Abstract
Nonalcoholic fatty liver disease (NAFLD) is now the most frequent chronic liver disease in Western Societies, affecting one in four adults in the USA and is strongly associated with hepatic insulin resistance, a major risk factor in the pathogenesis of type 2 diabetes. Although the cellular mechanisms underlying this relationship are unknown, hepatic accumulation of diacylglycerol (DAG) in both animals and humans has been linked to hepatic insulin resistance. In this Perspective, we discuss the role of DAG activation of protein kinase Cε as the mechanism responsible for NAFLD-associated hepatic insulin resistance seen in obesity, type 2 diabetes and lipodystrophy.
INTRODUCTION
NAFLD, now the most common chronic liver disease in the world with a prevalence of about 20–30% in Western countries, is a major risk factor in the development of type 2 diabetes most likely due to its strong association with hepatic insulin resistance (Angulo, 2002; Fabbrini et al., 2010; Shulman, 2000). In this Perspective, we briefly review recent studies in both rodents and humans supporting diacylglycerol-activation of protein kinase Cε (PKCε) as a key pathway responsible for causing NAFLD-associated hepatic insulin resistance.
Diacylglycerol-Induced Hepatic Insulin Resistance
Mice with targeted overexpression of lipoprotein lipase (LPL) in the liver develop liver specific steatosis associated with liver specific hepatic insulin resistance demonstrating that hepatic insulin resistance can occur independently of changes in circulating adipocytokines [tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), resistin, adiponectin, retinol binding protein-4 (RBP-4), etc.] (Kim et al., 2001a). Hepatic steatosis and hepatic insulin resistance can also be induced in mice and rats with three days of high-fat feeding before the development of obesity and increases in circulating adipocytokines (Samuel et al., 2004). In this model of hepatic insulin resistance, hepatic steatosis was associated with decreased insulin-stimulated insulin receptor substrate-2 (IRS-2) tyrosine phosphorylation by the insulin receptor kinase, leading to the inability of insulin to activate hepatic glycogen synthesis and suppress hepatic glucose production. In this case, hepatic insulin resistance was associated with an increase in hepatic DAG content. The link between hepatic DAG accumulation and hepatic insulin resistance could be attributed to activation of PKCε, which was the predominant PKC isoform activated in liver following fat feeding (Samuel et al., 2004). PKCε is a member of the PKC family, composed of three different groups: conventional (α, βI, βII and γ), novel (δ, ε, η and θ) and atypical (ζ and λ) (Newton, 2003). PKCε is a novel PKC isoform which has a much greater affinity for DAG than the conventional PKC isoforms (Dries et al., 2007), which are activated by calcium binding to the C2 domain, which increases the affinity of the C1 domain for DAG, subsequently leading to the removal of a pseudosubstrate from the catalytic domain. Phorbol esters have been shown to activate PKCs and impair activation of the insulin receptor in vitro (Pillay et al., 1990; Takayama et al., 1988). DAG has different stereoisomers, and it has been previously shown that activation of PKC was mostly due to the sn-1,2-DAG isoform (Rando and Young, 1984). The mechanism for lipid-induced insulin resistance is similar to what is observed in skeletal muscle where PKCθ has been shown to be the predominant novel PKC isoform activated during lipid-induced muscle insulin resistance (Griffin et al., 1999; Yu et al., 2002). The molecular mechanisms of DAG activation of PKCε in hepatic insulin resistance are summarized in Figure 1.
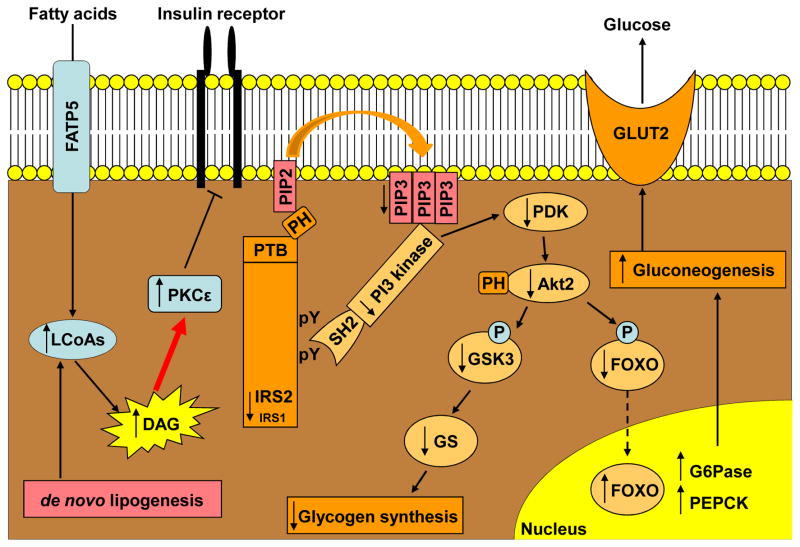
Figure 1. Molecular Mechanism of Diacylglycerol-PKCε Mediated Hepatic Insulin Resistance.
The accumulation of diacylglycerol (DAG) in the liver leads to the activation of protein kinase Cε (PKCε), which subsequently inhibits the insulin receptor kinase. This then leads to decreased insulin-stimulated tyrosine phosphorylation (pY) of insulin receptor substrate 1 and 2 (IRS1, IRS2), resulting in reduced insulin activation of 1-phosphoinositol 3-kinase (PI 3-kinase) and Akt2. Reduced Akt2 activation results in decreased glycogen synthase (GS)-mediated glycogen synthesis and decreased suppression of gluconeogenesis, which in turn leads to glucose release through glucose transporter 2 (GLUT2). FATP5, fatty acid transport protein 5; FOXO, forkhead box protein O; G6Pase, glucose-6-phosphatase; GSK3, glycogen synthase kinase-3; LCoAs, long chain fatty acids; PDK, pyruvate dehydrogenase kinase; PEPCK, phosphoenolpyruvate carboxykinase; PIP2, phosphatidylinositol bisphosphate; PIP3, phosphatidylinositol trisphosphate; PH, pleckstrin homology domain; PTB, phosphotyrosine binding domain; SH2, src homology domain.
Further evidence in support of intrahepatic lipid as the mediator of hepatic insulin resistance comes from studies in which high-fat fed rats were treated with low doses of 2,4-dinitrophenol (DNP) to promote mitochondrial energy uncoupling (Samuel et al., 2004). This treatment protected rats from developing hepatic steatosis as well as PKCε activation and hepatic insulin resistance (Samuel et al., 2004).
The specific role of PKCε in causing hepatic insulin resistance was directly examined using antisense oligonucleotides (ASO), which act preferentially in the liver and adipose tissue (Crooke, 2004). Using a specific PKCε antisense oligonucleotide, Samuel et al. were able to show that knocking down PKCε expression in liver protected rats from lipid-induced hepatic insulin resistance despite increases in hepatic lipid content (Samuel et al., 2007). Furthermore, they found that activation of PKCε caused hepatic insulin resistance by directly binding to and inhibiting insulin receptor kinase activity (Samuel et al., 2007). These results have recently been replicated in PKCε knockout mice, which were protected from high-fat feeding induced insulin resistance (Frangioudakis et al., 2009). Confirmation of this key interaction between DAG, activation of PKCε and hepatic insulin resistance has been demonstrated in numerous other rodent models of NAFLD associated hepatic insulin resistance (Birkenfeld et al., 2011a; Choi et al., 2007B; Erion et al., 2009; Jornayvaz et al., 2011; Jornayvaz et al., 2010a; Jornayvaz et al., 2012; Jornayvaz et al., 2010b; Lee et al., 2011; Nagai et al., 2009; Neschen et al., 2005; Savage et al., 2006; Zhang et al., 2007).
Mechanisms of Increased Hepatic Diacylglycerol Content
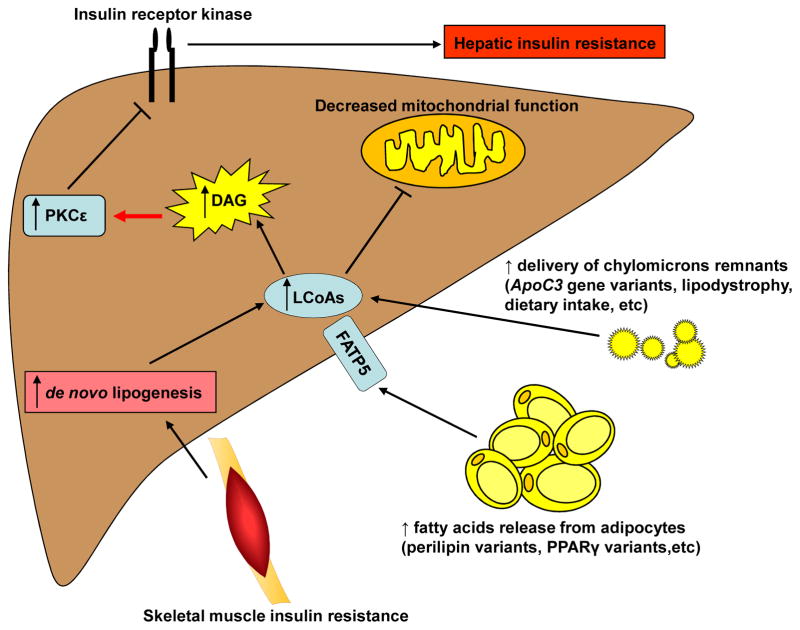
There are multiple causes for the net accumulation of DAGs in the liver and PKCε activation resulting in hepatic insulin resistance (Figure 2). First, DAGs can accumulate following an increased delivery of chylomicrons remnants secondary to increased dietary intake or depending on diet composition, like a high-fat diet, or in the case of lipodystrophy or some genetic factors. Second, hepatic accumulation DAGs can result from increased fatty acid release from adipocytes, for example in the case of certain gene variants. Third, skeletal muscle insulin resistance can result in de novo lipogenesis leading to an increase in hepatic DAG content. Finally, decreased mitochondrial function can also result in the net accumulation of DAGs.
Figure 2. Mechanisms of Hepatocellular Diacylglycerol Accumulation.
Increases in hepatic diacylglycerol (DAG) content results from an imbalance in rates of fatty acid delivery/uptake relative to rates of mitochondrial fatty acid oxidation and conversion of DAGs to triglycerides (TAGs). Increased energy intake exceeding rates of energy expenditure, is the most common cause of NAFLD and DAG-PKCε induced hepatic insulin resistance, which is most often seen in exogenous obesity. Predisposing genetic factors such as ApoC3 gene variants resulting in increased plasma ApoC3 concentrations results in suppression of lipoprotein lipase activity, increased postprandial chylomicron remnants and increased hepatic fat uptake, increased hepatic DAG content, PKCε-mediated hepatic insulin resistance. Defects in adipocyte fat storage as seen in lipodystrophy or due to genetic alterations (e.g. mutations in PPARγ or perilipin (Agostini et al., 2006; Gandotra et al., 2011) also can result in increased fat delivery to the liver, NAFLD and hepatic insulin resistance. Genetic or acquired defects in mitochondrial fatty acid oxidation may also predispose to NAFLD and DAG-PKCε mediated hepatic insulin resistance. Finally, fatty acids released from adipocytes, can enter the liver through the liver specific fatty acid transport protein 5 (FATP5), resulting in increased long chain fatty acids (LCoAs) (Schaffer and Lodish, 1994), which can then be converted to DAG.
Caloric Intake and Diet Composition
The most prevalent cause for NAFLD in Western Society can be attributed to energy imbalance where caloric intake exceeds caloric expenditure leading to an increase in the rate of substrate delivery to the liver, which exceeds the rate of hepatic fatty acid oxidation and the conversion of DAGs to neutral lipid [triacylglycerols (TAGs)] (Shulman, 2000). Mice fed a high-fat ketogenic diet develop severe hepatic steatosis and profound hepatic insulin resistance despite manifesting increased energy expenditure and weight loss (Jornayvaz et al., 2010a). In this case, hepatic DAG content was increased by 350% and resulted in PKCε activation, decreased insulinstimulated IRS2 tyrosine phosphorylation and decreased suppression of hepatic glucose production during a hyperinsulinemic-euglycemic clamp.
Decreased Mitochondrial Function
As noted hepatocellular DAG content reflects a balance between rates of lipogenesis, rates of mitochondrial fatty acid oxidation and conversion of DAGs to triglycerides (TAGs). It is therefore possible that decreased rates of mitochondrial oxidation relative to rates of hepatic lipogenesis by the liver can also be a predisposing condition to net DAG accumulation and hepatic insulin resistance. This mechanism has been well documented in the muscle of healthy, lean, insulin resistant elderly individuals (Petersen et al., 2003) and in the muscle of young, lean insulin-resistant offspring of parents with type 2 diabetes (IR offspring) (Befroy et al., 2007; Morino et al., 2005; Petersen et al., 2004) in which increased intramyocellular lipid content was associated with decreased basal rates of tricarboxylic acid flux (VTCA) and decreased basal rates of adenosine triphosphate (VATP) synthesis, assessed by 13C/31P magnetic resonance spectroscopy (MRS) respectively reflecting an imbalance between rates of fatty acid delivery/uptake into the muscle cell relative to rates of intramyocellular lipid utilization. In the latter situation this reduction in VTCA flux and VATP synthesis, could be attributed to a reduction in the ratio of slow twitch to fast twitch muscle fiber types and a reduction in mitochondrial content assessed by electron microscopy (Morino et al., 2005; Petersen et al., 2004). It is important to note that these observed reductions in mitochondrial function in both the elderly and young lean insulin resistant offspring occur independently of reductions in muscle ATP content and therefore must reflect reduced energy requirements of the muscle cell possibly due to reductions in the ratio of type 1 to type 2 muscle fiber types, as was observed in the IR offspring, and/or some other unknown factors.
It is also important to emphasize that increases in intramyocellular lipid reflect an imbalance between fatty acid uptake by the cell and intracellular lipid metabolism and can occur in the presence of low, normal or even increased rates of mitochondrial oxidation/phosphorylation activity. The best example of this is seen in muscle specific PGC-1α overexpressing mice which have increased muscle mitochondrial content and increased rates of basal mitochondrial VATP synthesis but are prone to muscle lipid and DAG accumulation associated with PKCθ activation and muscle insulin resistance when fed a high-fat diet (Choi et al., 2008). Increases in muscle DAG content in these mice can be attributed to increased CD36 expression in skeletal muscle leading to a situation where fatty acid uptake by the muscle cell exceeds rates of intramyocellular lipid utilization resulting in net increases in DAG content despite increased basal rates of mitochondrial function. Although reduced mitochondrial activity associated with muscle insulin resistance is clearly an acquired event associated with aging (Petersen et al., 2003) that can be prevented by targeted overexpression of catalase to the mitochondria (Lee et al., 2010) it remains to be determined if decreased mitochondrial function is a primary or a secondary event in the development of increased intramyocellular lipid uptake and muscle insulin resistance in young lean IR offspring (Befroy et al., 2007; Morino et al., 2005; Petersen et al., 2004). In this regard a recent study by Morino et al. has found reduced LPL expression leading to decreased activity of peroxisome proliferator-activated receptor (PPAR) δ in skeletal muscle of these young lean insulin resistant subjects suggesting that reductions in mitochondrial function and content may be an acquired event in these individuals due to reduced LPL expression in skeletal muscle (Morino et al., 2012). Nevertheless, given the potentially important role that intracellular lipids have in mediating muscle insulin resistance, any decrease in mitochondrial function would be expected to exacerbate lipid-induced muscle insulin resistance.
Genetic evidence demonstrating that reductions in liver mitochondrial function can be a predisposing factor to NAFLD and hepatic insulin resistance is observed in long chain acyl-CoA dehydrogenase (LCAD) knockout mice, which have reduced rates of hepatic mitochondrial fatty acid oxidation and are prone to develop hepatic steatosis associated with increased hepatic DAG content, PKCε activation and hepatic insulin resistance when fed a high-fat diet (Zhang et al., 2007). Whether similar reductions in hepatic mitochondrial oxidation is associated with NAFLD in humans is less clear with some studies demonstrating reduced (Cortez-Pinto et al., 1999; Schmid et al., 2011) and other studies suggesting increased rates of liver mitochondrial metabolism (Sunny et al., 2011).
The Role of Muscle Insulin Resistance in Promoting Hepatic Steatosis
Selective insulin resistance in skeletal muscle can lead to a redistribution of substrates away from muscle to the liver resulting in hepatic steatosis. An example of this occurs in mice with muscle specific deletion of the muscle insulin receptor (MIRKO) (Kim et al., 2000b). In these mice, insulin-stimulated muscle glucose transport and glycogen synthesis were decreased by ~80%, whereas insulin-stimulated glucose transport in adipocytes was increased by threefold, demonstrating that selective insulin resistance in muscle promotes redistribution of substrates toward increased adiposity (Kim et al., 2000b). These results were confirmed in mice lacking the muscle glucose transporter 4 (GLUT4), which had a ~90% decrease in insulin-stimulated muscle glucose uptake (Kim et al., 2001b) and were shown to have a redistribution of substrates to the liver making them prone to develop hepatic steatosis (Kotani et al., 2004). Petersen et al. translated these findings to humans by demonstrating that selective insulin resistance in skeletal muscle, which is observed in young, lean, healthy individuals who are in the bottom quartile of whole body insulin sensitivity, diverts the energy of ingested carbohydrates away from muscle glycogen synthesis towards hepatic de novo lipogenesis, thus predisposing these individuals to increased plasma triglycerides and reduced plasma high-density lipoproteins (Petersen et al., 2007). Notably, visceral fat mass measured with abdominal MRI was similar between insulin-resistant and insulin-sensitive subjects, suggesting that these features of the metabolic syndrome can develop independently of increased visceral adiposity. Moreover, no differences in plasma adipokines (adiponectin, IL-6, resistin, RBP-4 and TNFα) concentrations were observed between the insulin resistant and insulin sensitive individuals suggesting that these circulating adipocytokines were not responsible for causing insulin resistance in these individuals (Petersen et al., 2007). Consistent with these results, Stefan et al. reported that in a cohort of obese people, insulin-sensitive and insulin-resistant individuals were distinguished on the basis of lipid accumulation in the muscle and liver, but not according to visceral or subcutaneous adiposity (Stefan et al., 2008). This study was further supported by the finding that intrahepatic triglyceride content, but not visceral adiposity, was associated with insulin resistance and increased triglycerides secretion (Fabbrini et al., 2009). In order to directly examine the hypothesis that muscle insulin resistance can lead to a redistribution of substrates to the liver resulting in increased hepatic de novo lipogenesis, Rabøl et al. assessed the effect of a single-bout of exercise on hepatic de novo lipogenesis and hepatic triglyceride synthesis after the ingestion of a carbohydrate-rich meal in healthy, young, lean, insulin resistant individuals (Rabol et al., 2011). The rationale for this study came from previous studies by Perseghin et al. who found that a single 45-minute bout of exercise reversed defects in insulin-stimulated glucose transport/phosphorylation activity and muscle glycogen synthesis in young lean insulin-resistant offspring of parents with type 2 diabetes (Perseghin et al., 1996). Rabøl et al. showed that a single bout of exercise resulted in a 30% decrease in hepatic de novo lipogenesis and a 40% reduction in net hepatic triglyceride synthesis, without any changes in fasting or postprandial plasma glucose and insulin concentrations, demonstrating that skeletal muscle insulin resistance is an early therapeutic target for the prevention and treatment of atherogenic dyslipidemia and NAFLD in young insulin resistant individuals prone to develop the metabolic syndrome (Rabol et al., 2011).
Taken together, these studies in mice and humans support the concept that selective muscle insulin resistance is an important and early factor in the pathogenesis of atherogenic dyslipidemia and NAFLD in patients prone to develop the metabolic syndrome and type 2 diabetes.
Lipodystrophy
The assessment of the specific role of NAFLD in the development of hepatic insulin resistance is challenging as it is usually associated with obesity, so the changes in liver insulin action due to steatosis and those attributable to adiposity and related changes such as inflammation are therefore difficult to ascertain (Gastaldelli et al., 2004; Miyazaki et al., 2002a). The lipodystrophies offer the unique possibility to assess the role of hepatic lipid accumulation without any peripheral or visceral fat expansion. These conditions may be congenital or acquired and are characterized by partial or complete loss of adipose tissue and are associated with low plasma leptin concentrations and hyperphagia. The lack of subcutaneous fat causes hypertriglyceridemia, insulin resistance and ectopic fat deposition, which include the development of substantial hepatic steatosis.
Mice expressing the dominant negative protein A-ZIP/F-1 are virtually devoid of white adipose tissue (“fatless” mice) and develop fat accumulation in the liver and skeletal muscle, leading to profound peripheral and hepatic insulin resistance (Kim et al., 2000a). Interestingly, transplantation of wild-type fat in these mice reversed the hyperglycemia, lowered plasma insulin levels and improved liver and muscle insulin sensitivity (Kim et al., 2000a). Most of the metabolic defects associated with lipodystrophy in mice were also corrected by the administration of leptin (Shimomura et al., 1999).
Consistent with these mice studies, lipodystrophic patients benefit from the exogenous administration of recombinant leptin (Oral et al., 2002). A study by Petersen et al. provided important mechanistic insights into how leptin therapy was working in these patients by demonstrating that before leptin replacement, lipodystrophic patients had higher basal rates of glucose production than the control subjects matched for age, weight and sex (Petersen et al., 2002). Moreover, these patients had severe liver and muscle insulin resistance as reflected by the lack of suppression of hepatic glucose production and decreased stimulation of peripheral glucose uptake during a hyperinsulinemic-euglycemic clamp, which was associated with severe hepatic steatosis. However, after leptin replacement, there was a marker reduction in both liver and intramyocellular lipid content, which could be mostly be attributed to reduction in caloric intake, with concomitant improvement in both hepatic and peripheral insulin sensitivity (Petersen et al., 2002). Taken together, these studies in lipodystrophic patients and mouse models of lipoatrophy demonstrate that ectopic lipid accumulation in liver can lead to hepatic insulin resistance even in the absence of peripheral and visceral adiposity and that reversal of hepatic steatosis leads to reversal of hepatic insulin resistance.
Predisposing Genetic Factors for NAFLD
Importantly, there are ethnic differences in the prevalence of NAFLD. Notably, males of Asian-Indian ancestry have a higher risk of developing NAFLD, which is associated with marked hepatic insulin resistance (Petersen et al., 2006), despite having a normal body mass index (BMI) (<25 kg/m2). In this case, there was almost a doubling in the average liver triglyceride content in the lean Asian-Indian men when compared with age-weight-BMI matched Caucasian men. This increase in liver fat content in young Asian-Indian, normal-weight men put them at increased risk of developing type 2 diabetes, NAFLD and liver cirrhosis (Petersen et al., 2006).
Recently, Petersen et al. showed that common gene variants (C-482T/T-455C) in the insulin response element of the apolipoprotein C3 (ApoC3) gene are at higher risk of developing NAFLD and insulin resistance (Petersen et al., 2010). The carriers of these polymorphisms have an approximately 30% higher plasma concentrations of ApoC3 as well as postprandial hypertriglyceridemia compared to individuals who are wild type homozygotes for ApoC3 (C-482/T-455). ApoC3 inhibits LPL and as a consequence these carriers have decreased triglyceride clearance following an intravenous infusion of lipids and increased postprandial hypertriglyceridemia and postprandial chylomicrons remnants, leading to NAFLD and hepatic insulin resistance (Petersen et al., 2010). Furthermore, modest weight reduction in these subjects reversed the hepatic steatosis and insulin resistance (Petersen et al., 2010). It is important to note that these ApoC3 gene variants, which result in a 30% increase in plasma ApoC3 concentrations, do not directly cause hepatic steatosis but represent a predisposing condition for individuals who carry them. Therefore individuals with the ApoC3 gene variants (C-482T/T-455C) when exposed to a “toxic environment” of increased fat and calorie dense foods are more susceptible to develop NAFLD and hepatic insulin resistance. Furthermore, since this is a predisposing gene-environment interaction this association between increased ApoC3 concentrations and increased prevalence of hepatic steatosis will typically only be observed in lean individuals who normally have a low prevalence of NAFLD in contrast to overweight/obese individuals where some degree of hepatic steatosis is almost universally present. In support of this hypothesis it has recently been shown that mice with hepatic overexpression of ApoC3 had no metabolic phenotype (no hepatic steatosis or insulin resistance) when fed a regular chow diet but developed severe hepatic steatosis associated with increased hepatic DAG content, PKCε activation and hepatic insulin resistance when placed on a high-fat diet (Lee et al., 2011).
Another group at risk of developing NAFLD and hepatic insulin resistance are Hispanics adults and children (Liska et al., 2007). In this population, genetic screening identified an allele (Met148Ile) of the enzyme patatin-like phospholipase domain-containing protein 3, which is encoded by the PNPLA3 gene, to be strongly correlated with the development of NAFLD (Romeo et al., 2008). However, in contrast to the ApoC3 gene variants (T482/C455) described above this PNPLA3 gene variant was not associated with insulin resistance, suggesting that NAFLD can be dissociated from hepatic insulin resistance (Kantartzis et al., 2009). Whether hepatic DAG content in these individuals is increased along with hepatic TAG content is unknown. However it should be noted that in all studies to date that have examined the relationship between NAFLD in these PNPLA3 gene variants with NAFLD have used obese individuals as control subjects, who almost certainly have some degree of hepatic steatosis associated with hepatic insulin resistance therefore making it difficult to discern whether or not PNPLA3 gene variants with NAFLD truly manifest hepatic insulin resistance. Therefore, it will be very important to examine hepatic insulin sensitivity in lean PNPLA3 variants with NAFLD compared to lean healthy control subjects without NAFLD to get a definitive answer to this question.
Controversies and Alternative Hypotheses
NAFLD is strongly associated with hepatic insulin resistance and type 2 diabetes. However, some studies have reported a dissociation between NAFLD and hepatic insulin resistance. Among these, Monetti et al. reported that mice overexpressing acylCoA:diacylglycerol acyltransferase 2 (DGAT2) in the liver, the enzyme that catalyzes the conversion from DAG to TAG (Figure 3), have normal hepatic insulin sensitivity despite increased hepatic TAG, DAG and ceramide content (Monetti et al., 2007). In this study, insulin sensitivity was assessed using intraperitoneal glucose tolerance tests and the hyperinsulinemic-euglycemic clamp technique (Monetti et al., 2007). These results were surprising to us given the high hepatic DAG content in these DGAT2 transgenic mice and given previous studies demonstrating a key role of DAG-PKCε activation in mediating hepatic insulin resistance. Given this apparent paradox, we decided to reconsider the role of DAG in causing hepatic insulin resistance in this mouse model of severe hepatic steatosis. We therefore performed detailed studies to evaluate insulin sensitivity in awake mice by using the hyperinsulinemic-euglycemic clamp technique combined with radiolabeled glucose to assess rates of whole-body glucose turnover. We also assessed signaling events typically associated with an increase in hepatic DAG content, i.e. PKCε activation as well as potential alterations in insulin signaling downstream of the insulin receptor kinase. Consistent with the observations of Monetti et al. we found that DGAT2 transgenic mice had an increase in hepatic TAG, DAG and ceramide content (Jornayvaz et al., 2011). However, in contrast to the findings of Monetti et al. (Monetti et al., 2007), we found that the DGAT2 transgenic mice had severe liver resistance as reflected by the lack of suppression of hepatic glucose production during a hyperinsulinemic-euglycemic clamp. We also found that the increase in hepatic DAG content led to PKCε activation, resulting in decreased insulin-stimulated IRS2 tyrosine phosphorylation and Akt phosphorylation. Although the slight ~8% increase in ceramides may also have contributed to hepatic insulin resistance, it is likely that hepatic DAG accumulation was the major factor responsible for the development of hepatic insulin resistance in these mice given the much larger increases in hepatic DAG content compared to relatively small increases in hepatic ceramide content observed in these mice (Jornayvaz et al., 2011). While the main conclusion of these studies differs from that obtained by Monetti et al. (Monetti et al., 2007), several aspects of both studies were similar. We used the same strain of mice with the same housing and breeding conditions. The critical difference in our studies was the assessment of hepatic insulin response. The hyperinsulinemic-euglycemic clamp is the gold standard for directly quantifying hepatic insulin sensitivity, whereas glucose-tolerance reflects the coordinated response to a glucose load, but without measurements of plasma insulin concentrations as was done in the mice (Monetti et al., 2007), insulin sensitivity cannot be assessed (Ayala et al., 2010). It is likely that differences in clamp procedures are responsible for the critical differences between our studies. Monetti et al. performed clamp studies only 3 days following catheter implantation. However, 5 to 7 days are required for mice to fully recover from the stress of surgery and return to their pre-surgical body weight (Ayala et al., 2010). Consistent with this hypothesis, Monetti et al. observed high rates of hepatic glucose production during the hyperinsulinemic-euglycemic clamp [approximately 20 mg/(kg-min)], in both DGAT2 transgenic and control wild-type mice, and without a positive control group showing normal suppression of hepatic glucose production during the hyperinsulinemic-euglycemic clamp, it is not possible to discern hepatic insulin response in the DGAT2 transgenic group. Moreover, without reporting basal rates of hepatic glucose production prior to the insulin infusion, it is impossible to calculate the suppression in hepatic glucose production during the hyperinsulinemic-euglycemic clamp. Thus, while wild-type and DGAT2 transgenic mice had similar rates of hepatic glucose production during the hyperinsulinemic-euglycemic clamps done by Monetti et al., both their wild-type control mice and their DGAT2 transgenic mice appear to have severe hepatic insulin resistance (Monetti et al., 2007). In conclusion, increased hepatic DGAT2 expression increases hepatic DAG content and confirms the link between hepatic DAG, PKCε activation and hepatic insulin resistance (Jornayvaz et al., 2011). Moreover, the demonstration of insulin resistance in these mice strengthens the link between hepatic steatosis and hepatic insulin resistance and supports the hypothesis that DAG-induced PKCε activation plays a major role in NAFLD-associated hepatic insulin resistance.
Figure 3. Metabolic Pathways Leading to Hepatic Diacylglycerol Accumulation.
The glycerol 3-phosphate (or phosphatidic acid) pathway represents the de novo lipogenesis route in the synthesis of triglycerides (TAG) and phospholipids. Acyl-CoA:glycerol-sn-3-phosphate acyltransferase (GPAT) catalyzes the acylation of sn-glycerol-3-phosphate with acyl-coenzyme A (acyl-CoA) to generate lysophosphatidic acid (LPA). LPA is thought to be the rate-controlling step in TAG synthesis. Subsequently, the enzymes acyl-CoA:1-acylglycerol-sn-3-phosphate acyltransferase (AGPAT), phosphatidic acid phosphatase (PAP) and diacylglycerol:acyl-CoA acyltransferase (DGAT) generate phosphatidic acid (PA), diacylglycerol (DAG) and TAG. In the liver, TAG is either deposited in intracellular vacuoles or exported in very low-density lipoproteins (VLDL) particles. LPA and PA require translocation through the cytosol for TAG synthesis at the endoplasmic reticulum if they are not synthesized in the endoplasmic reticulum. DAG can be hydrolyzed to monoacylglycerol (MAG) by hormone-sensitive lipase (HSL) and subsequently to glycerol by monoglyceride lipase (MGL). These reactions release fatty acids. Glycerol can be used as a substrate for gluconeogenesis. The conversion from TAG to DAG is mediated by adipose triglyceride lipase (ATGL). Comparative Gene Identification-58 (CGI-58) is an activator of ATGL. DAG activate PKCε membrane translocation to inhibit the insulin receptor kinase. Phospholipase C can release DAG from membrane lipids. Whether DAG derived from the phospholipase C pathway and from lipid droplets can lead to PKCε activation and hepatic insulin resistance remains to be determined.
Importantly, in this study (Jornayvaz et al., 2011), hepatic insulin resistance was not associated with endoplasmic reticulum (ER) stress or inflammation, which are alternative views to explain insulin resistance in obesity and type 2 diabetes (Hotamisligil, 2006, 2008; Lazar, 2005; Ozcan et al., 2004; Taubes, 2009). Obesity combines insulin resistance and a proinflammatory state and mechanistically inflammatory signals are known to affect insulin action (Shoelson et al., 2006). Specifically, inflammatory signals such as IL-6 or TNFα activate kinases such as c-jun-N-terminal kinase (JNK) and Iκ kinase β, which are known to increase IRS1 serine phosphorylation and critical sites that block insulin-stimulated IRS1 tyrosine phosphorylation (Boden et al., 2005). ER stress might also activate inflammatory pathways to protect cells from producing aberrant proteins, often referred as the unfolded protein response (Hotamisligil, 2005). This activation has been shown to occur in human obesity (Boden et al., 2008) as well as in rodent models of obesity (Ozcan et al., 2004), and modulation of ER stress can ameliorate JNK activation and the development of insulin resistance (Ozcan et al., 2006). However, recent studies in X-box binding protein (XBP) knockout mice have disassociated inositol requiring enzyme (IRE)-1α mediated JNK-1 activation from hepatic insulin resistance (Jurczak et al., 2012).
Translation to the Bedside
Probably, the most critical question regarding all of the proposed cellular mechanisms of NAFLD associated hepatic insulin resistance is whether any of them will translate to explain hepatic insulin resistance in humans with NAFLD (Hotamisligil, 2006, 2008; Lazar, 2005; Ozcan et al., 2004; Shulman, 2000; Taubes, 2009). Evidence in support of an important role for DAG-mediated activation PKCε in this process comes from a recent study that found that hepatic DAG content in cytoplasmic lipid droplets was the best predictor of insulin resistance in obese, nondiabetic individuals with NAFLD and varying degrees of insulin resistance (Kumashiro et al., 2011). Furthermore hepatic DAG content and hepatic insulin resistance in these individuals was strongly correlated with PKCε activation, similar to what was observed in previous rodent studies (Samuel et al., 2004; Samuel et al., 2007). In contrast, there was no significant association between insulin resistance and plasma or hepatic markers of inflammation or hepatic ceramide content. Moreover, ER stress markers were only partly correlated with insulin resistance and there was no relationship between the IRE-1α/JNK ER stress pathway and insulin resistance. These data suggest that the IRE-1α/JNK1 ER stress pathway does not cause insulin resistance and is consistent with recent studies that have dissociated hepatic insulin resistance from activation of the IRE-1α/JNK1 ER stress pathway (Birkenfeld et al., 2011b; Jurczak et al., 2012). Taken together these studies show that hepatic DAG content associated with PKCε activation is the best predictor of insulin resistance in humans, and support the hypothesis that NAFLD-associated hepatic insulin resistance is caused by an increase in hepatic DAG content, which results in activation of PKCε and subsequent reductions in hepatic insulin signaling (Kumashiro et al., 2011).
DAG Compartmentation
Recent studies in both humans (Kumashiro et al., 2011) and rodents (Birkenfeld et al., 2011b; Jornayvaz et al., 2011; Jurczak et al., 2012) have clearly demonstrated that compartmentation of DAGs within the hepatocyte is a major factor in determining whether PKCε and hepatic insulin resistance occurs. Figure 3 summarizes the metabolic pathways leading to hepatic DAG accumulation. DAG can result from the glycerol 3-phosphate (or phosphatidic acid) pathway, which represents the lipogenesis route in the synthesis of TAG and phospholipids. Most studies to date have clearly implicated DAGs derived from this pathway in activation of PKCε and hepatic insulin resistance. However intracellular DAGs can also be derived from TAG hydrolysis of lipid droplets, mediated by ATGL, and activation of phospholipase C, which will release DAGs from membrane lipids. Whether DAGs derived from these latter two pathways can lead to PKCε activation and hepatic insulin resistance remains to be determined. Indeed compartmentation of DAGs in a neutral compartment where it cannot activate PKCε may explain why hepatic DAG content may not always correlate with hepatic insulin resistance in some mouse models. For example, mice treated with an ASO targeting Comparative Gene Identification-58 (CGI-58), develop NAFLD associated with increases in hepatic TAG, DAG and ceramide content but show improved glucose tolerance when assessed by an intraperitoneal glucose tolerance test (Brown et al., 2010). CGI-58 encodes a protein that acts as a coactivator of adipose triglyceride lipase (ATGL, also known as desnutrin), which hydrolyzes TAG to DAG (Figure 3). Interestingly, CGI-58 also possesses similar properties as the enzyme acyl-CoA:1-acylglycerol-sn-3-phosphate acyltransferase (AGPAT), which converts lysophosphatidic acid to phosphatidic acid, and may therefore be involved in neutral lipids and DAG synthesis (Montero-Moran et al., 2010). Although these results dissociate hepatic DAG content and hepatic insulin resistance, more careful studies are required. First, the effect of CGI-58 ASO on hepatic insulin sensitivity in mice needs to be directly assessed using hyperinsulinemic-euglycemic clamp studies as intraperitoneal glucose tolerance tests do not directly examine hepatic insulin sensitivity and studies in non body weight-matched conditions may results in misleading conclusions (Ayala et al., 2010). Furthermore, it will be important to understand where DAGs are localized within the hepatocyte in this model as reflected by recent studies demonstrating that DAGs in the cytosolic compartment of the hepatocyte best correlated with PKCε activation and insulin resistance in contrast to DAGs in other cellular compartments (Jornayvaz et al., 2011; Kumashiro et al., 2011). Clearly, further studies are required to describe how and where DAGs accumulate within the hepatocyte and lead to PKCε activation and hepatic insulin resistance. Moreover, it will be important to examine whether certain subspecies of DAGs are more potent at activating PKCε and causing hepatic insulin resistance.
Therapeutic Implications
Reduction in Hepatic Steatosis Reduces Hepatic Insulin Resistance
If hepatic lipid is an important mediator of hepatic insulin resistance it would be expected that reduction of hepatic steatosis in patients with NAFLD and type 2 diabetes would reduce hepatic insulin resistance. In order to test this hypothesis Petersen et al. subjected patients with NAFLD and type 2 diabetes to a hypocaloric diet (1200 kcal/d) for approximately two months during which time these individuals lost on average ~8kg of body weight (Petersen et al., 2005). Following weight stabilization this modest weight loss was associated with an ~80% reduction in hepatic TAG content, assessed by 1H MRS and was associated with normalization of fasting plasma glucose concentrations, rates of basal hepatic glucose production and hepatic sensitivity as reflected by normal suppression of hepatic glucose production during a hyperinsulinemic-euglycemic clamp (Petersen et al., 2005). Although hepatic DAG content was not assessed in this study, Kumashiro and coworkers have recently demonstrated a very strong relationship (R=0.90, P<0.001) between hepatic TAG content and hepatic DAG content measured in human liver biopsy studies (Kumashiro et al., 2011). Furthermore this normalization in hepatic insulin sensitivity occurred independently of any changes in circulating inflammatory adipocytokines (Petersen et al., 2005). These results have since been replicated in a recent study by Lim et al. who found that energy restriction (600 kcal/day) for 8 weeks resulted in modest weight loss, reduced hepatic TAG content and normalization of hepatic insulin sensitivity in patients with NAFLD and type 2 diabetes (Lim et al., 2011).
Among currently available anti-diabetic drugs, thiazolidinediones, which are potent peroxisome proliferator-activated γ (PPARγ) agonists, can also reduce hepatic steatosis. Rosiglitazone was shown to reduce hepatic TAG content by ~40% after three months of treatment in patients with NAFLD and type 2 diabetes (Mayerson et al., 2002). This effect was accompanied by a reduction of ~40% in intramyocellular TAG content and improved suppression of adipocyte lipolysis during a hyperinsulinemic-euglycemic clamp (Mayerson et al., 2002). Subsequent studies have confirmed that rosiglitazone treatment can decrease hepatic fat content in patients with NAFLD and type 2 diabetes and improve hepatic insulin sensitivity (Tiikkainen et al., 2004). Similar reductions in hepatic steatosis were also reported for pioglitazone treatment in patients with NAFLD and type 2 diabetes (Miyazaki et al., 2002b). Taken together, these data support the hypothesis that thiazolidinedione-associated improvement in insulin sensitivity is mediated mostly by shifting intracellular lipids from ectopic sites in liver and muscle into adipose tissue, as this is the main site of expression of PPARγ (Kim et al., 2003; Shulman, 2000).
Promoting Hepatic Mitochondrial Uncoupling and Hepatic Energy Dissipation
As discussed earlier, promoting hepatic mitochondrial uncoupling by treating rats with low dose 2,4-dinitrophenol, a potent mitochondrial uncoupler, decreased hepatic TAG content and protected rats from lipid-induced hepatic insulin resistance (Samuel et al., 2004). Similar observations have been made in skeletal muscle in transgenic mice with muscle specific overexpression of uncoupling protein 3 (UCP3) (Choi et al., 2007a). These results demonstrate that increasing mitochondrial energy uncoupling promotes mitochondrial substrate oxidation leading to reductions in tissue TAG/DAG content, decreased nPKC activation and prevention of insulin resistance in both liver and skeletal muscle. Besides providing important evidence in support of the DAG-PKCε hypothesis of hepatic insulin resistance these results also provide a novel pharmacologic approach to the treatment of NAFLD associated hepatic insulin resistance. Consistent with these findings Savage et al. have shown that promoting hepatic fatty acid oxidation and inhibiting fatty acid synthesis by knocking down hepatic expression of acetyl-CoA carboxylase 1 and 2 (ACC1 and ACC2) respectively using antisense oligonucleotides prevented hepatic steatosis, hepatic DAG accumulation, PKCε activation and hepatic insulin resistance in high-fat fed rats (Savage et al., 2006). However, it is important to note that simply promoting mitochondrial fat oxidation, at the expense of decreased glucose oxidation, will not likely solve the problem of NAFLD and hepatic insulin resistance unless one can promote mitochondrial energy uncoupling and dissipate the excess stored energy in the liver as DAGs and TAGs. Along with these lines Birkenfeld et al. have recently showed that mice lacking the INDY (acronym for I am Not Dead Yet) gene (mINDY−/−) were protected from high-fat diet-induced obesity and insulin resistance (Birkenfeld et al., 2011a). The rationale for this study in mice came from previous experiments in D. melanogaster and its homolog in C. elegans with reduced expression of the INDY gene that demonstrated increased longevity in a manner akin to caloric restriction. The liver of mINDY−/− mice had a reduced ATP/ADP ratio, which subsequently led to activation of AMP activated protein kinase (AMPK) and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), and to the inhibition of ACC2 and sterol regulatory element binding protein 1c (SREBP1c) protein levels. Therefore, mINDY−/− mice had improved mitochondrial biogenesis and increased hepatic lipid oxidation, with a subsequent reduction in hepatic TAG and DAG content. Consistent with the DAG-PKCε hypothesis mINDY−/− mice were protected from high-fat diet-induced hepatic insulin resistance and displayed decreased PKCε activation. Importantly, mINDY−/− mice had no difference in hepatic ceramide content compared to their wild-type littermate controls, excluding this lipid intermediate from being involved in hepatic insulin resistance in this mouse model (Birkenfeld et al., 2011a). Moreover, gene set enrichment analysis revealed markedly increased expression of pathways regulating mitochondrial genes and mitochondrial density assessed by electron microscopy in mINDY−/− mice.
Conclusions and Future Perspectives
In conclusion, there is increasing evidence not only in animal models of NAFLD but more importantly in humans with NAFLD associated with obesity, type 2 diabetes and lipodystrophy that hepatic steatosis is strongly linked with the development of hepatic insulin resistance. Moreover, these studies support the hypothesis that DAG-induced PKCε activation, resulting in inhibition of insulin-stimulated insulin receptor kinase activity, represents the root cause of hepatic insulin resistance in all of these conditions. Reducing hepatic DAG content by decreasing caloric intake and/or by increasing energy expenditure through exercise clearly represents the healthiest approach to treat NAFLD in these conditions. Based on animal studies novel therapies aimed at promoting hepatic mitochondrial energy uncoupling should also prove to be an effective treatment in lowering hepatic DAG content and reversing hepatic insulin resistance. Further studies aimed at understanding the potential role of DAG compartmentation in activation of PKCε may also lead to new therapeutic targets.
Acknowledgments
This work was supported by grants from the United States Public Health Service: DK-40936, DK-49230, DK-085638, DK-059635 and DK-45735. FRJ was funded by a grant from the Swiss National Science Foundation/Swiss Foundation for Grants in Biology and Medicine (PASMP3_132563).
Footnotes
DISCLOSURES
The authors have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agostini M, Schoenmakers E, Mitchell C, Szatmari I, Savage D, Smith A, Rajanayagam O, Semple R, Luan J, Bath L, et al. Non-DNA binding, dominant-negative, human PPARgamma mutations cause lipodystrophic insulin resistance. Cell Metab. 2006;4:303–311. doi: 10.1016/j.cmet.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, Wasserman DH, McGuinness OP. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech. 2010;3:525–534. doi: 10.1242/dmm.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Befroy DE, Petersen KF, Dufour S, Mason GF, de Graaf RA, Rothman DL, Shulman GI. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes. 2007;56:1376–1381. doi: 10.2337/db06-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenfeld AL, Lee HY, Guebre-Egziabher F, Alves TC, Jurczak MJ, Jornayvaz FR, Zhang D, Hsiao JJ, Martin-Montalvo A, Fischer-Rosinsky A, et al. Deletion of the Mammalian INDY Homolog Mimics Aspects of Dietary Restriction and Protects against Adiposity and Insulin Resistance in Mice. Cell Metab. 2011a;14:184–195. doi: 10.1016/j.cmet.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenfeld AL, Lee HY, Majumdar S, Jurczak MJ, Camporez JP, Jornayvaz FR, Frederick DW, Guigni B, Kahn M, Zhang D, et al. Influence of the hepatic eukaryotic initiation factor 2alpha (eIF2alpha) endoplasmic reticulum (ER) stress response pathway on insulin-mediated ER stress and hepatic and peripheral glucose metabolism. J Biol Chem. 2011b;286:36163–36170. doi: 10.1074/jbc.M111.228817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G, Duan X, Homko C, Molina EJ, Song W, Perez O, Cheung P, Merali S. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes. 2008;57:2438–2444. doi: 10.2337/db08-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G, She P, Mozzoli M, Cheung P, Gumireddy K, Reddy P, Xiang X, Luo Z, Ruderman N. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-kappaB pathway in rat liver. Diabetes. 2005;54:3458–3465. doi: 10.2337/diabetes.54.12.3458. [DOI] [PubMed] [Google Scholar]
- Brown JM, Betters JL, Lord C, Ma Y, Han X, Yang K, Alger HM, Melchior J, Sawyer J, Shah R, et al. CGI-58 knockdown in mice causes hepatic steatosis but prevents diet-induced obesity and glucose intolerance. J Lipid Res. 2010;51:3306–3315. doi: 10.1194/jlr.M010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CS, Befroy DE, Codella R, Kim S, Reznick RM, Hwang YJ, Liu ZX, Lee HY, Distefano A, Samuel VT, et al. Paradoxical effects of increased expression of PGC-1alpha on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proc Natl Acad Sci U S A. 2008;105:19926–19931. doi: 10.1073/pnas.0810339105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CS, Fillmore JJ, Kim JK, Liu ZX, Kim S, Collier EF, Kulkarni A, Distefano A, Hwang YJ, Kahn M, et al. Overexpression of uncoupling protein 3 in skeletal muscle protects against fat-induced insulin resistance. J Clin Invest. 2007a;117:1995–2003. doi: 10.1172/JCI13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CS, Savage DB, Abu-Elheiga L, Liu ZX, Kim S, Kulkarni A, Distefano A, Hwang YJ, Reznick RM, Codella R, et al. Continuous fat oxidation in acetyl-CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc Natl Acad Sci U S A. 2007b;104:16480–16485. doi: 10.1073/pnas.0706794104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez-Pinto H, Chatham J, Chacko VP, Arnold C, Rashid A, Diehl AM. Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis: a pilot study. JAMA. 1999;282:1659–1664. doi: 10.1001/jama.282.17.1659. [DOI] [PubMed] [Google Scholar]
- Crooke ST. Progress in antisense technology. Annu Rev Med. 2004;55:61–95. doi: 10.1146/annurev.med.55.091902.104408. [DOI] [PubMed] [Google Scholar]
- Dries DR, Gallegos LL, Newton AC. A single residue in the C1 domain sensitizes novel protein kinase C isoforms to cellular diacylglycerol production. J Biol Chem. 2007;282:826–830. doi: 10.1074/jbc.C600268200. [DOI] [PubMed] [Google Scholar]
- Erion DM, Ignatova ID, Yonemitsu S, Nagai Y, Chatterjee P, Weismann D, Hsiao JJ, Zhang D, Iwasaki T, Stark R, et al. Prevention of hepatic steatosis and hepatic insulin resistance by knockdown of cAMP response element-binding protein. Cell Metab. 2009;10:499–506. doi: 10.1016/j.cmet.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangioudakis G, Burchfield JG, Narasimhan S, Cooney GJ, Leitges M, Biden TJ, Schmitz-Peiffer C. Diverse roles for protein kinase C delta and protein kinase C epsilon in the generation of high-fat-diet-induced glucose intolerance in mice: regulation of lipogenesis by protein kinase C delta. Diabetologia. 2009;52:2616–2620. doi: 10.1007/s00125-009-1543-0. [DOI] [PubMed] [Google Scholar]
- Gandotra S, Le Dour C, Bottomley W, Cervera P, Giral P, Reznik Y, Charpentier G, Auclair M, Delepine M, Barroso I, et al. Perilipin deficiency and autosomal dominant partial lipodystrophy. N Engl J Med. 2011;364:740–748. doi: 10.1056/NEJMoa1007487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastaldelli A, Miyazaki Y, Pettiti M, Buzzigoli E, Mahankali S, Ferrannini E, DeFronzo RA. Separate contribution of diabetes, total fat mass, and fat topography to glucose production, gluconeogenesis, and glycogenolysis. J Clin Endocrinol Metab. 2004;89:3914–3921. doi: 10.1210/jc.2003-031941. [DOI] [PubMed] [Google Scholar]
- Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF, Shulman GI. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Role of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetes. Diabetes 54 Suppl. 2005;2:S73–78. doi: 10.2337/diabetes.54.suppl_2.s73. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int J Obes (Lond) 32 Suppl. 2008;7:S52–54. doi: 10.1038/ijo.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jornayvaz FR, Birkenfeld AL, Jurczak MJ, Kanda S, Guigni BA, Jiang DC, Zhang D, Lee HY, Samuel VT, Shulman GI. Hepatic insulin resistance in mice with hepatic overexpression of diacylglycerol acyltransferase 2. Proc Natl Acad Sci U S A. 2011;108:5748–5752. doi: 10.1073/pnas.1103451108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jornayvaz FR, Jurczak MJ, Lee HY, Birkenfeld AL, Frederick DW, Zhang D, Zhang XM, Samuel VT, Shulman GI. A high-fat, ketogenic diet causes hepatic insulin resistance in mice, despite increasing energy expenditure and preventing weight gain. Am J Physiol Endocrinol Metab. 2010a;299:E808–815. doi: 10.1152/ajpendo.00361.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jornayvaz FR, Lee HY, Jurczak MJ, Alves TC, Guebre-Egziabher F, Guigni BA, Zhang D, Samuel VT, Silva JE, Shulman GI. Thyroid Hormone Receptor-alpha Gene Knockout Mice Are Protected from Diet-Induced Hepatic Insulin Resistance. Endocrinology. 2012;153:583–591. doi: 10.1210/en.2011-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jornayvaz FR, Samuel VT, Shulman GI. The role of muscle insulin resistance in the pathogenesis of atherogenic dyslipidemia and nonalcoholic fatty liver disease associated with the metabolic syndrome. Annu Rev Nutr. 2010b;30:273–290. doi: 10.1146/annurev.nutr.012809.104726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurczak MJ, Lee AH, Jornayvaz FR, Lee HY, Birkenfeld AL, Guigni BA, Kahn M, Samuel VT, Glimcher LH, Shulman GI. Dissociation of Inositol-requiring Enzyme (IRE1alpha)-mediated c-Jun N-terminal Kinase Activation from Hepatic Insulin Resistance in Conditional X-box-binding Protein-1 (XBP1) Knockout Mice. J Biol Chem. 2012;287:2558–2567. doi: 10.1074/jbc.M111.316760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantartzis K, Peter A, Machicao F, Machann J, Wagner S, Konigsrainer I, Konigsrainer A, Schick F, Fritsche A, Haring HU, et al. Dissociation between fatty liver and insulin resistance in humans carrying a variant of the patatin-like phospholipase 3 gene. Diabetes. 2009;58:2616–2623. doi: 10.2337/db09-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Fillmore JJ, Chen Y, Yu C, Moore IK, Pypaert M, Lutz EP, Kako Y, Velez-Carrasco W, Goldberg IJ, et al. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc Natl Acad Sci U S A. 2001a;98:7522–7527. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Fillmore JJ, Gavrilova O, Chao L, Higashimori T, Choi H, Kim HJ, Yu C, Chen Y, Qu X, et al. Differential effects of rosiglitazone on skeletal muscle and liver insulin resistance in A-ZIP/F-1 fatless mice. Diabetes. 2003;52:1311–1318. doi: 10.2337/diabetes.52.6.1311. [DOI] [PubMed] [Google Scholar]
- Kim JK, Gavrilova O, Chen Y, Reitman ML, Shulman GI. Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J Biol Chem. 2000a;275:8456–8460. doi: 10.1074/jbc.275.12.8456. [DOI] [PubMed] [Google Scholar]
- Kim JK, Michael MD, Previs SF, Peroni OD, Mauvais-Jarvis F, Neschen S, Kahn BB, Kahn CR, Shulman GI. Redistribution of substrates to adipose tissue promotes obesity in mice with selective insulin resistance in muscle. J Clin Invest. 2000b;105:1791–1797. doi: 10.1172/JCI8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Zisman A, Fillmore JJ, Peroni OD, Kotani K, Perret P, Zong H, Dong J, Kahn CR, Kahn BB, et al. Glucose toxicity and the development of diabetes in mice with muscle-specific inactivation of GLUT4. J Clin Invest. 2001b;108:153–160. doi: 10.1172/JCI10294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani K, Peroni OD, Minokoshi Y, Boss O, Kahn BB. GLUT4 glucose transporter deficiency increases hepatic lipid production and peripheral lipid utilization. J Clin Invest. 2004;114:1666–1675. doi: 10.1172/JCI21341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumashiro N, Erion DM, Zhang D, Kahn M, Beddow SA, Chu X, Still CD, Gerhard GS, Han X, Dziura J, et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A. 2011;108:16381–16385. doi: 10.1073/pnas.1113359108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar MA. How obesity causes diabetes: not a tall tale. Science. 2005;307:373–375. doi: 10.1126/science.1104342. [DOI] [PubMed] [Google Scholar]
- Lee HY, Birkenfeld AL, Jornayvaz FR, Jurczak MJ, Kanda S, Popov V, Frederick DW, Zhang D, Guigni B, Bharadwaj KG, et al. Apolipoprotein CIII overexpressing mice are predisposed to diet-induced hepatic steatosis and hepatic insulin resistance. Hepatology. 2011;54:1650–1660. doi: 10.1002/hep.24571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Choi CS, Birkenfeld AL, Alves TC, Jornayvaz FR, Jurczak MJ, Zhang D, Woo DK, Shadel GS, Ladiges W, et al. Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell Metab. 2010;12:668–674. doi: 10.1016/j.cmet.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. 2011;54:2506–2514. doi: 10.1007/s00125-011-2204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liska D, Dufour S, Zern TL, Taksali S, Cali AM, Dziura J, Shulman GI, Pierpont BM, Caprio S. Interethnic differences in muscle, liver and abdominal fat partitioning in obese adolescents. PLoS One. 2007;2:e569. doi: 10.1371/journal.pone.0000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerson AB, Hundal RS, Dufour S, Lebon V, Befroy D, Cline GW, Enocksson S, Inzucchi SE, Shulman GI, Petersen KF. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes. 2002;51:797–802. doi: 10.2337/diabetes.51.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y, Glass L, Triplitt C, Wajcberg E, Mandarino LJ, DeFronzo RA. Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2002a;283:E1135–1143. doi: 10.1152/ajpendo.0327.2001. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Mahankali A, Matsuda M, Mahankali S, Hardies J, Cusi K, Mandarino LJ, DeFronzo RA. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2002b;87:2784–2791. doi: 10.1210/jcem.87.6.8567. [DOI] [PubMed] [Google Scholar]
- Monetti M, Levin MC, Watt MJ, Sajan MP, Marmor S, Hubbard BK, Stevens RD, Bain JR, Newgard CB, Farese RV, Sr, et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007;6:69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Montero-Moran G, Caviglia JM, McMahon D, Rothenberg A, Subramanian V, Xu Z, Lara-Gonzalez S, Storch J, Carman GM, Brasaemle DL. CGI-58/ABHD5 is a coenzyme A-dependent lysophosphatidic acid acyltransferase. J Lipid Res. 2010;51:709–719. doi: 10.1194/jlr.M001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morino K, Petersen KF, Sono S, Choi CS, Samuel VT, Lin A, Gallo A, Zhao H, Kashiwahi A, Goldberg IJ, et al. Regulation of mitochondrial biogenesis by lipoprotein lipase in muscle of insulin resistant offspring of parents with type 2 diabetes. Diabetes. 2012 doi: 10.2337/db11-1391. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Yonemitsu S, Erion DM, Iwasaki T, Stark R, Weismann D, Dong J, Zhang D, Jurczak MJ, Loffler MG, et al. The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab. 2009;9:252–264. doi: 10.1016/j.cmet.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neschen S, Morino K, Hammond LE, Zhang D, Liu ZX, Romanelli AJ, Cline GW, Pongratz RL, Zhang XM, Choi CS, et al. Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1 knockout mice. Cell Metab. 2005;2:55–65. doi: 10.1016/j.cmet.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem J. 2003;370:361–371. doi: 10.1042/BJ20021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perseghin G, Price TB, Petersen KF, Roden M, Cline GW, Gerow K, Rothman DL, Shulman GI. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med. 1996;335:1357–1362. doi: 10.1056/NEJM199610313351804. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Feng J, Befroy D, Dziura J, Dalla Man C, Cobelli C, Shulman GI. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc Natl Acad Sci U S A. 2006;103:18273–18277. doi: 10.1073/pnas.0608537103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Hariri A, Nelson-Williams C, Foo JN, Zhang XM, Dziura J, Lifton RP, Shulman GI. Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. N Engl J Med. 2010;362:1082–1089. doi: 10.1056/NEJMoa0907295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, Cline GW, Befroy D, Zemany L, Kahn BB, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104:12587–12594. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109:1345–1350. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay TS, Whittaker J, Siddle K. Phorbol ester-induced downregulation of protein kinase C potentiates insulin receptor tyrosine autophosphorylation: evidence for a major constitutive role in insulin receptor regulation. Biochem Soc Trans. 1990;18:494–495. doi: 10.1042/bst0180494. [DOI] [PubMed] [Google Scholar]
- Rabol R, Petersen KF, Dufour S, Flannery C, Shulman GI. Reversal of muscle insulin resistance with exercise reduces postprandial hepatic de novo lipogenesis in insulin resistant individuals. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1110105108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando RR, Young N. The stereospecific activation of protein kinase C. Biochem Biophys Res Commun. 1984;122:818–823. doi: 10.1016/s0006-291x(84)80107-2. [DOI] [PubMed] [Google Scholar]
- Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- Samuel VT, Liu ZX, Wang A, Beddow SA, Geisler JG, Kahn M, Zhang XM, Monia BP, Bhanot S, Shulman GI. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest. 2007;117:739–745. doi: 10.1172/JCI30400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage DB, Choi CS, Samuel VT, Liu ZX, Zhang D, Wang A, Zhang XM, Cline GW, Yu XX, Geisler JG, et al. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest. 2006;116:817–824. doi: 10.1172/JCI27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer JE, Lodish HF. Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell. 1994;79:427–436. doi: 10.1016/0092-8674(94)90252-6. [DOI] [PubMed] [Google Scholar]
- Schmid AI, Szendroedi J, Chmelik M, Krssak M, Moser E, Roden M. Liver ATP synthesis is lower and relates to insulin sensitivity in patients with type 2 diabetes. Diabetes Care. 2011;34:448–453. doi: 10.2337/dc10-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Haring HU. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- Sunny NE, Parks EJ, Browning JD, Burgess SC. Excessive Hepatic Mitochondrial TCA Cycle and Gluconeogenesis in Humans with Nonalcoholic Fatty Liver Disease. Cell Metab. 2011;14:804–810. doi: 10.1016/j.cmet.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S, White MF, Kahn CR. Phorbol ester-induced serine phosphorylation of the insulin receptor decreases its tyrosine kinase activity. J Biol Chem. 1988;263:3440–3447. [PubMed] [Google Scholar]
- Taubes G. Insulin resistance. Prosperity’s plague. Science. 2009;325:256–260. doi: 10.1126/science.325_256. [DOI] [PubMed] [Google Scholar]
- Tiikkainen M, Hakkinen AM, Korsheninnikova E, Nyman T, Makimattila S, Yki-Jarvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes. 2004;53:2169–2176. doi: 10.2337/diabetes.53.8.2169. [DOI] [PubMed] [Google Scholar]
- Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- Zhang D, Liu ZX, Choi CS, Tian L, Kibbey R, Dong J, Cline GW, Wood PA, Shulman GI. Mitochondrial dysfunction due to long-chain Acyl-CoA dehydrogenase deficiency causes hepatic steatosis and hepatic insulin resistance. Proc Natl Acad Sci U S A. 2007;104:17075–17080. doi: 10.1073/pnas.0707060104. [DOI] [PMC free article] [PubMed] [Google Scholar]