Abstract
The detection of West Nile virus (WNV) in areas endemic for Japanese encephalitis virus (JEV) is complicated by the extensive serological cross-reactivity between the two viruses. A testing algorithm was developed and employed for the detection of anti-WNV antibody in areas endemic for JEV. Using this differentiation algorithm, a serological survey of poultry (2004 through 2009) and horses (2007 through 2009) was performed. Among 2681 poultry sera, 125 samples were interpreted as being positive for antibodies against JEV, and 14 were suspected to be positive for antibodies against undetermined flaviviruses other than WNV and JEV. Of the 2601 horse sera tested, a total of 1914 (73.6%) were positive to the initial screening test. Of these positive sera, 132 sera (5.1%) had been collected from horses that had been imported from the United States, where WNV is endemic. These horses had WNV vaccination records, and no significant pattern of increasing titer was observed in paired sera tests. Of the remaining 1782 positive sera 1468 sera (56.4%) were also found to contain anti-JEV antibodies, and were interpreted to be JEV-specific antibodies by the differentiation algorithm developed in this study. The remaining 314 horses (12.1%) for which a fourfold difference in neutralizing antibody titer could not be demonstrated, were determined to contain an antibody against an unknown (unidentified or undetermined) flavivirus. No evidence of WNV infections were found during the period of this study.
Key Words: Algorithm, Differentiation, Japanese encephalitis virus, West Nile virus
Introduction
Because of the extensive serological cross-reactivity between flaviviruses, especially within the Japanese encephalitis virus (JEV) serocomplex, which includes West Nile virus (WNV), some studies have required that the neutralization titer for a specific virus be at least fourfold greater than that for other flaviviruses as a criterion for etiologic diagnosis (Farfan-Ale et al. 2006). Although cross-reactive anti-flavivirus antibodies may yield false-positive results in the diagnosis of WNV infections (Shirafuji et al. 2009; Hirota et al. 2010), there is little information concerning the serological diagnosis of WNV in areas endemic for JEV. Furthermore, there are no widely accepted guidelines to serologically differentiate WNV from JEV in serum samples that contain neutralizing antibodies to both flaviviruses.
Although WNV has not yet been detected in Korea, the perceived threat of its introduction has been highlighted by reports of WNV infection in a dead cinereous vulture (Aegypius monachus) in the Vladivostok region of Russia, which is adjacent to the Korean peninsula (Loktev 2004), and in several samples from cinereous vultures and cattle egrets (Bubulcus ibis) in the Russian Far East in 2002–2004 (Ternovoi et al. 2006). Furthermore, Saito and colleagues recently reported that several migrating birds captured in Japan tested positive for anti-flavivirus antibodies (Saito et al. 2009). This finding suggests that there is a threat of WNV introduction into Korea via migratory birds from WNV-infected areas, such as Russia, to naive hosts throughout the Korean peninsula, because many migratory birds share Korean and Japanese flyways (Lee et al. 2005). Moreover, several species of mosquitoes with the ability to transmit WNV, including Culex pipiens pallens (Lee et al. 1970,1984), Culex tritaeniorhynchus, Aedes vexans (Lee et al. 1984), Culex quinquefasciatus, and Aedes albopictus (Lee 1987), have been identified in Korea. It was recently reported that mosquitoes captured in Paju County, Gyeonggi Province, Republic of Korea, were highly susceptible to infection with WNV when allowed to feed on viremic chickens (Turell et al. 2006). In order to prepare for this possible threat of WNV introduction into Korea, a method for testing and interpreting the results to quickly and correctly identify WNV infection is required.
In the present study, a deterministic algorithm that can be used for anti-WNV antibody detection in areas endemic for JEV was developed and employed. In addition, to obtain a profile for WNV-neutralizing antibodies in poultry in South Korea, IgG ELISA and a plaque reduction neutralization test (PRNT) were used to investigate all of the poultry sera collected in this study, whereas IgM ELISA and PRNT were used to test all equine sera. Using the differentiation algorithm presented here, this study describes the serosurvey of a population of poultry from 2004 through 2009, and horses from 2007 through 2009, to evaluate the introduction or presence of WNV in South Korea.
Materials and Methods
Virus culture
WNV strain (strain NY385-99, lineage I, ATCC VR-1507) was obtained from the American Type Culture Collection (ATCC, Manassas, VA). The Anyang300 strain of JEV (Yang et al. 2005) was also used in this study. Experiments involving WNV were performed in a biosafety level three (BSL3) research laboratory at the National Veterinary Research and Quarantine Service (NVRQS; Anyang, the Republic of Korea) in accordance with the regulations of the Korean government.
Serological testing
Poultry serum samples were screened for WNV using an IgG ELISA assay developed in-house, according to the method described by Choi and associates (Choi et al. 2007). All poultry serum samples testing positive for WNV IgG were further investigated using a previously validated immunocapture WNV IgM ELISA assay to evaluate the time of infection (Johnson et al. 2003), which was modified by using WNV-reactive monoclonal antibody 5E8 (NVRQS) instead of SLE 6B6C-1.
Flavivirus-infected or vaccinated sera are known to exhibit cross-reactivity during the serodiagnosis of heterologous flavivirus infections (Williams et al. 2001; McLean et al. 2002; Koraka et al. 2002; Hirota et al. 2009), and furthermore, approximately 50% of horses are antibody-positive for JEV (Yang et al. 2008) in South Korea because of the ubiquitous use of the live JEV vaccine in horses, and because South Korea is endemic for JEV. To search for recent WNV infections, all horse sera submitted for anti-WNV antibody testing were evaluated using IgM antibody capture ELISAs (MAC-ELISA) developed in-house, according to the method described by Wagner and colleagues (Wagner et al. 2008).
All poultry and horse sera were tested by PRNT. JEV was included for testing by PRNT because of its occurrence in Asia and its known reactivity with anti-WNV antibodies (Burke and Monath, 2001; Kitai et al. 2007). To gain insight into the potential cross-reactivity of flaviviruses, the ability of serum samples from poultry and horses to neutralize WNV and JEV was determined by PRNT according to the method described by Blitvich and associates (Blitvich et al. 2003). However, in this study, JEV was used instead of St. Louis encephalitis virus (SLEV). To determine the end-point titers for WNV or JEV, samples with neutralizing antibodies were titrated in a twofold serial dilution series from 1:10 to 1:320. The number of plaques in each well was counted and PRNT titers (PRNT90 values) were expressed as the reciprocal of the highest dilution that yielded a ≥90% reduction in the number of plaques. Serum samples with a PRNT titer of ≥1:10 were considered positive.
A serological diagnostic algorithm to differentiate WNV from JEV infection
An acute WNV infection was suspected if the IgM antibody to WNV was present in the serum, or if there was a negative-to-positive seroconversion of the animal or a greater than fourfold increase between the neutralizing antibody titers in the paired serum samples assayed by PRNT. Sera with equivocal or positive WNV results in the IgM or IgG screening ELISAs were tested using PRNT for both WNV and JEV. Based on a review of previously reported studies in Africa, North America, Central America, and Europe, concerning serological differentiation between flaviviruses, such as WNV, SLEV, Ilhéus virus, Bussuquara virus, Murray Valley encephalitis virus, Usutu virus, Louping ill virus, and unidentified (undetermined) flaviviruses (Komar et al. 2001,2005; Buckley et al. 2003; Dupuis et al. 2003,2005; Lefrancois et al. 2005; Farfan-Ale et al. 2006; Bentler et al. 2007; Pant et al. 2006; Pupo et al. 2006), a new diagnostic strategy (Fig. 1) was designed for the serological identification and differentiation of WNV from JEV.
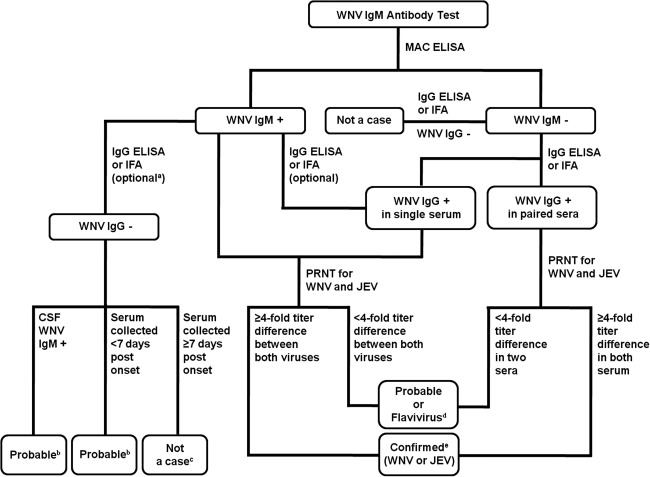
FIG. 1.
The scheme of West Nile virus (WNV) serological diagnosis streamlined starting from the IgM ELISA through the IgG ELISA to the PRNT. aOptional flow of the IgG ELISA test or IFA for IgM-positive samples. bConvalescent serum is necessary for confirmatory testing. Serum is confirmed to be positive for WNV if there is a fourfold or greater increase in titer in convalescent serum. cIf a titer difference of less than fourfold in two sera is observed, the sample could be determined to be “not positive for WNV (not a case).” dIf a fourfold difference could not be demonstrated, the sample was designated as containing an antibody against an unknown (unidentified or undetermined) flavivirus. History-taking, including vaccination record, clinical signs, or medical records indicative of WNV infection, is needed to confirm a diagnosis. eConfirmed (1) positive for WNV and negative for JEV if the neutralizing antibody titer against WNV was at least fourfold greater than that of the corresponding JEV antibody titer; (2) positive for JEV and negative for WNV if the neutralizing antibody titer against JEV was at least fourfold greater than that of the corresponding antibody titer to WNV (CSF, cerebrospinal fluid; ELISA, enzyme-linked immunosorbent assay; IFA, immunofluorescence assay; IgG, immunoglobulin G; IgM, immunoglobulin M; JEV, Japanese encephalitis virus; MAC-ELISA, IgM antibody capture ELISA; PRNT, plaque reduction neutralization test; WNV, West Nile virus).
Sera that exhibited positive PRNT90 titers for both WNV and JEV were categorized as follows.
WNV
Sera were considered to be positive for antibodies to WNV if the PRNT90 anti-WNV antibody titer was at least fourfold greater than that of the corresponding JEV antibody titer.
JEV
Sera were considered to be positive for antibodies to JEV if the PRNT90 JEV antibody titer was at least fourfold greater than that of its corresponding antibody titer to WNV.
Flavivirus
If a fourfold difference could not be demonstrated, the sample was designated as positive for an unknown (unidentified or undetermined) flavivirus antibody (Komar et al. 2001,2005; Dupuis et al. 2003).
Sera were considered to be negative for antibodies to WNV and JEV if sera exhibited no reactivity to WNV or JEV via PRNT90.
Serum samples for validation of the sensitivity and specificity of the WNV differentiation algorithm
Rabbits with an average body weight of 2.0 kg were randomly assigned to three groups of six animals, and 4-week-old chickens were also randomly assigned to three groups of four animals each. Animals were injected intramuscularly with one of the following: Vero cell-cultured WNV at a concentration of 106 plaque-forming units (PFU)/mL diluted in phosphate-buffered saline (PBS), Vero cell-cultured JEV at a concentration of 106 PFU/mL diluted in PBS, or PBS alone as a negative control. Serum samples from the inoculated animals were collected over a period of 3 weeks. Inoculations of animals with the live virus were performed in a BSL3 research laboratory at the NVRQS in accordance with the regulations of the government of the Republic of Korea. Reference horse sera were obtained from the OIE (World Organization for Animal Health) Reference Laboratory for WNV at the National Veterinary Services Laboratories (NVSL, Ames, IA), United States Department of Agriculture (W. NILE EQ AS VN336EDV0003BP192, WESTNILE EQ AS IgM 330EDV0201 Low Pos H351, WESTNILE EQ AS IgM 335EDV0002, WESTNILE EQ AS IgM 335EDV0003, Normal EQ Sera 305EDV0201 IgM, Normal EQ Sera 306EDV0201VN, and Normal EQ Sera 306EDV9801), and used to evaluate the algorithm. All of the reference sera and sera collected from experimental animals were confirmed by MAC-ELISA, IgG ELISA, IFA, and PRNT.
Field sera from poultry and horses for national WNV surveillance
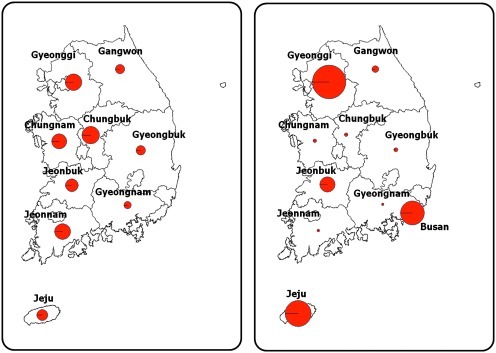
Poultry and horse serum samples were collected from across the country. Poultry samples were collected from 2681 live domestic chickens and ducks in nine regions for sentinel surveillance at a regional level during the peak period of vector activity from 2004 through 2009. Poultry sera from 2004 through 2006 were samples that were originally collected for serological survey of Newcastle disease and highly pathogenic avian influenza. Equine serum samples were collected by the Korea Racing Authority from 2601 horses during the period of vector activity from 2007 through 2009. Horses were selected from 10 regions in South Korea. Some horses were sampled twice in the event that a WNV-specific antibody was detected. The nationwide distribution and sampling numbers of the poultry and horses are illustrated in Figure 2.
FIG. 2.
Map of sampling sites in South Korea. Circle sizes represent the sample size. The sample size and location of poultry serum sampling for WNV detection is shown in the left-hand panel; equine serum sampling is shown in the right-hand panel (WNV, West Nile virus). (Color image available at www.liebertonline.com/vbz).
Results
The WNV serological testing algorithms described here provided good sensitivity (100%) and specificity (100%), as shown in Table 1. Based on this differential diagnostic algorithm, serological surveillance of poultry and horses was performed to evaluate the presence of WNV in South Korea.
Table 1.
Accuracy of West Nile Virus Differentiation Algorithm As Determined by Serological Screening and Confirmatory Diagnostic Tests Using Reference Sera and Serum Samples Collected from Animals Experimentally Infected with West Nile Virus (WNV) and Japanese Encephalitis Virus (JEV)
| |
|
WNV |
JEV |
Anti-WNV antibody screening test |
Interpretation by PRNT and WNV differentiation algorithm |
WNV differentiation algorithm accuracy |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | No. of samples | Pos | Neg | Pos | Neg | MAC- ELISA positive | IgG ELISA positive | Negative | WNV | JEV | WNV negative | Specificity (%) | Sensitivity (%) |
| Horse | 7 | 4 | 3 | 4 | 4 | 3 | 4 | 0 | 3 | 100 | 100 | ||
| 21 | 13 | 8 | 1 | 7 | 13 | 13 | 8 | 100 | |||||
| Rabbit | 66 | 41 | 25 | nt | 41 | 25 | 41 | 0 | 25 | 100 | 100 | ||
| 47 | 47 | nt | 37 | 10 | 0 | 37 | 10 | 100 | |||||
| Chicken | 50 | 30 | 20 | 26 | 29 | 20 | 30 | 0 | 20 | 100 | 100 | ||
| 27 | 27 | 21 | 19 | 6 | 0 | 21 | 6 | 100 | |||||
Equine reference serum samples obtained from the Reference Laboratory of the World Animal Health Organization (Office International des Epizooties) at the National Veterinary Services Laboratories, United States Department of Agriculture, Ames, Iowa.
IgG, immunoglobulin G; ELISA, enzyme-linked immunosorbent assay; PRNT, plaque reduction neutralization test; MAC-ELISA, immunoglobulin M antibody capture ELISA; Pos, positive; Neg, negative; nt, not tested.
In poultry, the overall prevalence of WNV antibodies by PRNT was 5.2% (139 of 2681 poultry serum samples), as shown in Table 2. Among these 139 sera, total of 125 samples (111 from ducks and 14 from chickens) were interpreted to be positive for anti-JEV antibodies (Table 2), and 14 samples from ducks were suspected to be positive for antibodies against undetermined flaviviruses other than WNV and JEV. All positive avian sera were negative for IgM, which potentially indicates that the flavivirus infections were not recent. Horses with WNV-reactive IgM in a single serum sample were classified as having a probable recent infection, and the overall prevalence for WNV-reactive IgM was 3.0% (78 of 2601 serum samples), as indicated in Table 3. All equine samples that were positive for IgM were confirmed to be negative for WNV and positive for JEV by the differentiation algorithm designed in this study. To search for WNV viremia, RT-PCR capable of identifying WNV RNA was performed on serum samples that were positive for WNV IgM according to the method described by Yeh and associates (Yeh et al. 2010). No amplified product was seen.
Table 2.
Results of IgG ELISA for West Nile Virus (WNV) and PRNT using WNV and Japanese Encephalitis Virus (JEV) in 2681 Poultry Serum Samples Collected During Sentinel Surveillance, 2004–2009
| Locality | No. of tested poultry serum samples | No. of positives determined by WNV IgG ELISA (%) | No. of positives confirmed by PRNT90 titer against WNV and JEV, respectively | Interpretation |
|---|---|---|---|---|
| Gangwon | 162 | 5 (3.1) | 5 | JEV |
| Gyeonggi | 448 | 27 (6.0) | 18 | JEV |
| 9 | Flavivirus | |||
| Jeonbuk | 284 | 18 (6.3) | 17 | JEV |
| 1 | Flavivirus | |||
| Jeonnam | 412 | 13 (3.2) | 13 | JEV |
| Chungbuk | 497 | 37 (7.4) | 34 | JEV |
| 3 | Flavivirus | |||
| Chungnam | 377 | 21 (5.6) | 21 | JEV |
| Gyeongbuk | 166 | 1 (0.6) | 1 | JEV |
| Gyeongnam | 112 | 0 (0.0) | 0 | |
| Jeju Island | 223 | 17 (7.6) | 16 | JEV |
| 1 | Flavivirus | |||
| Total | 2,681 | 139 (5.2) |
IgG ELISA, immunoglobulin G enzyme-linked immunosorbent assay; PRNT90, ≥90% reduction in the number of plaques induced by virus.
Table 3.
Prevalence of Immunoglobulin M Antibodies Against West Nile Virus (WNV) in 2601 Sera from Horses Between 2007 and 2009 in South Korea
| |
2007 |
2008 |
2009 |
Total |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Locality | No. of samples | WNV IgM+ | % | No. of samples | WNV IgM+ | % | No. of samples | WNV IgM+ | % | No. of samples | WNV IgM+ | % |
| Gangwon | 19 | 0 | 25 | 0 | 18 | 2 | 11.1 | 62 | 2 | 3.2 | ||
| Gyeonggi | 514 | 14 | 2.7 | 311 | 12 | 3.9 | 192 | 2 | 1.0 | 1017 | 28 | 2.8 |
| Gyeongnam | 0 | 0 | 0 | 0 | 10 | 0 | 10 | 0 | ||||
| Gyeongbuk | 9 | 0 | 2 | 0 | 15 | 0 | 26 | 0 | ||||
| Busan | 301 | 14 | 4.7 | 157 | 8 | 5.1 | 100 | 4 | 4.0 | 558 | 26 | 4.7 |
| Jeonnam | 0 | 0 | 4 | 0 | 9 | 0 | 13 | 0 | ||||
| Jeonbuk | 77 | 0 | 130 | 4 | 3.1 | 46 | 2 | 4.3 | 253 | 6 | 2.4 | |
| Jeju | 49 | 2 | 4.1 | 452 | 12 | 2.7 | 141 | 2 | 1.4 | 642 | 16 | 2.5 |
| Chungbuk | 0 | 0 | 4 | 0 | 16 | 0 | 20 | 0 | ||||
| Total | 969 | 30 | 3.1 | 1,085 | 36 | 3.3 | 547 | 12 | 2.2 | 2601 | 78 | 3.0 |
None of the WNV-IgM-positive samples was confirmed to be WNV infected during the period of this study. They were confirmed to be Japanese encephalitis virus-positive or other unknown (unidentified or undetermined) flavivirus-positive.
IgM, immunoglobulin M.
The prevalence of antibodies specific for WNV by PRNT in horses was 73.6% (1914 horses). Of these horses, 132 (5.1%) were from horses imported from the United States, where WNV is endemic. Most of these WNV-seropositive horses (n=129) had WNV vaccination records or had been imported in accordance with the national quarantine requirement for WNV, and furthermore, no significant pattern of increasing titer was observed in paired sera tests. However, for the imported horses (n=3) whose WNV vaccination histories were not known, which were imported before 2002 when horse quarantine regulations for WNV were first implemented in Korea, the date of onset of the WNV infection or vaccination was difficult to estimate due to lack of history for these horses, and a lack of published data concerning WNV IgM and IgG responses in naturally-infected or vaccinated horses. Only the persistence of IgG several years after the infection has been described (Murgue et al. 2001). However, it was considered that the infections or vaccinations most likely occurred in the United States. Of the remaining 1782 WNV-positive sera, which were not from horses known to be vaccinated for WNV nor from horses imported under national quarantine processes for WNV, total of 1468 horses (59.5%) exhibited WNV-neutralizing antibodies, which were found to be cross-reactive neutralizing antibodies against JEV, and were interpreted to be JEV-specific antibodies by the differentiation algorithm developed in this study. The remaining 314 horses (12.7%), for which a fourfold difference could not be demonstrated, were determined to contain an antibody against an unknown (unidentified or undetermined) flavivirus other than WNV or JEV. In all of these horses, no significant pattern of increasing titer was observed in paired sera tests. Also, none of the seropositive horses exhibited clinical signs or possessed medical records that were indicative of WNV infection, and no clinical signs of WNV were observed at the time of sample collection. When the serological diagnostic algorithm described here was applied, no samples from poultry or horses were confirmed as being WNV-infected during the period of this study.
Discussion
Screening tests (e.g., ELISA), are generally used to detect antibodies and frequently replace neutralization tests in many situations. IgM capture ELISA is used for anti-WNV antibody confirmation (Peiris and Amerasinghe 1994; Roehrig 2000). However, it is possible that if WNV infection follows infection with another flavivirus, then measuring anti-WNV IgM levels by MAC-ELISA may yield equivocal results (Tesh et al. 2002). The PRNT is a serological test for anti-WNV antibodies, and although expensive and technically challenging, it is useful for differentiation of infections with two antigenically related viruses (Roehrig 2000). Therefore, the PRNT is the confirmatory test for the identification of WNV-specific antibodies. Paired serum samples (acute phase and convalescent phase) are useful and allow the detection of an increase in antibody titer. In human patients from whom the acute-phase sample is obtained too early for IgM antibodies to be detected, both IgM and IgG antibodies are likely to be found in the convalescent-phase serum sample (Roehrig 2000).
Most horses born in South Korea that had records of JEV vaccination were confirmed to be JEV-positive, although the vaccination history of one-third of the horses was not available. However, 51 horses with records of vaccination against JEV only were identified to be flavivirus-positive in the present study. The differential diagnosis between WNV and JEV or other unidentified flaviviruses may be complicated by the antigenic cross-reactivity of these viruses (Williams et al. 2001; McLean et al. 2002; Koraka et al. 2002; Hirota et al. 2009). It has been reported that, in the serosurveillance of WNV, JEV-vaccinated horses can produce false-positive results in WNV IgG-ELISA, the hemagglutination inhibition test, and PRNT (Hirota et al. 2010). A recent study also demonstrated experimental WNV infections in JEV-vaccinated horses, and suggested that cross-reactive antibodies could cause confusion in the diagnosis of WNV-infected horses in areas endemic for JEV (Shirafuji et al. 2009). Based on the present field survey, it is clear that sera with flavivirus-positive antibodies cannot be diagnosed simply based on a single virus neutralization test or ELISA using single serum samples. It is important to compare neutralizing antibody titers against the flavivirus of interest with those against other flaviviruses that are present or endemic in certain regions.
Our results indicate that poultry and horses in South Korea were exposed to an antigenically closely related virus at some point in their lives. Alternatively, a virus closely related to WNV that cross-reacted in the highly specific virus-neutralizing test could be responsible for the observed serology. The prevalence of other flaviviruses as determined by the testing algorithm continued to increase from 2007 to 2009, whereas the rate of JEV infection declined (Table 4). Whether vector-borne flaviviruses, such as tick-borne encephalitis virus (TBEV), caused this rise in infection rate will require further investigation. Recently, TBEV, which is closely related to WNV, has been observed in South Korea (Kim, et al. 2008, 2009; Yun et al. 2009). It is possible that TBEV could be responsible for this cross-reactive neutralizing antibody; however, this possibility has yet to be investigated. Although 12.7% (314 horses) of the samples for which a fourfold difference could not be demonstrated were determined to be positive for an antibody against an unknown (unidentified or undetermined) flavivirus, a previous report proposed that this serological status could be the result of the animal being exposed to both JEV and WNV (Buckley et al. 2003). For this reason, paired tests were conducted with these samples, and no significant pattern of increasing titer was observed. Consequently, none of these sera were confirmed to have an active WNV infection during the period of this study. The false-positive rate of the WNV IgM assay used for this study was 3%, which emphasizes the need to use the algorithm presented in this study to compensate for the specificity of the assay.
Table 4.
Results of Testing for Neutralizing Antibodies Against West Nile Virus (WNV) and Japanese Encephalitis Virus (JEV) in Equine Serum Samples Collected in South Korea from 2007–2009 as Determined by a Plaque Reduction Neutralization Test Using WNV and JEV
| |
2007 |
2008 |
2009 |
Total |
||||
|---|---|---|---|---|---|---|---|---|
| Interpretationa | No. | % | No. | % | No. | % | No. | % |
| JEV | 622 | 66.1 | 594 | 57.4 | 252 | 51.0 | 1,468 | 59.5 |
| Flavivirusb | 60 | 6.4 | 153 | 14.8 | 101 | 20.4 | 314 | 12.7 |
| Negative to WNV and JEV | 259 | 27.5 | 287 | 27.8 | 141 | 28.5 | 687 | 27.8 |
| Total | 941 | 1034 | 494 | 2469 | ||||
Interpretation of the results was based on PRNT antibody titers that were at least fourfold greater for one virus than for another flavivirus.
If a fourfold difference could not be demonstrated, the sample was designated as positive for an antibody toward an unknown (unidentified or undetermined) flavivirus.
PRNT, plaque reduction neutralization test.
Collectively, the diagnostic algorithm described here enables the early diagnosis of WNV, provides case definition criteria for serological examination, and may also be useful in areas endemic for JEV as part of a serological surveillance or testing regimen for horses and human patients with viral encephalitis. The WNV differential algorithm presented here provides a clear diagnostic flow chart of WNV differentiation, permitting better monitoring of the disease in JEV-endemic areas. The present study also provides information regarding the prevalence of WNV and JEV infection in Korea among poultry and horses. When the algorithm was applied, none of the WNV-positive samples was confirmed to have an active WNV infection during the period of this study. This serological survey of horses and poultry supports the hypothesis that WNV had not been introduced during the period under investigation, which agrees with other studies conducted for the same period (Han et al. 2009; Tabei et al. 2007). To ensure early detection, the surveillance of horses and poultry as sentinels for WNV in Korea must continue.
Acknowledgments
This work was supported by a grant from the National Veterinary Research and Quarantine Service, Ministry for Food, Agriculture, Forestry, and Fisheries, the Republic of Korea (grant number 6235-320-210-13).
Author Disclosure Statement
No competing financial interests exist.
References
- Agricultural and Forestry Statistical Yearbook. Ministry of Agriculture & Forestry; Republic of Korea: 2007. [Google Scholar]
- Bentler KT. Hall JS. Root JJ, et al. Serologic evidence of West Nile virus exposure in North American mesopredators. Am J Trop Med Hyg. 2007;76:173–179. [PubMed] [Google Scholar]
- Blitvich BJ. Marlenee NL. Hall RA, et al. Epitope-blocking enzyme-linked immunosorbent assays for the detection of serum antibodies to West Nile virus in multiple avian species. J Clin Microbiol. 2003;41:1041–1047. doi: 10.1128/JCM.41.3.1041-1047.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley A. Dawson A. Moss SR, et al. Serological evidence of West Nile virus, Usutu virus and Sindbis virus infection of birds in the UK. J Gen Virol. 2003;84:2807–2817. doi: 10.1099/vir.0.19341-0. [DOI] [PubMed] [Google Scholar]
- Burke DS. Monath TP. Flavivirus. In: Knipe M, editor; Howley PM, editor. Fields Virology. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 1043–1125. [Google Scholar]
- Choi KS. Ko YJ. Nah JJ, et al. Monoclonal antibody-based competitive enzyme-linked immunosorbent assay for detecting and quantifying West Nile virus-neutralizing antibodies in horse sera. Clin Vaccine Immunol. 2007;14:134–138. doi: 10.1128/CVI.00322-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis AP., 2nd Marra PP. Kramer LD. Serologic evidence of West Nile virus transmission, Jamaica, West Indies. Emerg Infect Dis. 2003;9:860–863. doi: 10.3201/eid0907.030249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis AP 2nd Marra PP Reitsma R et al. Serologic evidence for West Nile virus transmission in Puerto Rico and Cuba. Am J Trop Med Hyg. 2005;73:474–476. [PubMed] [Google Scholar]
- Farfan-Ale JA. Blitvich BJ. Marlenee NL, et al. Antibodies to West Nile virus in asymptomatic mammals, birds, and reptiles in the Yucatan Peninsula of Mexico. Am J Trop Med Hyg. 2006;74:908–914. [PubMed] [Google Scholar]
- Han M-G. Lee H-I. Lee C-S, et al. The Microbiological Society of Korea's International Symposium. Jeju Island, Korea: The Microbiological Society of Korea; 2009. Surveillance of West Nile Viruses from 2006 to 2008, Korea: No Evidence of Infection; p. 237. [Google Scholar]
- Hirota J. Nishi H. Matsuda H, et al. Cross-reactivity of Japanese encephalitis virus-vaccinated horse sera in serodiagnosis of West Nile virus. J Vet Med Sci. 2010;72:369–372. doi: 10.1292/jvms.09-0311. [DOI] [PubMed] [Google Scholar]
- Hirota J. Nishi H. Matsuda H, et al. Cross-reactivity of Japanese encephalitis virus-vaccinated horse sera in serodiagnosis of West Nile Virus. J Vet Med Sci. 2009;72:369–372. doi: 10.1292/jvms.09-0311. [DOI] [PubMed] [Google Scholar]
- Johnson AJ. Langevin S. Wolff KL, et al. Detection of anti-West Nile virus immunoglobulin M in chicken serum by an enzyme-linked immunosorbent assay. J Clin Microbiol. 2003;41:2002–2007. doi: 10.1128/JCM.41.5.2002-2007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY. Jeong YE. Yun SM, et al. Molecular evidence for tick-borne encephalitis virus in ticks in South Korea. Med Vet Entomol. 2009;23:15–20. doi: 10.1111/j.1365-2915.2008.00755.x. [DOI] [PubMed] [Google Scholar]
- Kim SY. Yun SM. Han MG, et al. Isolation of tick-borne encephalitis viruses from wild rodents, South Korea. Vector Borne Zoonotic Dis. 2008;8:7–13. doi: 10.1089/vbz.2006.0634. [DOI] [PubMed] [Google Scholar]
- Kitai Y. Shoda M. Kondo T, et al. Epitope-blocking enzyme-linked immunosorbent assay to differentiate West Nile virus from Japanese encephalitis virus infections in equine sera. Clin Vaccine Immunol. 2007;14:1024–1031. doi: 10.1128/CVI.00051-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar N. Panella NA. Burns JE, et al. Serologic evidence for West Nile virus infection in birds in the New York City vicinity during an outbreak in 1999. Emerg Infect Dis. 2001;7:621–625. doi: 10.3201/eid0704.010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar O. Robbins MB. Contreras GG, et al. West Nile virus survey of birds and mosquitoes in the Dominican Republic. Vector Borne Zoonotic Dis. 2005;5:120–126. doi: 10.1089/vbz.2005.5.120. [DOI] [PubMed] [Google Scholar]
- Koraka P. Zeller H. Niedrig M, et al. Reactivity of serum samples from patients with a flavivirus infection measured by immunofluorescence assay and ELISA. Microbes Infect. 2002;4:1209–1215. doi: 10.1016/s1286-4579(02)01647-7. [DOI] [PubMed] [Google Scholar]
- Lee DS. Yoon HK. Kim HS, et al. [Studies on the life cycle of Culex pipiens pallens in Korea] Kisaengchunghak Chapchi. 1970;8:36–38. doi: 10.3347/kjp.1970.8.1.36. [DOI] [PubMed] [Google Scholar]
- Lee KW. [Checklist of mosquitoes (Culicidae) in Korea] Kisaengchunghak Chapchi. 1987;25:207–209. doi: 10.3347/kjp.1987.25.2.207. [DOI] [PubMed] [Google Scholar]
- Lee KW. Gupta RK. Wildie JA. Collection of adult and larval mosquitoes in U.S. army compounds in the Republic of Korea during 1979∼1983. Kisaengchunghak Chapchi. 1984;22:102–108. doi: 10.3347/kjp.1984.22.1.102. [DOI] [PubMed] [Google Scholar]
- Lee W-S. Gu T-H. Park J-Y. Seoul, Republic of Korea: Bon-Mu Gu (LG Foundation); 2005. A field guide to the birds of Korea; pp. 2–320. [Google Scholar]
- Lefrancois T. Blitvich BJ. Pradel J, et al. West Nile virus surveillance, Guadeloupe, 2003–2004. Emerg Infect Dis. 2005;11:1100–1103. doi: 10.3201/eid1107.050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loktev VB. West Nile virus, vulture—Russia (Vladivostok) ProMED-MAIL. 2004.
- Pant GR. Lunt RA. Rootes CL, et al. Serological evidence for Japanese encephalitis and West Nile viruses in domestic animals of Nepal. Comp Immunol Microbiol Infect Dis. 2006;29:166–175. doi: 10.1016/j.cimid.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Pupo M. Guzman MG. Fernandez R, et al. West Nile Virus infection in humans and horses, Cuba. Emerg Infect Dis. 2006;12:1022–1024. doi: 10.3201/eid1206.051235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean RG. Ubico SR. Bourne D, et al. West Nile virus in livestock and wildlife. Curr Top Microbiol Immunol. 2002;267:271–308. doi: 10.1007/978-3-642-59403-8_14. [DOI] [PubMed] [Google Scholar]
- Murgue B. Murri S. Zientara S, et al. West Nile outbreak in horses in southern France, 2000: the return after 35 years. Emerg Infect Dis. 2001;7:692–696. doi: 10.3201/eid0704.010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris JM. Amerasinghe F. Section B: Viral. In: Beran G, editor. Handbook of Zoonoses. 2nd. Boca Raton: CRC Press Inc.; 1994. pp. 139–148. [Google Scholar]
- Roehrig J. Clinical Virology Manual. In: Specter S, editor; Hodinka R, editor; Young S, editor. 3rd. Washington DC: USA ASM Press, American Society for Microbiology; 2000. pp. 356–373. [Google Scholar]
- Saito M. Osa Y. Asakawa M. Antibodies to flaviviruses in wild ducks captured in Hokkaido, Japan: risk assessment of invasive flaviviruses. Vector Borne Zoonotic Dis. 2009;9:253–258. doi: 10.1089/vbz.2008.0111. [DOI] [PubMed] [Google Scholar]
- Shirafuji H. Kanehira K. Kamio T, et al. Antibody responses induced by experimental West Nile virus infection with or without previous immunization with inactivated Japanese encephalitis vaccine in horses. J Vet Med Sci. 2009;71:969–974. doi: 10.1292/jvms.71.969. [DOI] [PubMed] [Google Scholar]
- Tabei Y. Hasegawa M. Iwasaki N, et al. Surveillance of mosquitoes and crows for West Nile virus in the Tokyo metropolitan area. Jpn J Infect Dis. 2007;60:413–416. [PubMed] [Google Scholar]
- Ternovoi VA. Protopopova EV. Surmach SG, et al. [The genotyping of the West Nile virus in birds in the far eastern region of Russia in 2002–2004] Mol Gen Mikrobiol Virusol. 2006:30–35. [PubMed] [Google Scholar]
- Tesh RB. Travassos da Rosa AP. Guzman H, et al. Immunization with heterologous flaviviruses protective against fatal West Nile encephalitis. Emerg Infect Dis. 2002;8:245–251. doi: 10.3201/eid0803.010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turell MJ. Mores CN. Dohm DJ, et al. Laboratory transmission of Japanese encephalitis, West Nile, and Getah viruses by mosquitoes (Diptera: Culicidae) collected near Camp Greaves, Gyeonggi Province, Republic of Korea 2003. J Med Entomol. 2006;43:1076–1081. doi: 10.1603/0022-2585(2006)43[1076:ltojew]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Wagner B. Glaser A. Hillegas JM, et al. Monoclonal antibodies to equine IgM improve the sensitivity of West Nile virus-specific IgM detection in horses. Vet Immunol Immunopathol. 2008;122:46–56. doi: 10.1016/j.vetimm.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Williams DT. Daniels PW. Lunt RA, et al. Experimental infections of pigs with Japanese encephalitis virus and closely related Australian flaviviruses. Am J Trop Med Hyg. 2001;65:379–387. doi: 10.4269/ajtmh.2001.65.379. [DOI] [PubMed] [Google Scholar]
- Yang DK. Kim BH. Kweon CH, et al. Serosurveillance for Japanese encephalitis, Akabane, and Aino viruses for Thoroughbred horses in Korea. J Vet Sci. 2008;9:381–385. doi: 10.4142/jvs.2008.9.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DK. Kweon CH. Kim BH, et al. Immunogenicity of baculovirus expressed recombinant proteins of Japanese encephalitis virus in mice. J Vet Sci. 2005;6:125–133. [PubMed] [Google Scholar]
- Yeh JY. Lee JH. Seo HJ, et al. Fast duplex one-step reverse transcriptase PCR for rapid differential detection of West Nile and Japanese encephalitis viruses. J Clin Microbiol. 2010;48:4010–4014. doi: 10.1128/JCM.00582-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun SM. Kim SY. Han MG, et al. Analysis of the envelope (E) protein gene of tick-borne encephalitis viruses isolated in South Korea. Vector Borne Zoonotic Dis. 2009;9:287–293. doi: 10.1089/vbz.2008.0085. [DOI] [PubMed] [Google Scholar]