Abstract
Progressive left ventricular (LV) dysfunction induces expression of the cytokine transforming growth factor-beta (TGFb1). Endoglin (CD105) is a TGFb1 co-receptor that is released into the circulation as soluble endoglin (sEng). The objective of this study was to assess serum levels of sEng in heart failure and to identify the predictive value of sEng for detecting elevated left ventricular end-diastolic pressures (LVEDP). We measured sEng levels in 82 consecutive patients with suspected LV dysfunction referred for determination of left heart filling pressures by cardiac catheterization. Among these subjects sEng levels correlated with LVEDP (R=0.689; p<0.0001) irrespective of LV ejection fraction (LVEF). Using a receiving operative characteristic (ROC) curve, sEng levels predicted an LVEDP≥16mmHg with an area-under-the-curve (AUC) of 0.85, exceeding measured AUCs for both atrial- and brain-natriuretic peptide, currently used biomarkers for heart failure diagnosis (ANP:0.68; BNP:0.65, p<0.01 vs sEng respectively). In 10 subjects receiving medical therapy for heart failure guided by invasive hemodynamic monitoring, decreased pulmonary capillary wedge pressure was associated with a reduced sEng level (R=0.75, p=0.008). Finally, compared to 25 healthy controls, sEng levels were elevated in subjects with suspected LV dysfunction (3589±588 vs 4257±966 pg/mL, respectively, p<0.005) and correlated directly with New York Heart Association class (NYHA; R=0.501, p<0.001). In conclusion, circulating levels of sEng are elevated in patients with increased LVEDP and NYHA class, irrespective of LVEF. Soluble endoglin levels also decrease in association with reduced cardiac filling pressure after diuresis. These findings identify circulating sEng as a sensitive measure of elevated left heart filling pressure.
Keywords: heart failure, cardiac filling pressure, soluble receptors
Background
Endoglin (CD105) is a 180-kDA Type III TGFb1 co-receptor that promotes binding of TGFb1 and TGFb3 to a Type II TGFb-receptor (1). In vascular tissue, endoglin modulates downstream TGFb1 signaling and regulates vascular tone (2). In cardiac tissue, endoglin is expressed by endothelial cells, fibroblasts in the connective tissue surrounding muscular fibers, and in fibroblast-like stromal cells of valve leaflets, while cardiac myocytes fail to demonstrate significant endoglin expression (3). Endoglin expression in heart failure has not been explored. The extracellular domain of endoglin can be released into the circulation as soluble endoglin (sEng), and has been associated with increased systemic vascular resistance observed in preeclampsia (4) and fibro-proliferative disorders (5–8). Since progressive heart failure is associated with increased systemic vascular resistance, increased TGFb1 activity, and cardiac fibrosis (9–11), we postulated that sEng levels may be increased in association with elevated LV filling pressure and may provide a sensitive, non-invasive measure of cardiac pressure overload. To explore this hypothesis, we prospectively measured sEng serum levels in patients referred for cardiac catheterization due to suspected LV dysfunction. Soluble endoglin levels significantly correlated with LV filling pressures and were more sensitive and specific predictors of cardiac pressure overload than biomarkers such as atrial-natriuretic peptide (ANP) or BNP. These findings suggest that elevated levels of sEng may serve as a marker of increased LV filling pressure.
Methods
In a prospective, observational study, we enrolled 82 consecutive patients referred for evaluation of suspected LV dysfunction by right and left-sided heart catheterization regardless of LV ejection fraction (EF) at Tufts Medical Center. Patients under 18 years of age and those presenting with an acute coronary syndrome, pregnancy, active or remote cancer, renal failure (estimated glomerular filtration rate ≤ 30), liver transaminases ≥ 2 times the upper limit of normal, non-sinus rhythm, or perceived interference with standard clinical care were excluded. All eligible patients who agreed to enroll had blood sampled at the time of arterial sheath insertion for diagnostic catheterization. LVEF was assessed by echocardiography or ventriculography at the time of catheterization. Data from the medical record and results of other blood tests were collected for subsequent analysis with SigmaStat 3.1 software. To study whether sEng levels reflect diuresis-induced reductions in cardiac filling pressure during medical therapy for heart failure, we enrolled 10 patients with systolic heart failure as defined by a pulmonary capillary wedge pressure ≥ 16mmHg and LVEF<50%, in whom follow-up pulmonary artery (PA) catheter measurements were deemed clinically necessary for hemodynamic monitoring after in-patient diuretic therapy. The same exclusion criteria listed above were applied to patients referred for follow-up right heart catheterization. Blood sampling was performed at the time of initial PA catheter placement, then 48 hours after in-patient therapy. At each time point, sEng, BNP, and ANP levels were measured. Control subjects consisted of 25 healthy, volunteers with no prior medical history, no active medical problems, and currently taking no medications as recorded by a screening questionnaire. Subjects were required to be between 21 and 80 years of age. Serum samples were obtained as a one-time lab draw in our clinical research center. All physicians were blinded to the results of the serum analysis. The institutional review board of Tufts Medical Center approved this study and all patients provided written informed consent. Blood samples were collected using serum separator tubes and allowed to clot for 30 minutes prior to centrifugation at 2000 × G for 15 minutes. Serum samples were immediately stored at −20° C. Human sEng, ANP, and BNP levels were measured in each serum sample in duplicate using commercially available quantitative sandwich enzyme immunoassay kits (Soluble Endoglin: R&D Systems; ANP and BNP: Phoenix Pharmaceuticals) according to the manufacturers instructions. The intra-assay and inter-assay coefficient of variation for each protein assay were: sEng: 3% and 6%, respectively; BNP: 8% and 4%, respectively; and ANP: 11% and 5%, respectively.
All values are expressed as the mean and standard deviation (SD). Differences between means were detected by Wilcoxon rank-sum test with two-tailed p values < 0.05. Soluble endoglin data were normally distributed, however, because the natriuretic peptide data were not normally distributed, log BNP and log ANP were used in the correlations and regression models. Stepwise multiple regression analysis was performed to examine predictors of LVEDP in LVD. Variables entered into the model included: age, sex, BSA, hypertension, diabetes, hypercholesterolemia, active tobacco use, and history of MI. Stepwise multiple regression analysis was further performed to examine biomarkers as predictors of LVEDP in all patients. Variables entered into the model included: sEng, ANP, and BNP. Blockwise multiple regression, using the enter method, was also employed to examine sEng as a predictor of LVEDP after entering BNP, ANP, NYHA class and LVEF into a single block. Stepwise multiple regression analysis was also performed to examine predictors of sEng. Variables entered into the model included: age, sex, BSA, hypertension, diabetes, hypercholesterolemia, active tobacco use, and history of MI. In a separate model, medication history was examined for the prediction of sEng levels. Medications entered into the model included: aspirin therapy, beta-blockers, ACE inhibitors, calcium channel blockers, angiotensin receptor blockers, aldosterone antagonist, anti-lipidemics, and diuretics. To evaluate the value of sEng, BNP, and ANP measurements in the diagnosis of heart failure, we compared the sensitivity, specificity, and accuracy of each biomarker with measurements of LVEDP. Finally, we constructed receiver-operating-characteristic curves to illustrate various cutoff values of sEng, ANP, and BNP. A p-value <0.05 was considered significant. Statistical analyses were performed using SigmaStat Software.
Results
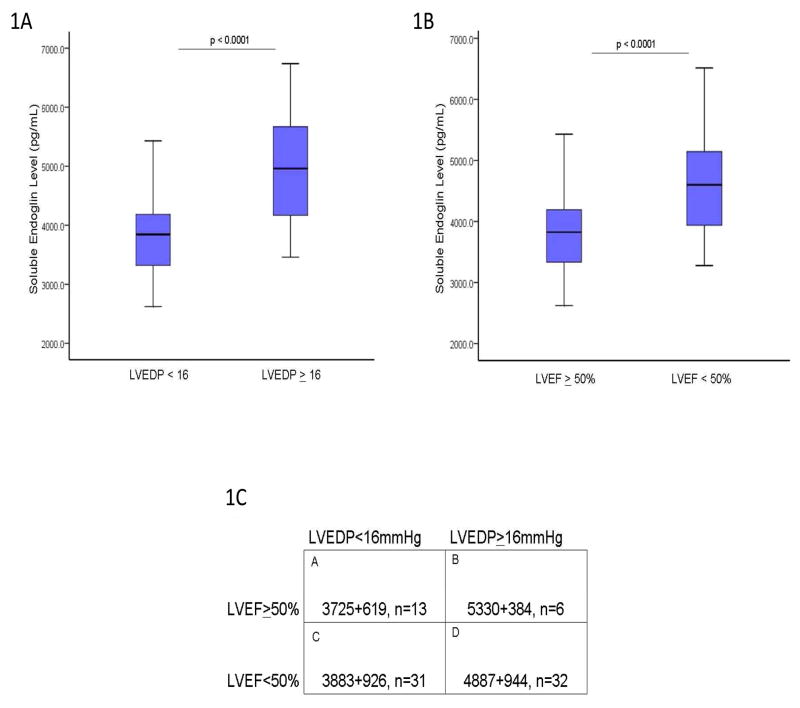
To determine whether sEng levels correlate with LVEDP, serum levels were measured by ELISA in 82 consecutive patients referred for cardiac catheterization to evaluate LV filling pressures regardless of LVEF. The clinical characteristics of study subjects were categorized according to LVEDP and are presented in Table 1. There were significant group differences in age, gender, prevalence of hypertension, smoking, diuretic use, creatinine and blood urea nitrogen (p<0.05). Among catheterization subjects, LVEDP directly correlated with LVEF (R:−0.588 ; r2:0.346 ; p<0.005). When categorized according to LV filling pressure, patients with a LVEDP≥16 mmHg had significantly higher sEng levels compared to individuals with a LVEDP <16 (p<0.001, Figure 1A). After adjusting for aforementioned group differences in age, gender, prevalence of hypertension, smoking, diuretic use, creatinine and blood urea nitrogen with ANCOVA, sEng remained higher in patients with LVEDP ≥ 16 compared to patients with LVEDP < 16 (adjusted means: LVEDP < 16 = 3848 pg/mL vs. LVEDP ≥ 16 = 4786 pg/mL; p<0.001). When grouped according to LVEF, study subjects with a low LVEF (<50%) had significantly higher sEng levels than patients with a LVEF ≥ 50% (p<0.0001, Figure 1B). Soluble endoglin levels correlated directly with elevated LVEDP in patients with either LVEF>50% (R=0.51, p<0.01) or LVEF<50% (R=0.50, p<0.01). When grouped by both variables, namely LVEF and LVEDP, sEng levels were significantly increased in subjects with elevated LVEDP>16 irrespective of LVEF (Figure 1C), suggesting that levels of sEng are also elevated in patients with cardiac pressure overload and preserved LV function.
Table 1.
Clinical Characteristics of Study Subjects grouped by LVEDP.
| Variable | LVEDP (mmHg)
|
|
|---|---|---|
| < 16 (n=47) | > 16 (n=35) | |
| Age (years) | 55 ± 15 | 61 ± 12† |
| Male | 26 (55%) | 27 (77%) † |
| Body Surface Area (kg/m2) | 1.97 ± 0.26 | 2.05 ± 0.20 |
| Hypertension | 37 (79%) | 18 (53%)† |
| Diabetes Mellitus | 9 (19%) | 10 (29%) |
| Active Smoking | 11 (23%) | 2 (6%)† |
| Peripheral Vascular Disease | 2 (4%) | 4 (12%) |
| Coronary Disease | 13 (28%) | 15 (44%) |
| Prior Myocardial Infarction | 8 (17%) | 11 (32%) |
| Cerebrovascular Disease | 3 (6%) | 1 (3%) |
| NYHA Class (scale 0–4) | 1 | 3† |
| LVEDP (mmHg) | 10 ± 2.7 | 21.6 ± 4.4† |
| LVEF (%) | 48 ± 16 | 25 ± 16† |
| Medications | ||
| Aspirin | 33 (70%) | 17 (50%) |
| Clopidogrel | 9 (19%) | 3 (9%) |
| β-blocker | 28 (60%) | 18 (53%) |
| ACE-inhibitor | 18 (38%) | 13 (38%) |
| Calcium channel blocker | 5 (11%) | 3 (9%) |
| ARB | 1 (2%) | 1 (3%) |
| Aldosterone antagonist | 3 (6%) | 7(21%) |
| Diuretic | 14 (30%) | 20 (59%)† |
| Anti-dyslipidemic agent | 19 (68%) | 14 (40%) |
| Admission Lab Values | ||
| Sodium (mEq/L) | 139 ± 3 | 137 ± 3 |
| Creatinine (mg/dl) | 1.0 ± 0.8 | 1.5 ± 0.9† |
| Blood Urea Nitrogen (mg/dL) | 17 ± 6 | 31 ± 18† |
| Glucose (mg/dL) | 118 ± 30 | 131 ± 43 |
| WBC Count (×10 cells/L) | 8.4 ± 3.3 | 8.1 ± 3.7 |
| Hemoglobin (g/dL) | 13.1 ± 1.5 | 12.6 ± 1.8 |
Significant group difference (p<0.05)
Values are mean +/− SD.
Figure 1.
Soluble Endoglin Expression and LV Filling Pressure. 1A) When grouped by LVEDP, sEng levels are significantly higher in patients with an elevated LVEDP>16 compared to a low LVEDP<16 (4912+922 vs 3785+724 pg/mL, respectively, p<0.0001). 1B) When grouped by LVEF, subjects with low LVEF<50% had significantly increased sEng levels compared to controls (4620+980 vs 3590+588, p<0.001, respectively) or subjects with LVEF>50% (4620+980 vs 3650+550, p<0.001, respectively). 1C) When grouped by both LVEF and LVEDP, sEng levels were significantly increased in subjects with an elevated LVEDP irrespective of LVEF (Group A vs B or D, p<0.001; Group A vs C, p=NS; Group C vs B or D, p<0.001; Group B vs D = NS). [LVEDP: left ventricular end-diastolic pressure; LVEF: left ventricular ejection fraction; sEng: soluble endoglin]
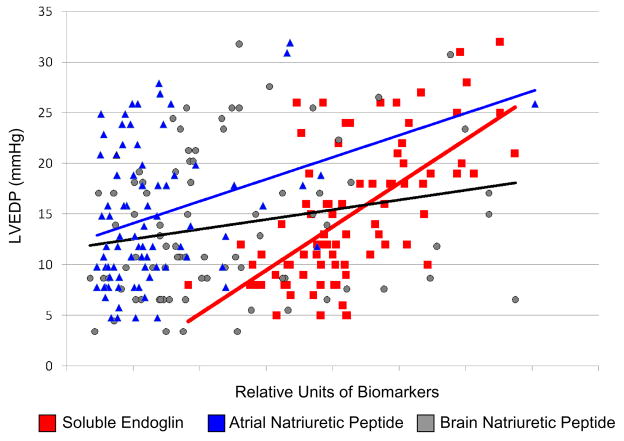
We next compared the sensitivity of sEng levels for predicting an elevated LVEDP to that of currently available biomarkers of heart failure including ANP and BNP. Study subjects with an elevated LVEDP ≥16mmHg exhibited increased ANP and BNP levels compared to subjects with a low LVEDP (ANP: 48±35 vs 26±14 ng/mL, p=0.01; BNP: 14±8 vs 10±7 ng/mL, p=0.03). Both ANP and BNP directly correlated with NYHA classification (ANP: R=0.298, p<0.01; BNP: R=0.309, p<0.01). Among study subjects, ANP levels correlated significantly with both LVEDP (p<0.01) and LVEF (p<0.01), while BNP levels correlated significantly with LVEDP (p=0.05), but demonstrated a weaker correlation with LVEF (p=0.09). In contrast, sEng levels exhibited a significant correlation with increased LVEDP (p<0.001) and inverse correlation with LVEF (p<0.001) (Table 2). Univariate regression plots for sEng, ANP, and BNP versus LVEDP are shown in Figure 2. sEng levels further demonstrated a significant correlation with ANP levels (R=0.234, p=0.03), however did not significantly correlate with BNP levels (p>0.05) among study subjects.
Table 2.
Univariate regression analysis of selected biomarkers as predictors of left ventricular end-diastolic pressure (LVEDP) or left ventricular ejection fraction (LVEF).
| LVEDP | LVEF | |||
|---|---|---|---|---|
| R | p-value | R | p-value | |
| Atrial Natriuretic Peptide | 0.322 | 0.003 | −0.309 | 0.005 |
| Brain Natiruretic Peptide | 0.219 | 0.05 | −0.183 | 0.09 |
| Soluble Endoglin | 0.628 | <0.001 | −0.399 | <0.001 |
Figure 2.
Univariate regression plots for sEng (red), ANP (blue), and BNP (gray) are shown. [ANP: atrial natriuretic peptide; BNP: brain natriuretic peptide; sEng: soluble endoglin].
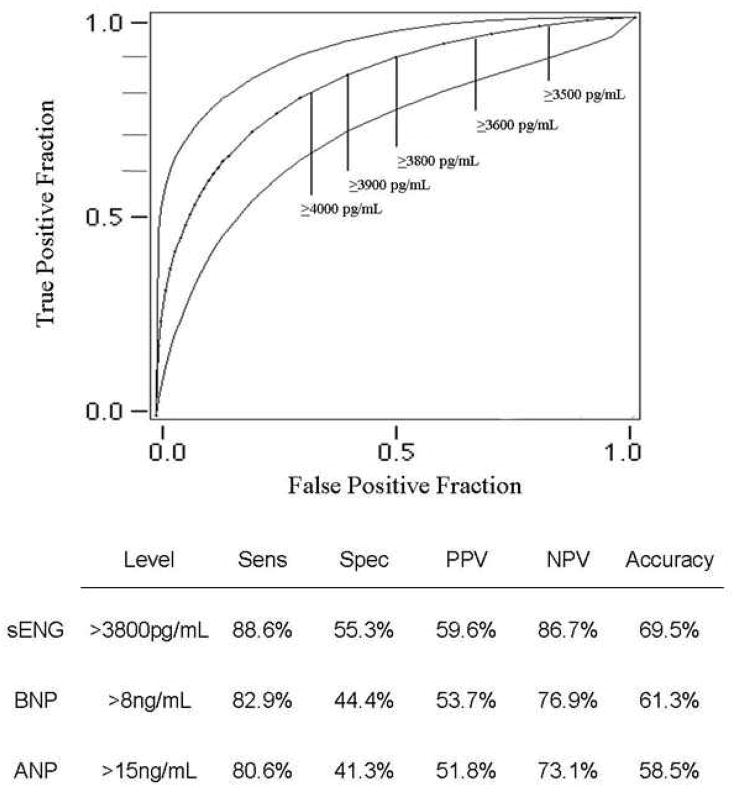
According to stepwise multiple regression, significant demographic predictors of LVEDP included a history of diabetes, hypertension and myocardial infarction. Overall, the model accounted for 17.7% of the variance in LVEDP. In a separate analysis, significant biomarker predictors of LVEDP included sEng (accounting for 39% of the variance) and ANP (accounting for an additional 3.2% of the variance) [Table 3]. Overall, the model accounted for 43% of the variance in LVEDP. According to blockwise multiple regression, a block consisting of BNP, ANP, NYHA class and LVEF accounted for 51% of the variance in LVEDP (p < 0.001). sEng accounted for a significant incremental 10% of the variance in LVEDP above that accounted for by these traditional predictors (p < 0.001). We next examined predictors of serum sEng levels among study subjects. Demographic predictors of sEng included a history of hypertension (accounting for 6.4% of the variance; β = −0.646, SE = 0.231, 95% CI = −1.107 – −0.185, p = 0.007) and diabetes (accounting for 5.7% of the variance; β = 0.556, SE = 0.253, 95% CI = 0.052 – 1.061, p = 0.031). No other co-morbidity was identified as a significant predictor of sEng. With respect to medication history, diuretic-use was a significant predictor of sEng (accounting for 19% of the variance; β = 0.836, SE = 0.262, 95% CI = 0.308 – 1.363, p = 0.003). No other medication was identified as a significant predictor of sEng. Using a receiving operative characteristic (ROC) curve, sEng levels predicted a LVEDP≥16 with an area-under-the-curve (AUC) of 0.851, exceeding the predictive value of either ANP (AUC: 0.68, p<0.01 vs sEng) or BNP (AUC: 0.65, p<0.01 vs sEng). Soluble endoglin also exhibited higher sensitivity, a greater negative predictive value and superior accuracy compared to BNP or ANP for predicting an elevated LVEDP (Figure 3).
Table 3.
Stepwise multiple regression analysis for predictors of left ventricular end-diastolic pressure (LVED) among study subjects.
| Demographic Variables | β-coefficient | Standard Error | p-value | 95% Confidence Interval | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Diabetes | 4.060 | 1.757 | 0.024 | 0.560 | 7.560 |
| Hypertension | −4.731 | 1.632 | 0.005 | −7.984 | −1.479 |
| Myocardial Infarction | 3.892 | 1.766 | 0.031 | 0.374 | 7.410 |
|
Biomarker Variables | |||||
| Soluble endoglin | 4.005 | 0.601 | <0.001 | 2.810 | 5.201 |
| Atrial natriuretic peptide | 0.032 | 0.015 | 0.038 | 0.002 | 0.061 |
Figure 3.
Predictive value of sEng as a determinant of elevated LVEDP. The receiving operator characteristic curve for sEng is shown with specific cut-points at various levels of sEng expression. [ANP: atrial natriuretic peptide; AUC: area-under-the-curve; BNP: brain natriuretic peptide; LVEDP: left ventricular end-diastolic pressure; LVEF: left ventricular ejection fraction; NPV: negative predictive value; PPV: positive predictive value; sEng: soluble endoglin; Sens: Sensitivity; Spec: specificity]
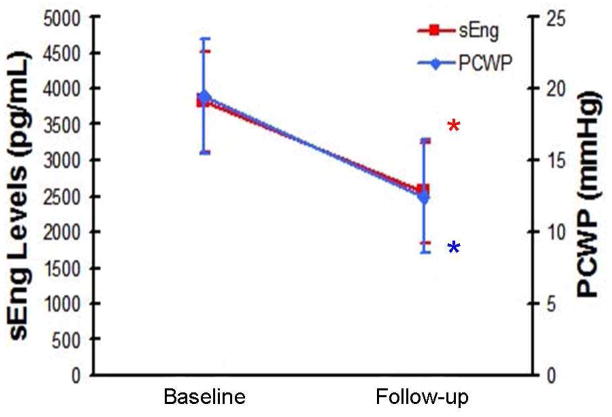
In a subgroup of 10 patients with systolic heart failure (LVEDP≥16 and LVEF<50%) receiving medical therapy for congestive heart failure in whom follow-up pulmonary artery (PA) catheter measurements were deemed clinically necessary for hemodynamic monitoring, ANOVA with repeated measures revealed a significant reduction in sEng levels after diuresis (p=0.013). Reduced sEng levels corresponded with decreased pulmonary capillary wedge pressure (PCWP) (Figure 4) as the percent change in sEng was strongly associated with the percent change in PCWP (R=0.75, p=0.008).
Figure 4.
Reduced sEng levels correlate with reduced PCWP. Compared to baseline values, sEng levels were significantly reduced after 48 hours of diuretic therapy (*3830+330 vs 2540+1060pg/mL, baseline vs follow-up, respectively, p<0.001) and corresponded with reduced PCWP (*19.5+3.1 vs 12.5+4.5mmHg, baseline vs follow-up, respectively, p<0.001). Percent change in sEng was strongly associated with the percent change in LVEDP (R=0.75, p=0.008). In this group, levels of ANP and BNP were also reduced after diuretic therapy (ANP: 10.5+4.7 vs 5.2+1.9 ng/mL, respectively p=0.02; BNP: 8.8+2.5 vs 6.2+1.7 ng/mL,, respectively, p=0.05). [ANP: atrial natriuretic peptide; BNP: brain natriuretic peptide; LVEDP: left ventricular end-diastolic pressure; LVEF: left ventricular ejection fraction; PCWP: pulmonary capillary wedge pressure; sEng: soluble endoglin]
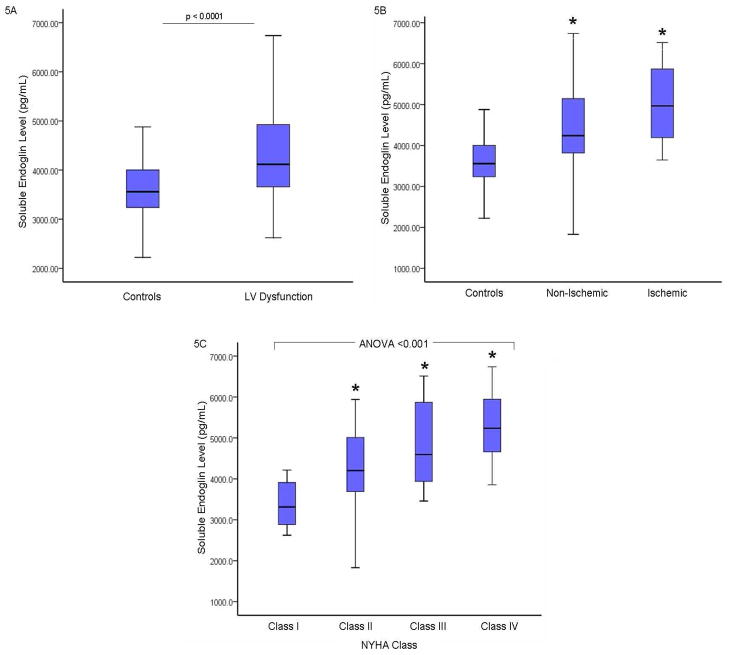
To further characterize sEng levels, twenty-five healthy volunteer subjects without any co-morbidities or currently taking any medications served as controls. Controls did not differ in age, gender, or race (p>0.05) from study subjects. Compared to healthy controls, sEng levels were significantly higher in the total group of 82 study subjects (3589±588 vs 4257±966 pg/mL, respectively, p<0.005; Figure 5A). Soluble endoglin levels did not differ between healthy controls and patients with LVEF ≥ 50% (3590±135 vs. 3837±117 pg/mL, p = 0.19) or patients with LVEDP < 16 mmHg (3590±135 vs. 3797±93 pg/mL, p = 0.22). Among study subjects with a low LVEF<50%, sEng levels were increased regardless of whether the underlying etiology was ischemic (n=17, 4979±881 pg/mL, p<0.0001 vs controls) or non-ischemic (n=35, 4431±1000 pg/mL, p=0.004, vs controls; Figure 5B). A trend toward higher sEng levels was observed in subjects with ischemic cardiomyopathy compared to patients with non-ischemic heart failure (p=0.06). Among study subjects, elevated sEng levels also correlated significantly with worsening NYHA classification (r=0.501, p<0.001; Figure 5C).
Figure 5.
Soluble Endoglin Expression in Subjects with Suspected LV Dysfunction Compared to healthy controls, sEng levels were significantly higher in the total group of 82 study subjects (3589+588 vs 4257+966 pg/mL, respectively, p<0.005; Figure 5A). Among study subjects with a low LVEF<50%, sEng levels were increased regardless of whether the underlying etiology was non-ischemic or ischemic (Figure 5B). Soluble endoglin levels were higher in subjects with ischemic cardiomyopathy compared to patients with non-ischemic heart failure (p=0.06). Among study subjects, worsening NYHA classification corresponded with increased sEng levels (NYHA Class 1: 3644.9+579, Class II: 4307+968, Class III: 4746+947, Class IV: 5089+1073 pg/mL; *p<0.01 vs Class 1; ANOVA p<0.001; Figure 5C). [LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; sEng: soluble endoglin].
Discussion
This is the first report to describe the relationship between endoglin expression and cardiac filling pressures. Compared to healthy controls, circulating levels of sEng were significantly increased in patients with suspected LV dysfunction referred for cardiac catheterization and strongly correlated with predictors of mortality in heart failure such as elevated LVEDP, reduced LVEF, and worsening NYHA class. Soluble endoglin levels were significantly increased in subjects with elevated LVEDP irrespective of LVEF, highlighting the association between endoglin expression and pressure overload. Furthermore, sEng levels demonstrated superior sensitivity, specificity, accuracy, and predictive value compared to ANP and BNP for identifying subjects with increased LVEDP. Finally, we demonstrated that sEng levels were reduced in patients receiving medical therapy for congestive heart failure and correlate with reduced cardiac filling pressures measured by a PA catheter. Taken together, our findings identify elevated sEng levels as a sensitive biomarker of increased cardiac filling pressure.
These findings have several implications. First, endoglin has been identified as a critical regulator of the cytokine TGFb1, yet has not been studied in the context of heart failure. While TGFb1 expression has been previously characterized in heart failure (12,13) in pre-clinical studies, the role of endoglin in heart failure has remained largely ignored. Since endoglin regulates vascular tone, increased sEng levels in patients with cardiac pressure overload suggests a potentially important role for this peptide in the pathophysiology of heart failure. Second, our findings may have important clinical implications that require further study in a larger population of patients. For example, the ability to accurately diagnose elevated cardiac filling pressures may reduce the need for invasive testing. Furthermore, since sEng levels correlate well with reduced cardiac filling pressures after diuretic therapy, this biomarker of cardiac pressure overload may be useful for monitoring therapeutic efficacy of both mechanical and pharmacologic approaches to decompensated heart failure. Third, in the heart, endoglin is expressed by endothelial cells, fibroblasts in the connective tissue surrounding muscular fibers, and in fibroblast-like stromal cells of valve leaflets, while cardiac myocytes fail to demonstrate significant endoglin expression (3, 14). Our findings suggest that proteins such as sEng, released from the heart including endothelial cells and cardiac fibroblasts, may exhibit enhanced clinical utility since these cell populations are viable in later stages of heart failure (15). Since endoglin is ubiquitously expressed by endothelial cells, we cannot exclude potential contributions to sEng levels in heart failure from the systemic or pulmonary vasculature.
Endoglin knockout mice die at embryologic day 10–11.5 due to impaired cardiovascular development (16), suggesting a critical role for this protein in cardiovascular development. Furthermore, deficient endoglin expression is responsible for the autosomal dominant, vascular dysplastic syndrome, Hereditary Hemorrhagic Telangiectasia (HHT-1) (17). Known regulators of endoglin expression include: Angiotensin-II, TGFb1, hypoxia-inducible factor-1 (HIF-1) (18), and stretch- mediated vascular injury (19). Each of these stimuli actively participates in the pathophysiology of congestive heart failure, suggesting several mechanisms by which endoglin expression may be altered by LV dysfunction. This finding is supported by our observations in patient referred for evaluation of left heart filling pressure, where sEng was identified as a sensitive predictor of LVEDP. Similar to previous reports (10, 20, 21, 22), BNP levels demonstrated a weaker association with LVEF and LVEDP, while ANP exhibited a significant correlation with both LVEF and LVEDP. Based on our observations, one may postulate that higher LVEDP may cause increased atrial wall tension resulting in higher circulating levels of both ANP and sEng. This may explain the strong association observed between ANP and sEng in our study population. The potential role for endoglin in the pathophysiology of heart failure is highlighted by previous studies showing that sEng disrupts nitric oxide-mediated vasodilation and contributes to increased systemic vascular resistance (4). In decompensated heart failure, elevated systemic vascular resistance is a natural adaptive response to maintain central perfusion due to a decline in cardiac output. In chronic heart failure, however, medical therapy is directed toward reducing vascular resistance, supporting exploration of therapeutics that inhibit sEng as a potential target to regulate vascular tone in heart failure.
The current study has several limitations. First, the number of subjects studied is small and requires further validation in a larger population. Second, since this was a prospective, observational study, the prognostic role of elevated sEng levels remains undetermined. Third, all subjects enrolled were selected based on the suspected diagnosis of LV dysfunction. The specificity of sEng for congestive heart failure as opposed to other forms of cardiovascular or non-cardiac disease remains unknown.
Acknowledgments
Grant Support: National Institutes of Health: K08 HL094909-01 awarded to Navin K. Kapur, MD
David DeNofrio, MD; Andrew Weintraub, MD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cheifetz S, Bellón T, Calés C, Vera S, Bernabeu C, Massagué J, Letarte M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992;267:19027–19030. [PubMed] [Google Scholar]
- 2.Lebrin F, Goumans MJ, Jonker L, Carvalho RL, Valdimarsdottir G, Thorikay M, Mummery C, Arthur HM, ten Dijke Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. P EMBO J. 2004;23:4018–4028. doi: 10.1038/sj.emboj.7600386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.St-Jacques S, Cymerman U, Pece N, Letarte M. Molecular characterization and in situ localization of murine endoglin reveal that it is a transforming growth factor-beta binding protein of endothelial and stromal cells. Endocrinology. 1994;134:2645–2657. doi: 10.1210/endo.134.6.8194490. [DOI] [PubMed] [Google Scholar]
- 4.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D’Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 5.Leask A, Abraham DJ, Finlay DR, Holmes A, Pennington D, Shi-Wen X, Chen Y, Venstrom K, Dou X, Ponticos M, Black C, Bernabeu C, Jackman JK, Findell PR, Connolly MK. Dysregulation of transforming growth factor beta signaling in scleroderma: overexpression of endoglin in cutaneous scleroderma fibroblasts. Arthritis Rheum. 2002;46:1857–1865. doi: 10.1002/art.10333. [DOI] [PubMed] [Google Scholar]
- 6.Rodríguez-Peña A, Eleno N, Düwell A, Arévalo M, Pérez-Barriocanal F, Flores O, Docherty N, Bernabeu C, Letarte M, López-Novoa JM. Endoglin upregulation during experimental renal interstitial fibrosis in mice. Hypertension. 2002;40:713–720. doi: 10.1161/01.hyp.0000037429.73954.27. [DOI] [PubMed] [Google Scholar]
- 7.Yagmur E, Rizk M, Stanzel S, Hellerbrand C, Lammert F, Trautwein C, Wasmuth HE, Gressner AM. Elevation of endoglin (CD105) concentrations in serum of patients with liver cirrhosis and carcinoma. Eur J Gastroenterol Hepatol. 2007;19:755–761. doi: 10.1097/MEG.0b013e3282202bea. [DOI] [PubMed] [Google Scholar]
- 8.Diez-Marques L, Ortega-Velazquez R, Langa C, Rodriguez-Barbero A, Lopez-Novoa JM, Lamas S, Bernabeu C. Expression of endoglin in human mesangial cells: modulation of extracellular matrix synthesis. Biochim Biophys Acta. 2002;1587:36–44. doi: 10.1016/s0925-4439(02)00051-0. [DOI] [PubMed] [Google Scholar]
- 9.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 10.Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res. 2004;63:423–432. doi: 10.1016/j.cardiores.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 11.Brutsaert DL. Role of endocardium in cardiac overloading and failure. Eur Heart J. 1990;11:G8–G16. doi: 10.1093/eurheartj/11.suppl_g.8. (intro) [DOI] [PubMed] [Google Scholar]
- 12.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 13.Khan R. Examining potential therapies targeting myocardial fibrosis through the inhibition of transforming growth factor-beta 1. Cardiology. 2007;108:368–380. doi: 10.1159/000099111. [DOI] [PubMed] [Google Scholar]
- 14.Valeria B, Maddalena G, Enrica V, Onofrio T, Gaetano B. Endoglin (CD105) expression in the human heart throughout gestation: an immunohistochemical study. Reprod Sci. 2008;15:1018–1026. doi: 10.1177/1933719108322429. [DOI] [PubMed] [Google Scholar]
- 15.Foo RS, Mani K, Kitsis RN. Death begets failure in the heart. J Clin Invest. 2005;115:565–571. doi: 10.1172/JCI24569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP. Defective angiogenesis in mice lacking endoglin. Science. 1999;284:1534–1537. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- 17.McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 1994;8:345–351. doi: 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Y, Sun Y, Xie L, Jin K, Sheibani N, Greenberg DA. Hypoxic induction of endoglin via mitogen-activated protein kinases in mouse brain microvascular endothelial cells. Stroke. 2003;34:2483–2488. doi: 10.1161/01.STR.0000088644.60368.ED. [DOI] [PubMed] [Google Scholar]
- 19.Botella LM, Sánchez-Elsner T, Sanz-Rodriguez F, Kojima S, Shimada J, Guerrero-Esteo M, Cooreman MP, Ratziu V, Langa C, Vary CP, Ramirez JR, Friedman S, Bernabéu C. Transcriptional activation of endoglin and transforming growth factor-beta signaling components by cooperative interaction between Sp1 and KLF6: their potential role in the response to vascular injury. Blood. 2002;100:4001–4010. doi: 10.1182/blood.V100.12.4001. [DOI] [PubMed] [Google Scholar]
- 20.Wu AH, Smith A, Wieczore S. Biological Variation for N-terminal pro- and B-type natriuretic peptides and implications of the therapeutic monitoring of patients with congestive heart failure. Am J Cardiol. 2003;92:628–631. doi: 10.1016/s0002-9149(03)00741-0. [DOI] [PubMed] [Google Scholar]
- 21.Forfia PR, Watkins SP, Rame JE. Relationship between B-Type natriuretic peptides and pulmonary capillary wedge pressure in the intensive care unit. J Am Col Card. 2005;45:1667–1671. doi: 10.1016/j.jacc.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 22.Larsen AI, Dickstein K, Ahmadi NS, Aarsland T, Kvaløy JT, Hall C. The effect of altering hemodynamics on the plasma concentrations of natriuretic peptides in heart failure. Eur J Heart Fail. 2006;8:628–633. doi: 10.1016/j.ejheart.2005.11.018. [DOI] [PubMed] [Google Scholar]