthritis. J Rheumatol Suppl. 2004;70:77–80. [PubMed] [Google Scholar]
- 19.Bedelbaeva K, Snyder A, Gourevitch D, Clark L, Zhang XM, Leferovich J, et al. Lack of p21 expression links cell cycle control and appendage regeneration in mice. Proc Natl Acad Sci U S A. 2010;107:5845–50. doi: 10.1073/pnas.1000830107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark LD, Clark RK, Heber-Katz E. A new murine model for mammalian wound repair and regeneration. Clin Immunol Immunopathol. 1998;88:35–45. doi: 10.1006/clin.1998.4519. [DOI] [PubMed] [Google Scholar]
- 21.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18 (Suppl 3):S17–23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 22.Odgaard A, Gundersen HJ. Quantification of connectivity in cancellous bone, with special emphasis on 3-D reconstructions. Bone. 1993;14:173–82. doi: 10.1016/8756-3282(93)90245-6. [DOI] [PubMed] [Google Scholar]
- 23.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–34. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 24.Aigner T, Kurz B, Fukui N, Sandell L. Roles of chondrocytes in the pathogenesis of osteoarthritis. Curr Opin Rheumatol. 2002;14:578–84. doi: 10.1097/00002281-200209000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Dedrick DK, Goulet R, Huston L, Goldstein SA, Bole GG. Early bone changes in experimental osteoarthritis using microscopic computed tomography. J Rheumatol Suppl. 1991;27:44–5. [PubMed] [Google Scholar]
- 26.Muraoka T, Hagino H, Okano T, Enokida M, Teshima R. Role of subchondral bone in osteoarthritis development: a comparative study of two strains of guinea pigs with and without spontaneously occurring osteoarthritis. Arthritis Rheum. 2007;56:3366–74. doi: 10.1002/art.22921. [DOI] [PubMed] [Google Scholar]
- 27.Wu Q, Kim KO, Sampson ER, Chen D, Awad H, O’Brien T, et al. Induction of an osteoarthritis-like phenotype and degradation of phosphorylated Smad3 by Smurf2 in transgenic mice. Arthritis Rheum. 2008;58:3132–44. doi: 10.1002/art.23946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wachsmuth L, Engelke K. High-resolution imaging of osteoarthritis using microcomputed tomography. Methods Mol Med. 2004;101:231–48. doi: 10.1385/1-59259-821-8:231. [DOI] [PubMed] [Google Scholar]
- 29.Botter SM, van Osch GJ, Waarsing JH, Day JS, Verhaar JA, Pols HA, et al. Quantification of subchondral bone changes in a murine osteoarthritis model using micro-CT. Biorheology. 2006;43:379–88. [PubMed] [Google Scholar]
- 30.Botter SM, Glasson SS, Hopkins B, Clockaerts S, Weinans H, van Leeuwen JP, et al. ADAMTS5−/− mice have less subchondral bone changes after induction of osteoarthritis through surgical instability: implications for a link between cartilage and subchondral bone changes. Osteoarthritis Cartilage. 2009;17:636–45. doi: 10.1016/j.joca.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 31.Brandt KD, Myers SL, Burr D, Albrecht M. Osteoarthritic changes in canine articular cartilage, subchondral bone, and synovium fifty-four months after transection of the anterior cruciate ligament. Arthritis Rheum. 1991;34:1560–70. doi: 10.1002/art.1780341214. [DOI] [PubMed] [Google Scholar]
- 32.Wohl GR, Shymkiw RC, Matyas JR, Kloiber R, Zernicke RF. Periarticular cancellous bone changes following anterior cruciate ligament injury. J Appl Physiol. 2001;91:336–42. doi: 10.1152/jappl.2001.91.1.336. [DOI] [PubMed] [Google Scholar]
- 33.Leppala J, Kannus P, Natri A, Pasanen M, Sievanen H, Vuori I, et al. Effect of anterior cruciate ligament injury of the knee on bone mineral density of the spine and affected lower extremity: a prospective one-year follow-Up study. Calcif Tissue Int. 1999;64:357–63. doi: 10.1007/s002239900632. [DOI] [PubMed] [Google Scholar]
- 34.Patel V, Issever AS, Burghardt A, Laib A, Ries M, Majumdar S. MicroCT evaluation of normal and osteoarthritic bone structure in human knee specimens. J Orthop Res. 2003;21:6–13. doi: 10.1016/S0736-0266(02)00093-1. [DOI] [PubMed] [Google Scholar]
- 35.Hayami T, Pickarski M, Zhuo Y, Wesolowski GA, Rodan GA, Duong le T. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone. 2006;38:234–43. doi: 10.1016/j.bone.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Chappard C, Peyrin F, Bonnassie A, Lemineur G, Brunet-Imbault B, Lespessailles E, et al. Subchondral bone micro-architectural alterations in osteoarthritis: a synchrotron micro-computed tomography study. Osteoarthritis Cartilage. 2006;14:215–23. doi: 10.1016/j.joca.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Reich MS, Jarvis JP, Silva MJ, Cheverud JM. Genetic relationships between obesity and osteoporosis in LGXSM recombinant inbred mice. Genet Res (Camb) 2008;90:433–44. doi: 10.1017/S0016672308009798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McBrearty BA, Clark LD, Zhang XM, Blankenhorn EP, Heber-Katz E. Genetic analysis of a mammalian wound-healing trait. Proc Natl Acad Sci U S A. 1998;95:11792–7. doi: 10.1073/pnas.95.20.11792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheverud JM, Routman EJ, Duarte FA, van Swinderen B, Cothran K, Perel C. Quantitative trait loci for murine growth. Genetics. 1996;142:1305–19. doi: 10.1093/genetics/142.4.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kenney-Hunt JP, Vaughn TT, Pletscher LS, Peripato A, Routman E, Cothran K, et al. Quantitative trait loci for body size components in mice. Mamm Genome. 2006;17:526–37. doi: 10.1007/s00335-005-0160-6. [DOI] [PubMed] [Google Scholar]
- 41.Cheverud JM. A simple correction for multiple comparisons in interval mapping genome scans. Heredity. 2001;87:52–8. doi: 10.1046/j.1365-2540.2001.00901.x. [DOI] [PubMed] [Google Scholar]
- 42.Cheverud JM, Pletscher LS, Vaughn TT, Marshall B. Differential response to dietary fat in large (LG/J) and small (SM/J) inbred mouse strains. Physiol Genomics. 1999;1:33–9. doi: 10.1152/physiolgenomics.1999.1.1.33. [DOI] [PubMed] [Google Scholar]
- 43.Cheverud JM, Ehrich TH, Hrbek T, Kenney JP, Pletscher LS, Semenkovich CF. Quantitative trait loci for obesity- and diabetes-related traits and their dietary responses to high-fat feeding in LGXSM recombinant inbred mouse strains. Diabetes. 2004;53:3328–36. doi: 10.2337/diabetes.53.12.3328. [DOI] [PubMed] [Google Scholar]