Abstract
Epigenetic alterations are strongly associated with cancer development. We conducted a phase I/II trial of combined epigenetic therapy with azacitidine and entinostat, inhibitors of DNA methylation and histone deacetylation, respectively, in extensively pretreated patients with recurrent metastatic non-small cell lung cancer. This therapy is well tolerated, and objective responses were observed, including a complete response and a partial response in a patient who remains alive and without disease progression approximately 2 years after completing protocol therapy. Median survival in the entire cohort was 6.4 months (95% CI: 3.8–9.2), comparing favorably with existing therapeutic options. Demethylation of a set of four epigenetically silenced genes known to be associated with lung cancer was detectable in serial blood samples in these patients, and was associated with improved progression-free (p=0.034) and overall survival (p=0.035). Four of 19 patients had major objective responses to subsequent anti-cancer therapies given immediately following epigenetic therapy.
Keywords: azacitidine, entinostat, demethylation, histone deacetylase inhibitor
INTRODUCTION
Aberrant epigenetic regulation of gene expression plays a fundamental role in oncogenesis and cancer progression (1). Recent data have strongly implicated epigenetic changes as a key determinant in maintenance of subpopulations of cancer cells with high-level resistance to cytotoxic therapy and potent tumorigenic capacity (2). Multiple levels of epigenetic silencing have been defined, among which two of the most fundamental are DNA methylation and chromatin deacetylation (3). Unlike oncogenic mutations, which are fixed in the cancer genome, epigenetic alterations are potentially reversible, offering a unique therapeutic opportunity. Nonetheless, initial clinical evaluations of epigenetically targeted agents in patients with solid tumors have, in general, been disappointing.
Two cytidine analogs, azacitidine and decitabine, inhibit DNA methyltransferase activity upon incorporation into DNA, resulting in loss of DNA methylation. Both of these agents are approved for use in patients with myelodysplastic syndrome (MDS). Initial studies of these agents in solid tumor patients were associated with extensive toxicity and minimal efficacy when used at or near the maximally tolerated dose (4). However, concentrations required for reversal of tumor-specific DNA methylation are much lower than those producing maximal cytotoxicity. The cell cycle arrest and cytotoxicity associated with high-level exposure to cytidine analogs may actually limit the reversal of DNA methylation state, which requires replicative incorporation into genomic DNA. For azacitidine and decitabine, both clinical efficacy and long-term tolerance improved in MDS patients when doses well below maximally tolerated dose levels were employed (5).
Multiple histone deacetylase (HDAC) inhibitors are also in clinical development, and two have now been approved in the treatment of another hematologic malignancy, cutaneous T-cell lymphoma (6). Single agent activities of HDAC inhibitors in solid tumor patients have been modest (7, 8). Current clinical development efforts are primarily focused on combination studies with cytotoxic agents, based in part on the ability of these agents to modify the acetylation state of a large number of non-histone targets (9). While expression of many genes is influenced by HDAC inhibition alone, re-expression of densely hypermethylated, epigenetically silenced genes is not typically observed in the absence of a demethylating agent (10, 11). Preclinical models suggest that combinatorial epigenetic targeted therapy inhibiting both DNA methyltransferase and histone deacetylase activity can synergistically reactivate gene expression and result in tumor response (10–13). Recent clinical data in MDS/leukemia patients treated with low-dose azacitidine and HDAC inhibitors have defined well-tolerated combination regimens, and suggest promising activity (14, 15).
Entinostat is an orally bioavailable benzamidine HDAC inhibitor. In contrast to several other HDAC inhibitors in clinical development, entinostat has relatively focused inhibitory specificity for the class I HDACs 1 and 3 (16). Class I HDACs are found preferentially in the nucleus, and regulate the acetylation state of DNA-associated histones. Other classes of HDACs shuttle between the nucleus and cytoplasm, or are predominantly cytoplasmic, and target a much broader array of protein targets (17, 18). Combination studies involving entinostat and a variety of anticancer agents are ongoing.
We recently evaluated the prognostic implications of promoter hypermethylation of a series of genes previously implicated as targets of tumor-specific epigenetic silencing in early stage non-small cell lung cancer (NSCLC) (19). Analysis of DNA methylation in tumors and mediastinal lymph nodes from a series of patients with surgically resected stage I NSCLC defined several prognostic markers associated with rapid tumor recurrence. Four gene targets of tumor-specific epigenetic silencing, CDKN2a, CDH13, APC, and RASSF1a, were identified as strongly associated with disease recurrence and death, both singly and in combination. Methylation of any 2 of these 4 target genes in tumor and mediastinal lymph nodes conferred a markedly worse prognosis in patients with stage I lung cancer (p < 0.001), similar to patients with stage III disease (19).
Together these observations suggest that reversal of aberrant tumor-specific epigenetic silencing, using low-dose azacitidine and entinostat as defined in patients with MDS (15), might have clinical activity in advanced lung cancer, and that this activity might correlate with effects on these defined epigenetically regulated target genes. We initiated a phase I/II study to test these hypotheses in patients with progressive, metastatic NSCLC.
RESULTS
Patient Characteristics
Ten patients participated in the phase I portion of this study; three patients received 30 mg/m2/d azacitidine and seven received 40 mg/m2/d azacitidine. Entinostat was administered to all patients at a fixed dose of 7 mg on days 3 and 10 of each cycle. In total, 42 patients, including the seven patients from the phase I portion, were treated at the phase II azacitidine dose of 40 mg/m2. This paper summarizes data on all 45 phase I and II patients. Demographics are provided in Table 1.
Table 1.
Demographic Characteristics
| Characteristic | (N = 45) |
|---|---|
| Age | |
| Median (Range) | 64 (46–80) |
|
| |
| Sex | |
| Male/Female | 23/22 |
|
| |
| Race | |
| White/Black/Asian | 39/8/1 |
|
| |
| Smoking Status | |
| Active | 2 |
| Former | 38 |
| Never | 5 |
|
| |
| Histology | |
| Adenocarcinoma | 34 |
| Squamous | 9 |
| NSCLC not otherwise specified | 2 |
|
| |
| Median Number of Prior Therapies | 3 |
The most common reason for study discontinuation of all patients receiving combination therapy was disease progression (39 of 45; 87%). The median number of treatment cycles was 2 (range, 1 to 18) and the median treatment duration was 52 days (range, 7 to 507).
Safety
Dose-limiting toxicities (DLTs)
None of the 3 patients in the 30 mg/m2 azacitidine cohort experienced DLTs. One patient in the 40 mg/m2 azacitidine cohort withdrew from the study during the first week due to decreasing performance status and was replaced. None of the remaining 6 phase I patients had DLTs. The maximal dose planned in the phase I dose escalation was 40 mg/m2 in an effort to remain at an epigenetically targeted dose rather than a directly cytotoxic dose. The recommended phase II dose was therefore defined as 40 mg/m2 azacitidine given on days 1–6 and 8–10 plus 7 mg entinostat given on days 3 and 10 of each 28 day cycle.
Adverse events
All patients experienced at least 1 treatment-related adverse event. Toxicity attributions were assigned by the principal investigator. The most common treatment-related, non-hematologic adverse events included low-grade skin/injection-site reactions (93%), fatigue (71%), nausea (73%), vomiting (40%), constipation (36%), anorexia (29%), electrolyte disturbances (29%), and hyperglycemia (22%). These adverse events are anticipated toxicities of either azacitidine or entinostat and generally required no intervention except anti-emetics and other agents for gastrointestinal side effects, which were easily medically managed. Anemia was the most common hematologic toxicity (40%). Lymphopenia and thrombocytopenia were each seen in 27% of patients.
Grade 3 or 4 toxicities were seen in 28% of patients during cycle 1 (Table 2). The most common grade 3 or 4 toxicity was fatigue. Grade 3 or 4 hematologic toxicities were transient and generally asymptomatic. Four patients with anemia all improved following a one-time red blood cell transfusion. There were no correlations between the worst grade of toxicity and azacitidine or entinostat exposure (p > 0.05).
Table 2.
Grade 3 and 4 Toxicities
| Toxicities | Gr. 3 | Gr. 3 | Gr. 4 | Gr. 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Cycle 1 | Cycle ≥ 2 | Cycle 1 | Cycle ≥ 2 | |||||
| N = 45 | N = 32 | N = 45 | N = 32 | |||||
|
| ||||||||
| N | % | N | % | N | % | N | % | |
| Hematologic Toxicities | ||||||||
| Anemia | 3 | 6.7 | 1 | 3.1 | - | - | - | - |
| Leukopenia | 1 | 2.2 | 2 | 6.3 | 1 | 2.2 | - | - |
| Lymphopenia | 4 | 8.9 | 3 | 9.4 | 1 | 2.2 | - | - |
| Neutropenia | - | - | 3 | 9.4 | 1 | 2.2 | - | - |
| Thrombocytopenia | 1 | 2.2 | - | - | - | - | - | - |
|
| ||||||||
| Gastrointestinal Symptoms | ||||||||
| Abdominal pain | 1 | 2.2 | - | - | - | - | - | - |
| Constipation | 1 | 2.2 | 1 | 3.1 | - | - | - | - |
| Diarrhea | 1 | 2.2 | - | - | - | - | - | - |
| Nausea | 2 | 4.4 | - | - | - | - | - | - |
| Vomiting | 1 | 2.2 | - | - | - | - | - | - |
|
| ||||||||
| Electrolyte Disturbances | ||||||||
| Hypokalemia | - | - | 1 | 3.1 | - | - | - | - |
| Hyponatremia | 3 | 6.7 | - | - | - | - | - | - |
|
| ||||||||
| Generalized Symptoms | ||||||||
| Anorexia | 2 | 4.4 | - | - | - | - | - | - |
| Dyspnea | 1 | 2.2 | - | - | - | - | - | - |
| Fatigue | 2 | 4.4 | 4 | 12.5 | - | - | - | - |
| Weakness | - | - | 1 | 3.1 | - | - | - | - |
Pharmacokinetics
Pharmacokinetic data were obtained from 40 of the 42 patients treated at an azacitadine dose level of 40 mg/m2/d. As previously reported, azacitidine was rapidly absorbed and eliminated with the time to maximal concentration (Tmax) occurring at 0.50 hours (median; range 0.25 - 2.00 hours) and half-life (t1/2) of 1.12 ± 1.06 hours (average ± sd). The large variability in t1/2 is in part attributable to individuals who had detectable azacitidine concentrations at 6 (n = 11) or 8 (n = 3) hours. Maximum concentration (Cmax) and area under the curve (AUC0-∞) for azacitidine were 468 ± 241 ng/mL and 675 ± 290 ng*hr/mL. Entinostat concentrations in cycle 1 were 0.84 ± 0.23 ng/mL on day 10 (pretreatment) and 1.10 ± 0.34 ng/mL on day 17. Entinostat concentrations were detectable in 30% of patients prior to starting cycle 2 (day 29). In those patients, the residual concentrations were 0.66 ± 0.18 ng/mL.
Efficacy
One patient had a complete response that lasted 14 months (Figure 1). A second patient had a partial response that lasted 8 months (Figure 2). Ten patients had stabilization of disease of at least 12 weeks. One of these patients had stable disease for 18 months and another for 14 months, both with prolonged symptomatic improvement. Twenty-two patients had progressive disease after two cycles of therapy. Eleven patients were considered non-evaluable for efficacy because they did not complete one cycle of therapy. Two of these patients withdrew consent due to difficulty with traveling based on the schedule. Four of these patients came off study for decreased performance status. The remaining five patients experienced early disease progression including one patient with unsuspected brain metastases diagnosed ten days into treatment.
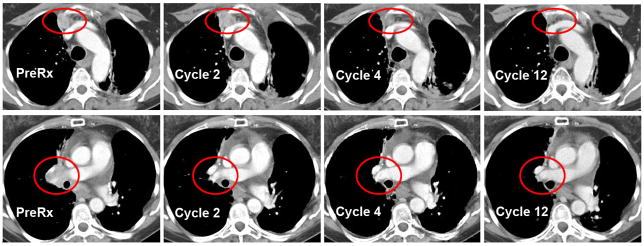
Figure 1. Serial CT scan images from patient with a complete response.
Scans show progressive resolution of a prevascular tumor (top) and a right hilar mass (bottom) over the course of one year on therapy. Red circles indicate areas of measurable disease.
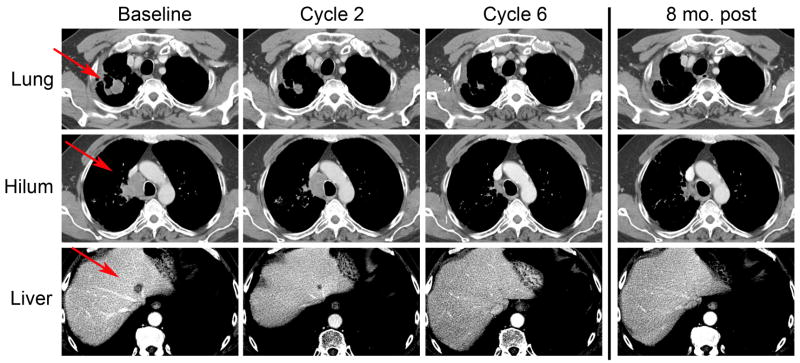
Figure 2. Serial CT scan images from patient with a partial response.
Scans demonstrate gradual resolution of sites of disease including a lung mass, matted hilar lymph nodes, and one of the liver metastases. Although he was taken off study for treatment of an intercurrent small cell lung cancer, he remains alive and was without evidence of disease recurrence nearly 2 years after completion of this therapy. Red arrrows indicate areas of measurable disease.
The patient with the complete response was initially diagnosed with a stage IA adenocarcinoma, but had rapid disease recurrence within 6 months of surgical resection. Analysis of the patient’s original stage I tumor and mediastinal lymph nodes demonstrated a pattern of target gene hypermethylation previously defined as strongly predictive of early recurrence, with methylation of each of the four target genes detectable in either tumor, mediastinal lymph nodes, or both (19). In the 13 months between post-surgical recurrence and study enrollment, the patient experienced progressive disease on three chemotherapy regimens. On combination epigenetic therapy she experienced gradual improvement over a prolonged period of time, achieving maximal response after about 6 months. After 14 months on treatment she was noted to have an enlarging solitary pulmonary nodule, which had been present as a 2 mm lesion at the time of study entry. The nodule was resected and appeared to represent a second primary NSCLC, molecularly distinct from her initial primary in that it harbored a KRAS mutation while her original tumor did not. Surgical exploration with biopsy at the time of resection revealed no viable tumor at prior sites of disease. She continued with no evidence of disease after the resection for nearly 12 months, followed by relapse and ultimately fatal progression of her KRAS-mutant tumor.
The patient with a partial response, also with 3 prior therapies for metastatic disease, had gradual complete radiographic resolution of multiple liver metastases and partial resolution of lung and mediastinal disease over 8 cycles of therapy. He ultimately developed a new mediastinal nodule, which on biopsy proved to be a second primary tumor: small cell lung cancer. This was treated with etoposide, carboplatin, and concomitant radiation. He had no evidence of disease progression for 22 months after discontinuing epigenetic therapy, off all anticancer treatment for approximately 18 months, but has recently had localized disease recurrence of NSCLC in the chest. His liver metastases have completely resolved, and remain undetectable over 2 years after completion of therapy.
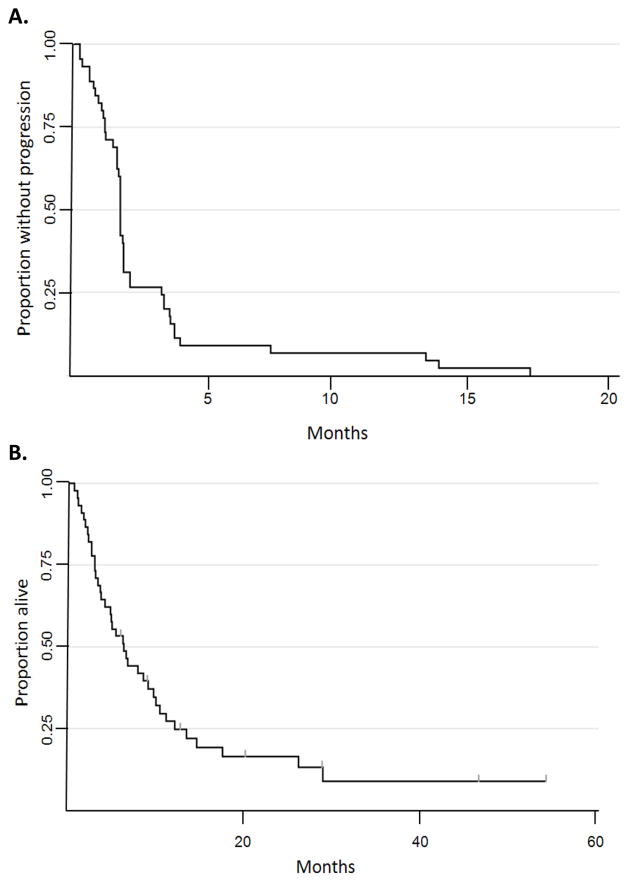
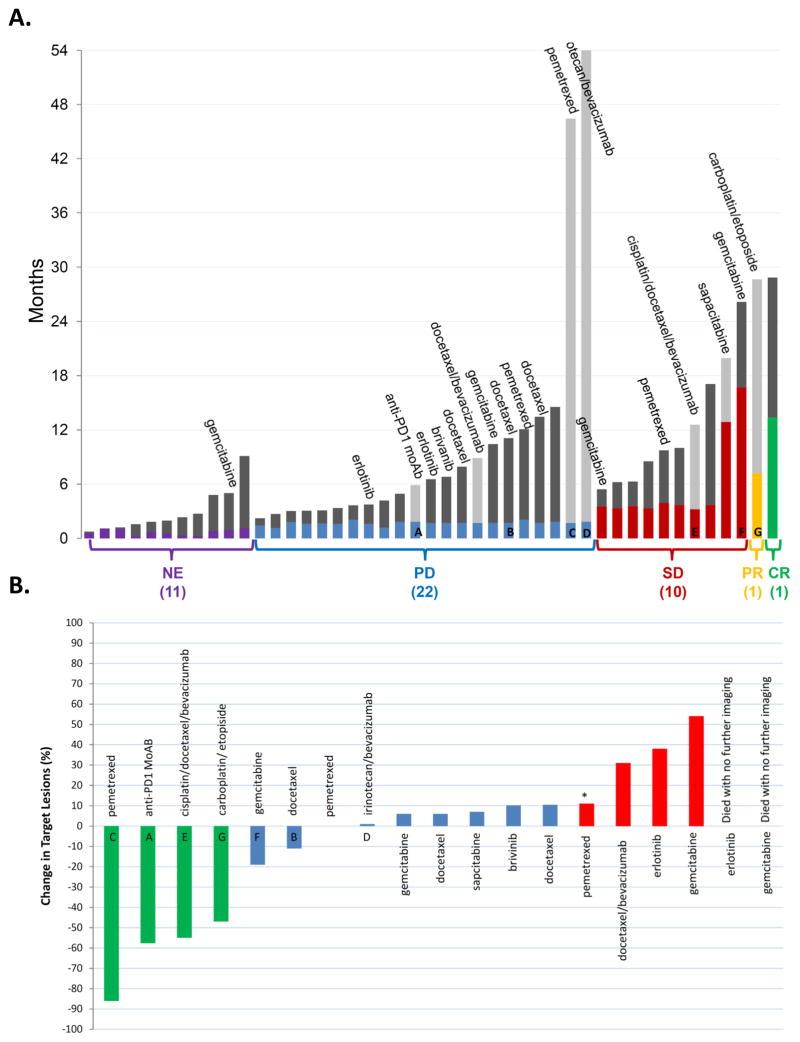
In an intent-to-treat analysis of all patients enrolled, median progression-free survival was 7.4 weeks (95% CI: 7.0–8.0 weeks) and median overall survival was 6.4 months (95% CI: 3.8–9.2 months) (Figure 3). Median survival among patients who completed at least one cycle of epigenetic therapy was 8.6 months (95% CI: 5.5-12.2 months). The prolonged survival in patients with extensively pretreated metastatic NSCLC prompted an assessment of subsequent treatment. A total of 19 patients received at least one subsequent systemic treatment in the 6 months after going off study (Figure 4A) including two long-term survivors of 44 and 52 months post-epigenetic therapy having received only one post-study treatment regimen. Interestingly, four of the patients who received subsequent chemotherapy (21%) had major objective responses to the immediate subsequent therapy (Figure 4B).
Figure 3. Kaplan Meier survival analyses (intent-to-treat).
All patients on study are included in these analyses. A. Progression-free survival. B. Overall survival.
Figure 4. Survival, subsequent therapies, and response.
A Duration of survival on and after protocol therapy. The height of the gray bar indicates duration of survival. Light gray bars indicate patients who are still alive. The colored portion of the bar represents the duration of therapy received on trial. NE: non-evaluable for response, PD: progressive disease, SD: stable disease, PR: partial response, CR: complete response. If a patient received subsequent chemotherapy within 6 months, it is listed above the patient’s survival bar. Letters identify corresponding patients in panel B. B. Waterfall plot of response to immediate subsequent therapy. The best change in defined target lesions to subsequent systemic anti-cancer treatment following epigenetic therapy is shown. Green: PR, blue: SD, red: PD. (*) indicates progression defined by a new lesion. Two patients, indicated at right, died without follow-up imaging.
Biomarker Analyses
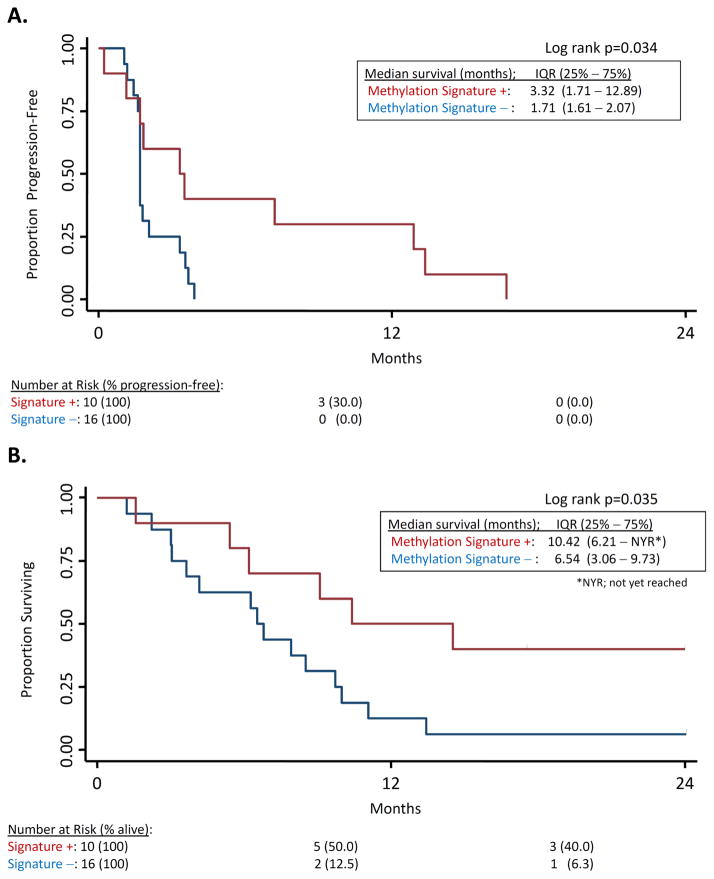
Our prior analysis of methylation status of tumor and mediastinal lymph nodes from patients with stage I NSCLC revealed 4 genes (APC, RASSF1A, CDH13, and CDKN2A) as negative prognostic factors for survival (19). Promoter hypermethylation of at least 2 of these 4 genes in tumor and histologically normal lymph nodes was a strong predictor of early disease recurrence (p < 0.001). We hypothesized that the effect on methylation of these same 4 genes might define a population of lung cancer patients responsive to epigenetically targeted therapy. We did not have available pre-treatment tumor biopsy material from patients on this study, as pretreatment biopsies were not mandated, but we did have pre-treatment and post-cycle 1 plasma samples from study subjects. Free plasma DNA concentration is known to be elevated in cancer patients, and has proved to be a valuable source of tumor DNA for biomarker development, including detection of hypermethylated tumor suppressor genes in NSCLC patients (20–23). We therefore examined promoter methylation status in circulating DNA from patient plasma collected prior to therapy (day 0) and after 1 cycle of therapy (day 29). A total of 26 patients had sufficient circulating free DNA at both timepoints for analysis. Ten patients had at least two methylated target genes on day 0, and had a decrease in the level of methylation of two or more of these genes by day 29. Of these 10 “methylation signature”-positive patients, 8 (80%) had either stable disease or objective responses to epigenetic therapy. In contrast, of the 16 methylation signature-negative patients, only 4 (25%) had stable disease and there were no objective responses. The median overall survival was 10.42 months for the methylation signature-positive cohort vs. 6.54 months for the methylation signature-negative (p = 0.035; Figure 5A). Progression-free survival was 3.32 months vs. 1.71 for methylation signature-positive and negative cohorts, respectively (p = 0.034; Figure 5B).
Figure 5. Survival stratified by target gene methylation status.
Promoter methylation of APC, CDH13, RASS1a, and CDKN2A were evaluated in circulating plasma DNA from patients at pre-treatment and on day 29. Red: patients with pre-treatment methylation of ≥2 of these 4 genes that demonstrate demethylation by day 29. Blue: all other patients with detectable circulating DNA (total N = 26). A. Overall survival. B. Progression-free survival.
DISCUSSION
We report the first objective, durable responses in solid tumor patients using combined epigenetic therapy with a DNA methyltransferase inhibitor and a histone deacetylase inhibitor. Objective responses to this therapy in this heavily pretreated population occurred in only 4% of the patients, but the anti-tumor responses that were observed were impressive. Several observations from this cohort of patients suggest that this therapy is quite distinct from prior experience with high-dose azacitidine, and merits additional focused investigation.
Unlike participants in older clinical trials of azacitidine or decitabine in solid tumor patients (24–28), our patients received doses far below the maximally tolerated dose, permitting repetitive dosing over many months, and avoiding the cytotoxicity associated with the high-dose regimens. Low-dose azacitidine or decitabine regimens have led to successful treatment of MDS (5, 14, 29, 30) with improved survival (31). Consistent with clinical trial observations in MDS (5), and in contrast to typical responses to cytotoxic chemotherapy, tumor responses in NSCLC patients improved gradually and progressively over several months of treatment. Intriguingly, the clinical responses produced were sustained even after cessation of epigenetic therapy. There was no evidence of relapse of the complete responder’s original wild-type KRAS metastatic disease at the time of her death, sixteen months after discontinuing epigenetic therapy. Similarly, at present there has been no evidence of recurrence of the partial responder’s hepatic metastases, over 2 years after stopping epigenetic treatment.
Another potential contributor to the responses seen in these patients is the methylation status of certain key genes in the tumor. Multiple studies have demonstrated the relevance of DNA methylation in lung cancer (32). The patient with a complete response demonstrated tumor-specific promoter hypermethylation in primary tumor and mediastinal lymph nodes in a pattern prognostic of poor survival in early stage lung cancer, which may define a subset of lung cancers driven by epigenetic mechanisms (19). The patient with partial response did not have baseline tumor available for analysis, but circulating DNA analysis from this patient confirms target gene methylation at baseline, and de-methylation with treatment. Both of these patients had methylation of 3 of 4 genes detectable in circulating DNA, with demethylation in all 3 with epigenetic therapy.
Analysis of free-circulating tumor DNA in the plasma of patients supports early de-methylation as a potential predictor of clinical benefit from this therapy, and is consistent with an on-target epigenetic mechanism of action. Evaluation of methylation changes in tumor DNA during cycle 1 of therapy as a predictor of clinical benefit should be included in future trials of epigenetically directed therapy. Other putative biomarkers of response to epigenetically targeted agents defined in recent studies could also be explored in this context (33).
Two of the patients described here developed molecularly and histologically distinct second lung cancers on therapy. This has not been observed in other patients in this study. Second primary cancers are common in lung cancer patients, associated with similar carcinogenic exposure throughout the lung field (34). While it is difficult to entirely rule out the possibility of therapy-related adverse effects, secondary malignancies have not been reported in much larger series of patients receiving similar therapies for MDS (35, 36).
A limitation of the general applicability of this therapy is the need for subcutaneous injection of azacitidine on a daily basis. This was also a common cause of low grade toxicities (e.g. injection site reactions with localized erythema) on this study. Activity and bioavailability of an oral formulation of azacitidine has been recently reported in patients with hematologic malignancies (37). Other novel oral demethylating agents including zebularine derivatives have shown activity in experimental cancer models (38, 39). If bioequivalence can be demonstrated, these oral agents may have significant advantages over subcutaneous administration in terms of patient tolerance over prolonged courses of drug administration.
An important feature of this trial is combinatorial targeting of epigenetic silencing adding the HDAC inhibitor, entinostat. Preclinical data have demonstrated that while re-expression of epigenetically silenced target genes can be induced with inhibition of DNA methyltransferase alone, additional targeting of histone deacetylation results in more robust and persistent changes in gene expression (12, 13). Previous trials combining demethylating agents and HDAC inhibitors have used much less potent HDAC inhibitors (40, 41). We hypothesize that these key features contributed to the responses observed.
The median survival of 6.4 months on azacitidine and entinostat in extensively pretreated NSCLC (median number of prior therapies = 3) is similar to the median survival previously noted for patients with 1 or 2 prior therapies treated with the only FDA-approved drug for this patient population, erlotinib (median survival 6.7 months, vs. 4.7 months for placebo control) (42). Stable changes in gene expression induced by epigenetically directed therapy could alter cancer cell sensitivity to subsequent cytotoxic therapy. Recent data from the Settleman laboratory have defined drug resistant “persisters” within clonal cancer cell populations highly sensitive to targeted therapy, and that maintenance of these drug resistant persisters is epigenetically regulated (2). Major objective responses to immediate subsequent therapies, even among patients with primary RECIST progression on epigenetic therapy, have been observed and may have contributed to the exceptionally long survival among some patients on this study. Interestingly, a previous study also observed long-term survival in a patient following chemotherapy given after the DNA methyltransferase inhibitor, decitabine (24, 43). These observations suggest a testable hypothesis, that epigenetic therapy could prime cancers for response to subsequent cytotoxic therapy. Efforts to further refine characteristics of patients likely to benefit from this novel therapeutic approach, and to optimize this regimen to benefit additional cancer patients, are ongoing.
METHODS
Patient population
This study enrolled adults with metastatic NSCLC with disease progression after at least one prior anti-cancer regimen for metastatic disease. Any number of prior therapies was allowed, and patients with treated brain metastases were included. Additional eligibility requirements included measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST) 1.0 (44), Eastern Cooperative Oncology Group performance status of 0 to 2, life expectancy of ≥3 months, and adequate liver, renal and bone marrow function. Patients were excluded if they had uncontrolled brain metastases or liver metastases replacing > 30% of the liver parenchyma. Patients with HIV on anti-retroviral therapy were also excluded. This study was conducted according to the Declaration of Helsinki and with full Institutional Review Board approval. All participants provided written informed consent before participating. The study was registered with the National Institutes of Health (NCT00387465).
Study design
The phase I component used a standard 3 + 3 patient cohort design to assess adverse events of the combination of azacitidine at 30 mg/m2 or 40 mg/m2 on days 1–6 and 8–10 with entinostat at a 7 mg fixed dose on days 3 and 10 of each 28 day cycle. Based on the strategy of exploring doses of azacitidine well below previously defined maximally tolerated doses, dose levels in phase I were prespecified as limited to no more than 40 mg/m2 daily. The phase II component was a single-arm two-stage open label study designed to assess the response rate of the combination at the 40 mg/m2/d dose of azacitidine.
Safety
Safety assessments included history and physical examinations, vital signs, Eastern Cooperative Oncology Group performance status, adverse events, serum chemistry and blood counts. Physical exams were performed at screening, every other week during cycle 1, monthly during cycle 2 and subsequent cycles, and at the final study visit. Laboratory analyses were performed at screening, every other week while on treatment, and at the final study visit. Adverse event severity was graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events, version 3.0 (45). Adverse Events (AEs) judged possibly or probably related to azacitidine and entinostat administration were considered dose-limiting toxicities (DLTs) if they satisfied any of the following criteria: non-hematologic grade 3/4 toxicity except grade 3 nausea or vomiting only if unresponsive to therapy; grade 4 neutropenia, leukopenia, lymphopenia; and decreased hemoglobin. A delay in treatment of greater than 2 weeks was also considered a DLT. The phase II dose was defined as the dose at which ≤30% of patients experienced DLTs during cycle 1 up to a pre-specified maximal dose of 40 mg/m2 of azacitidine.
Pharmacokinetics
For azacitidine, blood samples were obtained prior to treatment and at 0.25, 0.5, 1, 2, 4, 6, 8, and 24 hours after the first dose of azacitidine. Plasma samples were stored and processed as previously described utilizing liquid chromatography/tandem mass spectrometry (46, 47). Pharmacokinetic parameters were determined as previously described (46).
For entinostat, blood samples were collected on cycle 1 day 1 and then approximately 7 days after entinostat administration on days 10 and 17. Plasma concentrations of entinostat were measured using liquid chromatography/tandem mass spectrometry as previously described (48). Samples were considered trough concentrations (Cmin) if they were collected pre-treatment concentrations on days 10 and 17.
Pharmacodynamic correlates
Free-circulating plasma tumor DNA was evaluated for DNA methylation in each patient. Samples were collected pre-treatment and on days 10 and 29 on the study. Blood samples for methylation analysis were collected into citrate vacutainer cell preparation tubes.
DNA methylation, for all lung cancer, lymph node, and plasma DNA samples, was determined by methylation-specific PCR (MSP) and performed as previously described (49). Plasma samples were also analyzed by quantitative MSP using Applied Biosystems StepOne qPCR machines, and qPCR or molecular beacon primer or primer and probe sets. CDH13 and RASSF1A methylation was analyzed using MSP primers for quantitative analysis on the AB platform with 60°C as the annealing temperature. Molecular beacon assays were used to analyze APC and CDKN2A methylation, with β-Actin for gene normalization, all using 57°C as the annealing temperature.
All analyses were performed by investigators blinded to the origin of the samples. All reactions were performed in triplicate. Ctgenemax is equal to the earliest cycle threshold for any sample in the dataset and Ctgene1, 2, and 3 are used to denote samples run in triplicate. Relative methylation values were generated using the following equation:
Efficacy
Efficacy variables including objective response rate, progression-free survival, and overall survival were evaluated for all patients treated at the phase II dose. Tumor response was assessed using RECIST 1.0 after every 2 cycles of therapy (44). Response assessment to subsequent therapy was similarly based on RECIST criteria and was performed by a single radiologist blinded to clinical data.
Statistical Analysis
Simple descriptive statistics were utilized to display the data on toxicity seen from azacitidine and entinostat in this patient population. Pharmacokinetic parameters were summarized using descriptive statistics. The distributions of progression-free and overall survival were estimated using the Kaplan-Meier method (50). A Kruskal-Wallis test was used to determine the association between azacitidine and entinostat exposure and response and worst grade of toxicity. The method of Tukey-Kramer was used to adjust for multiple comparisons of mean values. The association between categorical variables was assessed using Fisher's exact test. All tests of hypotheses were conducted at two-sided α = .05 level.
Significance.
This study demonstrates that combined epigenetic therapy with low-dose azacitidine and entinostat results in objective, durable responses in solid tumor patients, and defines a blood-based biomarker that correlates with clinical benefit.
Acknowledgments
This study was sponsored through the Cancer Therapy Evaluation Program with grant support from the National Institutes of Health (R21 CA126265, UO1 CA70095, P50 CA058184, P30 CA006973, S10RR026824, and UL1 RR 025005), the Flight Attendant Medical Research Institute, and an Entertainment Industry Foundation (EIF)- American Association for Cancer Research (AACR) Stand Up to Cancer award.
Abbreviations
- CI
confidence interval
- sd
standard deviation
Footnotes
Potential conflicts of interest: RAJ and CMR have consulted for Syndax. JGH has consulted for MDx Health, JGH and MVB have research support from MDxHealth, and JGH, SBB, and MVB hold a patent licensed to MDx Health. These arrangements are managed by the Johns Hopkins University in accordance with its conflict of interest policies.
References
- 1.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–92. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma SV, Lee DY, Li B, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–54. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 4.Glover AB, Leyland-Jones BR, Chun HG, Davies B, Hoth DF. Azacitidine: 10 years later. Cancer Treat Rep. 1987;71:737–46. [PubMed] [Google Scholar]
- 5.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–40. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 6.Howman RA, Prince HM. New drug therapies in peripheral T-cell lymphoma. Expert Rev Anticancer Ther. 2011;11:457–72. doi: 10.1586/era.11.4. [DOI] [PubMed] [Google Scholar]
- 7.Vansteenkiste J, Van Cutsem E, Dumez H, et al. Early phase II trial of oral vorinostat in relapsed or refractory breast, colorectal, or non-small cell lung cancer. Invest New Drugs. 2008;26:483–8. doi: 10.1007/s10637-008-9131-6. [DOI] [PubMed] [Google Scholar]
- 8.Schrump DS, Fischette MR, Nguyen DM, et al. Clinical and molecular responses in lung cancer patients receiving Romidepsin. Clin Cancer Res. 2008;14:188–98. doi: 10.1158/1078-0432.CCR-07-0135. [DOI] [PubMed] [Google Scholar]
- 9.Marks PA. Histone deacetylase inhibitors: a chemical genetics approach to understanding cellular functions. Biochim Biophys Acta. 2010;1799:717–25. doi: 10.1016/j.bbagrm.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoemaker AR, Mitten MJ, Adickes J, et al. Activity of the Bcl-2 family inhibitor ABT-263 in a panel of small cell lung cancer xenograft models. Clin Cancer Res. 2008;14:3268–77. doi: 10.1158/1078-0432.CCR-07-4622. [DOI] [PubMed] [Google Scholar]
- 11.Jain HV, Meyer-Hermann M. The molecular basis of synergism between carboplatin and ABT-737 therapy targeting ovarian carcinomas. Cancer Res. 2011;71:705–15. doi: 10.1158/0008-5472.CAN-10-3174. [DOI] [PubMed] [Google Scholar]
- 12.Belinsky SA, Klinge DM, Stidley CA, et al. Inhibition of DNA methylation and histone deacetylation prevents murine lung cancer. Cancer Res. 2003;63:7089–93. [PubMed] [Google Scholar]
- 13.Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21:103–7. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- 14.Gore SD, Baylin S, Sugar E, et al. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res. 2006;66:6361–9. doi: 10.1158/0008-5472.CAN-06-0080. [DOI] [PubMed] [Google Scholar]
- 15.Fandy TE, Herman JG, Kerns P, et al. Early epigenetic changes and DNA damage do not predict clinical response in an overlapping schedule of 5-azacytidine and entinostat in patients with myeloid malignancies. Blood. 2009;114:2764–73. doi: 10.1182/blood-2009-02-203547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu E, Dul E, Sung CM, et al. Identification of novel isoform-selective inhibitors within class I histone deacetylases. J Pharmacol Exp Ther. 2003;307:720–8. doi: 10.1124/jpet.103.055541. [DOI] [PubMed] [Google Scholar]
- 17.Johnstone RW, Licht JD. Histone deacetylase inhibitors in cancer therapy: is transcription the primary target? Cancer Cell. 2003;4:13–8. doi: 10.1016/s1535-6108(03)00165-x. [DOI] [PubMed] [Google Scholar]
- 18.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–84. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 19.Brock MV, Hooker CM, Ota-Machida E, et al. DNA methylation markers and early recurrence in stage I lung cancer. N Engl J Med. 2008;358:1118–28. doi: 10.1056/NEJMoa0706550. [DOI] [PubMed] [Google Scholar]
- 20.Esteller M, Sanchez-Cespedes M, Rosell R, Sidransky D, Baylin SB, Herman JG. Detection of aberrant promoter hypermethylation of tumor suppressor genes in serum DNA from non-small cell lung cancer patients. Cancer Res. 1999;59:67–70. [PubMed] [Google Scholar]
- 21.Sozzi G, Conte D, Leon M, et al. Quantification of free circulating DNA as a diagnostic marker in lung cancer. J Clin Oncol. 2003;21:3902–8. doi: 10.1200/JCO.2003.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Sirera R, Bremnes RM, Cabrera A, et al. Circulating DNA is a useful prognostic factor in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2011;6:286–90. doi: 10.1097/JTO.0b013e31820189a5. [DOI] [PubMed] [Google Scholar]
- 23.Lee YJ, Yoon KA, Han JY, et al. Circulating cell-free DNA in plasma of never smokers with advanced lung adenocarcinoma receiving gefitinib or standard chemotherapy as first-line therapy. Clin Cancer Res. 17:5179–87. doi: 10.1158/1078-0432.CCR-11-0400. [DOI] [PubMed] [Google Scholar]
- 24.Momparler RL, Bouffard DY, Momparler LF, Dionne J, Belanger K, Ayoub J. Pilot phase I-II study on 5-aza-2'-deoxycytidine (Decitabine) in patients with metastatic lung cancer. Anticancer Drugs. 1997;8:358–68. doi: 10.1097/00001813-199704000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Vogler WR, Arkun S, Velez-Garcia E. Phase I study of twice weekly 5-azacytidine (NSC-102816) Cancer Chemother Rep. 1974;58:895–9. [PubMed] [Google Scholar]
- 26.Lomen PL, Baker LH, Neil GL, Samson MK. Phase I study of 5-azacytidine (NSC-102816) using 24-hour continuous infusion for 5 days. Cancer Chemother Rep. 1975;59:1123–6. [PubMed] [Google Scholar]
- 27.Weiss AJ, Stambaugh JE, Mastrangelo MJ, Laucius JF, Bellet RE. Phase I study of 5-azacytidine (NSC-102816) Cancer Chemother Rep. 1972;56:413–9. [PubMed] [Google Scholar]
- 28.Quagliana JM, O'Bryan RM, Baker L, et al. Phase II study of 5-azacytidine in solid tumors. Cancer Treat Rep. 1977;61:51–4. [PubMed] [Google Scholar]
- 29.List AF. Treatment strategies to optimize clinical benefit in the patient with myelodysplastic syndromes. Cancer Control. 2008;15 (Suppl):29–39. doi: 10.1177/107327480801504s04. [DOI] [PubMed] [Google Scholar]
- 30.Lubbert M, Bertz H, Ruter B, et al. Non-intensive treatment with low-dose 5-aza-2'-deoxycytidine (DAC) prior to allogeneic blood SCT of older MDS/AML patients. Bone Marrow Transplant. 2009;44:585–8. doi: 10.1038/bmt.2009.64. [DOI] [PubMed] [Google Scholar]
- 31.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–32. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anglim PP, Alonzo TA, Laird-Offringa IA. DNA methylation-based biomarkers for early detection of non-small cell lung cancer: an update. Molecular cancer. 2008;7:81. doi: 10.1186/1476-4598-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simo-Riudalbas L, Melo SA, Esteller M. DNMT3B gene amplification predicts resistance to DNA demethylating drugs. Genes Chromosomes Cancer. 50:527–34. doi: 10.1002/gcc.20877. [DOI] [PubMed] [Google Scholar]
- 34.Brock MV, Alberg AJ, Hooker CM, et al. Risk of subsequent primary neoplasms developing in lung cancer patients with prior malignancies. J Thorac Cardiovasc Surg. 2004;127:1119–25. doi: 10.1016/j.jtcvs.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 35.Gandhi L, Camidge DR, Ribeiro de Oliveira M, et al. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol. 2011;29:909–16. doi: 10.1200/JCO.2010.31.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudin CM, Mauer A, Smakal M, et al. Phase I/II study of pemetrexed with or without ABT-751 in advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2011;29:1075–82. doi: 10.1200/JCO.2010.32.5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Manero G, Gore SD, Cogle C, et al. Phase I study of oral azacitidine in myelodysplastic syndromes, chronic myelomonocytic leukemia, and acute myeloid leukemia. J Clin Oncol. 29:2521–7. doi: 10.1200/JCO.2010.34.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng JC, Matsen CB, Gonzales FA, et al. Inhibition of DNA methylation and reactivation of silenced genes by zebularine. J Natl Cancer Inst. 2003;95:399–409. doi: 10.1093/jnci/95.5.399. [DOI] [PubMed] [Google Scholar]
- 39.Herranz M, Martin-Caballero J, Fraga MF, et al. The novel DNA methylation inhibitor zebularine is effective against the development of murine T-cell lymphoma. Blood. 2006;107:1174–7. doi: 10.1182/blood-2005-05-2033. [DOI] [PubMed] [Google Scholar]
- 40.Braiteh F, Soriano AO, Garcia-Manero G, et al. Phase I study of epigenetic modulation with 5-azacytidine and valproic acid in patients with advanced cancers. Clin Cancer Res. 2008;14:6296–301. doi: 10.1158/1078-0432.CCR-08-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin J, Gilbert J, Rudek MA, et al. A phase I dose-finding study of 5-azacytidine in combination with sodium phenylbutyrate in patients with refractory solid tumors. Clin Cancer Res. 2009;15:6241–9. doi: 10.1158/1078-0432.CCR-09-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shepherd FA, Pereira J, Ciuleanu TE, et al. A randomized placebo-controlled trial of erlotinib in patients with advanced non-small cell lung cancer following failure of 1st line or 2nd line chemotherapy. A National Cancer Institute of Canada Clinical Trials Group trial. Proc Am Soc Clin Oncol. 2004;22:622s. [Google Scholar]
- 43.Momparler RL, Ayoub J. Potential of 5-aza-2'-deoxycytidine (Decitabine) a potent inhibitor of DNA methylation for therapy of advanced non-small cell lung cancer. Lung Cancer. 2001;34 (Suppl 4):S111–5. doi: 10.1016/s0169-5002(01)00397-x. [DOI] [PubMed] [Google Scholar]
- 44.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 45.Trotti A, Colevas AD, Setser A, et al. CTCAE v3. 0: development of a comprehensive grading system for the adverse effects of cancer treatment. Seminars in radiation oncology. 2003;13:176–81. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 46.Rudek MA, Zhao M, He P, et al. Pharmacokinetics of 5-azacitidine administered with phenylbutyrate in patients with refractory solid tumors or hematologic malignancies. J Clin Oncol. 2005;23:3906–11. doi: 10.1200/JCO.2005.07.450. [DOI] [PubMed] [Google Scholar]
- 47.Zhao M, Rudek MA, He P, et al. Quantification of 5-azacytidine in plasma by electrospray tandem mass spectrometry coupled with high-performance liquid chromatography. Journal of chromatography. 2004;813:81–8. doi: 10.1016/j.jchromb.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 48.Zhao M, Rudek MA, Mnasakanyan A, Hartke C, Pili R, Baker SD. A liquid chromatography/tandem mass spectrometry assay to quantitate MS-275 in human plasma. Journal of pharmaceutical and biomedical analysis. 2007;43:784–7. doi: 10.1016/j.jpba.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A. 1996;93:9821–6. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]